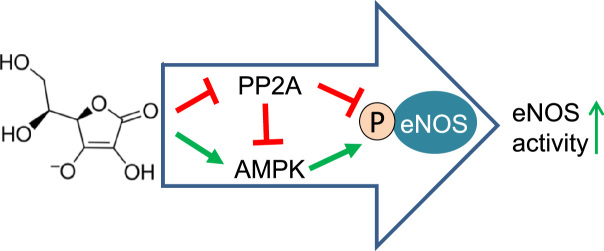
Abstract
Long-term exposure to ascorbate is known to enhance endothelial nitric oxide synthase (eNOS) activity by stabilizing the eNOS cofactor tetrahydrobiopterin (BH4). We investigated acute effects of ascorbate on eNOS function in primary (HUVEC) and immortalized human endothelial cells (EA.hy926), aiming to provide a molecular explanation for the rapid vasodilatation seen in vivo upon administration of ascorbate. Enzymatic activity of eNOS and intracellular BH4 levels were assessed by means of an arginine–citrulline conversion assay and HPLC analysis, respectively. Over a period of 4 h, ascorbate steadily increased eNOS activity, although endothelial BH4 levels remained unchanged compared to untreated control cells. Immunoblot analyses revealed that as early as 5 min after treatment ascorbate dose-dependently increased phosphorylation at eNOS-Ser1177 and concomitantly decreased phosphorylation at eNOS-Thr495, a phosphorylation pattern indicative of increased eNOS activity. By employing pharmacological inhibitors, siRNA-mediated knockdown approaches, and overexpression of the catalytic subunit of protein phosphatase 2A (PP2A), we show that this effect was at least partly owing to reduction of PP2A activity and subsequent activation of AMP-activated kinase. In this report, we unravel a novel mechanism for how ascorbate rapidly activates eNOS independent of its effects on BH4 stabilization.
Abbreviations: AMPK, AMP-activated protein kinase; BH4, tetrahydrobiopterin; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; eNOS, endothelial nitric oxide synthase; FBS, fetal bovine serum; HA-tag, hemagglutinin tag; Hepes, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; HIV, human immunodeficiency virus; HPLC, high-performance liquid chromatography; HUVEC, human umbilical vein endothelial cell; PI3K, phosphatidylinositol 3-kinases; PKC, protein kinase C; PP2A, protein phosphatase 2A; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; TLC, thin-layer chromatography
Keywords: Ascorbate, Endothelial NO synthase activity, Endothelial NO synthase phosphorylation, AMP-activated kinase, Protein phosphatase 2A, Free radicals
Graphical abstract
Highlights
► Ascorbate can enhance the activity of endothelial NO synthase (eNOS) within minutes. ► This effect occurs independent of stabilization of tetrahydrobiopterin. ► Ascorbate modulates eNOS phosphorylation in a PP2A- and AMPK-dependent manner.
Introduction
Endothelial dysfunction is a primary cause of the development of atherosclerosis, the main pathology underlying cardiovascular diseases [1]. Endothelial dysfunction is characterized by impaired endothelium-dependent vasodilation owing to decreased activity of the enzyme endothelial nitric oxide synthase (eNOS)2 or reduced bioavailability of its synthesis product, nitric oxide (NO) [1,2]. NO not only is the main vasodilatory substance released by the endothelium but also exerts important antiproliferative, antithrombotic, and anti-inflammatory functions in the vasculature [3]. The enzymatic conversion of l-arginine and molecular oxygen to NO and l-citrulline by eNOS is dependent on calcium and controlled by cofactors such as tetrahydrobiopterin (BH4) and nicotinamide adenine dinucleotide phosphate [4]. Further levels of regulation include eNOS expression and posttranslational modifications [5]. Phosphorylation is a key mechanism of eNOS regulation, allowing for rapid changes in eNOS activity in response to diverse hormonal and metabolic cues [6]. Several kinases and phosphatases control eNOS phosphorylation, including protein kinase C (PKC), Akt, AMP-activated kinase (AMPK), protein phosphatase 1 and protein phosphatase 2A (PP2A) [6]. Tight temporal and spatial regulation of NO production is essential because of the short half-life of the NO radical.
Ascorbate, the deprotonated form of ascorbic acid (vitamin C), acts as a water-soluble reducing agent and antioxidant in biological systems and is an essential micronutrient for humans. Mean plasma levels of ascorbate are between 50 and 60 μM for healthy, well-nourished, nonsmoking individuals and can be increased up to 100 μM by oral supplementation [7–11]. Low levels of plasma ascorbate are observed in several diseases linked to increased oxidative stress, such as cancer, diabetes mellitus, cataract, HIV infection, and sepsis, or in smokers [12–19].
In cultured endothelial cells ascorbate was shown to stabilize the eNOS cofactor BH4 [20,21]. Low levels of BH4 compromise eNOS function by promoting electron transfer to molecular oxygen instead of l-arginine. As a result, eNOS generates superoxide instead of NO, a situation commonly referred to as “eNOS uncoupling” [22,23]. The therapeutic potential of ascorbate for the prevention of eNOS uncoupling under conditions of oxidative stress has been investigated in several clinical studies. Whereas oral supplementation of vitamin C has proven largely unsuccessful [24], infusions rapidly improved endothelial-dependent vasodilatation in conditions such as diabetes [25,26], hypertension [27–31], hypercholesterolemia [32,33], experimental sepsis [18,34], and smoking [35–37] without affecting healthy control groups.
Whether the stabilization of BH4 can explain such rapid effects of ascorbate is unknown. We therefore investigated the molecular mechanisms underlying acute effects of ascorbate on eNOS in cultured endothelial cells.
Materials and methods
Chemicals and cell culture reagents
Dulbecco's modified Eagle's medium (DMEM) without phenol red containing 4.5 g/L glucose, endothelial growth medium EBM, EBM SingleQuots, glutamine, amphotericin B, benzylpenicillin, and streptomycin were purchased from Lonza (Belgium); HAT supplement (100 μM hypoxanthine, 0.4 μM aminopterin, and 16 μM thymidine) was from Biochrom (Germany); and trypsin was from Cambrex (Belgium). Fetal bovine serum (FBS) was obtained from Gibco via Invitrogen (UK). A23187 and okadaic acid were bought from Alexis Biochemicals (Switzerland) and l-[14C]arginine (346 mCi/mmol) and l-[1-14C]ascorbic acid (5.35 mCi/mmol) from New England Nuclear (USA). Hydrogen peroxide was purchased from Roth (Germany). Antibodies were obtained from the following companies: eNOS from BD (USA); phospho-eNOS-Ser1177, phospho-eNOS-Thr495, phospho-AMPK-Thr172, AMPK, and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody from Cell Signaling (USA); anti-tubulin from Santa Cruz (USA); and horseradish peroxidase-conjugated goat anti-mouse secondary antibody from Upstate (Millipore, Austria). All other chemicals were bought from Sigma–Aldrich (Austria). TLC plates were bought from Machery–Nagel (Austria).
Cell culture
The human endothelial cell line EA.hy926 [38] (kindly provided by Dr. C.-J.S. Edgell, University of North Carolina, Chapel Hill, NC, USA) was grown in DMEM without phenol red supplemented with 2 mM glutamine, 100 U/ml benzylpenicillin, 100 μg/ml streptomycin, HAT supplement, and 10% heat-inactivated fetal bovine serum until passage 26. For experiments, cells were seeded in six-well plates at a density of 5×105 cells/well and treated with test compounds at confluence after approximately 72 h. HUVECs were obtained from Lonza and cultivated in EBM growth medium supplemented with 10% FBS, EBM SingleQuots, 100 U/ml benzylpenicillin, 100 μg/ml streptomycin, and 1% amphotericin until passage 5. For experiments cells were seeded in gelatin-coated six-well plates at a density of 105 cells/well. Ascorbic acid was dissolved in ultrapure water. The pH was adjusted to 7.4 with NaOH, and the solution was filtered through a 0.22-μm filter and stored at −80 °C as 1000-fold stock solution. Okadaic acid, STO 609, and compound C were dissolved in dimethyl sulfoxide (DMSO) and stored at −80 °C. Final DMSO concentrations did not exceed 0.1%, unless indicated. Control cells were treated with an equal volume of solvent.
l-[14C]Arginine/l-[14C]citrulline conversion assay
The enzymatic reaction catalyzed by eNOS converts the amino acid arginine into citrulline and NO. l-[14C]Citrulline production can thus serve as a surrogate marker of NO production. The assay was performed as previously described [39].
Determination of BH4 levels
BH4 was quantified by HPLC after oxidation with iodine in acid and base as described [21], by methods modified from Fukushima and Nixon [40]. Briefly, cells were homogenized using an Ultra Turrax microhomogenizer (IKA, Stauffen, Germany) in distilled water containing 5 mM dithiothreitol and centrifuged at 13,000g for 10 min at 4 °C. To 100 μl of the supernatant, 20 μl of a 1:1 (v/v) mixture of HCl (0.1 M) and iodine (0.1 M in 0.25 M KI) or NaOH (0.1 M) and iodine (0.1 M in 0.25 M KI) was added, mixed, and incubated for 60 min in the dark. HCl (20 μl of 0.1 M) was then added to the alkaline solution only, and insoluble material was removed from both incubations by centrifugation (5 min, 13,000g), followed by addition of 20 μl of freshly prepared ascorbic acid (0.1 M in water) to both incubations. The mixtures were then analyzed on an Agilent 1200 HPLC System (Agilent, Vienna, Austria). Twenty microliters of the final mixture was injected onto a Nucleosil 10 SA column (Machery–Nagel) isocratically eluted with 100 mM potassium phosphate buffer, pH 3.0, at a flow rate of 1.5 ml/min, thermostated to 35 °C. Biopterin was detected by fluorescence (Jasco FP 920; Jasco, Tokyo, Japan), with excitation 350 nm and emission 440 nm and a detection limit of 1 nmol/L. The amount of tetrahydrobiopterin was calculated from the difference between the oxidation in acid and base, respectively. Values were normalized to the protein content of extracts determined by the Bradford method.
Gel electrophoresis and immunoblot analysis
Preparation of cell extracts, SDS–PAGE, immunoblot analyses, and densitometric evaluations were performed as described previously [41]. For detection of multiple proteins with similar molecular weights in one sample, two or more identical membranes were processed in parallel.
siRNA-mediated knockdown of AMPKα
HUVECs were seeded in six-well plates at a density of 0.3×106 cells/well and transfected 1 day later with 33 pmol AMPKα siRNA (Santa Cruz) or scrambled control (Invitrogen), using the OptiMEM/Oligofectamine system (Invitrogen). Seventy-two hours after transfection the cells were used for experiments. Successful knockdown of the target proteins was confirmed by Western blot analysis.
Overexpression of PP2Ac
HUVECs were seeded in six-well plates at a density of 0.3×106 cells/well and transfected 1 day later with 1 μg of an expression vector for the catalytic subunit of PP2A (pCMV-HA-PP2Ac; kindly provided by Dr. Verin, Medical College of Georgia, Atlanta, GA, USA) or empty control vector (pCMV) using Fugene HD transfection reagent (Roche Applied Science) according to the manufacturer's instructions.
Determination of hydrogen peroxide (H2O2) levels
Extracellular H2O2 levels were determined with the Amplex red assay (molecular Probes/Invitrogen) according to the manufacturer's instructions. to ensure specificity of the assessed fluorescence signal, all values were corrected for the non-catalase-blockable signal.
Ascorbate uptake assay
EA.hy926 cells or HUVECs were seeded in 12-well plates at a density of 0.16×106 cells/well or 0.08×106 cells/well, respectively, and were used for experiments at confluence after approximately 72 h. The cells were washed twice with KRH buffer (20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, 1.4 mM CaCl2). Then they were incubated for the indicated time points at 37 °C with KRH buffer containing 5 mM d-glucose, 0.5 mM glutathione, and 100 μM l-[1-14C]ascorbic acid. The supernatant was aspirated and the cell layer was washed twice with ice-cold KRH buffer before the cells were treated for 30 min with 0.5 ml 0.05 N NaOH in phosphate-buffered saline. The cell lysate (350 μl) was then added to 5 ml Ultima Gold liquid scintillation fluid (PerkinElmer). The radioactivity of duplicate samples was measured in a Packard TRI-CARB 2100TR liquid scintillation analyzer after at least 1 h, to allow decay of chemiluminescence. Results were normalized to protein content of the cells as determined by the Bradford method. l-[1-14C]Ascorbic acid was dissolved in 0.1 mM acetic acid and stored in multiple aliquots at −20 °C.
Statistics
Statistical analysis was done using GraphPad Prism software version 4.03 (GraphPad Software, La Jolla, CA, USA). One-way or two-way ANOVA was used for comparison of different treatment groups and Student's t test for comparison of two groups. p values <0.05 were considered significant. In figures with bar graphs, these show means±SEM of at least three independent experiments unless stated otherwise.
Results
Rapid elevation of NO synthesis in endothelial cells by ascorbate is independent of chemical stabilization of BH4
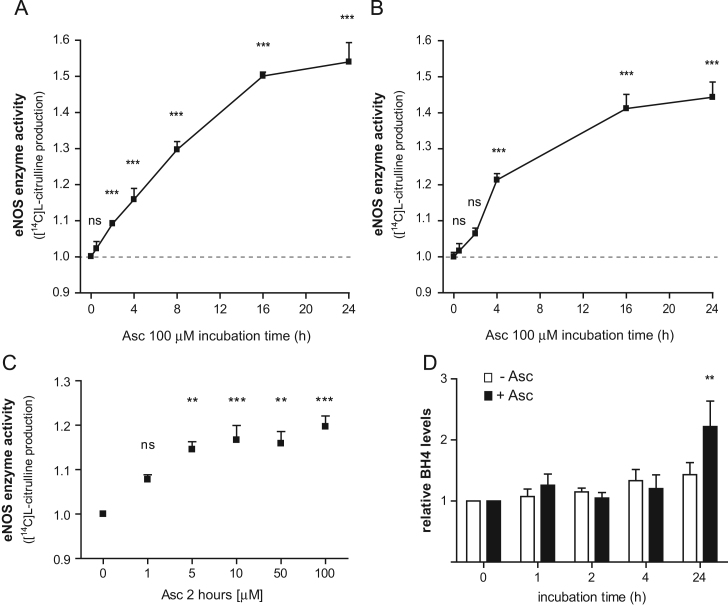
To characterize the response of endothelial cells to ascorbate, we first performed a time-course experiment and measured eNOS activity in cultured HUVECs and HUVEC-derived EA.hy926 cells (a stable endothelial cell line [38]). Cells were treated with 100 μM ascorbate for up to 24 h. In line with published data, ascorbate led to a gradual increase of eNOS enzyme activity (Fig. 1A and B) in both cell types [42]. This increase was detectable in our assay 30 min after the addition of ascorbate and reached statistical significance within 2–4 h, depending on the cell type. Experiments with various concentrations of ascorbate revealed that the rise in eNOS enzyme activity is already observable at concentrations as low as 5 μM and that the effect of ascorbate saturates at 10–100 μM (Fig. 1C). Previous studies have shown that ascorbate enhances eNOS activity after prolonged treatment of 24 h owing to stabilization of the eNOS cofactor BH4 [20,21]. We therefore investigated whether the gradual increase in enzyme activity correlates with BH4 stabilization. As expected, intracellular BH4 concentrations were increased 24 h after addition of ascorbate to EA.hy926 cells; total biopterin levels were unchanged. However, BH4 levels did not change within the first four hours when eNOS activity was already steadily increasing (Fig. 1D). Comparable results were obtained in HUVECs (data not shown).
Fig. 1.
Time-dependent effects of ascorbate on eNOS activity and endothelial BH4 levels. (A) EA.hy926 cells were treated with 100 μM ascorbate for 0.5–24 h. Then an l-[14C]arginine/l-[14C]citrulline conversion assay was performed as described. l-[14C]Citrulline production was normalized to the untreated control (⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=3). (B) HUVECs were incubated with 100 μM ascorbate for 0.5–24 h, and eNOS activity was determined as for (A) (⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=3). (C) EA.hy926 cells were treated for 2 h with the indicated concentrations of ascorbate and eNOS activity was determined as for (A) (⁎⁎p<0.01; ⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=3). For (A–C), note the start of the y axis at 0.9 to better visualize the effect of ascorbate. (D) EA.hy926 cells were treated with 100 μM ascorbate for 0–24 h. Then intracellular BH4 levels were assessed as described under Materials and methods. The values obtained (pmol BH4/μg cellular protein) for each treatment condition were normalized to the cellular BH4 level at time point 0 (⁎⁎p<0.01; mean±SEM, n=3).
Rapid activation of eNOS by ascorbate is associated with changes in eNOS phosphorylation
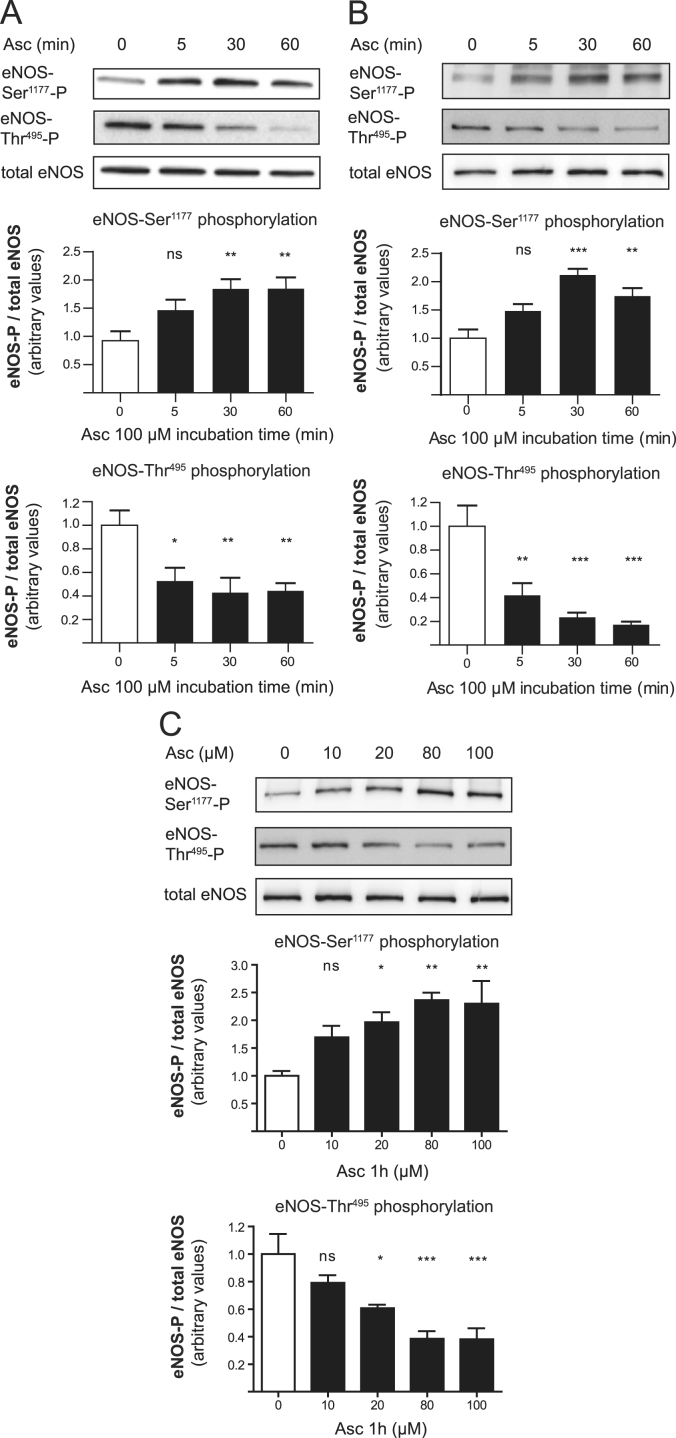
Fast changes in eNOS enzyme activity as seen in Fig. 1 could be mediated by modulation of eNOS phosphorylation. We next investigated the phosphorylation of eNOS at Ser1177 and Thr495, two key regulatory sites of eNOS activity [6]. Treatment of HUVECs and EA.hy926 cells with ascorbate resulted in eNOS dephosphorylation at Thr495 and increased phosphorylation at Ser1177 within 5 min (Fig. 2A and B). This phosphorylation pattern, which is known to increase eNOS activity, was maintained for at least 8 h and was elicited in a dose-dependent manner by ascorbate (Fig. 2C). Ascorbate still altered eNOS phosphorylation when endothelial cells were supplemented with the BH4 precursor sepiapterin, supporting the notion that regeneration or stabilization of BH4, even of small amounts, is unlikely to have a role in the observed rapid eNOS activation (Supplementary Fig. 1).
Fig. 2.
Rapid activation of eNOS by ascorbate is linked to changes in eNOS phosphorylation. (A) EA.hy926 cells and (B) HUVECs were treated with 100 μM ascorbate for 5 min to 1 h. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) eNOS protein levels. One representative blot is shown. Band intensities are normalized to tubulin and expressed as fold untreated control (⁎p<0.05; ⁎⁎p<0.01; ⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=5 for (A) and n=3 for (B)). (C) EA.hy926 cells were treated for 1 h with the indicated concentrations of ascorbate and subjected to Western blot analysis for the detection of (phospho-) eNOS levels. One representative blot is shown. Band intensities are normalized to actin and expressed as fold untreated control (⁎p<0.05; ⁎⁎p<0.01; ⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=4).
Ascorbate does not alter cellular H2O2 levels or Akt activation
Ascorbate can promote formation of H2O2 in cell culture media [43,44] and in interstitial fluids after infusion [45]. Micromolar concentrations of H2O2, in turn, activate the PI3K/Akt/eNOS signaling pathway in endothelial cells [46]. In view of the above, we tested the hypothesis that ascorbate may alter eNOS-Ser1177 phosphorylation via modulation of extracellular H2O2 levels. Co-incubation with catalase, which degrades H2O2, did not abrogate the stimulatory effect of ascorbate on eNOS activity in EA.hy926 cells (Supplementary Fig. 2A). Moreover, extracellular H2O2 levels produced by ascorbate in the medium were lower than 250 nM under our experimental conditions and therefore unlikely to play a role in the observed eNOS activation (Supplementary Fig. 2B). The phosphorylation status of Akt at Ser473, indicative of PI3K/Akt activity, was not altered by ascorbate and, consequently, inhibition of PI3K with wortmannin failed to overcome the changes in eNOS phosphorylation elicited by ascorbate (Supplementary Fig. 2C). All these data render extracellular H2O2 and the PI3K/Akt pathway unlikely mediators of the altered eNOS phosphorylation in response to ascorbate.
Modulation of eNOS phosphorylation seems to be dependent on cellular uptake of ascorbate
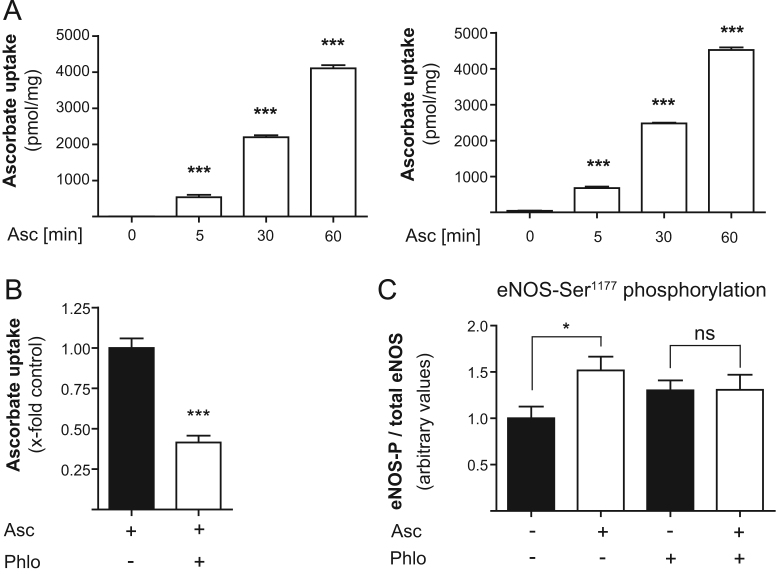
Next we addressed the question whether our observations are dependent on a rise in intracellular ascorbate levels. Assessing the cellular uptake of ascorbate revealed that ascorbate rapidly entered endothelial cells, with significant amounts detectable already after 5 min. Intracellular ascorbate levels increased linearly over time. Values ranged thereby from ∼500 pmol/mg cellular protein after 5 min up to ∼4 nmol/mg after 1 h with no significant difference between EA.hy926 cells and HUVECs (Fig. 3A). Moreover, administration of phloretin, used to interfere with the sodium-dependent endothelial ascorbate transporter (SVCT2; SLC23A2) [47,48], reduced the ascorbate uptake by 59% and abolished the altered eNOS phosphorylation seen in control cells upon ascorbate treatment (Fig. 3B and C). Experiments performed with ouabain, another reported ascorbate transport inhibitor, yielded comparable results (data not shown). These data indicate that ascorbate is rapidly entering endothelial cells to modulate eNOS phosphorylation.
Fig. 3.
Uptake of ascorbate into cultured endothelial cells occurs within minutes and is required for modulation of eNOS phosphorylation. (A) EA.hy926 cells and HUVECs were treated with 100 μM l-[1-14C]ascorbic acid for 5 min to 1 h. Ascorbate uptake was assayed as described. The measured radioactivity was normalized to cellular protein content (⁎⁎⁎p<0.001; mean±SEM, n=4). (B) EA.hy926 cells were pretreated with 10 μM phloretin before treatment with 100 μM l-[1-14C]ascorbic acid. Then the assay was performed as for (A) (⁎⁎⁎p<0.001; mean±SEM, n=3). (C) EA.hy926 cells were pretreated with 10 μM phloretin and then incubated with 100 μM ascorbate for 1 h. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) eNOS protein levels (⁎p<0.05; mean±SEM, n=3).
Ascorbate promotes phosphorylation of eNOS-Ser1177 via activation of AMPK-Thr172
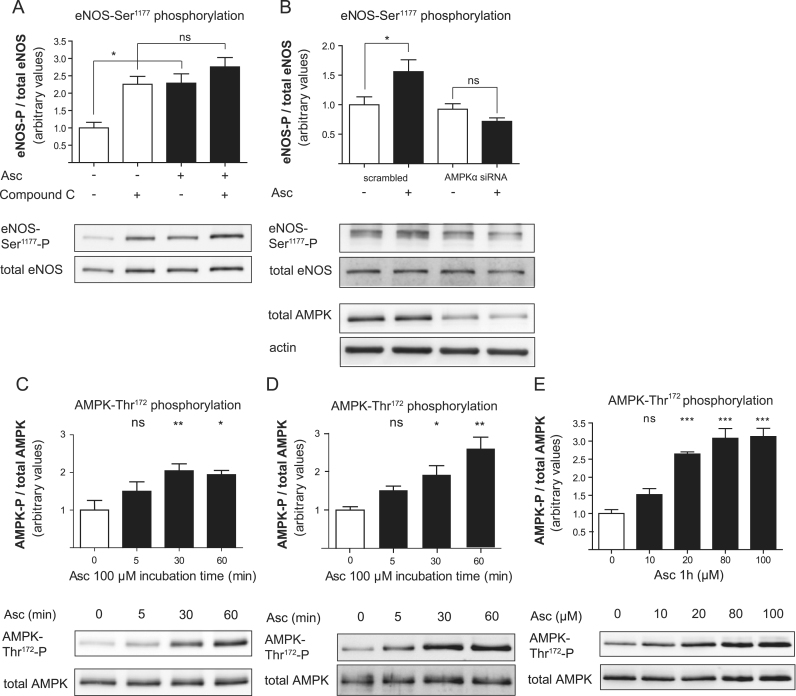
AMPK can phosphorylate eNOS at Ser1177 [49]. In EA.hy926 cells treated with compound C, an AMPK inhibitor, ascorbate failed to increase phosphorylation of eNOS-Ser1177 (Fig. 4A), suggesting that AMPK may be responsible for eNOS phosphorylation in response to ascorbate. However, compound C alone already led to an unexpected elevated basal eNOS phosphorylation at Ser1177, presumably due to off-target effects or compensatory mechanisms triggered by compound C. To unambiguously corroborate involvement of AMPK in our findings we therefore additionally chose an siRNA approach. In HUVECs in which AMPK levels were successfully downregulated by transfection with a specific siRNA, ascorbate failed to elicit enhanced eNOS-Ser1177 phosphorylation (Fig. 4B). To investigate whether ascorbate activates AMPK, phosphorylation of AMPK at Thr172 was determined at various time points after treatment. Ascorbate led to a visible increase in AMPK-Thr172 phosphorylation as early as 5 min after exposure in EA.hy926 cells (Fig. 4C) and in HUVECs (Fig. 4D). Moreover, activation of AMPK by ascorbate occurred in a dose-dependent fashion (Fig. 4E). Based on these findings one can conclude that ascorbate rapidly changes AMPK and consequently eNOS phosphorylation and activity.
Fig. 4.
Ascorbate leads to increased eNOS phosphorylation via AMPK activation. (A) EA.hy926 cells were pretreated with 20 μM compound C for 30 min and then incubated with 100 μM ascorbate for 1 h. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) eNOS protein levels. One representative blot is shown. Band intensities were normalized to tubulin and expressed as fold untreated control (⁎p<0.05; ns, not significant; mean±SEM, n=3). (B) HUVECs were transfected with AMPKα siRNA or scrambled control before treatment with 100 μM ascorbate for 1 h as indicated. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) eNOS and AMPK protein. One representative blot is shown. Band intensities were normalized to actin and expressed as fold untreated control (⁎p<0.05; ns, not significant; mean±SEM, n=4). (C) EA.hy926 cells or (D) HUVECs were treated with 100 μM ascorbate for 5 min to 1 h. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) AMPK protein levels. One representative blot is shown. Band intensities were normalized to tubulin and expressed as fold untreated control (⁎p<0.05; ⁎⁎p<0.01; ns, not significant; mean±SEM, n=3). (E) EA.hy926 cells were treated for 1 h with the indicated concentrations of ascorbate and subjected to Western blot analysis for the detection of (phospho-) AMPK levels. One representative blot is shown. Band intensities were normalized to actin and expressed as fold untreated control (⁎⁎⁎p<0.001; ns, not significant; mean±SEM, n=4).
Ascorbate inhibits PP2A
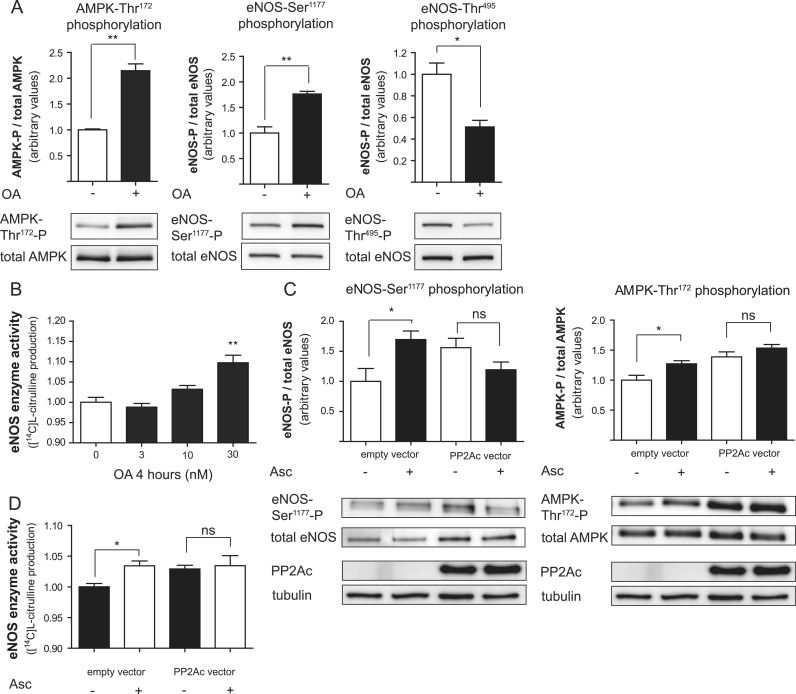
PP2A is known to dephosphorylate both AMPK-Thr172 and eNOS-Ser1177 [50,51] and can be inhibited selectively by okadaic acid (when used in concentrations below 1 μM [52]). Okadaic acid elicited a phosphorylation pattern of AMPK and eNOS strikingly similar to that of ascorbate (Fig. 5A). Moreover, treatment with okadaic acid concentration-dependently increased eNOS enzyme activity (Fig. 5B). These findings suggested that PP2A inhibition by ascorbate might explain the observations in ascorbate-treated cells. Overexpression of the HA-tagged catalytic subunit of PP2A (PP2Ac) in HUVECs completely blocked the enhancing effect of ascorbate on both eNOS-Ser1177 and AMPK-Thr172 phosphorylation (Fig. 5C) as well as on eNOS enzyme activity (Fig. 5D). Compared with untreated cells, HUVECs transfected with the empty control vector display an attenuated response to ascorbate, possibly owing to cellular stress imposed by the transfection procedure. The stimulatory effect of ascorbate on eNOS activity, however, was fully abrogated when cells overexpressed PP2Ac. This finding indicates that inhibition of PP2A activity or activation is a crucial event that mediates the rapid changes in AMPK and eNOS phosphorylation and the subsequent increase in eNOS activity induced by ascorbate in the endothelium.
Fig. 5.
Interference with protein phosphatase 2A activity is involved in the ascorbate-induced alterations in AMPK and eNOS phosphorylation. (A) EA.hy926 cells were treated with 30 nM okadaic acid (OA) for 1.5 h before total cell lysates were subjected to Western blot determination of (phospho-) AMPK and (phospho-) eNOS levels. One representative blot is shown. Band intensities were normalized to tubulin and expressed as fold untreated control (⁎p<0.05; ⁎⁎p<0.01; mean±SEM, n=3). (B) EA.hy926 cells were treated for 4 h with the indicated concentrations of OA. Then an l-[14C]arginine/l-[14C]citrulline conversion assay was performed as described. l-[14C]Citrulline production was normalized to the untreated control (⁎⁎p<0.01; mean±SEM, n=3). (C) HUVECs were transfected with empty vector or HA-tagged PP2Ac expression vector before treatment with 100 μM ascorbate for 1 h as indicated. Western blot and subsequent densitometric analyses were performed to detect and quantify (phospho-) eNOS and (phospho-) AMPK protein. One representative blot is shown. Band intensities were normalized to tubulin and expressed as fold untreated control (⁎p<0.05; ns, not significant; mean±SEM, n=3). Successful transfection was ensured by a positive signal in the HA immunoblot. (D) HUVECs were transfected with empty vector or HA-tagged PP2Ac expression vector before treatment with 100 μM ascorbate for 4 h as indicated. Then an l-[14C]arginine/l-[14C]citrulline conversion assay was performed as described. l-[14C]Citrulline production was normalized to the untreated control (⁎p<0.05; ns, not significant; mean±SEM, n=3). Note the start of the y axis at 0.9 to better visualize the small but significant effect of ascorbate in the vector-transfected control cells.
Discussion
Our study reveals a novel mechanism by which ascorbate rapidly enhances eNOS activity in cultured endothelial cells through alterations in eNOS phosphorylation. These alterations are dependent on PP2A and AMPK and clearly precede increases in endothelial BH4 levels. The extent of the ascorbate-triggered changes is in a range comparable with that of known eNOS activators (see Supplementary Fig. 3).
Pioneering work by Heller and Huang showed about a decade ago that long-term ascorbate exposure of endothelial cells in culture (for 24 h) enhances eNOS activity because of chemical stabilization of the essential eNOS cofactor BH4 [20,21]. However, the question whether this mechanism can also explain rapid increases in eNOS activity observed in cell culture experiments or improved endothelial-dependent vasodilatation upon infusion of ascorbate has so far escaped attention. Here we show that in response to elevated intracellular ascorbate, eNOS is rapidly phosphorylated at eNOS-Ser1177 in cultured endothelial cells. Using pharmacological inhibitors and knockdown approaches we identified AMPK as the responsible upstream kinase and ruled out participation of the PI3K/Akt pathway. Both events were blocked successfully upon overexpression of the phosphatase PP2A, suggesting that a decrease in PP2A activity may underlie these phenomena. Consequently, pharmacological inhibition of PP2A by okadaic acid induced a strikingly similar phosphorylation pattern. Given that AMPK and eNOS are dephosphorylation targets of PP2A, our findings imply that ascorbate mediates its effects on the endothelium at least partially by reducing PP2A activity or activation. This hypothesis is supported by two recent studies in which ascorbate improved endothelial barrier function in an experimental model of sepsis through inhibition of PP2A activation [53] and increased NO production [54]. Our data could provide a molecular explanation for the ascorbate-mediated PP2A inhibition and the elevated NO levels observed in the context of endothelial permeability and sepsis. The impact of PP2A on endothelial function and whether this protein holds potential for manipulation in a therapeutic context should not be overlooked.
As PP2A is activated by oxidative stress [53], incubation with ascorbate might interfere with PP2A activation by antagonizing increased levels of oxidative stress often observed in cell culture, especially when the ascorbate levels in the culture medium are low [55]. Interestingly, these conditions resemble the situation in patients with endothelial dysfunction and this mechanism could explain why infusions of ascorbate have no effect in patients with normal endothelial function. However, how such a rapid effect on cellular reactive oxygen species (ROS) production would be mediated on the molecular level remains unclear. One possibility would be a general antioxidant action of ascorbate by which preferential oxidation of ascorbate preserves stores of the intracellular antioxidant glutathione and thereby counteracts pro-oxidant signaling [56,57]. Nonetheless, neither did we observe reduced intracellular ROS levels in our experimental settings (100 μM ascorbate, short incubation times, and assessed by oxidized dihydrofluorescein) nor did other antioxidants (such as N-acetylcysteine, trolox) mimic the effect of ascorbate.
In addition to these acute actions, ascorbate also corrects the BH4 deficiency after longer incubation periods, thereby alleviating oxidative stress due to eNOS uncoupling [55]. Although ascorbate and other antioxidants can promote generation of H2O2 in some cell culture media [44], H2O2 is unlikely to mediate the effects observed in our system. This is because, first, the amount of H2O2 generated in the medium was in the nanomolar range and therefore 2–3 orders of magnitude below the concentrations needed to activate eNOS [46], and, second, addition of catalase, which detoxifies H2O2, to the medium did not avert the rapid increase of eNOS activity upon addition of ascorbate.
Whereas we identified the signaling pathway behind phosphorylation of eNOS-Ser1177, the mechanism mediating parallel dephosphorylation at eNOS-Thr495 remains enigmatic. Although PP2A is reported to directly dephosphorylate eNOS at Thr495, PP2A inhibition had no enhancing effect on the Thr495 phosphorylation state (as expected from a phosphatase inhibitor) of eNOS in our experiments. This finding indicates that PP2A is predominantly acting on eNOS-Ser1177 and AMPK-Thr172 rather than on eNOS-Thr495, which is line with previous reports [41,50]. We also found no evidence for modulation of PKC, a common regulator of this phosphorylation site, by ascorbate (data not shown). Although at least one study identified pharmacological means to selectively alter eNOS phosphorylation at Thr495 [41], this site is often considered to be intrinsically linked to Ser1177, mirroring its phosphorylation status reciprocally through mechanisms that are incompletely understood [58].
Summary
This study provides evidence for a novel mechanism for how ascorbate rapidly activates eNOS independent of its effect on BH4 stabilization. The enzymatic activation of eNOS is achieved by modulating activities of PP2A and AMPK, resulting in specific changes in eNOS phosphorylation at Ser1177 and Thr495. Based on our observations we propose a new model according to which activation of eNOS in response to ascorbate occurs in two phases: first, rapid changes in eNOS phosphorylation enhance eNOS activity within minutes; second, long-lasting improvements of eNOS function are achieved through chemical stabilization of BH4 after several hours. Whether these effects are uniquely observed in cells in culture or also in vivo upon ascorbate infusion warrants further investigation.
Acknowledgments
The authors thank Nina Madl and Hortenzia Beres for excellent technical assistance. This project was partly funded by the Austrian Science Fund (J2957-B11 to C.A.S., P22289 to E.R.W, and P23317 to E.H.H.) and the Hochschuljubiläumsstiftung der Stadt Wien (H-01509/2007 to E.H.H.).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2012.03.022.
Appendix A. Supplementary material
Supplementary material.
References
- 1.Chatterjee A., Black S.M., Catravas J.D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vasc. Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davignon J., Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 3.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol. Chem. 2006;387:1521–1533. doi: 10.1515/BC.2006.190. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro L.J., Cirino G., Casini A., Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J. Cardiovasc. Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt C.A., Dirsch V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Mount P.F., Kemp B.E., Power D.A. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J. Mol. Cell. Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Woollard K.J., Loryman C.J., Meredith E., Bevan R., Shaw J.A., Lunec J., Griffiths H.R. Effects of oral vitamin C on monocyte: endothelial cell adhesion in healthy subjects. Biochem. Biophys. Res. Commun. 2002;294:1161–1168. doi: 10.1016/S0006-291X(02)00603-4. [DOI] [PubMed] [Google Scholar]
- 8.Astley S.B., Elliott R.M., Archer D.B., Southon S. Evidence that dietary supplementation with carotenoids and carotenoid-rich foods modulates the DNA damage: repair balance in human lymphocytes. Br. J. Nutr. 2004;91:63–72. doi: 10.1079/bjn20031001. [DOI] [PubMed] [Google Scholar]
- 9.Choi S.W., Benzie I.F., Collins A.R., Hannigan B.M., Strain J.J. Vitamins C and E: acute interactive effects on biomarkers of antioxidant defense and oxidative stress. Mutat. Res. 2004;551:109–117. doi: 10.1016/j.mrfmmm.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Brennan L.A., Morris G.M., Wasson G.R., Hannigan B.M., Barnett Y.A. The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. Br. J. Nutr. 2000;84:195–202. doi: 10.1017/s0007114500001422. [DOI] [PubMed] [Google Scholar]
- 11.Szeto Y.T., Kwok T.C., Benzie I.F. Effects of a long-term vegetarian diet on biomarkers of antioxidant status and cardiovascular disease risk. Nutrition. 2004;20:863–866. doi: 10.1016/j.nut.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Padayatty S.J., Levine M. Vitamin C and coronary microcirculation. Circulation. 2001;103:E117. doi: 10.1161/01.cir.103.23.e117. [DOI] [PubMed] [Google Scholar]
- 13.Mandl J., Szarka A., Banhegyi G. Vitamin C: update on physiology and pharmacology. Br. J. Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor A., Hobbs M. 2001 Assessment of nutritional influences on risk for cataract. Nutrition. 2001;17:845–857. doi: 10.1016/s0899-9007(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 15.Bodansky O., Wroblewski F., Markardt B. Concentrations of ascorbic acid in plasma and white blood cells of patients with cancer and noncancerous chronic disease. Cancer. 1952;5:678–684. doi: 10.1002/1097-0142(195207)5:4<678::aid-cncr2820050404>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. Free Radical Biol. Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 17.Favier A., Sappey C., Leclerc P., Faure P., Micoud M. Antioxidant status and lipid peroxidation in patients infected with HIV. Chem. Biol. Interact. 1994;91:165–180. doi: 10.1016/0009-2797(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson J.X. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35:5–13. doi: 10.1002/biof.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galley H.F., Davies M.J., Webster N.R. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radical Biol. Med. 1996;20:139–143. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 20.Huang A., Vita J.A., Venema R.C., Keaney J.F., Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 21.Heller R., Unbehaun A., Schellenberg B., Mayer B., Werner-Felmayer G., Werner E.R. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 22.Guzik T.J., Harrison D.G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discovery Today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bendall J.K., Alp N.J., Warrick N., Cai S., Adlam D., Rockett K., Yokoyama M., Kawashima S., Channon K.M. Stoichiometric relationships between endothelial tetrahydrobiopterin, endothelial NO synthase (eNOS) activity, and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTP cyclohydrolase 1 and eNOS overexpression. Circ. Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 24.Stanner S.A., Hughes J., Kelly C.N., Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7:407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 25.Timimi F.K., Ting H.H., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998;31:552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 26.Heitzer T., Finckh B., Albers S., Krohn K., Kohlschutter A., Meinertz T. Beneficial effects of α-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radical Biol. Med. 2001;31:53–61. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A., Giugliano D., Quatraro A., Lefebvre P.J. Anti-oxidants show an anti-hypertensive effect in diabetic and hypertensive subjects. Clin. Sci. (London) 1991;81:739–742. doi: 10.1042/cs0810739. [DOI] [PubMed] [Google Scholar]
- 28.Taddei S., Virdis A., Ghiadoni L., Magagna A., Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 29.Hirooka Y., Eshima K., Setoguchi S., Kishi T., Egashira K., Takeshita A. Vitamin C improves attenuated angiotensin II-induced endothelium-dependent vasodilation in human forearm vessels. Hypertens. Res. 2003;26:953–959. doi: 10.1291/hypres.26.953. [DOI] [PubMed] [Google Scholar]
- 30.Natali A., Sironi A.M., Toschi E., Camastra S., Sanna G., Perissinotto A., Taddei S., Ferrannini E. Effect of vitamin C on forearm blood flow and glucose metabolism in essential hypertension. Arterioscler. Thromb. Vasc. Biol. 2000;20:2401–2406. doi: 10.1161/01.atv.20.11.2401. [DOI] [PubMed] [Google Scholar]
- 31.Solzbach U., Hornig B., Jeserich M., Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.cir.96.5.1513. [DOI] [PubMed] [Google Scholar]
- 32.Perticone F., Ceravolo R., Maio R., Cloro C., Candigliota M., Scozzafava A., Mongiardo A., Mastroroberto P., Chello M., Mattioli P.L. Effects of atorvastatin and vitamin C on endothelial function of hypercholesterolemic patients. Atherosclerosis. 2000;152:511–518. doi: 10.1016/s0021-9150(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 33.Ting H.H., Timimi F.K., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 34.Tyml K., Li F., Wilson J.X. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit. Care Med. 2008;36:2355–2362. doi: 10.1097/CCM.0b013e31818024f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindler T.H., Magosaki N., Jeserich M., Olschewski M., Nitzsche E., Holubarsch C., Solzbach U., Just H. Effect of ascorbic acid on endothelial dysfunction of epicardial coronary arteries in chronic smokers assessed by cold pressor testing. Cardiology. 2000;94:239–246. doi: 10.1159/000047324. [DOI] [PubMed] [Google Scholar]
- 36.Hirai N., Kawano H., Hirashima O., Motoyama T., Moriyama Y., Sakamoto T., Kugiyama K., Ogawa H., Nakao K., Yasue H. Insulin resistance and endothelial dysfunction in smokers: effects of vitamin C. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1172–H1178. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- 37.Heitzer T., Just H., Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 38.Edgell C.J., McDonald C.C., Graham J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt C.A., Handler N., Heiss E.H., Erker T., Dirsch V.M. No evidence for modulation of endothelial nitric oxide synthase by the olive oil polyphenol hydroxytyrosol in human endothelial cells. Atherosclerosis. 2007;195:e58–e64. doi: 10.1016/j.atherosclerosis.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima T., Nixon J.C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 1980;102:176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt C.A., Heiss E.H., Aristei Y., Severin T., Dirsch V.M. Norfuraneol dephosphorylates eNOS at threonine 495 and enhances eNOS activity in human endothelial cells. Cardiovasc. Res. 2009;81:750–757. doi: 10.1093/cvr/cvn326. [DOI] [PubMed] [Google Scholar]
- 42.Heller R., Munscher-Paulig F., Grabner R., Till U. l-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J. Biol. Chem. 1999;274:8254–8260. doi: 10.1074/jbc.274.12.8254. [DOI] [PubMed] [Google Scholar]
- 43.Garry A., Edwards D.H., Fallis I.F., Jenkins R.L., Griffith T.M. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc. Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halliwell B., Clement M.V., Ramalingam J., Long L.H. Hydrogen peroxide: ubiquitous in cell culture and in vivo? IUBMB Life. 2000;50:251–257. doi: 10.1080/713803727. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E., Choyke P.L., Pooput C., Kirk K.L., Buettner G.R., Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas S.R., Chen K., Keaney J.F., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 47.May J.M. The SLC23 family of ascorbate transporters: ensuring that you get and keep your daily dose of vitamin C. Br. J. Pharmacol. 2011;164:1793–1801. doi: 10.1111/j.1476-5381.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlachodimitropoulou E., Sharp P.A., Naftalin R.J. Quercetin–iron chelates are transported via glucose transporters. Free Radical Biol. Med. 2011;50:934–944. doi: 10.1016/j.freeradbiomed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z.P., Mitchelhill K.I., Michell B.J., Stapleton D., Rodriguez-Crespo I., Witters L.A., Power D.A., Ortiz de Montellano P.R., Kemp B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 50.Michell B.J., Chen Z., Tiganis T., Stapleton D., Katsis F., Power D.A., Sim A.T., Kemp B.E. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J. Biol. Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 51.Witczak C.A., Sharoff C.G., Goodyear L.J. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell. Mol. Life Sci. 2008;65:3737–3755. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millward T.A., Zolnierowicz S., Hemmings B.A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 53.Han M., Pendem S., Teh S.L., Sukumaran D.K., Wu F., Wilson J.X. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radical Biol. Med. 2010;48:128–135. doi: 10.1016/j.freeradbiomed.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.May J.M., Qu Z.C. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem. Biophys. Res. Commun. 2011;404:701–705. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith A.R., Visioli F., Hagen T.M. Vitamin C matters: increased oxidative stress in cultured human aortic endothelial cells without supplemental ascorbic acid. FASEB J. 2002;16:1102–1104. doi: 10.1096/fj.01-0825fje. [DOI] [PubMed] [Google Scholar]
- 56.May J.M., Qu Z.C., Juliao S., Cobb C.E. Ascorbic acid decreases oxidant stress in endothelial cells caused by the nitroxide tempol. Free Radical Res. 2005;39:195–202. doi: 10.1080/10715760400019661. [DOI] [PubMed] [Google Scholar]
- 57.May J.M., Qu Z.C., Li X. Ascorbic acid blunts oxidant stress due to menadione in endothelial cells. Arch. Biochem. Biophys. 2003;411:136–144. doi: 10.1016/s0003-9861(02)00715-4. [DOI] [PubMed] [Google Scholar]
- 58.Fleming I., Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.