Abstract
Skeletal muscle is a dynamic composite of proteins that responds to both internal and external cues to facilitate muscle adaptation. In cases of disease or altered use, these messages can be distorted resulting in myopathic conditions such as fibrosis. In this work, we describe a mild and progressive fibrotic adaptation in skeletal muscle lacking the cytoskeletal intermediate filament protein desmin. Muscles lacking desmin become progressively stiffer, accumulate increased collagen, and increase expression of genes involved in extracellular matrix turnover. Additionally, in the absence of desmin, skeletal muscle is in an increased state of inflammation and regeneration as indicated by increased centrally nucleated fibers, elevated inflammation and regeneration related gene expression, and increased numbers of inflammatory cells. These data suggest a potential link between increased cellular damage and the development of fibrosis in muscles lacking the cytoskeletal support of the desmin filament network.
Keywords: extracellular matrix, adaptation
skeletal muscle is a composite tissue composed of multinucleated muscle cells embedded in a connective tissue matrix. The interaction between active muscle force generating components and passive extracellular matrix (ECM) force transmitting components provides skeletal muscle with its characteristic biomechanical properties, which enable it to produce work and resist extension. The amount of ECM is surprisingly consistent across individuals and across muscles, presumably due to the physiological balance between the passive and active mechanical properties of a muscle. However, in cases of disease (e.g., muscular dystrophies and diabetes) or altered use (e.g., immobilization, aging, exercise, and denervation), the amount of ECM can increase dramatically relative to muscle fibers, resulting in a condition known as fibrosis (11, 12, 15, 19, 35). Tissue fibrosis represents a tremendous clinical problem, not only in muscles where it restricts range of motion and may be related to the process of contracture formation but also other tissues such as liver, kidney, lung, and heart (22), highlighting the diversity and relevance of this adaptation.
Skeletal muscle cells are very mechanosensitive, as are the other cells that reside in the matrix (10, 13). One putative pathway for the transmission of forces between the intracellular environment and the extracellular scaffolding is the intermediate filament protein desmin (21). The desmin filament network comprises a significant portion of the cytoskeleton in skeletal muscle fibers and thus is likely to play a role in intracellular stabilization and organization. However, desmin also links to the ECM at costameres, suggesting it could have some influence on the organization and regulation of the matrix as well. Functional alterations to the active properties of desmin knockout (des−/−) muscle have been well documented (24, 25, 34), but much less is known about how the passive properties of muscle are affected, despite the fact that maintenance of proper passive extensibility is equally vital to skeletal muscle function (14). This is easily appreciated in cases of muscle fibrosis, which can leave patients debilitated.
In this study, we demonstrate alterations in the passive mechanical properties of des−/− muscle at both the cellular level and in the ECM. Using a combination of protein assay, immunohistochemistry, and gene expression, we demonstrate a progressive fibrosis in response to the absence of desmin. Then, based on increases in inflammatory and regenerative markers, we suggest a potential causal relationship among loss of force transmission, cellular damage, and the proliferative response of the ECM cellular constituents. This model may prove particularly useful in understanding mechanisms of fibrosis in muscle since the mechanical and structural changes develop relatively slowly and uniformly along and across the muscle, in contrast to other methods of creating fibrosis that produce relatively complicated spatial injury patterns and often disrupt the microvasculature, thus complicating data interpretation.
METHODS
Experimental design.
Experiments were performed on muscles from male wild-type (wt) 129/Sv (Taconic Farms, Germantown, NY) and desmin knockout (des−/−) 129/Sv (28) mice. Mice were age matched in three age groups: “neonatal” (10–20 days), “young” (7–9 wk), and “adult” (12–14 mo). Animals were anesthetized with 2% isoflurane at 2 l/min and then euthanized by cervical dislocation. Hindlimbs were transected proximal to the knee and placed in a mammalian Ringer solution composed of the following (in mM): NaCl (137), KCl (5), NaH2PO4 (1), Na HCO3 (24), CaCl2 (2), MgSO4 (1), and glucose (11) with 10 mg/l curare for dissection. Experiments were performed on extensor digitorum longus (EDL), tibialis anterior (TA), gastrocnemius, quadriceps, or a combination of these muscles. All of these muscles are composed of ≥90% fast fibers (7), and stress production deficiency in des−/− muscle is consistent between the EDL and TA (unpublished data) suggesting the des−/− phenotype is not unique to a single muscle. Care was taken to maximize tissue usage and experimental data available from each mouse. All procedures were performed in accordance with the National Institutes of Health's Guide for the Use and Care of Laboratory Animals and were approved by the University of California and Department of Veterans Affairs Committees on the Use of Animal Subjects in Research.
Muscle mechanical testing.
Passive mechanical testing was performed on single fibers and fiber bundles from the fifth toe of the EDL muscle. Samples were isolated from at least five muscles per group (2 genotypes × 3 ages) with approximately three fibers and three bundles per muscle subjected to testing (n = 97 total fibers; n = 84 total bundles). After dissection, muscles were stored in a glycerinated storage solution overnight composed of the following (in mM): K-propionate (170), K3EGTA (5), MgCl2 (5.3), imidazole (10), Na2ATP (21.2), NaN3 (1), glutathione (2.5), 50 μM leupeptin, and 50% (vol/vol) glycerol. Before mechanical testing, muscles were removed from storage solution and transferred to a relaxing solution at pCa 8.0 and pH 7.1 consisting of the following (in mM): imidazole (59.4), KCH4O3S (86), Ca(KCH4O3S)2 (0.13), Mg(KCH4O3S)2 (10.8), K3EGTA (5.5), KH2PO4 (1), Na2ATP (5.1), and 50.0 μM of the protease inhibitor leupeptin.
Single fiber or fiber bundle segments (2–3 mm in length) were carefully dissected and mounted in a custom chamber. They were secured using 10–0 monofilament nylon suture on one end to a force transducer (Aurora Scientific 405A; Aurora, ON, Canada) and on the other end to a titanium wire rigidly attached to a rotational bearing (Newport MT-RS; Irvine, CA). Sarcomere length provided an objective assessment of internal specimen strain and was measured by transilluminating the specimen with a low power laser diode. Segments displaying obvious abnormalities were not used.
Single fibers were brought to slack length (L0), which was determined by the knot-to-knot length at which passive tension was just measurable above the noise level of the force transducer (∼1 mN). Sample length and diameter were measured with a cross-hair reticule mounted on a dissecting microscope and micromanipulators on an x-y mobile stage. The fiber was deformed incrementally in 10% L0 steps at a strain rate of 20 FL/s and then allowed to stress relax for 3 min. Fibers were strained either to failure or to 100% L0, whichever was reached first. Fiber Cauchy stress was determined by dividing the tension by the fiber cross-sectional area at the end of each stretch. Fiber cross-sectional area was calculated based on the measured initial fiber diameter and the assumption that the fiber was cylindrical and isovolumic (38). Fiber bundles were tested in the same manner as single fibers and consisted of 10–20 fibers and their constitutive ECM.
Passive mechanical data were acquired via customized LabView software throughout the 3-min stress relaxation and analyzed in Matlab. Stress was plotted against sarcomere length at each stretch and fit with quadratic regression (r2 > 0.98 for all samples). Tangent modulus was computed as the slope of the regression line at a sarcomere length of 3.9 μm (the end of the predicted length-tension curve for mouse muscle).
ECM material properties were determined using the theory of composites, which states that the elastic modulus of a composite material is the sum of the elastic modulus of each component multiplied by its volume fraction (18). This relationship is given mathematically by the rule of mixtures, stated below, and has been used previously to define the contribution of the ECM to muscle passive mechanics (33).
| (1) |
The composite elastic modulus is given by Ec, where Em and Ef are the matrix and fiber elastic moduli and Vm and Vf are the matrix and fiber volume fractions, respectively.
Microarray processing.
To gain insights into the biological processes associated with fibrosis, microarray analysis was performed on the TA muscle from wt and des−/− mice. RNA was extracted from whole muscles (∼30 mg tissue) using a combination of standard TRIzol (Invitrogen, Carlsbad, CA) and RNeasy (Qiagen, Valencia, CA) protocols. Muscles were homogenized for 60 s in a rotor-stator homogenizer on ice in 0.5 ml TRIzol, 0.1 ml of chloroform was then added, and the sample was vigorously vortexed for 15 s followed by centrifugation. The supernatant was removed and combined with an equal volume of 70% ethanol, and the mixture was filtered through the RNeasy spin column. The column was then washed, incubated with RNAse-free DNAse (Qiagen), washed again, and eluted as described in the manufacturer's protocol. RNA concentration was determined by the absorbance at 260 nm, and the 260-to-280 nm absorbance ratio was calculated to define RNA purity.
Individual Affymetrix microarrays (“GeneChip” Mouse Genome 430A 2.0 Array; Affymetrix, Santa Clara, CA) were used for each muscle. A total of 19 chips were used with 4–5 chips per group (2 genotypes × 2 ages). RNA processing for the GeneChip, including stringent quality control measures, was performed by the Gene Chip Core at the Department of Veterans Affairs San Diego Health Care System (San Diego, CA).
Genespring software (SiliconGenetics, Redwood City, CA) was used to identify genes that were differentially expressed as a function of genotype (wt or des−/−) at two ages (young and adult). Three independent probe set algorithms were used for background subtraction and normalization (MAS5, RMA, and GCRMA), and each feature was normalized per chip and per gene as previously described (39). Normalized expressions of identified genes, excluding putative genes and expressed sequence tags, were subjected to two-way ANOVA with a significance level (α) set to 0.05 and a Benjamini and Hochberg false discovery rate multiple testing correction for present features. In accordance with Minimum Information About a Microarray Experiment (MIAME) standards, microarray data and annotations have been deposited via Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/) and can be accessed with accession number GSE34388.
To investigate the biological context of transcriptional changes, the role of significant genes in various muscle pathways was investigated. Gene ontology (GO) analyses were performed using a web-based gene set analysis toolkit (WebGestalt; bioinfo.vanderbilt.edu/webgestalt/). In this analysis, a P value is generated for each pathway based on hypergeometric comparison of the number of genes present in that list to the number of genes expected to be present based on the size of the list.
Quantitative real-time PCR.
In addition to microarray processing, isolated RNA samples were subjected to quantitative real-time PCR (qPCR) to provide validation of GeneChip expression values. After RNA was extracted from the muscle (as described for microarray processing) and diluted 1:5 with DNase/RNase free water, 1 μl of each sample was reverse transcribed using standard protocols (Superscript III; Invitrogen). cDNA was amplified with the Eppendorf MasterCycler GradientS (Hamburg, Germany) with primers specific to the genes of interest. All primers were tested for cross-reactivity with other transcripts using nBLAST and Oligo (version 6.6; Molecular Biology Insights, Cascade, CO). All samples were run at least in triplicate on a 96-well plate. Each well contained 10-μl volume made up of the KAPA SYBR FAST Master Mix (2×) Universal (KAPA Biosystems) and forward and reverse primers.
Amplification conditions were as follows: an initial hold at 95°C for 2 min was followed by 40 cycles of denaturing at 95°C for 15 s, followed by annealing/extension at 68°C for 40 s. The success of each reaction was deduced based on the observation of a single reaction product on an agarose gel and a single peak on the DNA melting temperature curve determined at the end of the reaction. To express qPCR results, the standard curve method was used with the “cycles to threshold” value representing the PCR cycle number at which the SYBR green signal was increased above the threshold. Expression of each gene was normalized to its mean value.
Hydroxyproline assay for collagen content.
Total collagen content was determined in the TA muscle using a colorimetric assay for hydroxyproline content (16). Samples were taken from ≥5 muscles per group (n = 45 total muscles). TA muscles were dissected from the hindlimb and immediately flash frozen in liquid nitrogen cooled isopentane. Portions of the muscle that contained no internal tendon were isolated and hydrolyzed in 6 N HCl at 110°C for 18 h. After hydrolysis, samples were neutralized and treated with a chloramine T solution for 20 min at room temperature followed by a solution of p-diaminobenzaldehyde for 30 min at 60°C. Sample absorbance was read from three aliquots of each sample at 550 nm. Hydroxyproline content was converted to collagen content using the extinction coefficient for hydroxyproline and dividing by the number of hydroxyproline residues in a molecule of collagen.
Immunohistochemistry.
Cross sections (10 μm thick) from flash-frozen muscle were cut from the midbelly of the TA muscle on a cryostat at −20°C (Microm HM500, Waldorf, Germany). Serial sections were stained with hematoxylin and eosin (H&E) to view overall fiber appearance (n = 16 muscles). Centrally nucleated fibers were identified and manually counted as a fraction of all fibers in the cross section by an observer blinded to experimental identity. To visualize ECM components in the muscle, serial sections were immuonolabeled with primary antibodies to laminin (rabbit polyclonal; Sigma, St. Louis, MO) and type I collagen (rabbit polyclonal; Rockland, Gilbertsville, PA). Muscle sections were treated with BSA as a blocking agent and incubated with antibodies overnight. An Alexa Fluor 594 goat anti-rabbit immunoglobulin G (Invitrogen) secondary antibody was used for visualization.
Determination of fiber size was performed on laminin-stained sections (n = 16 muscles) as previously described using a custom macro in ImageJ (NIH, Bethesda, MD; Ref. 29). Filtering criteria required that fibers have an area >50 μm2 but <5,600 μm2 to eliminate neurovascular structures, and optically fused fibers and fibers touching the edge of the field were excluded. Additionally, circularity was required to be between 0.3 and 1.0 to prevent inclusion of obliquely sectioned fibers. Area fraction of ECM was also determined from laminin stained sections (n = 20 total muscles). A background subtraction algorithm was applied to each image to normalize intensity values and remove any effects of background noise. Fiber centers, considered image background, were set to a value of 0 in the RGB channel, and image intensity was rescaled from 0 to 255. A threshold was then applied to each image and the relative number of white (stained) pixels vs. black (unstained) pixels was computed using ImageJ. Image processing settings were standardized across all sections, and computation of stained area fraction was automated eliminating any user error or bias. Systematic sampling across the section (3 fields per section) was performed in accordance with established stereological principles (40). Before analysis, each image was inspected, and areas with sectioning artifacts, large blood vessels, or poor staining quality were omitted from quantification.
Flow cytometry.
TA, gastrocnemius, and quadriceps muscle groups were dissected from both hindlimbs of wt and des−/− mice. Muscles were incubated in a digestive solution consisting of the following: collagenase type I (2.67 g/l), dispase II (75 g/l), penicillin (50 units/ml), and streptomycin (50 units/ml) in DMEM. Cells were then strained through a 70-μm nylon filter, centrifuged, and resuspended in a buffer solution consisting of: EDTA (1 mM) and normal goat serum (2.5%) in sterile PBS. Cell preparations were incubated with primary antibodies on ice for 20 min. Compensation beads (BD CompBead Plus) were used as compensation controls for the fluorophores, and fluorescence-minus-one controls were generated by combining cells from each sample population with appropriate antibodies. After incubation, samples and controls were centrifuged, the supernatant was removed, and the pellet was resuspended in buffer solution. Two of the antibodies used (ER-TR7 and α-smooth muscle actin) were specific for intracellular structures, and thus cells used for this gating were fixed and permeabilized to allow the antibodies access to the cell interior. Cells were fixed by suspending them in a 70% ethanol solution on ice for 20 min and permeabilized by resuspending them for an additional 10 min in a blocking solution consisting of the following: BSA (2%), FBS (5%), Triton X-100 (0.2%), and sodium azide (0.1%).
Analysis was performed on a Special Order LSRFortessa (BD Biosciences, San Jose) with four lasers (405 nm, 50 mW; 488 nm, 50 mW; 561 nm, 100 mW; and 640 nm 50 mW). Fluors were detected at the following wavelengths: FITC, 497–523 nm; PE, 575–589 nm; Alexa Fluor 700, 710–750 nm; eFluor 450, 425–475 nm; PerCP, 675–715 nm; and APC, 663–677 nm. Cytometer performance and laser delay settings were verified daily with the Cytometer Setup and Tracking system (CST; BD Biosciences, San Jose) according to the manufacturer's directions. Data were collected using FacsDiVa (BD Biosciences, San Jose, CA) software version 6.2 and analyzed with FlowJo 9.3.1. All gating was performed on fluorescence-minus-one controls with a 1% error rate.
Data processing and statistical analysis.
For comparisons across genotype and age, two-way ANOVA was used with a significance level (α) set to 0.05 and with a Tukey multiple corrections post hoc test where appropriate. Results in the text and in tables are presented as means ± SE.
RESULTS
Desmin knockout fibers and bundles have altered material properties.
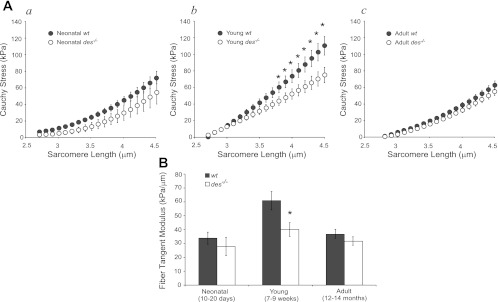
Cauchy stress of isolated single fibers computed at the end of stress relaxation was fit with quadratic regression as a function of sarcomere length. Average fits of young wt fibers resulted in significantly higher stresses than young des−/− at long sarcomere lengths (Fig. 1A). At the neonatal time point, when desmin levels first reach their mature level, wt and des−/− fiber stresses and moduli were not significantly different (P > 0.1; Fig. 1, Aa and B, neonatal). However, as the muscle matured, des−/− fibers failed to develop normal biomechanical properties and were significantly more compliant compared with wt (P < 0.05; Fig. 1, Ab and B, young). As the muscle continued to age, des−/− fibers maintained their modulus, while wt fibers became significantly more compliant (Fig. 1, Ac and B, adult).
Fig. 1.
Desmin knockout (des−/−) fibers from young muscle were more compliant compared with wild type (wt). A: passive Cauchy stress-sarcomere length quadratic curve fits for young wt (n = 17) and des−/− (n = 14) fibers. Aa: neonatal mice. Ab: young mice. Ac: adult mice. Although raw data were fit with quadratic regression, fiber stress-sarcomere length curves were nearly linear. Young des−/− fibers had significantly lower stress compared with wt at sarcomere lengths >3.7 μm as determined by ANOVA with repeated measures. B: tangent moduli calculated from curve fits at a sarcomere length of 3.9 μm for des−/− and wt fibers in 3 age groups. Young des−/− fibers were significantly more compliant compared with wt. However, there was no difference between neonatal or adult fiber stiffness values with genotype. *P < 0.05.
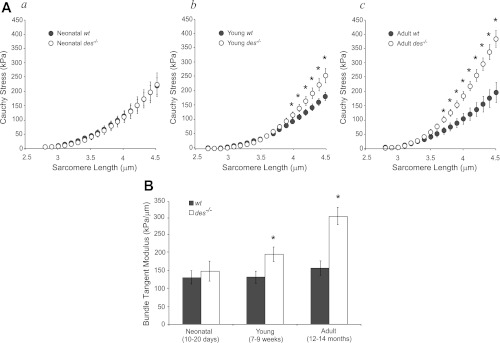
In contrast to the finding that young and adult des−/− fibers were more compliant or equally compliant as wt, young and adult des−/− bundles had higher stresses (Fig. 2, Ab and Ac) and were stiffer compared with wt bundles (P < 0.05; Fig. 2B). This difference in biomechanical properties occurred in spite of the fact that bundles showed no significant genotype differences at the neonatal time point (P > 0.5; Fig. 2, Aa and B). Additionally, des−/− bundles exhibited increasing modulus with age, a trend that was absent in wt bundles. Two-way ANOVA revealed a significant effect of genotype (P < 0.01), age (P < 0.01), and a significant interaction (P < 0.01), explicitly demonstrating an age-dependent effect of genotype on modulus. In other words, the des−/− muscle became disproportionately stiffer with age.
Fig. 2.
Des−/− bundles from young and adult muscle were stiffer compared with wt. A: passive Cauchy stress-sarcomere length quadratic curve fits for young wt (n = 10) and des−/− (n = 12) bundles. Aa: neonatal mice. Ab: young mice. Ac: adult mice. Bundle curves were more nonlinear than fiber curves (compare with Fig. 1A) with increased nonlinearity in the young des−/− curve relative to young wt. Young and adult des−/− bundles had significantly higher stress than wt at sarcomere lengths greater than 4.1 μm and 3.7 μm, respectively, as determined by ANOVA with repeated measures. B: tangent moduli calculated from curve fits at a sarcomere length of 3.9 μm for des−/− and wt bundles in three age groups. Young and adult des−/− bundles were significantly stiffer compared wt. However, there is no difference in neonatal bundle stiffness with genotype. Additionally, there was a significant genotype-age interaction as determined by two-way ANOVA due to continually increasing bundle stiffness with age in des−/− bundles that was absent in wt bundles. *P < 0.05.
In light of a recent report (27) demonstrating that differences in the material properties of single fibers and fiber bundles are due to the contribution of the ECM bundle modulus data suggest that des−/− muscle is chronically altering its ECM. There is support for this hypothesis based on the shapes of the stress-sarcomere length curves as well. Bundles have significantly more nonlinearity in their stress-sarcomere length relationships (Fig. 2A) than fibers (Fig. 1A). This nonlinearity is likely due to the contribution of the ECM which is known to have distinctly nonlinear material properties (33). In addition to the fact that the young des−/− bundles sustain higher passive stress at longer sarcomere lengths, an increase in the nonlinearity of the stress-sarcomere length relationship is also evident (Fig. 2A, des−/−).
No significant differences were detected in either degree or rate of stress relaxation between genotype and age in either fibers or bundles. Thus the observed relationships between fully relaxed stress and sarcomere length are representative of the entire spectrum of stress relaxation. Additionally, no significant differences were observed in slack sarcomere length and fiber and bundle mechanical properties were qualitatively the same as a function of sarcomere length or specimen strain (data not shown).
Desmin knockout muscles have increased expression of ECM constituents.
To investigate the basis for altered ECM properties in des−/− muscle, gene expression in the TA from young and adult mice was investigated. Of the 9,161 identified genes on the mouse chip, 1,018 (11%) genes in the young muscle and 3,172 (35%) genes in the adult muscle were differentially expressed with genotype. Only 452 of these genes were shared by both ages indicating that not only was there a larger genotype effect in the adult muscle (over twice the number of significant genes) but the effect was different between young and adult muscles.
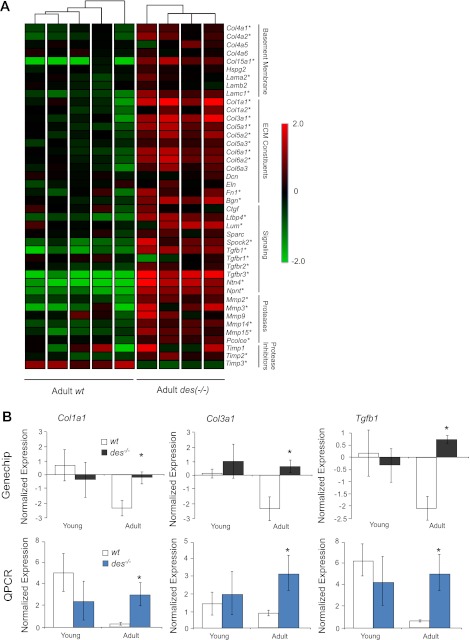
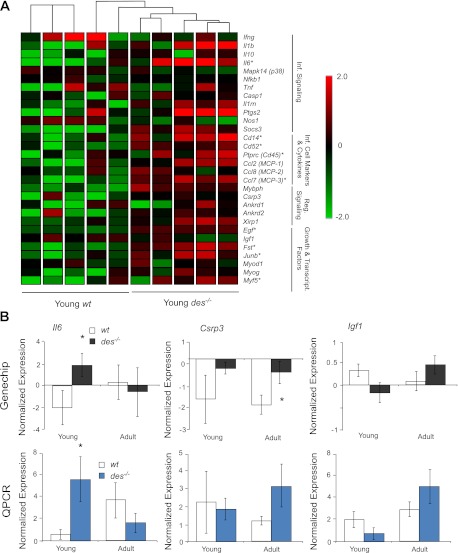
The primary genes involved in ECM structure, organization, and remodeling in muscle were identified based on published pathways and literature searches (9, 36, 39). A hierarchical gene clustering condition tree generated based on these genes did not cluster young des−/− separately from young wt but did result in the adult des−/− samples being clustered separately from the adult wt, indicating that the ECM was differentially regulated in these samples (Fig. 3A). The condition tree clusters samples with similar expression patterns of genes of interest, which can be visualized by plotting gene expression by color with red indicating high expression and green indicating low expression. The four adult des−/− samples were clearly differentiated by their red color scheme indicating most of the genes involved in muscle ECM had higher normalized expression in adult des−/− muscle (Fig. 3A). Indeed, 29 out of the 42 genes were significantly upregulated in the adult des−/− compared with the adult wt (P < 0.01), while only 5 were significantly upregulated in the young des−/−, compared with the young wt (P < 0.01).
Fig. 3.
Adult des−/− muscle showed increased extracellular matrix (ECM) specific gene expression. A: normalized gene expression for 42 genes involved in ECM structure and maintenance is shown on a colored scale with green representing low expression and red representing high expression. Hierarchical clustering is represented by connecting lines at top of the grid with lines closest to the grid denoting the most similar samples. The clustering algorithm grouped samples according to genotype with adult des−/− in 1 group at right, indicating that adult des−/− samples had a distinct expression pattern of ECM genes compared with wt. Adult des−/− samples show higher expression of the majority of the listed genes indicated by their red color scheme. ECM-related genes are subdivided into 4 categories based on function: Basement Membrane, ECM Constituents, Proteases and Protease Inhibitors. *Significantly higher expression values, as determined by two-way ANOVA. B: normalized expression as determined by GeneChip and quantitative (q)PCR for 3 important ECM genes: Col1a1 (type I collagen), Col3a1 (type III collagen), and Tgfb1 (transforming growth factor-β). Consistent with GeneChip data, qPCR revealed significantly higher expression for all 3 genes in the adult des−/− muscle. *P < 0.05.
The expression of three genes in the ECM list was confirmed by qPCR for adult muscle (Fig. 3B). Consistent with GeneChip data, qPCR indicated that the expression of collagen type I (Col1a1), collagen type III (Col3a1), and transforming growth factor-β (Tgfb1) was significantly elevated in the adult des−/− muscle compared with wt muscle.
Although the genes presented in Fig. 3 are thought to be the primary contributors to ECM structure and maintenance, the list is by no means exhaustive. The GO pathway for ECM provides a more extensive list and a means to determine whether the pathway itself is overrepresented in adult des−/− muscle. Consistent with the expression results (Fig. 3A), the ECM cellular component pathway (GO:0031012) was significantly overrepresented in adult des−/− muscle compared with adult wt (P < 0.05) but not in the young des−/− (P > 0.1).
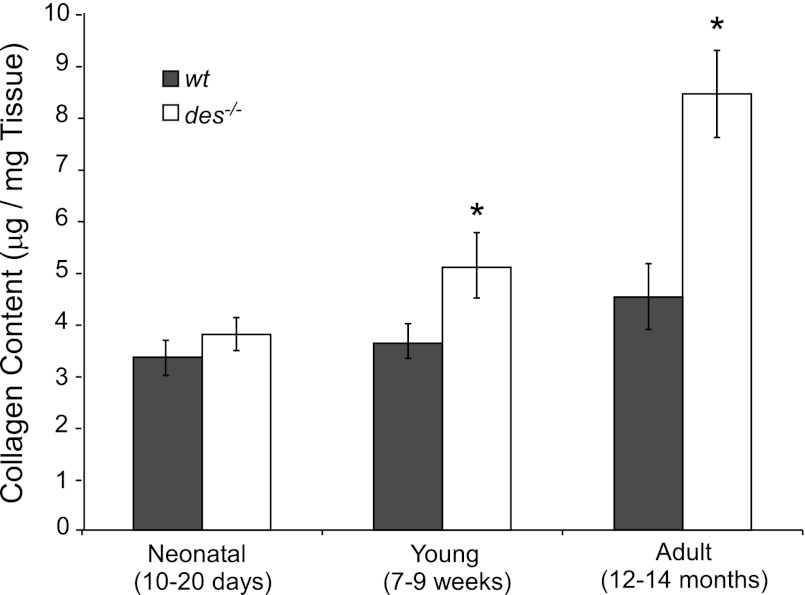
To confirm that gene expression changes result in an accumulation of protein in the ECM, intramuscular collagen content was measured. Collagen content was similar between genotypes in neonatal muscle but was significantly increased in young and adult des−/− muscle (P < 0.05; Fig. 4). Additionally, collagen content increased with age in des−/− muscle, a trend absent from wt muscle but similar to the increasing des−/− bundle stiffness (Fig. 2). Two-way ANOVA revealed a significant effect of genotype (P < 0.05) and age (P < 0.05) as well as a significant interaction (P < 0.05), indicating that collagen content varied between genotypes in an age-dependent manner. This provides a biochemical explanation for the differential functional response of des−/− muscle to age.
Fig. 4.
Young and adult des−/− muscle had increased collagen content compared with wt. There was an increase in collagen content in the des−/− muscle with age that mirrored the increase in stiffness seen in the bundle data (compare with Fig. 2B). Collagen content nearly doubles in the adult des−/− compared with the adult wt as does bundle tangent modulus. There is a significant genotype-age interaction determined by two-way ANOVA. *P < 0.05.
Although higher collagen content and gene expression were detected in des−/− muscle, flow cytometry data indicate no significant increase in the number of reticular (collagen producing) fibroblasts present in the muscle and no switch to a myofibroblastic phenotype (data not shown).
Desmin knockout muscle alters the amount and type of ECM with age.
Although fiber bundle mechanics combined with gene and protein expression data demonstrated changes to the ECM in des−/− muscle with age, it is unclear whether there is simply excessive ECM accumulation or whether the properties of existing ECM are being altered (collagen arrangement, cross-linking, swelling, etc.). It is possible to calculate the material properties of the ECM from fiber and bundle data using the theory of composites, but first the volume fraction of ECM relative to muscle fibers must be defined. Since fiber bundles were manually dissected from the midbelly of the muscle away from either tendon, there is likely little variation in collagen content with length. Additionally, since all bundles were similar lengths, it is reasonable to assume that the volume fraction of ECM will scale with area fraction or, in other words, that the changes in ECM volume fraction will all occur laterally in the spaces between fibers. Thus ECM area fraction can be determined histologically and substituted for volume fraction into Eq. 1 since both values are unitless.
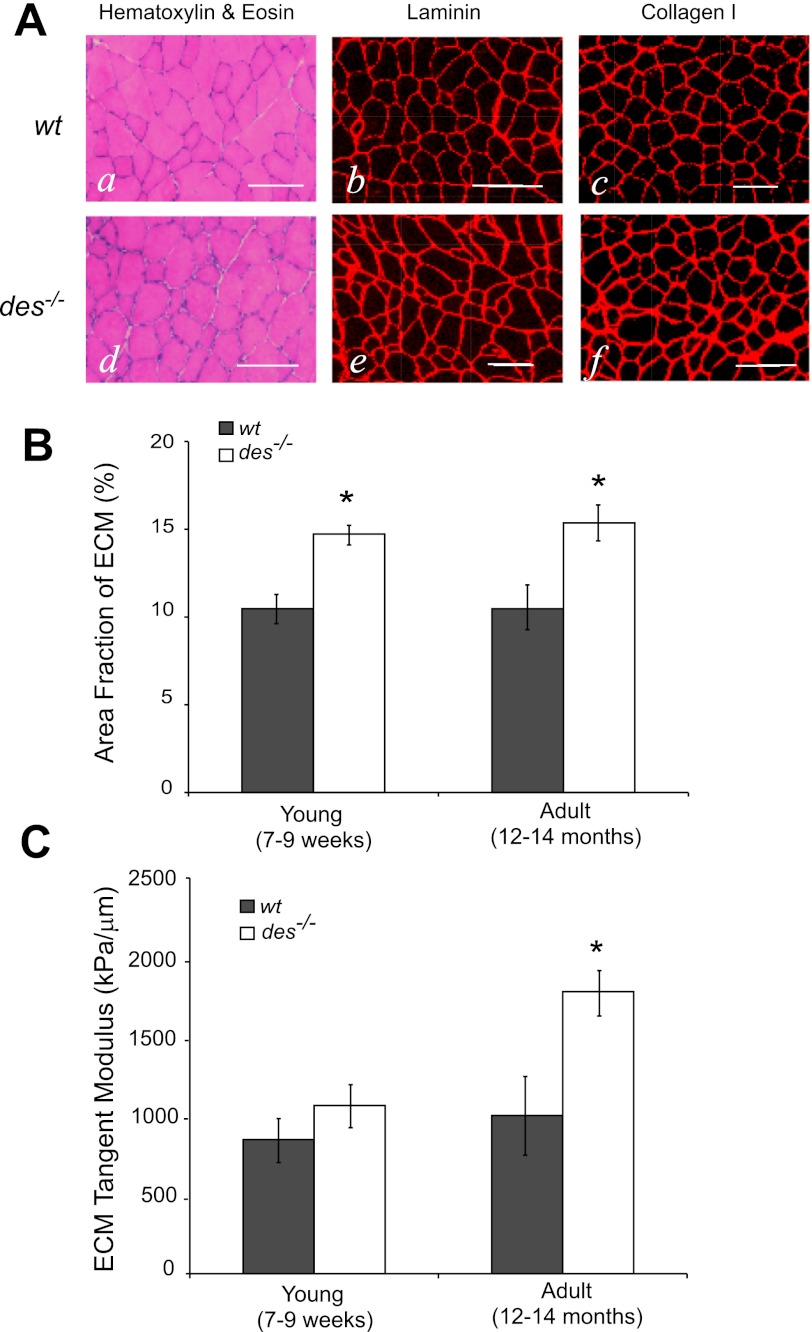
On H&E stained cross sections, a small increase in interfiber spacing can be seen in the des−/− muscle compared with the wt muscle (Fig. 5, Aa and Ad). Presumably this increased spacing is due to accumulation of ECM, but to ensure that it was not a sectioning artifact, sections were immunostained for two important components of the ECM: laminin and type I collagen. In immunostained sections, a small increase in staining apparent in des−/− compared with wt muscle demonstrated that the increased interfiber spacing was indeed ECM (Fig. 5, Ab, Ac, Ae, and Af). The area fraction of laminin staining was slightly, but significantly greater in the des−/− compared with wt muscle, from ∼10–15% in both young and adult sections, suggesting an accumulation of ECM in des−/− muscle (P < 0.05; Fig. 5B). Laminin staining was chosen for quantification so as not to exclude the basement membrane from the ECM definition, but area fraction measures on H&E and type I collagen stained sections yielded similar results (data not shown).
Fig. 5.
Des−/− muscle contained a higher fraction of ECM compared with wt. A: representative images from wt and des−/− muscle stained for hematoxylin & eosin (H&E; a and d), laminin (b and e), and collagen type I (c and f). H&E staining revealed a slight but notable increase in inter-fiber spacing in des−/− sections, which correlated with thicker and brighter staining for laminin and collagen I, suggesting an increase in ECM area. Scale bars = 100 μm. B: area fraction of ECM quantified from laminin-stained sections. There was a small, but significant, increase in the area fraction of the ECM in both young and adult des−/− muscle. C: although there was an increase in the area fraction of the ECM in both young and adult des−/− muscle, it was not enough to account for the measured changes in adult bundle stiffness, and calculated ECM tangent modulus was significantly higher in adult des−/− muscle. *P < 0.05.
With the use of the ECM area fraction calculated from the laminin images (Fig. 5B), des−/− and wt fiber moduli (Fig. 1B) and des−/− and wt bundle moduli (Fig. 2B), a modulus value for des−/− and wt ECM was calculated using composite theory. Although there was a significant increase in the area fraction of ECM in both young and adult des−/− muscle, it did not quantitatively account for the altered adult fiber bundle properties. Since ECM modulus was significantly higher in adult des−/− muscle (P < 0.01; Fig. 5C), but not in young, these data suggest that the ECM in des−/− muscle first proliferated and then altered its mechanical properties with age.
Desmin knockout muscle shows signs of inflammation and regeneration.
While these results indicate a progressive change in des−/− ECM with age, the mechanism for this alteration is unknown. Frequently, muscle fibrosis is preceded by inflammation, potentially caused by injury and cycles of regeneration (32, 36). Injury studies in des−/− muscle have yielded conflicting results with some noting an increased susceptibility to injury, some noting a decreased susceptibility, and some noting no difference (24, 25, 34). To investigate the potential role of inflammation and regeneration in des−/− muscle fibrosis, gene expression profiles in these categories were investigated. A gene list composing 18 genes involved in inflammation and 11 genes involved in muscle regeneration was generated based on previously published physiological pathways and literature results (4, 8, 39). A condition tree of expression data generated based on these genes clustered young des−/− separately from young wt (P < 0.05; Fig. 6) but did not cluster the adult des−/− samples separately from the adult wt (P > 0.1). This result suggests that inflammation and regeneration could play a significant role in young des−/− muscle ECM remodeling. Six of the 18 genes identified as being involved in inflammation (33%) were significantly upregulated in young des−/− samples over wt compared with only 3 in adult des−/−. Expression of three genes involved in inflammation, and regeneration was again confirmed by qPCR (Fig. 6B). Consistent with GeneChip data, qPCR expression of interleukin-6 (Il6) was significantly higher in the young des−/− compared with wt, and expression of muscle LIM protein (Csrp3) and expression insulin-like growth factor (Igf1) were both increased (though not significantly) in the adult des−/− compared with wt.
Fig. 6.
Young des−/− muscle showed increased inflammation and regeneration specific gene expression. A: normalized gene expression for 18 genes involved in inflammation signaling and inflammatory cell migration and 11 genes involved in regeneration shown on a colored scale with green representing low expression and red representing high expression. Hierarchical clustering is represented by connecting lines at top of the grid with lines closest to the grid noting the most similar samples. The clustering algorithm separated samples according to genotype with young des−/− to at right, indicating that young des−/− samples had a distinct expression pattern of inflammation/regeneration genes compared with wt. Young des−/− samples showed higher expression of the majority of the listed genes. *Significantly higher expression values, as determined by two-way ANOVA. B: normalized expression as determined by GeneChip and qPCR for three important inflammation/regeneration genes: Il6 (interleukin 6), Csrp3 (muscle LIM protein), and Igf1 (insulin like growth factor). Consistent with GeneChip data, qPCR revealed significantly higher expression for Il6 in the young des−/− muscle. *P < 0.05.
Consistent with these results, GO pathways involved in inflammation and response to stress were overrepresented in the young des−/− compared with the young wt including response to wounding (GO: 0009611; P < 0.05), inflammatory response (GO: 0006954; P < 0.005), and regulation of tumor necrosis factor (GO: 0032680; P < 0.05), none of which were significantly different in the adult muscle groups.
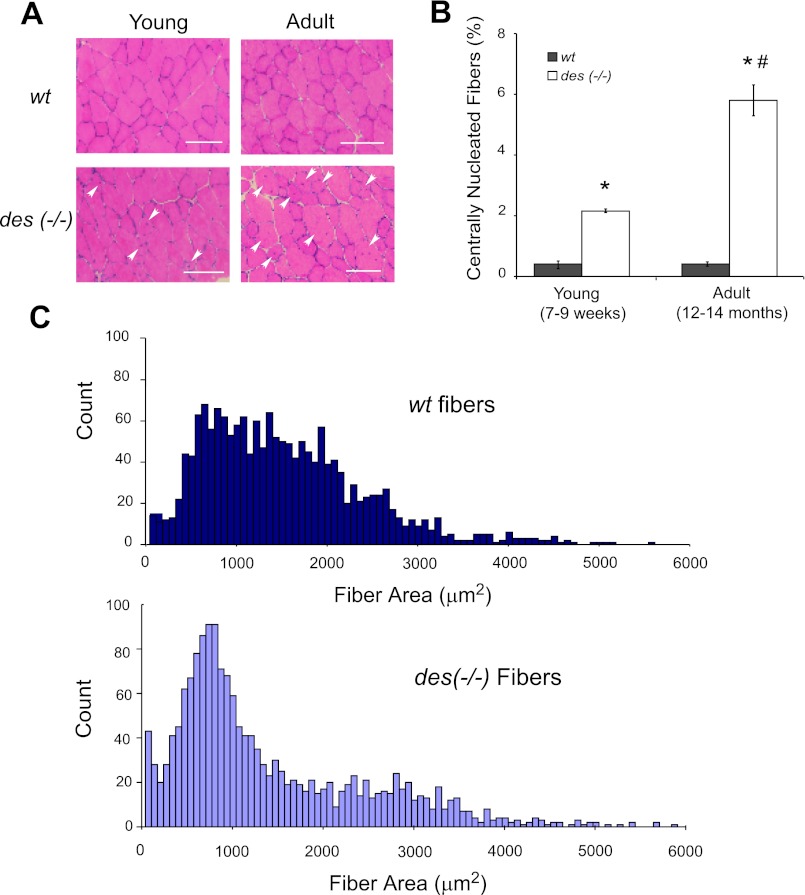
Two hallmarks of regenerating fibers in muscle are small cross-sectional areas and centrally located nuclei. Centrally nucleated fibers were identified in des−/− and wt muscle using H&E-stained sections (Fig. 7A). Quantification of the number of centrally nucleated fibers as a fraction of total fibers in the cross section showed a significant increase in young des−/− muscle compared with wt (2.23 ± 0.06 and 0.37 ± 0.10%, respectively; P < 0.001; Fig. 7B). Interestingly, the number of centralized nuclei continued to increase in the des−/− muscle with age, but remained the same in wt (5.85 ± 0.49 and 0.42 ± 0.05%, respectively; Fig. 7B). Consistent with this result, a histogram of fiber sizes in both young and adult des−/− cross sections shows a leftward shift toward smaller fiber diameters, with over twice the number of fibers with areas <100 μm2 in the des−/− sections compared with wt (Fig. 7C).
Fig. 7.
Des−/− muscle showed signs of regeneration. A: sections from the tibialis anterior midbelly of young and adult des−/− muscle stained with H&E showed an increased number of centrally nucleated fibers (arrows) compared with wt, which is a hallmark of muscle regeneration. Scale bars = 100 μm. B: quantification of H&E-stained sections showed a significant increase in the number of centrally nucleated fibers in both young and adult des−/− sections compared with wt where <1% of fibers were centrally nucleated. Additionally, the number of centrally nucleated fibers increased in the des−/− muscle with age but did not in the wt. Two-way ANOVA yielded a significant effect of genotype and a significant genotype-age interaction. C: histograms of young and adult des−/− fiber sizes quantified from laminin-stained sections revealed a leftward shift toward smaller fiber sizes compared with wt. *P < 0.05.
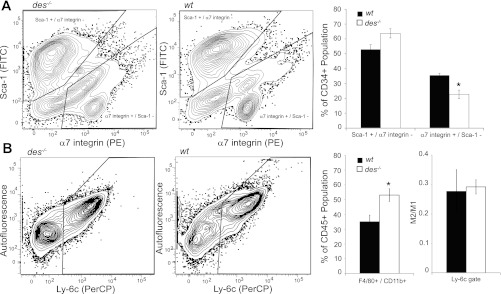
Flow cytometry data support an increased state of injury and inflammation as well. Muscle-resident stem cells can be classified in two primary populations, muscle progenitor (MP) cells and fibroadipogenic progenitor (FAP) cells, the dynamics of which have been shown to change with injury. Specifically, in response to injury, the MP population decreases while the FAP population increases (17). Stem cell populations in des−/− muscle show a similar shift in population dynamics. Cell populations identified by the MP marker α7-integrin were significantly decreased in des−/− muscle compared with wt while cell populations identified by the FAP marker Sca-1 were elevated in des−/− muscle compared with wt though not significantly (Fig. 8A). Additionally, cell populations identified by the macrophage markers CD11b and F4/80 were significantly elevated in des−/− muscle compared with wt indicating increased macrophage infiltration (Fig. 8B). The macrophage population can be further subdivided into classically activated macrophages (M1), which are inflammatory and initially recruited to the injury site, and alternatively activated macrophages (M2), which are fibrotic and appear at a later stage (3). Although both populations were elevated in the des−/− muscle, the relative contribution of each population remained the same as in wt (Fig. 8B). Together, these data indicate an increased state of inflammation and regeneration in des−/− muscle that resembles an injury response.
Fig. 8.
Flow cytometery indicated signs of increased injury in the des−/− muscle. A: cell populations positive for the muscle progenitor (MP) marker α7-integrin and negative for the fibroadipogenic (FAP) marker Sca-1 are significantly reduced in des−/− muscle. Cell populations positive for Sca-1 but negative for α7-integrin are elevated in des−/− muscle, although not significantly so. Populations were first gated on CD45, CD31, and CD34 to identify a muscle-resident stem cell population. B: cell populations positive for the immune cell marker CD11b and positive for the macrophage marker F4/80 are significantly elevated in des−/− muscle. However, the fraction of this population that is negative for Ly-6c (M2), a marker of classically activated macrophages, relative to those that are positive (M1) remains unchanged.
DISCUSSION
The main result of this study is that while des−/− and wt muscles are “born” with identical fiber and ECM material properties, these properties diverge becoming quite different with age. Specifically, des−/− fibers become more compliant compared with wt fibers (Fig. 1) while des−/− bundles become progressively stiffer with age compared with wt bundles (Fig. 2). This result suggests that des−/− muscles are chronically altering their ECM and likely increasing the fraction of the load that is born by the ECM rather than the muscle fibers themselves. Evidence supporting this hypothesis was consistently observed using a number of measurement methods including direct measurement of fiber and bundle biomechanical properties, biochemical assay of collagen content, morphological measurement of ECM area fraction, gene expression patterns and flow cytometry.
The general notion that muscle fibers and their ECM properties are somehow complimentary is already deeply rooted in the muscle physiology literature. For example, it has been established in animal models and human experiments that the compliance of muscle connective tissue is extremely consistent and has a specific relation to the properties of the contractile tissue within the muscle. Connective tissue compliance is also interpreted in terms of functional differences, as variations between sprinters and endurance runners has been documented in terms of locomotion efficiency (20) or body acceleration (6). In all cases, it is not known whether such different connective tissue properties “develop” or are “born” into the system. The results of the current study suggest that either process is possible. It is interesting that wt fibers became stiffer and then more compliant with age while des−/− fibers maintained their properties. The mechanism for this difference is unknown, but GeneChip data indicate a significant decrease in desmin expression in the adult wt samples compared with young (data not shown), so the wt fiber cytoskeleton may be developing normally but then becoming compromised with age. More studies are required to investigate this hypothesis.
Additionally, the observed decrease in collagen gene expression in wt muscle between the young and adult age groups is interesting as it contrasts with our observation that bundle stiffness and collagen content continue to increase. This discrepancy may be due to the fact that collagen accumulation in muscle is a function both of its production and of its breakdown, and thus collagen gene expression does not necessarily correlate with collagen content. We note that in adult des−/− samples, there is also a significant increase in matrix metalloproteinase (MMP) expression (proteases involved in collagen breakdown) and differential expression of some tissue inhibitors of MMP activity (TIMPs). We think that the overall picture of ECM-related gene expression in adult des−/− muscle compared with adult wt is increased ECM turnover. It is only when combined with hydroxyproline and immunohistochemical measures of collagen content that changes in collagen expression can be related to collagen content in muscle. ECM area fraction from micrographs must also be interpreted with caution as it may not adequately represent the complexity of the load-bearing portion of the ECM. Since there are as yet no universally accepted methods to estimate this actual number, we have used area fraction as defined in stereology (40) as a first approximation.
It is interesting that, in the absence of the muscle specific intermediate filament desmin, alterations to the cytoskeleton lead to whole muscle adaptation. Our gene expression data provide some insight into a potential mechanism of muscle adaptation in this particular system. We noted that in young des−/− muscle, genes involved in the inflammatory pathway were upregulated (Fig. 6). We speculate that the loss of desmin results in a loss of intermyofibrillar mechanotransduction, which leads to muscle injury during development, as the muscle is less equipped to distribute strains imposed by everyday activity. In response to this injury, the muscle then experiences an inflammatory response that eventually results in regeneration. However, since the regenerated fibers still lack desmin, they will still be susceptible to further injury. Since the inflammatory pathway is already known to be fibrosis inducing (23, 36), we speculate that the mechanism of “stiffening” of the ECM is mediated through these inflammatory pathways. Importantly, this type of response would provide a negative feedback loop in that, after stiffening, the ECM would prevent excessive fiber strain, which is believed to be related to muscle fiber injury (2, 30, 31), which would then relieve the inflammatory stimulus and stabilize the ECM system. Of course, this suggested mechanism is speculative at this point but can be experimentally tested.
Although there is some existing evidence for increased stiffness (1) and regeneration (24, 25) in desmin knockout muscle, this study provides a potential mechanistic link between these findings. Additionally, The current data have implications for interpreting our previous results on des−/− muscle injury with age. Previously, we reported that des−/− muscles generated lower isometric stress were less susceptible to injury compared with their wt counterparts (34). These results were interpreted exclusively in terms of the mechanical effects of desmin on the cytoskeletal lattice. This finding was then followed by reports demonstrating increased myofibrillar compliance (37) and by a theoretical model that provided the same results (26). We used these approaches to suggest that des−/− muscles generate lower stress because of less efficient force transmission across the myofibrillar lattice and experience less injury because of lower shear stresses across the fiber. The current results suggest that mechanical explanations alone do not explain the desmin knockout model. Our results suggest that lower stress generated by des−/− muscles is a result of the inflammatory process that has compromised either the excitation-contraction coupling system, the force transmitting system, or the force generating system. Further, it is possible that the “lower” injury to des−/− muscles simply results from the fact that they are already in a state of inflammation and regeneration from everyday activity. The fact that such divergent mechanisms can be invoked to explain the current experimental results highlights, in part, the complex nature of the skeletal muscle tissue force-generating system.
Skeletal muscle fibrosis is a highly sensitive but nonspecific adaptation to a multitude of altered use paradigms. Nearly all primary myopathies have fibrosis as a defining parameter, which is obvious to the practicing pathologist and diagnostic for disease. However, the development of therapies for prevention and reversal of fibrosis are still in their infancy. There is an increased focus on the development of antifibrotic therapies (5, 23); however, most of these are accomplished indirectly by suppression of the muscle inflammatory response. In light of the current studies, we suggest that inhibition of fibrosis might ultimately be detrimental to the muscle since no structure would be present that could prevent the excessive sarcomere strain that leads to muscle fiber injury. More “physiological” therapies might be envisioned that mechanically stabilize the muscle tissue without preventing the excessive fibrosis that can lead to contracture or tissue dysfunction. Future studies are required in this area that take into account both the biological biomechanical complexity of skeletal muscle tissue.
GRANTS
This work was supported by grants from the National Institute of Health (AR-40050 and HD-050837) and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.A.M. and R.L.L. conception and design of research; G.A.M. performed experiments; G.A.M. analyzed data; G.A.M. and R. L. L. interpreted results of experiments; G.A.M. prepared figures; G.A.M. and R. L. L. drafted manuscript; G.A.M. and R.L.L. edited and revised manuscript; G.A.M. and R.L.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Lucas Smith, Dr. Samuel Ward, and Dr. Andrew McCulloch for helpful discussion and guidance and Mary Burrows, Evie Lin, Randy Gastwirt, and Shannon Bremner for technical assistance. This work was completed at the University of California, San Diego.
REFERENCES
- 1. Anderson J, Li Z, Goubel F. Passive stiffness is increased in soleus muscle of desmin knockout mouse. Muscle Nerve 24: 1090–1092, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol 54: 80–93, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286: C355–C364, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med 36: 1548–1554, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Biewener AA, Farley CT, Roberts TJ, Temaner M. Muscle mechanical advantage of human walking and running: implications for energy cost. J Appl Physiol 97: 2266–2274, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Cannon JG, Pierre BA. Cytokines in exertion-induced skeletal muscle injury. Mol Cell Biochem 179: 159–167, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29: 191–197, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta 1793: 911–920, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Ferreira R, Neuparth MJ, Ascensao A, Magalhaes J, Vitorino R, Duarte JA, Amado F. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first wk of hindlimb suspension. Eur J Appl Physiol 97: 340–346, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Foidart M, Foidart JM, Engel WK. Collagen localization in normal and fibrotic human skeletal muscle. Arch Neurol 38: 152–157, 1981 [DOI] [PubMed] [Google Scholar]
- 13. Frey JW, Farley EE, O'Neil TK, Burkholder TJ, Hornberger TA. Evidence that mechanosensors with distinct biomechanical properties allow for specificity in mechanotransduction. Biophys J 97: 347–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon) 16: 87–101, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grant RA. Estimation of hydroxyproline by the autoanalyser. J Clin Pathol 17: 685–686, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones RM. Mechanics of Composite Materials. Taylor & Francis, 1999 [Google Scholar]
- 19. Kang L, Ayala JE, Lee-Young RS, Zhang Z, James FD, Neufer PD, Pozzi A, Zutter MM, Wasserman DH. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes 60: 416–426, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Elastic properties of muscle-tendon complex in long-distance runners. Eur J Appl Physiol 81: 181–187, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature 283: 249–256, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139: 129–144, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovering RM, O'Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ. Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 300: C803–C813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer GA, Kiss B, Ward SR, Morgan DL, Kellermayer MS, Lieber RL. Theoretical predictions of the effects of force transmission by desmin on intersarcomere dynamics. Biophys J 98: 258–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol 134: 1255–1270, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minamoto VB, Hulst JB, Lim M, Peace WJ, Bremner SN, Ward SR, Lieber RL. Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Dev Med Child Neurol 49: 907–914, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J 57: 209–221, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel TJ, Das R, Friden J, Lutz GJ, Lieber RL. Sarcomere strain and heterogeneity correlate with injury to frog skeletal muscle fiber bundles. J Appl Physiol 97: 1803–1813, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11: 263–272, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil 15: 299–308, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279: C1116–C1122, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Savolainen J, Myllyla V, Myllyla R, Vihko V, Vaananen K, Takala TE. Effects of denervation and immobilization on collagen synthesis in rat skeletal muscle and tendon. Am J Physiol Regul Integr Comp Physiol 254: R897–R902, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316: 3050–3058, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J 86: 2993–3008, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith LR, Gerace-Fowler L, Lieber RL. Muscle extracellular matrix applies a transverse stress on fibers with axial strain. J Biomech 44: 1618–1620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith LR, Ponten E, Hedstrom Y, Ward SR, Chambers HG, Subramaniam S, Lieber RL. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med Genomics 2: 44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weibel ER. Measuring through the microscope: development and evolution of stereological methods. J Microsc 155: 393–403, 1989 [DOI] [PubMed] [Google Scholar]