Abstract
The chemokine-like receptor-1 (CMKLR1) is a G protein-coupled receptor that is activated by chemerin, a secreted plasma leukocyte attractant and adipokine. Previous studies identified that CMKLR1 is expressed in skeletal muscle in a stage-specific fashion during embryogenesis and in adult mice; however, its function in skeletal muscle remains unclear. Based on the established function of CMKLR1 in cell migration and differentiation, we investigated the hypothesis that CMKLR1 regulates the differentiation of myoblasts into myotubes. In C2C12 mouse myoblasts, CMKLR1 expression increased threefold with differentiation into multinucleated myotubes. Decreasing CMKLR1 expression by adenoviral-delivered small-hairpin RNA (shRNA) impaired the differentiation of C2C12 myoblasts into mature myotubes and reduced the mRNA expression of myogenic regulatory factors myogenin and MyoD while increasing Myf5 and Mrf4. At embryonic day 12.5 (E12.5), CMKLR1 knockout (CMKLR1−/−) mice appeared developmentally delayed and displayed significantly lower wet weights and a considerably diminished myotomal component of somites as revealed by immunolocalization of myosin heavy chain protein compared with wild-type (CMKLR1+/+) mouse embryos. These changes were associated with increased Myf5 and decreased MyoD protein expression in the somites of E12.5 CMKLR1−/− mouse embryos. Adult male CMKLR1−/− mice had significantly reduced bone-free lean mass and weighed less than the CMKLR1+/+ mice. We conclude that CMKLR1 is essential for myogenic differentiation of C2C12 cells in vitro, and the CMKLR1 null mice have a subtle skeletal muscle deficit beginning from embryonic life that persists during postnatal life.
Keywords: C2C12 cells, chemerin, skeletal muscle, differentiation, myogenic regulatory factors
the chemokine-like receptor-1 (CMKLR1) protein is a G protein-coupled receptor that is expressed in immune cells, including macrophages and dendritic cells (37–39). CMKLR1 mRNA is also highly expressed in white adipose tissue, with intermediate levels in heart, lung, and placenta and lower levels in most other tissues, including skeletal muscle (7, 8). The endogenous ligand for CMKLR1 is chemerin, a secreted protein that is present in plasma, serum, and inflammatory fluids (7, 23, 37, 40). While it is clear that white adipose tissue and liver are important sources of circulating active chemerin (8, 21, 23, 28, 32, 36), chemerin is also produced by skeletal muscle, fetal intestinal epithelial cells, platelets, keratinocytes, and vascular endothelial cells where it may affect local physiological and pathological processes (6–8, 14, 19, 24, 29, 30, 35).
In addition to its role as a leukocyte chemoattractant, our studies have identified a second important function for chemerin/CMKLR1 signaling in the terminal differentiation of cells of mesenchymal origin. Initially, we reported that CMKLR1 is required for the differentiation of 3T3-L1 preadipocytes into lipid-containing adipocytes (8, 20). Furthermore, in a more physiological model of adipogenesis, primary mouse bone marrow stromal cells (bMSCs) lacking functional CMKLR1 or chemerin were also impaired in adipogenesis, and instead were diverted to differentiate into osteoblastogenic-lineage cells (21). Together these results reflect the importance of functional chemerin/CMKLR1 signaling in adipogenesis and in directing the terminal differentiation of multipotent bMSCs.
A recent study by Harewood et al. (12) characterized CMKLR1 expression in the developing embryonic limb bud. CMKLR1 was first detected at embryonic (E) day 9.5 in the mesenchyme and myotome, followed by expression in migratory myoblasts at E10.5. At E11.5–12.5, CMKLR1 was expressed around the forming bone of the limbs. At E14.5, CMKLR1 was expressed within the muscle of the developing limb as well as within other muscle tissues, including the intercostal and facial muscles, tongue, diaphragm, and the body cavity wall (12). Given the site and stage-specific expression of CMKLR1 in developing muscle, and its role in the terminal differentiation of adipocytes and osteoblasts, we explored the hypothesis that CMKLR1 regulates the differentiation of myoblasts into skeletal muscle myotubes in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Mouse C2C12 myoblast cell culture.
C2C12 myoblasts were obtained from the American Tissue Culture Collection (Manassas, VA). C2C12 cells were grown in Dulbecco's modified Eagles medium (DMEM, phenol red-free) supplemented with 10% fetal bovine serum (Thermo Scientific, Ottawa, ON, Canada), 100 IU/ml penicillin, 250 μg/ml streptomycin (Invitrogen, Burlington, ON, Canada), and 1 mM sodium pyruvate (Sigma, St. Louis, MO). The growth medium was changed every 2 or 3 days, and the cells were maintained in a standard humidified atmosphere supplemented with 5% CO2 at 37°C.
RNA isolation and quantitative polymerase chain reaction analysis.
Adult male mice were killed with 90 mg/kg pentobarbital sodium. Skeletal muscle and white adipose tissue were isolated and snap-frozen in liquid nitrogen. Total RNA was isolated from tissues or from C2C12 cell lysates using the RNeasy Mini Kit (Qiagen, Mississauga, ON, Canada) according to the manufacturer's instructions. Total RNA (1 μg) from tissues or cells was reverse-transcribed using Stratagene Reverse Transcriptase (Stratagene, Cedar Creek, TX). One microliter of the cDNA product was amplified by quantitative PCR using 125 nM gene-specific primers (Table 1) in a total volume of 20 μl using the QuantiFast SYBR Green PCR Kit (Qiagen) and a Stratagene MX3000p Thermocycler. Relative gene expression was normalized to cyclophylin A using the ΔΔCT method (15).
Table 1.
Quantitative PCR primers
| Gene | Accession No. | PCR Forward and Reverse Primers | Product Size, bp |
|---|---|---|---|
| CyclophilinA | NM_008907 | Fw GAGCTGTTTGCAGACAAAGTTC | 124 |
| Rv CCCTGGCACATGAATCCTGG | |||
| Chemerin | NM_027852 | Fw TACAGGTGGCTCTGGAGGAGTTC | 196 |
| Rv CTTCTCCCGTTTGGTTTGATTG | |||
| CMKLR1 | NM_008153 | Fw CCGAGCCTCTACAACAGGAG | 165 |
| Rv GGTAACTTCCTCACCCACGA | |||
| Myogenin | NM_031189 | Fw CCGAGCCTCTACAACAGGAG | 175 |
| Rv GGTAACTTCCTCACCCACGA | |||
| MyoD1 | NM_010866 | Fw GACAGGGAGGAGGGGTAGAG | 219 |
| Rv TGCTGTCTCAAAGGAGCAGA | |||
| Mrf4 | NM_008657 | Fw GGCTGGATCAGCAAGAGAAG | 212 |
| Rv AAGAAAGGCGCTGAAGACTG | |||
| Myf5 | NM_008656 | Fw AGGAAAAGAAGCCCTGAAGC | 151 |
| Rv GCAAAAAGAACAGGCAGAGG | |||
| MyH1 | NM_030679 | Fw CTTCAACCACCACATGTTCG | 241 |
| Rv AGGTTTGGGCTTTTGGAAGT | |||
| Vimentin | NM_011701 | Fw ATGCTTCTCTGGCACGTCTT | 206 |
| Rv AGCCACGCTTTCATACTGCT | |||
| Desmin | NM_010043 | Fw GTGAAGATGGCCTTGGATGT | 212 |
| Rv TGTGTAGCCTCGCTGACAAC | |||
| β-Actin | NM_007393 | Fw AGCCATGTACGTAGCCATCC | 228 |
| Rv CTCTCAGCTGTGGTGGTGAA | |||
| α-Actin | NM_009606 | Fw CTGAGCGTGGCTATTCCTTC | 280 |
| Rv ACAGGTCCTTCCTGATGTCG | |||
| Integrin-β1 | NM_010578 | Fw TGGACAATGTCACCTGGAAA | 164 |
| Rv TGTGCCCACTGCTGACTTAG | |||
| Calpain3 | NM_007601 | Fw ACCCAAGTGGCATCTATTCG | 220 |
| Rv TGTTGGCTCCACCAATGATA |
CMKLR1, chemokine-like receptor-1; Mrf, myogenic regulatory factor; Fw, forward; Rv, reverse.
Quantitative analysis of bioactive chemerin in C2C12 cell media.
To quantify bioactive chemerin in the cell media, we utilized a cell-based CMKLR1 reporter assay that specifically and quantitatively measures CMKLR1 activation by chemerin (1, 23). C2C12 cell growth media from the confluent myoblast stage (day 0) and at days 3, 5, and 8 of differentiation were replaced with serum-free media. After a period of 24 h, this conditioned media was collected for the chemerin bioassay. Conditioned media (serum free, 24 h incubation) from mature 3T3-L1 adipocytes (day 8) were used as a positive control (8, 9). Recombinant mouse chemerin (R&D Systems, Minneapolis, MN) was used to generate a standard curve. The apparent concentration of chemerin in C2C12 media, [S]app, was calculated for each sample by Eq. 1 where V is the measured sample activity and Km and Vmax are the constants determined by nonlinear regression fit of the chemerin standards to the Michaelis-Menten equation.
| (1) |
Adenoviral small-hairpin RNA interference.
CMKRL1 small-hairpin RNA (CR-shRNA) vectors were used to decrease the expression of CMKLR1 in C2C12 cells as previously described (8, 9, 21). A LacZ small-hairpin RNA (LZ-shRNA) adenovirus vector expressing an small-hairpin RNA (shRNA) targeting bacterial β-galactosidase was used as a control for nonspecific effects of adenoviral shRNA. For the CMKLR1 knockdown experiments, C2C12 cells were plated on 12-well plates (Corning, NY) and allowed to grow to confluence. A polybrene (Sigma)-assisted procedure was used to transduce confluent C2C12 myoblasts with the CR- and LZ-shRNA vectors. The adenoviral transduction media was prepared by adding CR- and LZ-shRNA adenoviral lysates [multiplicity of infection (MOI) = 500–1,000] to serum-free media that contained 6 μg/ml of polybrene. The viral transduction media was preincubated for 5 min at room temperature before applying it to the C2C12 cells. C2C12 growth media was aspirated, and the cells were washed one time with 500 μl of PBS followed by the addition of 500 μl of the viral transduction media to each well. After 24 h, 1 ml of normal growth media was added to each well and incubated for another 24 h, after which the cells were maintained in the growth media.
Immunofluorescence detection of myosin heavy chain and phalloidin staining of F-actin in C2C12 cells.
C2C12 cells were plated on 96-well thin bottom plates (Corning) and upon reaching confluence were transduced with CR- and LZ-shRNA. On days 0, 3, 5, and 8 after shRNA treatment, the cells were fixed in 40 μl of 4% paraformaldehyde (PFA). The PFA-fixed cells were rinsed with 40 μl of PBS for 5 min, permeabilized with 0.1% vol/vol Triton X-100 for 5 min, and incubated in standard blocking solution (10% vol/vol goat serum, 1% wt/vol BSA in PBS) for 1 h. The cells were then incubated for 2 h with a monoclonal anti-skeletal myosin heavy chain (MHC) (M4276, Clone MY-32; Sigma) primary antibody. Cells were washed three times with 40 μl of PBS for 5 min and then incubated for 1 h with a 1:200 dilution of Alexa Fluor 546 goat anti-mouse IgG (Invitrogen) secondary antibody. For detection of F-actin, day 8 C2C12 cells were incubated with a 1:500 dilution of tetramethylrhodamine-phalloidin (Invitrogen) for 1 h. The cell nuclei were counterstained with 1 μg/ml Hoescht 33258 (Sigma) in PBS for 5 min. Images were captured on a Zeiss Axiovert 200 inverted fluorescence microscope equipped with an AxioCam camera system (Zeiss Canada, Toronto, ON, Canada).
Myogenic index.
The myogenic index is defined as the number of nuclei within the MHC-positive cells containing three or more nuclei, divided by the total number of nuclei in the field. Two individuals counted the nuclei in four sample replicates for each treatment group. The counts from both individuals were averaged and expressed as the percentage of total nuclei incorporated into myotubes (34, 41).
Creatine kinase activity.
Confluent C2C12 myoblasts grown on 12-well plates were transduced with CR- and LZ-shRNA according to the protocol described above. On days 0, 3, 5, and 8, C2C12 cells were washed one time with 1 ml of PBS, 500 μl of serum-free DMEM were added to each well, and the cells were scraped from the wells and stored at −20°C. The cell suspensions were homogenized, and creatine kinase activity was measured according to the manufacturer's instructions (Bioassay Systems, Hayward, CA). The creatine kinase activity was recorded as units per liter and normalized to total homogenate protein, independently determined by the Lowry assay (16).
Glucose uptake assay.
The cellular uptake of 2-[3H]deoxyglucose (1 mCi/ml; Perkin-Elmer Life and Analytical Sciences, Waltham, MA) was measured as previously described (9). The radioactivity in each sample was counted using a β-scintillation counter (LS6500 Liquid Scintillation Counter; Beckman Coulter, Fullerton, CA). The counted radioactivity was recorded as disintegrations per minute and normalized to the total protein in each sample, independently determined by the Lowry assay.
Animals and housing.
Procedures involving mice were approved by the Dalhousie University Committee on Laboratory Animals and performed according to the guidelines of the Canadian Council on Animal Care. CMKLR1 knockout mice were originally obtained from Deltagen and fully backcrossed (nine generations) onto the C57BL/6 background (10). Homozygous (CMKLR1−/−), heterozygous (CMKLR1+/−), and wild-type (CMKLR1+/+) mice were bred in-house in the Carleton Campus Animal Care Facility. The mice were kept on a 12:12-h day-night cycle, were housed in cages lined with pine bedding, and had free access to water and Purina mouse chow.
Generation of wild-type and CMKLR1-deficient mouse embryos.
Timed matings were set up between CMKLR1−/− female × CMKLR1−/− male, CMKLR1−/− female × CMKLR1+/+ male, and CMKLR1+/+ female × CMKLR1+/+ male mice to respectively generate homozygous CMKLR1−/− knockout, heterozygous CMKLR1+/−, and homozygous CMKLR1+/+ wild-type mice. The animals were set for mating in the evening and inspected for vaginal plugs the following day. Noon on the day of vaginal plug detection was considered as 0.5 gestational day and thus the age of the embryo was designated as E0.5. Females were killed, and the embryos were harvested at E12.5 and E20.5. CMKLR1−/−, CMKLR1+/−, and CMKLR1+/+ embryos were individually weighed and analyzed.
Immunohistochemical localization of MHC, MyoD, and Myf5 proteins in mouse embryos.
For immunofluorescent detection of Myf5, MyoD, and MHC proteins, E12.5 embryos were fixed in 10% neutral buffered formalin for 48 h, dehydrated, embedded in paraffin, and sectioned at 5 μm thickness. The sections were deparaffinized, rehydrated, and microwaved to boil in 10 mM citrate buffer two times for 30 s and cooled at room temperature for 20 min for antigen retrieval. The sections were blocked by incubation with 10% goat serum and 1% BSA in PBS for 1 h. To detect embryonic Myf5, MyoD, and MHC, the slides were incubated with the primary Myf5 (SAB4501943; Sigma), MyoD (sc-32758; Santa Cruz Biotechnology), and MHC (M4276, Clone MY-32; Sigma) antibodies overnight at 4°C in a humidified chamber followed by a series of three washes in PBS and incubation with the goat anti-mouse IgG (A11018; Invitrogen) and goat anti-rabbit (A11008; Invitrogen) secondary antibodies conjugated with Alexa Fluor 546 and Alexa Fluor 488 for 1 h at room temperature. After this, the sections were washed again in PBS three more times and counterstained with Hoechst 33258. The sections were visualized under a fluorescent microscope, and images were captured using a Leica DFC 500 image capturing system. Normal rabbit IgG (sc-2027; Santa Cruz Biotechnology) and normal mouse IgG1 (sc-3877; Santa Cruz Biotechnology) isotype controls did not show any nonspecific fluorescence, confirming the specificity of the antibodies (data not shown).
Dual-energy X-ray absorptiometry.
Dual-energy X-ray absorptiometry (DEXA) was used to measure body composition of 9-wk-old male mice according to previously established procedures (22). Anesthetic induction was performed with 4% isoflurane/O2, and anesthesia was maintained during the procedure with 2% isoflurane/O2. The anesthetized mouse was placed on the scanner platform of the DEXA (Lunar PIXImus; GE Medical Systems) and arranged so that forearms and hind legs were facing caudal toward the tail. The tail itself was positioned to sit tightly beside the right forearm. After the DEXA scan was complete, a region of interest (ROI) was established encompassing the animal body (i.e., subcranial body composition), thus eliminating the high lipid content of the brain from analysis. A report of bone-free lean mass and fat mass was determined utilizing the ROI.
Data analysis.
For each cell culture experiment, the individual treatments were performed in triplicate or quadruplicate. Unless otherwise stated, each cell culture experiment was repeated two or three times. All data are expressed as means ± SE. A one-way ANOVA was used for multiple comparisons in experiments with one independent variable. A two-way ANOVA was used for multiple-comparison procedures in experiments with two independent variables. A Bonferroni test was used for post hoc analysis of the significant ANOVA. A difference in mean values between groups was considered to be significant when P ≤ 0.05.
RESULTS
CMKLR1 and chemerin mRNA expression increase with myogenic differentiation of C2C12 skeletal myoblasts.
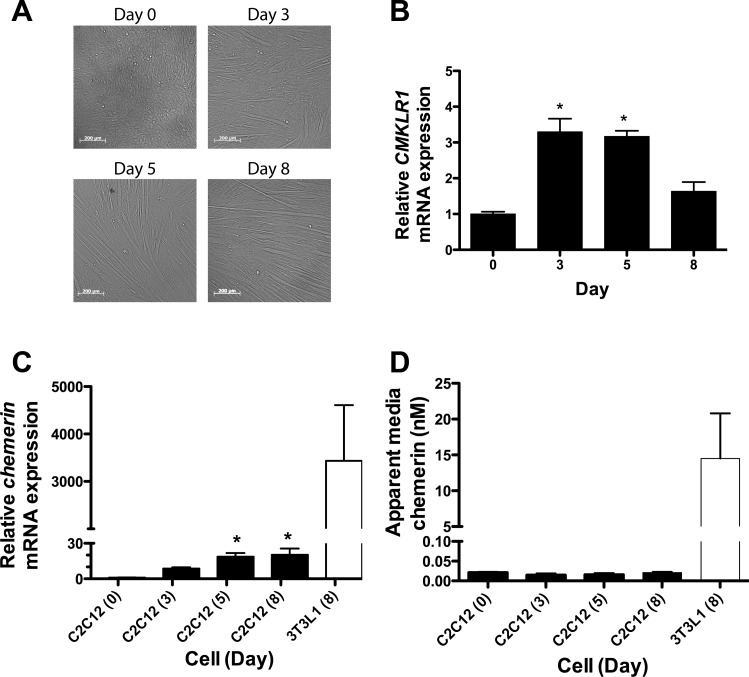
The C2C12 skeletal myoblast cells, upon reaching confluence (day 0), spontaneously undergo differentiation (days 3–5) into elongated skeletal myotubes (day 8) (Fig. 1A). CMKLR1 expression was low but detectable in confluent myoblasts (day 0), increased approximately threefold by days 3 and 5, and then declined toward basal levels on day 8 (Fig. 1B). Chemerin expression was lowest in confluent myoblasts and increased progressively by ∼20-fold as the cells differentiated into myotubes (Fig. 1C). Although chemerin mRNA expression increased significantly with myogenesis, its relative levels in myotubes were 150-fold less than the level detected in mature 3T3-L1 adipocytes (Fig. 1C). The apparent bioactive chemerin protein concentration in conditioned media from C2C12 cells was ∼0.02 nM in undifferentiated myoblasts (Fig. 1D). Despite the differentiation-associated increase in chemerin mRNA, there was no significant change in the media bioactive chemerin protein through C2C12 cell differentiation into myotubes (Fig. 1D). Furthermore, the bioactive chemerin level in conditioned media from myotubes was ∼1,000-fold less than the level of bioactive chemerin detected in media from mature adipocytes (Fig. 1D).
Fig. 1.
Chemerin and chemokine-like receptor-1 (CMKLR1) mRNA expression increase with myogenesis. A: phase-contrast images of confluent C2C12 myoblasts (day 0), differentiating myocytes (days 3 and 5), and fully differentiated myotubes (day 8). B: relative CMKLR1 mRNA expression in confluent undifferentiated C2C12 myoblasts (day 0) and in myoblasts harvested at the indicated differentiation time points. C: relative chemerin expression in C2C12 cells through differentiation and in differentiated (day 8) 3T3-L1 adipocytes. Relative gene expression was determined by quantitative polymerase chain reaction (QPCR) and normalized to the reference gene cyclophilin A. Gene expression in the undifferentiated myoblast (day 0) cells served as the reference (expression = 1.0) to which the other samples were compared. D: the apparent concentrations of bioactive chemerin in 24-h conditioned media obtained from C2C12 cells through differentiation and differentiated (day 8) 3T3-L1 adipocytes was determined using a CMKLR1 cell-based bioassay. Each bar represents the mean ± SE of 4 samples and is representative of 2 to 3 independent experiments that produced similar results. *P < 0.05, significantly different compared with the day 0 control by one-way ANOVA followed by Bonferroni multiple-comparison test.
CMKLR1 knockdown impairs myogenesis in vitro.
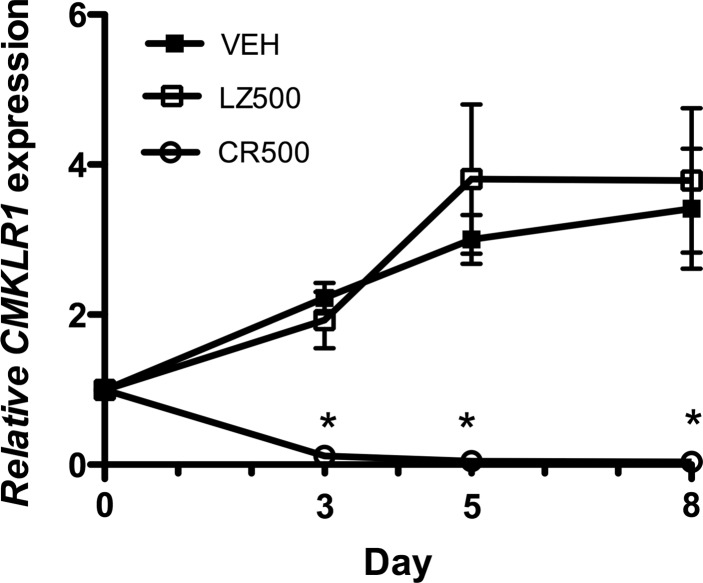
To address the hypothesis that CMKLR1 regulates the differentiation of C2C12 myoblasts into myotubes, we used an established adenoviral shRNA approach to reduce CMKLR1 expression (8, 21). An MOI of 500 provided the optimal dose of CR-shRNA to knock down CMKLR1 in C2C12 myoblasts while minimizing off-target effects (data not shown). The 500 MOI CR-shRNA treatment on day 0 (confluent myoblasts) abolished the expression of CMKLR1 mRNA in C2C12 cells, with nearly complete (>95%) gene knockdown persisting through days 3–8 of differentiation (Fig. 2).
Fig. 2.
Inhibition of CMKLR1 expression by adenoviral-mediated delivery of CMKLR1 small-hairpin RNA (shRNA). Confluent C2C12 myoblasts (day 0) were transduced with adenoviral lysates that contained 500 multiplicity of infection of CMKLR1-shRNA (CR500) or control LacZ-shRNA (LZ500) or transduction vehicle control (VEH) and then cultured under differentiation-permissive conditions. CMKLR1 was measured by QPCR on days 0, 3, 5, and 8 and normalized to the expression of the reference gene cyclophilin A. Gene expression in the day 0 cells served as the reference (expression = 1.0) to which the other treatments were compared. Each symbol represents the mean ± SE of 6–8 samples pooled from two independent experiments. *P < 0.05 compared with the VEH and LacZ shRNA (LZ-shRNA)-treated cells at the indicated time points, two-way ANOVA followed by Bonferroni multiple-comparison test.
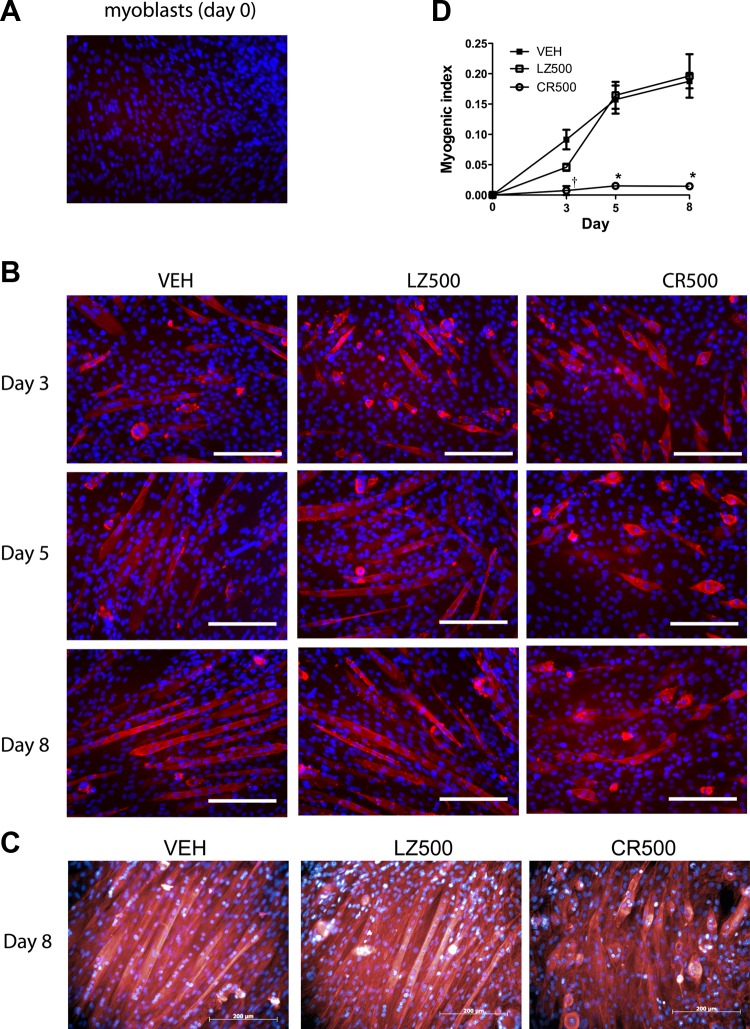
Using MHC immunostaining, we examined the effects of CR-shRNA knockdown on the differentiation of C2C12 myoblasts into multinucleated myotubes. MHC protein was not detected in confluent myoblasts at day 0 (Fig. 3A). Vehicle control and LZ-shRNA-treated cells showed a similar progressive increase from day 3 to day 8 posttreatment of elongated and multinucleated myotubes that stained positive for MHC (Fig. 3B). In contrast to the control groups, the MHC-positive cells detected in the CR-shRNA-treated group failed to fuse into myotubes and had a short, swollen, spindle-shaped appearance (Fig. 3B). To confirm the defect in myotube formation induced by CMKLR1 knockdown, we stained the shRNA-transduced C2C12 cells on day 8 of differentiation with tetramethylrhodamine phalloidin, an F-actin-specific stain. Vehicle control and LZ-shRNA transduced cells formed organized, elongated multinucleated myotubes (Fig. 3C). In contrast, CR-shRNA-transduced cells were substantially shorter in length, rounded in appearance, and unorganized relative to the vehicle or knockdown control cells (Fig. 3C). This profound defect in myotube formation was reflected quantitatively in the 90% reduction in myogenic index in the CR-shRNA-treated cells compared with the vehicle and LZ-shRNA controls (Fig. 3D).
Fig. 3.
CMKLR1 knockdown impairs fusion of C2C12 myoblasts into myotubes. An anti-myosin heavy chain (MHC) antibody (red) was used to detect MHC protein in undifferentiated C2C12 myoblasts (A) and in differentiating C2C12 cells at the indicated differentiation time points following transduction with 500 multiplicity of infection of CMKRL1 shRNA (CR500)- and LZ-shRNA (LZ500) or vehicle (VEH) treatment (B). The cell nuclei were counterstained with Hoechst 33258 (blue). Each image is representative of 4 independent samples; scale bar = 200 μm. On day 8, F-actin filaments were stained with phalloidin (red) to visualize the formation of myotubes, and the cells were counterstained with Hoechst 33258 nuclear stain (blue) (C). Each image is representative of a total of 6 samples obtained from 2 independent experimental replicates; scale bar = 200 μm. The myogenic index was calculated as the ratio of nuclei in MHC-positive myotubes/total nuclei in the field of view (D). P < 0.05 compared with the VEH and LZ-shRNA controls at the indicated time point (*) and compared with the respective LZ-shRNA control only (†), two-way ANOVA followed by Bonferroni multiple-comparison test.
CMKLR1 knockdown alters the expression of muscle-specific genes during myogenesis.
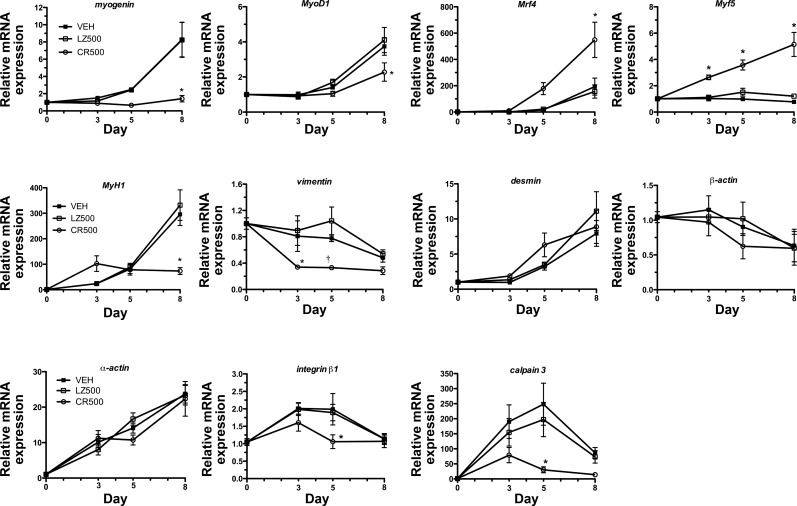
We next determined if CMKLR1 knockdown altered the expression of key myogenic genes during C2C12 myoblast differentiation (Fig. 4). Expression of the myogenic regulatory factors (MRFs) myogenin, MyoD1, and Mrf4 increased throughout myogenesis in the vehicle control and LZ-shRNA transduced cells, whereas CR-shRNA significantly reduced the differentiation-dependent expression of myogenin by 70% and MyoD1 by 35% in day 8 myotubes. In contrast, CMKLR1 knockdown increased expression of Mrf4 and Myf5 by three- and fivefold, respectively, compared with the vehicle and LZ-shRNA controls in day 8 myotubes. CMKLR1 knockdown reduced expression of muscle contractile genes MyH1 in day 8 myotubes by 70% and vimentin in day 3 and day 5 myoblasts compared with the vehicle and LZ-shRNA controls. CMKLR1 knockdown had no significant effect on the expression of muscle filament genes β-actin, desmin, or α-actin throughout myogenesis. Compared with controls, CR-shRNA treatment inhibited the expression of cell fusion genes integrin-β1 and calpain 3 on day 5 of myogenic differentiation.
Fig. 4.
CMKLR1 knockdown alters the expression of myogenic genes. Confluent C2C12 myoblasts were transduced with 500 multiplicity of infection of CR-shRNA (CR500) and LZ-shRNA (LZ500) or treated with the transduction vehicle control (VEH) and cultured for 8 days under differentiation-permissive conditions. The relative expression of myogenin, MyoD1, myogenic regulatory factor (Mrf) 4, Myf5, MyH1, vimentin, desmin, β-actin, α-actin, integrin-β1, and calpain 3 was measured by QPCR on days 0, 3, 5, and 8 and normalized to the expression of the reference gene cyclophilin A. Gene expression in the day 0 VEH-treated cells served as the reference (expression = 1.0) to which the other treatments were compared. Each bar represents the mean ± SE of 6–8 samples pooled from two independent experiments. P < 0.05 compared with the VEH and LZ-shRNA controls at the indicated time point (*) and compared with the respective LZ-shRNA control only (†), two-way ANOVA followed by Bonferroni multiple-comparison test.
The effects of CMKLR1 knockdown on metabolic pathways in C2C12 cells.
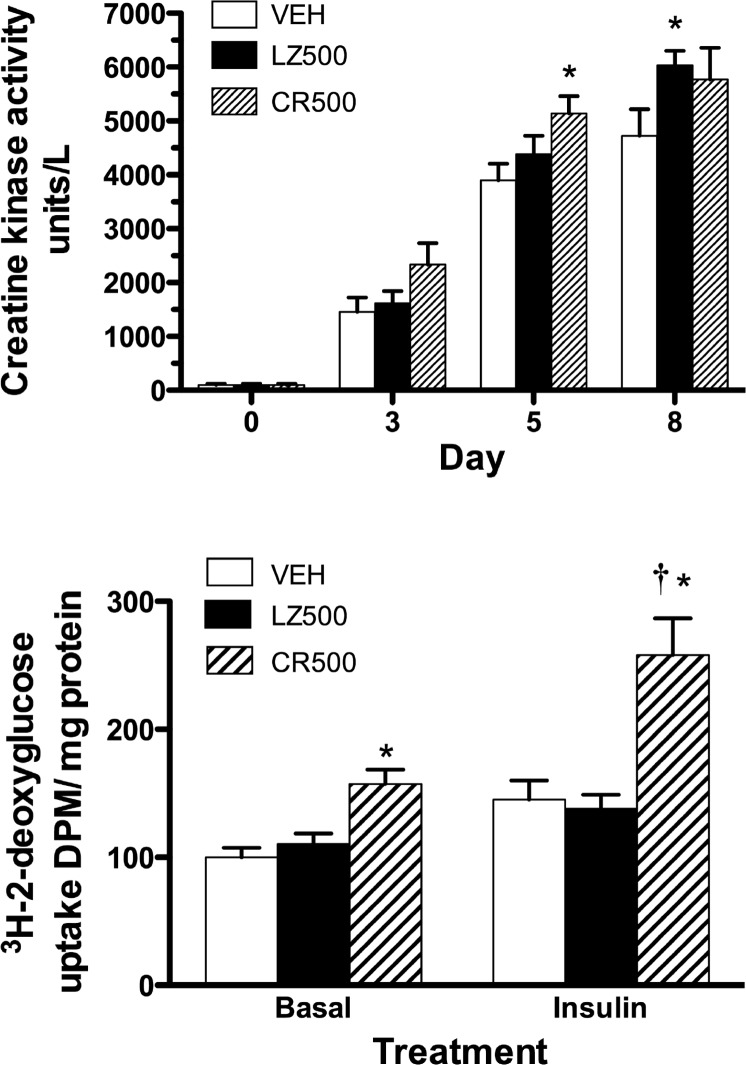
We next investigated if the loss of CMKLR1 affected energy production or glucose uptake in C2C12 cells. Creatine kinase is a marker of energy production and activity in myogenic cells (Fig. 5A) (34). As expected, creatine kinase activity was minimal in undifferentiated myoblasts and increased with myogenesis. However, CMKLR1 knockdown did not significantly affect creatine kinase activity compared with the LZ-shRNA controls at any time point. In comparison, CMKLR1 knockdown enhanced the basal and insulin-stimulated uptake of glucose (Fig. 5B).
Fig. 5.
CMKLR1 knockdown enhances basal and insulin-stimulated glucose uptake in C2C12 cells. Confluent C2C12 myoblasts were transduced with 500 multiplicity of infection of CR-shRNA (CR500) and LZ-shRNA (LZ500) or treated with the transduction vehicle control (VEH) and cultured for 8 days under differentiation-permissive conditions. A: creatine kinase activity was measured on the day of treatment (day 0) and thereafter on days 3, 5, and 8. B: basal and insulin-stimulated glucose uptake was measured in myotubes 8 days posttransduction. Each bar represents the mean ± SE of 7–8 sample replicates pooled from two independent experiments. *P < 0.05, significantly different compared with creatine kinase activity in the corresponding VEH control on day 5 and day 8 (A) or compared with the basal and insulin-stimulated glucose uptake in the respective VEH or LZ controls (B) and †P < 0.05 compared with basal glucose uptake in the CR-shRNA-treated cells, two-way ANOVA followed by Bonferroni multiple-comparison test.
Effect of CMKLR1 deficiency on embryonic mouse weight and MHC and MRF expression.
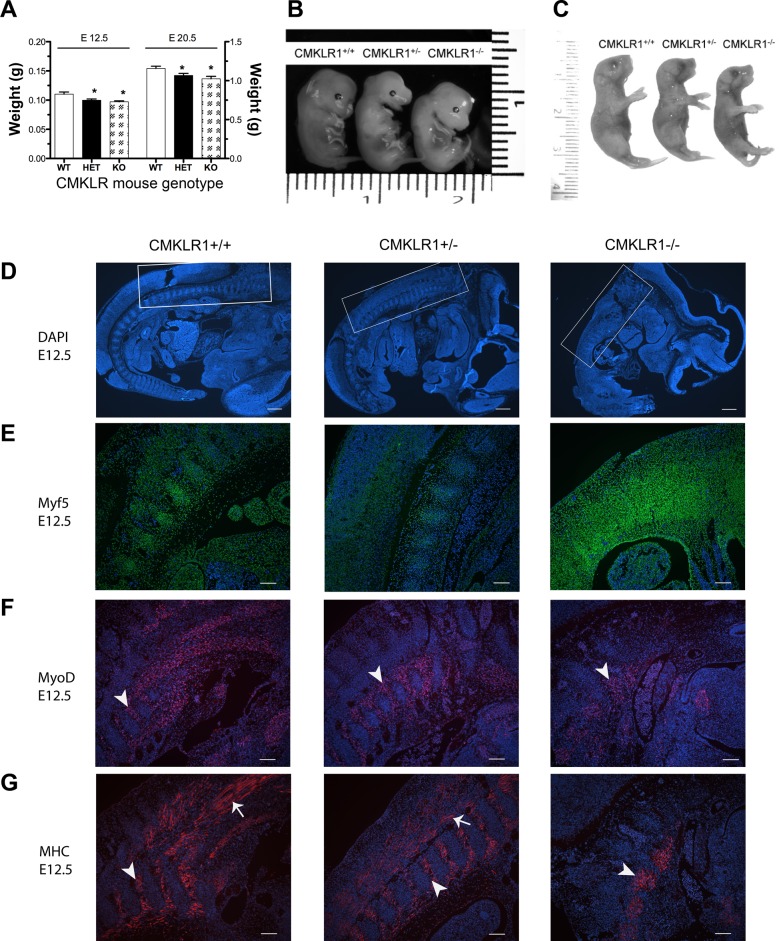
Given the severe defect in C2C12 myoblast differentiation triggered by CMKLR1 knockdown in vitro, we next asked if CMKLR1-deficient mice presented with muscle defects in vivo. The offspring of crosses between homozygous CMKLR1−/− mice or between CMKLR1−/− and CMKLR1+/+ mice were viable homozygous CMKLR1−/− and heterozygous CMKLR1+/− offspring, respectively, as previously reported (10). When we examined the CMKLR1+/− and CMKLR1−/− embryos at various stages of development, we found that the wet weights of CMKLR1+/− and CMKLR1−/− embryos were 9.6 and 12% lower at E12.5 and 7.8 and 13% lower at E20.5, respectively, compared with wild-type controls (Fig. 6A). The CMKLR1 heterozygous and homozygous knockout embryos were noticeably smaller than wild-type controls as shown by the representative images of the E12.5 and E20.5 embryos (Fig. 6, B and C). Furthermore, the significantly reduced E12.5 and E20.5 embryo mass appeared to persist into adulthood, since 9-wk-old male CMKLR1−/− mice had lower total body mass and bone-free lean body mass than CMKLR1+/+ mice (Table 2).
Fig. 6.
Effect of CMKLR1 deficiency on embryonic mouse weight and embryonic MHC and MRF expression. The wet weights of CMKLR1+/+ [wild-type (WT)], CMKLR1+/− (HET), and CMKLR1−/− [knockout (KO)] embryos were measured at E12.5 and E20.5 (A). Each bar is the mean ± SE of 6 embryos. Representative photographs of the embryos at E12.5 (B) and E20.5 (C). The scales shown in B and C are in cm. *P < 0.05, significantly lower weight compared with the CMKLR1+/+ mice, one-way ANOVA followed by the Bonferroni multiple-comparison test. Myf5, MyoD, and MHC proteins were detected by immunostaining in E12.5 longitudinal embryonic sections from CMKLR1+/+, CMKLR1+/−, and CMKLR1−/− mice. The cell nuclei were counterstained with Hoechst 33258. Representative photomicrographs of DAPI-counterstained embryonic sections at E12.5 (D) illustrate gross morphology. The area bounded by the white squares (D) encompasses the trunk somites in the hind-thoracic region of embryos and corresponds to the magnified regions shown for Myf5 (green, E), MyoD (red, F), and MHC (red, G) protein expression by immunostaining. The white arrowheads show examples of the myotomal components of somites, and the white arrows show elongated myotubes. Each image for Myf5, MyoD, and MHC staining is representative of 3 embryos with similar results. Scale bars = 800 μm (D) and 100 μm (E–G).
Table 2.
Dual-energy X-ray absorptiometry analysis of body composition of adult male CMKLR1+/+ and CMKLR1−/− mice
| Characteristic | CMKLR1+/+ Mice | CMKLR1−/− Mice |
|---|---|---|
| Age, days | 68 ± 1 | 68 ± 1 |
| Total body wt, g | 25.8 ± 0.2 | 22.7 ± 0.4* |
| ROI | ||
| Weight, g | 24.6 ± 0.3 | 21.4 ± 0.3* |
| Lean mass, g | 20.5 ± 0.6 | 18.1 ± 0.2* |
| Fat mass, g | 4.1 ± 0.7 | 3.3 ± 0.2 |
Each value represents the mean ± SE of 5 individual animals. ROI, region of interest for detection and includes the entire mouse minus the head.
P < 0.05, significantly lower than the CMKLR1+/+ mice, unpaired t-test.
We next examined E12.5 whole embryo sections from CMKLR1+/+, CMKLR1+/−, and CMKLR1−/− mice for evidence of abnormal skeletal muscle development by staining for Myf5, MyoD, and MHC proteins in the myotomal region of trunk somites. In representative images of DAPI-counterstained E12.5 embryonic cross sections, the CMKLR1−/− embryos appear smaller and generally less developed compared with the CMKLR1+/+ and CMKLR1+/− mice at the same embryonic stage (Fig. 6D). Immunohistochemical staining of Myf5 was localized to cell nuclei, and the Myf5-positve cells appeared generally dispersed throughout the somites. In the somites of the CMKLR1−/− mice, there were more Myf5-positive cells, and the Myf5 staining appeared more intense compared with the CMKLR1+/+ and CMKLR1+/− embryos (Fig. 6E). The cellular localization of MyoD was primarily nuclear, but, unlike Myf5, the MyoD-positive cells were restricted to the myotomal component of somites and were reduced in the CMKLR1 knockout compared with wild-type and heterozygous E12.5 embryos (Fig. 6F). At E12.5, MHC showed a similar localization of staining compared with MyoD (Fig. 6G). The cellular localization of MHC was cytoplasmic, and the presence of differentiated myotubes is evident from the appearance of many long spindle-like shaped cells (Fig. 6G). Like for MyoD, the area of MHC-positive staining in the CMKLR1−/− embryos is markedly limited in distribution compared with wild-type and heterozygous embryos.
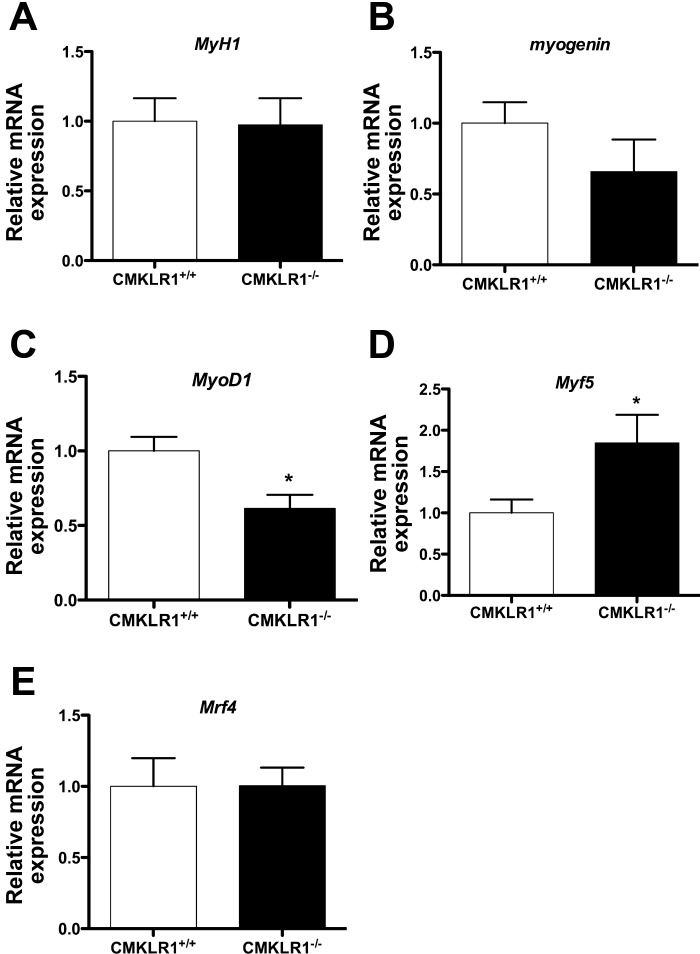
Finally, we examined the gene expression profile of key myogenic genes in adult mouse skeletal muscle. Similar to C2C12 myoblasts, skeletal muscle from 12-wk-old adult CMKLR1−/− mice expressed significantly lower levels of MyoD1, significantly higher levels of Myf5, and showed a trend toward lower myogenin expression compared with the wild-type controls (Fig. 7).
Fig. 7.
Altered MRF gene expression in CMKLR1−/− mice. The relative expression of MyH1, Myogenin, MyoD1, Myf5, and Mrf4 genes in the gastrocnemius muscle from 12-wk-old WT and CMKLR1 KO mice was measured by QPCR and normalized to the expression of the reference gene cyclophilin A. Each bar represents the mean ± SE of 5 samples. *P < 0.05 compared with the CMKLR1+/+ mice, unpaired t-test.
DISCUSSION
Herein we report that the loss of CMKLR1 expression by C2C12 myoblasts impairs effective myogenesis in vitro and that CMKLR1 deficiency in vivo is associated with alterations in embryonic musculature, reduced embryo mass, and reduced lean mass and body weight in adult mice. In previous studies, we showed that CMKLR1 is required for proper adipogenesis in 3T3-L1 cells, primary bMSCs, and preosteoblast 7F2 cells (8, 21). CMKLR1 knockdown also increased the propensity of bMSCs to adopt an osteoblastogenic phenotype (21). Taken together with these earlier findings, our current results support a general role for CMKLR1 in directing the terminal differentiation of multiple cell lineages, including osteoblasts, adipocytes, and now myoblasts.
C2C12 cells are a committed myoblast cell population that is predetermined to undergo terminal differentiation, activation of muscle-specific genes (e.g., myosin, actin, desmin, and troponin), and fusion to form elongated multinucleated myotubes (2). Our findings that the CR-shRNA-treated cells displayed normal myogenic-dependent changes in α-actin, desmin, and β-actin mRNA and increases in creatine kinase activity indicate that C2C12 cells are capable of initiating some aspects of myogenic differentiation in the absence of CMKLR1. In contrast, lower plateau levels of MyH1 mRNA, a reduced myogenic index, and an earlier decline in vimentin, an intermediate filament protein that normally decreases during myogenesis, would indicate that CMKLR1 influences the complete terminal differentiation into myotubes (26). The unique phenotype produced by CMKLR1 knockdown in C2C12 cells in our study is not unlike that previously observed in the mouse-derived skeletal muscle-like BC3H1 cell line. Under myogenic differentiation conditions, the BC3H1 cells turn on a number of muscle-specific genes, including creatine kinase, insulin receptors, MHC, and α-actin but are intrinsically defective for commitment to fusion and terminal differentiation into myotubes (3, 31, 33). The ability of the C2C12 cells to retain some characteristics of skeletal muscles cells in the face of reduced myogenesis also suggests that CMKLR1 function is important in specific myogenic pathways.
While we detected low apparent concentrations (20 pM) of bioactive chemerin in the C2C12 cell media relative to 3T3-L1 adipocyte media, chemerin in the low picomolar to low nanomolar mediates biological responses through CMKLR1 (4, 6, 8, 17, 37). Thus, the chemerin concentration in the C2C12 media should be high enough for basal activation of the receptor providing for a mechanism whereby CMKLR1 knockdown inhibits C2C12 cell myogenesis. The finding that knockdown of CMKLR1 in C2C12 cells impairs myoblast differentiation in the presence of low endogenous bioactive chemerin is consistent with our previous finding that knockdown of CMKLR1 in preadipocytes (when bioactive chemerin levels are very low or undetectable) prevents those cells from undergoing adipogenesis (8).
Myogenesis is executed by the conserved family of muscle-specific basic helix-loop-helix transcription factors called MRFs, which include MyoD, Myf5, myogenin, and Mrf4 (25). Work completed by Dedieu and coworkers (5) identified that inhibition of MyoD, Myf5, and myogenin with antisense oligonucleotides in C2C12 cells blocks myogenic differentiation to varying degrees. Compared with the forced expression of MyoD, Myf5, Mrf4, and myogenin which convert nonmuscular cells into cells capable of myocyte fusion and muscle-specific gene expression (18). Therefore, following CMKLR1 knockdown, it is possible that the elevation in Myf5, which precedes the changes in other MRFs, could prompt the cells to begin terminal differentiation. However, this effect is then opposed by a loss in myogenin and MyoD at later points leading to a failure of myocyte fusion, as demonstrated by the reduced myogenic index following CMKLR1 knockdown. In support of this idea, inhibition of myogenin in C2C12 cells partially arrests myogenic fusion, whereas inhibition of MyoD completely arrests myogenic fusion in the presence of normal Myf5 expression (5). The reduction of integrin β1 and calpain 3, two genes implicated in skeletal muscle cell fusion, on day 5 post-CMKLR1 knockdown further supports a role for CMKLR1 signaling in late-stage myogenic differentiation (27).
In addition to the C2C12 cell line, we have performed a preliminary analysis of myogenic differentiation of primary myoblasts isolated from skeletal muscle of 5-day-old CMKLR1+/+ and CMKLR1−/− mice (data not shown). Myotube formation appeared to be somewhat lower in the CMKLR1−/− vs. CMKLR1+/+ myoblast cultures. We also detected clumps of aggregated cells on the edges of the fused CMKLR1−/− myotubes, a phenotype that was not apparent in the CMKLR1+/+ myoblast cultures. The initial data support an altered myogenic phenotype in the absence of CMKLR1, but further experiments are required to confirm the findings.
Chemerin signaling through CMKLR1 has been reported to modify glucose homeostasis in vitro and in vivo. Sell et al. (28) found that chemerin-treated skeletal muscle cells displayed a minor decrease in basal glucose uptake and a significant decrease in insulin-stimulated glucose uptake (28). We previously demonstrated that chemerin treatment exacerbated glucose intolerance in leptin and leptin receptor-deficient mice by decreasing skeletal muscle glucose uptake (7). Coincidently, skeletal muscle CMKLR1 expression is significantly higher in skeletal muscle of ob/ob and db/db mouse models (7). Our present results, which show that CMKLR1 knockdown increased basal and insulin-stimulated glucose uptake in C2C12 myotubes, are in agreement with these previous studies. Together, these results support the notion that activation of CMKLR1 signaling negatively modulates, whereas removal of CMKLR1 positively modulates, insulin-mediated glucose uptake into skeletal muscle cells.
Given the severe impairment of myoblast differentiation observed in vitro and previous reports of embryonic CMKLR1 expression in the limb bud mesenchyme and myotome (E9.5), migratory myoblasts (E10.5), and various other muscle tissue depots (E14.5), we investigated CMKLR1-deficient embryos and adult mice for muscular abnormalities (12). The recent study by Harewood et al. (12) reported that CMKLR1−/− knockout mouse embryos had no obvious limb phenotype. Similarly, our evaluation of embryonic mice revealed no overt limb phenotype in either the heterozygous or homozygous CMKLR1 knockout mice. However, our results are indicative of a generalized embryonic developmental delay in the absence of CMKLR1. Furthermore, we observed a diminished total area of MHC immunolocalization in the myotomal component of somites of E12.5 CMKLR1−/− embryonic mice, which indicated the possibility of a subtle muscle developmental abnormality. Reduced skeletal muscle likely contributes to the low comparative weight of the CMKLR1+/− and CMKLR1−/− embryos; however, at present, we cannot rule out accompanying changes in other tissues during development. Our observations that adult CMKLR1−/− mice are smaller, have less bone-free lean mass, and, like CMKLR1-deficient C2C12 cells, express higher Myf5, lower MyoD, and a trend to lower myogenin expression would suggest that the changes initiated in the embryonic stages persist into adulthood. A limitation of the CMKLR1 knockout mouse model is that we do not know for certain if the in vivo phenotypic changes to skeletal muscle specifically arise due to CMKLR1 ablation in skeletal muscle progenitors. There is the possibility of indirect effects due to loss of CMKLR1 in mesenchymal stem cells, hematopoietic cells, and/or other developing organs (8, 21, 37, 39). This question may be resolved in the future through conditional CMKLR1 knockout in the myogenic lineage.
There is considerable redundancy in the function of myogenic transcription factors during skeletal muscle development. For instance, mice deficient in MyoD or Myf5 are viable and, respectively, only show subtle developmental delays in hypaxial and epaxial muscle development (13). More recently, the overlapping functions of Myf5 and MyoD have been attributed to the presence of distinct Myf5-dependent and Myf5-independent cellular lineages in myogenesis. When the Myf5 lineage is depleted, myogenesis is sustained by MyoD-expressing myoblasts (11). We observed a decrease in MyoD protein and an increase in Myf5 protein expression in the trunk somites at E12.5. Therefore, it is possible that the absence of a severe muscle deficiency in the homozygous CMKLR1 knockout mice reflects cellular compensation through an increase in the Myf5-myogenic cell lineage.
In conclusion, the results of this study reveal a novel involvement for CMKLR1 in myogenic differentiation in vitro and suggest an important role for CMKLR1 in proper muscle development in vivo. Ongoing research in our laboratories is focused on understanding how the embryonic alterations caused by CMKLR1 deficiency affect the development, function, and regeneration of skeletal muscle during postnatal life.
GRANTS
The research project was funded by grants from the Canadian Foundation for Innovation (K. B. Goralski), the Canadian Institutes of Health Research (K. B. Goralski and C. J. Sinal), the Nova Scotia Health Research Foundation (K. B. Goralski), the Pharmacy Endowment (K. B. Goralski), the Dalhousie Medical Research Foundation (K. B. Goralski), and the Faculty of Health Professions, Dalhousie University (K. B. Goralski). B. A. Zabel is supported by National Institute of Allergy and Infectious Diseases Grant AI-079320. E. C. Butcher is supported by grants from the National Institutes of Health and a Merit Award from the Department of Veterans Affairs. Trainees are supported through Nova Scotia Health Research Foundation Scholarships (M. C. Ernst., S. D. Parlee, and S. Muruganandan) and the Canadian Institute for Health Research Canada Graduate Scholarship (S. D. Parlee).
DISCLOSURES
The authors have no disclosures or conflicts of interest to report.
AUTHOR CONTRIBUTIONS
Author contributions: M.E.I., B.A.Z., E.C.B., C.S., and K.B.G. conception and design of research; M.E.I., S.M., M.C.E., S.D.P., and K.B.G. performed experiments; M.E.I., S.M., M.C.E., S.D.P., and K.B.G. analyzed data; M.E.I., S.M., M.C.E., S.D.P., B.A.Z., and K.B.G. interpreted results of experiments; M.E.I., S.M., and K.B.G. prepared figures; M.E.I. drafted manuscript; M.E.I., S.M., M.C.E., S.D.P., B.A.Z., C.S., and K.B.G. edited and revised manuscript; M.E.I., S.M., M.C.E., S.D.P., B.A.Z., E.C.B., C.S., and K.B.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tanya McCarthy and Kathleen Nason for assisting with quantitative polymerase chain reaction analysis and Dr. Kishore Pasumarthi for use of the fluorescence microscope.
Preliminary results of the study have been presented as an abstract and poster at Experimental Biology, New Orleans, LA, April 19-24, 2009, and at the annual meeting of the Association of Faculties of Pharmacy of Canada, Halifax, Nova Scotia, June 5, 2009.
REFERENCES
- 1. Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA 105: 64–69, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science 230: 758–766, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Brennan TJ, Edmondson DG, Olson EN. Aberrant regulation of MyoD1 contributes to the partially defective myogenic phenotype of BC3H1 cells. J Cell Biol 110: 929–937, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 205: 767–775, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dedieu S, Mazeres G, Cottin P, Brustis JJ. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int J Dev Biol 46: 235–241, 2002 [PubMed] [Google Scholar]
- 6. Du XY, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J Biol Chem 284: 751–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 151: 1998–2007, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282: 28175–28188, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Goralski KB, Sinal CJ. Elucidation of chemerin and chemokine-like receptor-1 function in adipocytes by adenoviral-mediated shRNA knockdown of gene expression. Methods Enzymol 460: 289–312, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Graham KL, Zabel BA, Loghavi S, Zuniga LA, Ho PP, Sobel RA, Butcher EC. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J Immunol 183: 6717–6723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haldar M, Karan G, Tvrdik P, Capecchi MR. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev Cell 14: 437–445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harewood L, Keeling JW, Fantes JA, Opitz JM, FitzPatrick DR. “Crommelin-type” symmetrical tetramelic reduction deformity: a new case and breakpoint mapping of a reported case with de-novo t(2;12) (p25.1;q233). Clin Dysmorphol 19: 5–13 [DOI] [PubMed] [Google Scholar]
- 13. Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 124: 4729–4738, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, Severa M, Parmentier M, Ristori G, Salvetti M, Aloisi F, Coccia EM. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol 67: 388–401, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 17. Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol 183: 6489–6499, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB J 9: 1595–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, Dimmitt R, Saeed S, Randolph DA, Aprahamian C, Datta G, Ohls RK. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol 297: G1–G10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muruganandan S, Parlee SD, Rourke JL, Ernst MC, Goralski KB, Sinal CJ. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem 286: 23982–23995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muruganandan S, Roman AA, Sinal CJ. Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res 25: 222–234, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8: 392–398, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Parlee SD, Ernst MC, Muruganandan S, Sinal CJ, Goralski KB. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-α. Endocrinology 151: 2590–2602, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, Moretta A, Sozzani S. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109: 3625–3632, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet 57: 16–25, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Sax CM, Farrell FX, Zehner ZE. Down-regulation of vimentin gene expression during myogenesis is controlled by a 5′-flanking sequence. Gene 78: 235–242, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell 4: 673–685, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 58: 2731–2740, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skrzeczynska-Moncznik J, Stefanska A, Zabel BA, Kapinska-Mrowiecka M, Butcher EC, Cichy J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim Pol 56: 355–360, 2009 [PMC free article] [PubMed] [Google Scholar]
- 30. Skrzeczynska-Moncznik J, Wawro K, Stefanska A, Oleszycka E, Kulig P, Zabel BA, Sulkowski M, Kapinska-Mrowiecka M, Czubak-Macugowska M, Butcher EC, Cichy J. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem Biophys Res Commun 380: 323–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Standaert ML, Schimmel SD, Pollet RJ. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem 259: 2337–2345, 1984 [PubMed] [Google Scholar]
- 32. Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O'Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 58: 1971–1977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taubman MB, Smith CW, Izumo S, Grant JW, Endo T, Andreadis A, Nadal-Ginard B. The expression of sarcomeric muscle-specific contractile protein genes in BC3H1 cells: BC3H1 cells resemble skeletal myoblasts that are defective for commitment to terminal differentiation. J Cell Biol 108: 1799–1806, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Velica P, Khanim FL, Bunce CM. Prostaglandin D2 inhibits C2C12 myogenesis. Mol Cell Endocrinol 319: 71–78, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, Sironi M, Mantovani A, Parmentier M, Facchetti F, Sozzani S. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 201: 509–515, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol (Oxf) 72: 342–348, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198: 977–985, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, Guido DG, Handel TM, Butcher EC. Chemokine-like receptor 1 expression by macrophages in vivo: Regulation by TGF-beta and TLR ligands. Exp Hematol 34: 1106–1114, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol 174: 244–251, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Zabel BA, Zuniga L, Ohyama T, Allen SJ, Cichy J, Handel TM, Butcher EC. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol 34: 1021–1032, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Zhang W, Zhao L, Mulholland MW. Ghrelin stimulates myocyte development. Cell Physiol Biochem 20: 659–664, 2007 [DOI] [PubMed] [Google Scholar]