Abstract
Transient receptor potential melastatin 7 (TRPM7) channels were originally identified electrophysiologically when depletion of cytosolic Mg2+ resulted in the gradual development of an outwardly rectifying cation current. Conversely, inclusion of millimolar Mg2+ in internal solutions prevented activation of these channels in whole cell patch clamp. We recently demonstrated that the Jurkat T-cell whole cell TRPM7 channels are inhibited by internal Mg2+ in a biphasic manner, displaying high [IC50(1) ≈ 10 μM] and low [IC50(2) ≈ 165 μM] affinity inhibitor sites. In that study, we had characterized the dependence of the maximum cell current density on intracellular Mg2+ concentration. To characterize Mg2+ inhibition in Jurkat T cells in more detail and compare it to whole cell results, we recorded single TRPM7 channels in cell-free membrane patches and investigated the dependence of their activity on Mg2+ added on the cytoplasmic side. We systematically varied free Mg2+ from 265 nM to 407 μM and evaluated the extent of channel inhibition in inside-out patch for 58 patches. We found that the TRPM7 channel shows two conductance levels of 39.0 pS (γ1) and 18.6 pS (γ2) and that both are reversibly inhibited by internal Mg2+. The 39.0-pS conductance is the dominant state of the channel, observed most frequently in this recording configuration. The dose-response relation in inside-out patches shows a steeper Mg2+ dependence than in whole cell, yielding IC50(1) of 25.1 μM and IC50(2) of 91.2 μM.. Single-channel analysis shows that the primary effect of Mg2+ in multichannel patches is a reversible reduction of the number of conducting channels (No). Additionally, at high Mg2+ concentrations, we observed a saturating 20% reduction in unitary conductance (γ1). Thus Mg2+ inhibition in whole cell can be explained by a drop in individual participating channels and a modest reduction in conductance. We also found that TRPM7 channels in some patches were not sensitive to this ion at submaximal Mg2+ concentrations. Interestingly, Mg2+ inhibition showed the property of use dependence: with repeated applications, Mg2+ effect became gradually more potent, which suggests that Mg2+ sensitivity of the channel is a dynamic characteristic that depends on other membrane factors.
Keywords: inside-out patch, electrophysiology, magnesium-inhibited cation channel, divalent cation, charge screening, transient receptor potential melastatin 7
transient receptor potential melastatin 7 (TRPM7) proteins belong to the small group of human ion channels fused to functional cytoplasmic enzymes, which also includes TRPM6 and TRPM2. In the case of TRPM6 and TRPM7, which possess close primary sequence similarity, the enzyme portion is an atypical serine/threonine kinase (2, 36, 45, 50, 59), whereas in TRPM2, the enzyme entity is a ADP ribose pyrophosphatase (16, 40). The channel portion of these proteins belongs to the TRP superfamily of cation-conducting channels (43).
Jurkat T lymphocytes have been widely used in studies of human T-lymphocyte signal transduction events that underlie T-cell receptor-dependent activation and proliferation. Thus several key steps in T-lymphocyte signaling were initially identified in Jurkat T cells: the requirement for calcium influx for efficient activation, discoveries of nuclear factor of activated T-cell transcription factor, its calcineurin dependence, and regulation of gene expression by calcium oscillations (8, 12, 13, 28, 56). The ion channel expression profile in Jurkat T cells is also similar to that of normal human peripheral blood lymphocytes and includes Orai1–3/STIM1–2 store-operated Ca2+ channels, Kv1.3 voltage-gated potassium channels, volume-activated chloride channels, and voltage-activated proton channels; however, some differences have also been documented (5, 49). Like normal human T lymphocytes, Jurkat T cells lack voltage-gated Ca2+ channels. Among TRP family channels, expression of functional TRPM2 and TRPM4 has been reported (25, 48). In some instances, Jurkat T cells express higher levels of normal T-cell channels, as is the case with TRPM7 and calcium-relase activated calcium channels, making their study easier technically (21, 31, 39, 61; Kozak JA, unpublished observations). It is thought that calcium- and voltage-activated K+ channels and calcium-release activated calcium channels are necessary for T-cell activation by mitogens and antigens by virtue of their hyperpolarizing action on the plasma membrane and augmented Ca2+ entry (28). Jurkat T cells undergo many of the same early steps of activation as human peripheral blood T lymphocytes and are employed in the studies of immunological synapse structure and formation dynamics in vitro (9, 18, 60). In their capacity as a human leukemic line (52), Jurkat T cells are often used for testing anticancer small molecule compounds and biologics (e.g., Refs. 10, 22).
In the present study, we focused on the properties of the most highly expressed TRP channel in human T cells, TRPM7 (5, 21, 42). The physiological hallmark of TRPM7 is its sensitivity to intracellular Mg2+. Upon depletion of cellular Mg2+, TRPM7 channels open gradually giving rise to Mg2+-inhibited cation (MIC) currents (20, 42). We (6) recently characterized the Mg2+ dependence of Jurkat T-cell native TRPM7 channels in the whole cell configuration. We showed that TRPM7 channels are inhibited by cytosolic Mg2+ in a biphasic manner, with IC50 values of 10.2 and 165 μM free Mg2+. We also showed that despite these relatively low values compared with estimates of T-cell Mg2+ content, most Jurkat T cells show some degree of preactivated TRPM7 current even when intracellular Mg2+ depletion has not taken place. The reasons for this remain unknown and point to additional cellular factors regulating this channel. The presence of active TRPM7 conductance could result in regulation of resting membrane potential and consequently of cellular Ca2+ metabolism. In addition to Mg2+, TRPM7 channels are sensitive to intracellular pH and organic cations, such as polyamines. We (21) have suggested that inhibition occurs by a charge screening mechanism, since cations with poor screening ability are also ineffective in inhibiting TRPM7 channels. This view of Mg2+ and polyamine inhibitory action has since been adopted by other investigators studying other ion channels (e.g., Refs. 1, 41).
Removal of extracellular divalent cations results in a drastic change of TRPM7 current-voltage relation: from steeply outwardly rectifying it becomes semilinear (7, 19, 20, 42). Addition of micromolar concentrations of Mg2+ or other cations to a divalent-free buffer demonstrates that TRPM7 channels are also extremely sensitive to extracellular polyvalent cations. However, unlike the case for intracellular cations, this interaction represents ion conduction pore block and does not involve surface charge screening (19–21, 23). Moreover, the extracellular cation blocking sites are not accessible from the cytoplasmic side, and therefore, inhibition by internal Mg2+ or a polyamine molecule does not cause modifications of current-voltage relation (21). Conversely, at millimolar concentrations, extracellular Mg2+ does not elicit slow voltage-independent current inhibition (19, 21). In the case of l-type calcium channels, for example, the Mg2+ blocking site can be reached both from inside and outside (23). In summary, the processes of internal and external blockade of TRPM7 channels by Mg2+ at first approximation do not interact.
We (21) previously demonstrated that for the recombinant TRPM7 channels Mg2+ inhibits reversibly in inside-out macropatches pulled from Chinese hamster ovary cells. Channel activity ran down when intracellular solutions contained micromolar Mg2+, but the presence of EDTA, a strong Mg2+ chelator, prevented rundown. In addition to EDTA, channel rundown could be reversed by alkalinizing the cytoplasm or by applying phospholipid phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] in its short chain form to membrane patches (21, 46). Heterologously expressed TRPM7 channels can be readily recorded in inside-out patches as macroscopic current. However, since most cell lines commonly used in overexpression studies have substantial endogenous TRPM7 channels (discussed in Ref. 6), unitary channel resolution becomes problematic because it is difficult to determine with certainty if the detected single-channel activity is due to the overexpressed or endogenous channels. In view of this methodological issue, we set out to characterize Mg2+ sensitivity of human TRPM7 channels at the single-channel level in a native system, the Jurkat T lymphocyte.
Mg2+ sensitivity of native TRPM7 channels in cell-free patches has not been studied in detail. To characterize the mechanism of TRPM7 channel inhibition by Mg2+, we performed current measurements in inside-out configuration of patch-clamp and constructed Mg2+ dose-response relations by systematically increasing the free Mg2+ concentration applied to patches. Superimposition of cell-free and whole cell dose-response data showed an overall correspondence with an increased variability for cell-free patches. Mg2+ dependence in isolated membrane patches appears to be steeper than in whole cell; thus, in whole cell, Mg2+ inhibited with IC50 values of 10.2 and 165.0 μM, whereas in cell-free patches two IC50 sites of 25.1 μM and 91.2 μM were evident. We found that TRPM7 channels in Jurkat T cells exhibit two conductance states: 39.0 ± 2.47 pS (γ1 ± SD) and 18.6 ± 2.19 pS (γ2 ± SD). The γ1 conductance was observed most frequently and was reduced by ∼20% in Mg2+ with a single IC50 value of 93.5 μM. The main effect of Mg2+ in cell-free patches appears to be a reversible reduction in the number of conducting channels.
MATERIALS AND METHODS
Cell Culture
Human Jurkat T lymphocytes (kindly provided by Dr. Tom L. Brown) were grown in a humidified atmosphere of 95% air-5% CO2 (Napco 8000 CO2 incubator; Thermo) in RPMI-1640 medium supplemented with l-glutamine (Lonza, Wakersville, MD) and 10% FBS (BioWest, Nuaille, France). Antibiotics were not used. Cells were passaged twice a week by diluting cell suspensions 10- to 20-fold in a fresh medium. Tests of mycoplasma presence were not conducted.
Patch-Clamp Electrophysiology
Patch-clamp recordings were conducted in the inside-out (cell-free) patch mode (57) using EPC10 patch clamp amplifier (HEKA Elektronik, Lambrecht, Germany). Pipettes were manufactured from borosilicate capillary glass (Warner Instruments, Hamden, CT) on a robotic puller (Zeitz Instruments, Martinsried, Germany). Glass capillaries had an outer diameter of 1.2 mm and an inner diameter of 0.93 mm. Patch electrode resistances were 1.5–3.5 MΩ.
In normal Ca2+- and Mg2+-containing bathing buffers, TRPM7 maintains its outwardly rectifying current-voltage relation with small inward component as a consequence of tonic blockade by these cations. In the absence of external divalent cations, TRPM7 (magnesium-inhibited cation) channel unitary conductance in the inward direction jumps to ∼40 pS (19, 20). The TRPM7 channel is steeply outwardly rectifying in the presence of external millimolar Ca2+ and Mg2+ and unitary channel activity cannot be resolved at hyperpolarized potentials due to its small amplitude. For these reasons, we performed all experiments using divalent cation-free pipette (extracellular) solutions and at negative membrane potentials. This gave us the ability to detect single-channel activity in cell-free patches from Jurkat T cells. Most recordings were performed at −90 mV, a voltage at which the patch remains stable for long periods of time, but some recordings were done at −100 and −120 mV with similar results. Data traces were analyzed with WinEDR (v. 3.2.6) available from the Strathclyde Electrophysiology Software suite (11) and Origin v.8 software (OriginLab, Northampton, MA). Current recordings were low-pass filtered at 2.9 kHz and digitized at a sampling rate of 6 kHz. For depiction of raw traces, the data were filtered offline to 0.1–0.4 Hz. In presented raw current recordings, gaps (not shown to scale) represent stops in acquisition due to the perfusion system that lasted ∼4 s. The pipette (external) solution contained 10 mM HEDTA, 106 mM Cs aspartate, and 10 mM HEPES, pH 7.3. Cs+, rather than Na+ was used as the main cation because TRPM7 channel conducts this ion slightly better than Na+ measured in the absence of divalent metal ions based on reversal potential comparison (20). Additionally, the use of Cs+ is expected to reduce contamination from K+ channels endogenous to Jurkat T lymphocytes. Osmolalities of the bath and pipette recording solutions were measured with a freezing point depression osmometer (Precision Systems, Natick, MA) and adjusted to 290–300 mosmol/kgH2O with d-mannitol. Gigaohm seals were formed on Jurkat T cell bathed in divalent-free bath solution by applying brief gentle suction. Suction was relieved after gigaohm seal formation, and patches were excised by lifting the pipette away from the cell with a motorized manipulator. Solutions on the inner face of the patch were exchanged by a gravity-fed rapid perfusion system SF-77B equipped with a motor-driven stepper (Warner Instruments, Hamden, CT). We estimated the speed of exchange with this system at <1 s. After excision, the patch was positioned facing sequentially one of the two glass barrels supplying the control and Mg2+-containing buffers. These solutions were applied repeatedly to the same patch, and each patch was exposed to one concentration of Mg2+ only, similar to the situation that exists in whole cell recording. Nitric acid-washed glass coverslips were coated with 1 mg/ml poly-l-lysine (Sigma-Aldrich, St. Louis, MO) . On the day of experiment, suspensions of Jurkat cells were placed on the coverslips for attachment. For patch-clamp recordings, the glass coverslip with attached cells was transferred to a glass-bottom plexiglass recording chamber mounted on a mechanical stage of a Nikon Diaphot inverted microscope. The microscope, micromanipulators, and the perfusion system were enclosed in a grounded Faraday cage made of copper mesh to reduce electrical noise. All experiments were performed at room temperature.
The basal intracellular (bath) solution was similar to the pipette solution we previously used to characterize the whole cell TRPM7 current and contained 112 mM Cs-glutamate, 8 mM NaCl, 10 mM HEDTA, and 10 mM HEPES pH 7.3 (6). Aspartate and glutamate salts were used to reduce contamination by chloride channels (5, 29). Patches were exposed to Mg2+-containing solutions for 10–30 s, sufficient time to observe an inhibitory effect. To distinguish inhibition from channel rundown, only experiments in which Mg2+ inhibition was fully or partially reversible upon washout were considered in the present study (20, 21). Free Mg2+ concentrations in HEDTA-containing solutions were estimated using MaxChelator software available at http://www.stanford.edu/∼cpatton/webmaxcS.htm. The extent of Mg2+ inhibition for dose-response relation data (see Fig. 6A) was determined by taking the area under a 5-s duration segment of channel open probability (NPo) plot immediately before Mg2+ application and dividing by a 5-s area taken immediately before washout (53). In the text, we refer to changes in Mg2+ sensitivity of TRPM7 channel with repeated application of the same Mg2+ concentration as “use dependence,” a term used by others to describe changes in drug potency during repeated activation of channels (e.g., Refs. 24, 55).
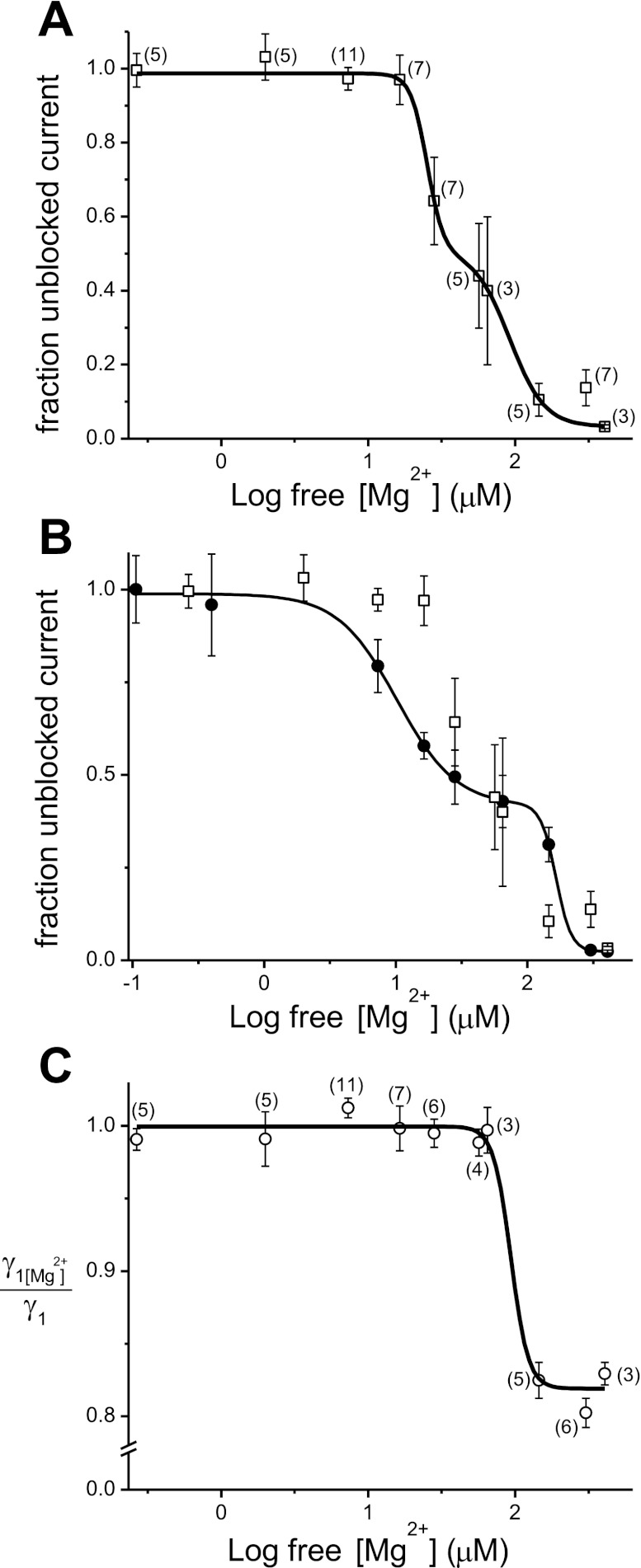
Fig. 6.
Dose-response relations for Mg2+ inhibition of TRPM7 channels in cell-free patches. Comparison to whole cell dose-response curve. A: channel activity plotted against free [Mg2+] as fraction unblocked current for the first application of Mg2+. Data were fitted with biphasic dose-response curve (adjusted R-square 0.99) giving IC50(1) = 25.1 μM and IC50(2)= 91.2 μM and Hill coefficients of 3.65 and 7.78.. Each point was generated from pooled and averaged inhibition data obtained from different patches exposed to the same Mg2+ concentration. Membranes were held at −90 mV. B: superimposed Mg2+ dose-response curves obtained from single-channel (□) and whole cell (●) recordings (after Fig. 2 in Ref. 6) using a logarithmic x-axis. Fitted curve represents a biphasic dose-response curve with IC50(1) = 10.2 and IC50(2) = 165.0 μM and Hill coefficients of 1.92 and 7.62 for whole cell data. Note that the whole cell dose-response relation was obtained from measurements taken at +85 mV in the presence of extracellular calcium and sodium. C: dependence of single-channel conductance γ1 on Mg2+ analyzed from recordings used in A. Ratios of unitary current amplitudes in Mg2+ and after washout were determined for each [Mg2+] applied multiple times. Data are fitted with a single dose-response curve (adjusted R-square 0.98) with IC50 93.47 μM and Hill coefficient of 7.36. Vertical bars represent SE. Number in brackets near the symbol represents the number of patches tested for the given [Mg2+]. Patches used to test 265.8 nM (n = 5) and 2 μM (n = 5) Mg2+ effects were exposed to inhibitory concentration of 303 μM at the end of each experiment to verify sensitivity to higher Mg2+ concentrations.
The measured reversal potential of whole cell TRPM7 current in internal and external solutions used for single-channel recording was −1.86 mV (SE = 0.12, n = 3 cells), and this value was used to calculate unitary channel conductance. Dependence of γ1 (39.0 pS) conductance on Mg2+ was estimated by determining the mean unitary current amplitude at −90 mV in the presence of a given Mg2+ concentration for each membrane patch and dividing this value by the amplitude in the same patch after washout of Mg2+. The ratios for each Mg2+ concentration were then averaged (see Fig. 6C). Data were fitted with biphasic (see Fig. 6A) and single dose-response (see Fig. 6C) curves using Origin 8 software.
Water with resistivity of ≥17.4 MΩ/cm (Barnstead Nanopure) was used for solution preparation. Salts were purchased from Acros Organics (Geel, Belgium) and Sigma-Aldrich. A 1.0 M MgCl2 standard solution (Fluka) was diluted to obtain the desired MgCl2 concentration.
RESULTS
Internal Mg2+ Sensitivity of Monovalent TRPM7 Channels in Inside-Out Patches
Mg2+ effect on NPo.
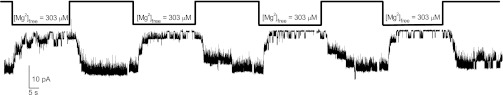
We showed previously that that the native TRPM7 current of Jurkat T lymphocytes (MIC) is inhibited in a biphasic manner by intracellular Mg2+ with IC50 values of 10 and 165 μM (6). Free Mg2+ concentrations of 303 μM and higher inhibit the whole cell current almost completely. We set out to investigate the Mg2+ sensitivity of TRPM7 channels in the inside-out patch configuration, enabling us to explore this phenomenon in more detail and compare to our macroscopic data. In whole cell recording, when internal Mg2+ concentration is in low micromolar range, the MIC currents undergo time-dependent reduction of activity that we refer to as rundown (20). Reduction of free Mg2+ down to nanomolar abolishes rundown (21). In whole cell recording method, however, reversible inhibition by increasing Mg2+ concentrations cannot be distinguished from rundown of activity in the presence of Mg2+. The time course of Mg2+ inhibition cannot be resolved in whole cell either, since the cytoplasm is exposed to one internal Mg2+ concentration at all times starting with break-in: thus channels open, close, and run down in the presence of submaximal concentrations of Mg2+. Figure 1 shows an inside-out patch recording of TRPM7 channel activity. In our experiments, most patches pulled from Jurkat T cells contained more than one active channel. In pilot experiments, we observed quick rundown when 12 mM EGTA was used in the bath (with no Mg2+ added), presumably due to residual contaminating micromolar Mg2+, which is not removed by this relatively weak Mg2+ chelator. In the present study, therefore, we used 10 mM HEDTA, a strong Mg2+ chelator, in all our bath (intracellular) solutions that drastically reduced spontaneous channel rundown, as in the case of whole cell recordings. The patch in Fig. 1 was excised into 10 mM HEDTA with no added Mg2+ (see materials and methods) and repeatedly exposed to 303 μM free Mg2+. Each Mg2+ application produced a fast and drastic inhibition of the current manifested as sequential closings of active channels in the patch. Mg2+ inhibition was reversible upon washout with the basal Mg2+-free solution, manifested as stepwise reopening of presumably the same individual channels. We conclude from these data that Mg2+ inhibits TRPM7 channels reversibly, rather than reducing current magnitude by causing monotonic rundown. This lack of quick rundown in patches exposed to Mg2+ multiple times was somewhat surprising to us. Mg2+ reportedly induces channel rundown by activating Mg2+-sensitive lipid phosphatases and depleting membrane phosphoinositides (e.g., Refs. 27, 30). In Jurkat T-cell patches, however, Mg2+ effect was almost fully reversible in the absence of ATP (Fig. 1), which is necessary for membrane PI(4,5)P2 resynthesis (14, 17, 58).
Fig. 1.
Reversible inhibition of transient receptor potential melastatin 7 (TRPM7) channels by 303 μM Mg2+ in an inside-out patch excised from a Jurkat T lymphocyte. Mg2+ was applied multiple times directly to the inner face of the membrane. Current reduction was fully reversible after each application. Note that with third and fourth applications the current magnitude is gradually reduced due to slow rundown. Membrane was held at −90 mV. Representative experiment chosen from n = 7 patches. [Mg2+]free, free Mg2+ concentration.
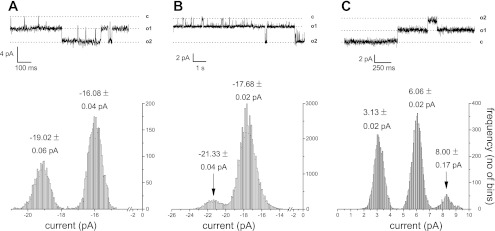
In Jurkat T cells, TRPM7 channel unitary conductance in the absence of external divalent cations has been estimated at 40–44 pS (19, 42). We examined channel activity in Jurkat T-cell patches at negative and positive holding potentials in the absence of Mg2+. Figure 2A shows a recording from a membrane patch bathed in the standard Mg2+-free solution. An all points histogram of the recorded trace shows peaks corresponding to conducting (open) and nonconducting (closed) states of the channel. The peaks could be fitted with Gaussian curves (not shown), and the mean current values of each state are given above. Channel amplitudes at −90 mV were −3.43 ± 0.22 pA (means ± SD; n = 718 direct measurements from a total of 36 patches), corresponding to mean unitary conductance of 39.0 pS (γ1) and a smaller subconductance state of 1.64 ± 0.19 pA (means ± SD; determined in total of 94 measurements in 5 patches; Fig. 2B). The smaller subconductance state has an extrapolated mean conductance of 18.6 pS (γ2). The slightly reduced apparent conductance of 39.0 pS is most likely due to reduced monovalent cation concentrations used in the present study compared with references (19, 42). Thus an additional, smaller conductance channel substate is evident in Jurkat patches, which was not reported previously (19). Both high and low conductance channels were sensitive to Mg2+ (see below). In Fig. 2C, the patch was stepped to +90 mV to demonstrate that the TRPM7 channel conducts also in the outward direction, as expected from our whole cell studies (19, 20). We directly measured unitary outward currents at various depolarized potentials to estimate outward conductance values. Measurements taken in nine patches (n = 272 measurements) could be fitted with two Gaussian distributions with peaks corresponding to 18.05 ± 0.3 and 26.6 ± 0.12 pS (means ± SE). The large conductance state was most often observed. Therefore, we find that TRPM7 channels show two conductance states and conduct both in the inward and outward direction.
Fig. 2.
Microscopic properties of Mg2+ -inhibited cation channel in Jurkat T lymphocytes. Channel activity in 10 HEDTA basal solution with no added Mg2+ in inside-out patch configuration. A: 2 large conductance (γ1) open channel states are seen. Measurement taken at −90 mV. B: membrane patch showing the activity of one large conductance (γ1) and one small conductance (γ2) channel activity measured at −100 mV. C: large conductance TRPM7 channel unitary currents in the outward direction measured at +90 mV. All points histograms corresponding to the current traces are at bottom. Numbers shown in histograms represent means ± SD obtained from Gaussian fits.
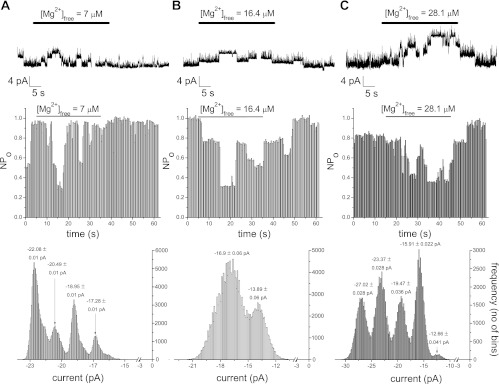
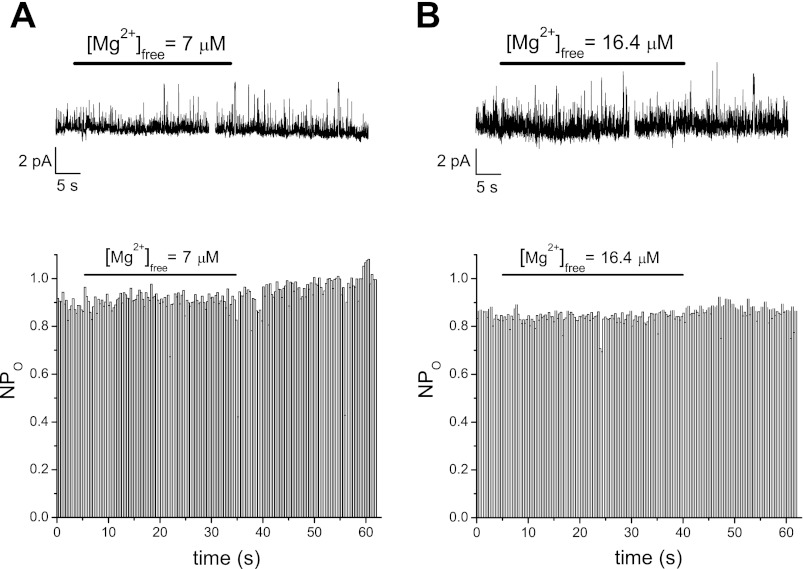
We next focused our attention at constructing a dose-response relation for patch TRPM7 activity. We proceeded to test the effect of systematically increasing Mg2+ concentration on the TRPM7 single-channel activity measured at −90 mV. The concentration was increased in steps of approximately twofold or smaller to obtain detailed Mg2+ dependence information. Figure 3 shows the effect of addition of low Mg2+ concentrations that lie in the range of the high affinity inhibitor site, i.e., IC50 = 10.2 μM in the whole cell dose-response curve (6). Then, 1, 2, and 3 mM MgCl2 were added to the 10 mM HEDTA basal solution and perfused on the internal side of the patch, followed by washout. These amounts of MgCl2 are expected to yield free Mg2+ concentrations of 7, 16.4, and 28.1 μM, respectively. As shown in Fig. 3, 7 ,16.4, and 28.1 μM Mg2+ inhibited TRPM7 channels reversibly. Channel activity was measured by constructing NPo plots and all points histogram in each case, depicted below the raw traces.
Fig. 3.
Reversible inhibition of TRPM7 channel activity at low concentrations of Mg2+; 7 μM (A), 16.4 μM (B), and 28.1 μM (C) Mg2+ were applied to inside-out patches held at −90 mV, followed by washout. Representative patches from total of 7–11 for each concentration of Mg2+. Channel inhibition was quantified as changes in single channel open probability (NPo) in the presence of Mg2+ (middle row). Bottom row: all points amplitude histograms obtained from the raw trace. Numbers on top of peaks represent means ± SD of individual Gaussian fits (not shown). Note that the patch in B contains both large and small conductance channels.
Fig. 7.
Use dependence of Mg2+ inhibition. Response of TRPM7 channels in inside-out patch to multiple (n = 8) applications of Mg2+. A: 28.1 μM Mg2+ effect in second application on a Jurkat T cell membrane patch containing active TRPM7 channels. B: eighth exposure of the patch to the same [Mg2+] resulted in a nearly complete inhibition of channel activity. First application was without effect (not shown). In A and B, inhibition was reversible upon washout of Mg2+.
We also tested lower Mg2+ concentrations of 2 μM (n = 5 patches) and 265.8 nM (n = 5 patches), and in all cases these concentrations did not measurably inhibit TRPM7 channels (not shown).
We found great variability between patches in the extent of inhibition by a given Mg2+ concentration. Interestingly, at these concentration values, we also recorded from patches that were insensitive to Mg2+ applications. Figure 4A shows an example of a membrane patch treated with 7 μM Mg2+. The channel activity was not noticeably diminished as seen in the corresponding NPo plot. We also observed Mg2+-insensitive patches for Mg2+ concentration of 16.4 μM (Fig. 4B). The existence of TRPM7 channels that are not Mg2+-sensitive has not been reported before and suggests that the Mg2+ binding sites are not located on the channel protein itself but depend on other factors in the plasma membrane (see discussion).
Fig. 4.
Examples of patches with Mg2+-insensitive TRPM7 channels; 7 μM (A) and 16.4 μM (B) free [Mg2+] were applied to a Jurkat T cell inside-out patch with one active channel producing no current inhibition. Recordings from two different membrane patches. Holding potential = −90 mV.
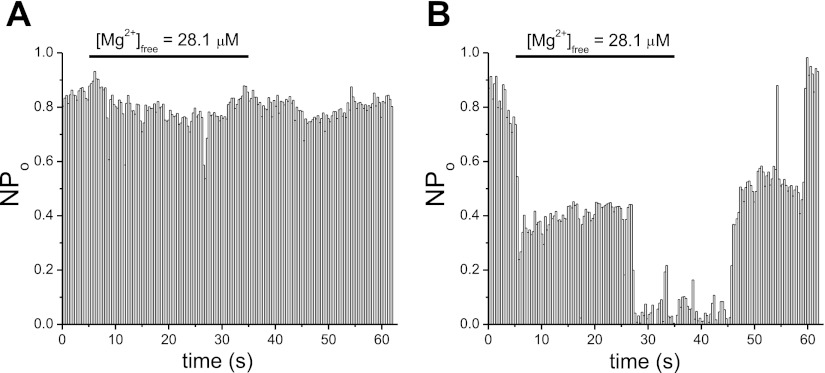
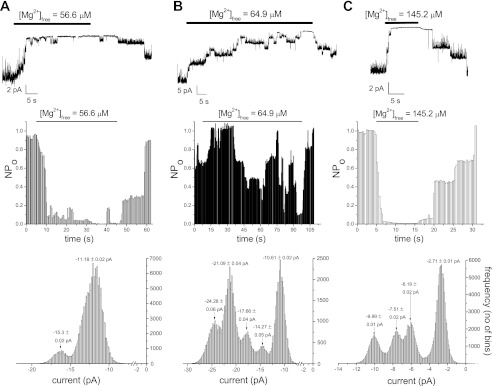
We investigated further the characteristics of Mg2+ inhibition at higher concentrations of 56.6–145.2 μM, which are in the range of the second inhibitor site of IC50165 μM in the whole cell dose-response relation (6). In Fig. 5, solutions containing 4, 5, and 7 MgCl2 (corresponding calculated free Mg2+ concentration = 56.6, 64.9, 145.2 μM) were applied repeatedly and reversible inhibition was observed. In two out of six total patches, only small inhibition (<40%) was observed for 56.6 μM Mg2+; 56.6 μM were effective in potently inhibiting TRPM7 channels in the remaining four patches. The onset of inhibition was slow, taking >5 s to reach maximum.
Fig. 5.
Inhibition of TRPM7 channel activity by high Mg2+ concentrations; 56.5–145.2 μM free Mg2+ were applied to the patches followed by washout. Degree of inhibition was quantified as in Fig. 4. Representative traces chosen from three to seven patches for each Mg2+ concentration. Holding potential = −90 mV.
The concentration-response of our whole cell currents showed only partial inhibition at these concentrations (56–145 μM; Ref. 6). Thus we find that even though TRPM7 channels in most cell-free patches preserve their Mg2+ sensitivity, the dependence appears to be steeper: submaximal concentrations exhibit stronger inhibition (Fig. 5), whereas lower micromolar concentrations exert a weaker inhibitory effect (Fig. 3).
The effect of 407.1 μM Mg2+ was similar to 303 μM, fully inhibiting channels (not shown; n = 3 patches).
We constructed a full range concentration-response relationship for Mg2+ concentration = 265.8 nM to 407.1 μM by calculating percent inhibition for each concentration from the NPo plots and plotting the fraction of unblocked current against Mg2+ concentration. The resulting dose-response relation from 58 different membrane patches is presented in Fig. 6A. The relation can be fitted with a biphasic dose-response curve giving IC50 of 25.1 and 91.2 μM and Hill coefficients of 3.65 and 7.78.
For ease of comparison, we plotted the inside-out and whole cell dose-response relations in the same graph (Fig. 6B). It clearly shows that the inside-out and whole cell dose-response relations overlap at most tested Mg2+ concentrations. At the same time in cell-free patches, Mg2+ dependence shows greater variability between individual patches and is significantly steeper, giving two IC50 values that are closer to each other compared with whole cell (3.6-fold vs. 16-fold difference).
Mg2+ effect on unitary conductance.
We also compared γ1 single-channel conductance values for the full range of Mg2+ concentrations. Fraction of conductance in presence of Mg2+ normalized to conductance measured after washout and plotted against Mg2+ concentration is shown in Fig. 6C. We found that channel conductance remains unchanged for Mg2+ concentration of 265.8 nM to 64.9 μM. At concentrations of 145.2 μM and higher, γ1 conductance drops steeply to approximately 80–83% of its former value, with no further reductions in γ1 at higher Mg2+ concentrations. The change in conductance was statistically significant (one-way ANOVA, P < 0.0001, Tukey's pairwise tests at α = 0.05). The reduction in conductance was reversible upon washout of Mg2+. The data could be fitted with a Hill equation with IC50 of 93.5 μM and Hill coefficient of 7.36. Thus, in cell-free patches, Mg2+ affects both the number of active channels and the large single-channel conductance.
Mg2+ sensitivity of TRPM7 channels during multiple applications.
In standard whole cell patch clamp, the channels are exposed to the same Mg2+ concentration throughout the recording. In view of our observation that not all patches responded to Mg2+ (Fig. 3), we tested if multiple applications of Mg2+ to the same patch exert the same extent of inhibition. We applied Mg2+ at submaximal concentrations repeatedly and compared the degree of inhibition between applications; 28.1 μM Mg2+ were applied to the inner face of the TRPM7 channels n = 10 times (Fig. 7). The second application (Fig. 7A) reduced channel activity only modestly. Subsequent applications of the same concentration, surprisingly, resulted in substantially stronger inhibition than early ones. Figure 7B shows the response of the channels to the eighth application of 56.6 μM, which results in almost complete inhibition. In all cases, Mg2+ inhibition was reversible. We observed increased inhibition with multiple applications (use dependence) for 16.4 to 64.9 μM Mg2+. For 16.4 μM, out of total seven patches where Mg2+ was applied multiple times, three showed a strong effect, two showed moderate use dependence, and two showed none. For 28.1 and 56.6 μM, four out of four and five out of five patches showed use dependence, respectively. For two patches exposed to 64.9 μM multiple times, both showed a strong effect. For higher concentrations, the first application was sufficient to inhibit most of the channels. We conclude that in the majority of patches tested with multiple Mg2+ applications, later applications elicit more pronounced reductions in channel activity than earlier ones. Rundown of whole cell TRPM7 current observed with low micromolar intracellular Mg2+ during prolonged recordings (21, 33), therefore, can be explained simply as a manifestation of progressively stronger Mg2+ inhibition due to use dependence.
DISCUSSION
We report here experiments investigating the Mg2+ dependence of human TRPM7 channels in cell-free patches. The present study explores the properties of the native TRPM7 channels in Jurkat T cells in their natural environment and physiological levels of surface expression. We have constructed a dose-response curve for TRPM7 channel Mg2+ inhibition from NPo plots, which yields IC50 values of 25.1 and 91.2 μM. We find that the native TRPM7 channel exhibits two conductance states of 39.0 pS (γ1) and 18.6 pS (γ2). The γ1 conductance is modestly reduced by Mg2+ with a single IC50 value of 93.5 μM. In agreement with our earlier study of recombinant murine TRPM7 channels, we find that native TRPM7 channels of Jurkat T lymphocytes are inhibited by Mg2+ in a reversible manner. We also noticed that the onset of Mg2+ inhibition was slower at lower concentrations and increased at higher concentrations. We have not quantified this effect rigorously, however.
In the study presented here, our main goal was to compare the macroscopic (whole cell) current concentration-response relation generated for TRPM7 (6) to that generated from single-channel measurements in this cell type. We find that even though the single channels preserve their property of Mg2+ sensitivity, the profile of this dependence is not identical to the whole cell case. The main obvious difference is that the contribution of the high affinity inhibitor site of 10.2 μM is weakened. In contrast to this reduction of sensitivity, the low affinity Mg2+ inhibitor site (165 μM in whole cell) became stronger. The resulting dose-response relation overlaps with the whole cell curve yielding IC50 values of 25.1 and 91.2 μM (Fig. 6).
The main effect of Mg2+ in inside-out patch appears to be a reduction in the number of conducting channels, No, by an abrupt reduction in the probability of opening of individual channels. In addition to its effect on channel NPo parameter, Mg2+ reduced the major single-channel conductance of 39.0 pS by ∼20% (Fig. 6C). The effect became significant at concentrations of 145 μM and above and was monophasic. The IC50 determined in these experiments was 93.5 μM, almost identical to IC50(2) value of 91.2 μM, determined from NPo measurements (compare Fig. 6, A and C). It is therefore tempting to speculate that these two inhibitory processes result from a common underlying mechanism. For rabbit sacroplasmic reticulum and bacterial potassium channels reconstituted in lipid bilayers, it has been demonstrated that negatively charged lipids interacting with the ion channel change the electrostatic profile of the protein by a Gouy-Champan surface charge modification mechanism and attract cations to the vicinity of the channel pore, increasing its unitary conductance (3, 4, 34). In view of Mg2+ and protons exerting their inhibitory action on TRPM7 by screening negatively charged phospholipids (6, 21), it is reasonable to expect these ions to restrict the accumulation of permeant cations near the channel pore entrance, thus reducing its apparent conductance. The decrease in TRPM7 channel γ1 conductance does not involve the high affinity Mg2+ inhibitor site (Fig. 6C), suggesting that this site may involve screening of annular phospholipids surrounding the channel and therefore distant from the ion conduction pathway (see Ref. 26), although a direct effect of Mg2+ on the channel protein cannot be ruled out. Mg2+ reduction of the number of available TRPM7 channels does not seem to require decreases in conductance, since significant reductions in NPo are observed at concentrations below 145 μM, necessary for an effect on the conductance. We have not investigated in detail if the smaller conductance γ2 of TRPM7 channels is also sensitive to Mg2+; however, preliminary results from membrane patches exposed to 303 μM Mg2+ show no significant reduction in γ2 unitary current amplitude (7 patches; data not shown). We did not observe differential sensitivity of the two conductance states of the channel to Mg2+; however, this possibility cannot be ruled out and will require further investigation.
We find that at low micromolar concentrations Mg2+ inhibited in most patches yet in others there was no noticeable effect (compare Figs. 3 and 4). This finding suggests to us that Mg2+ inhibition requires additional modulatory molecules in the membrane and does not involve direct bimolecular Mg2+-channel reaction. In our studies of extracellular cation block of TRPM7, we (19, 20) observed blockade for every application without exception, which in this case involves Mg2+ binding in the ion conduction pathway of the channel pore. Such direct interactions of ion with the channel would therefore be expected to occur every time. In the case of TRPM7 inhibition by internal Mg2+, however, some channels appear to be lacking the receptor conveying Mg2+ sensitivity (current study). This can be explained by groups of channels clustered in microdomains of distinct ordered anionic lipids (26). It is also possible that the less sensitive channels are exposed to phospholipids, which are themselves less sensitive to screening by Mg2+. At high concentrations of 145.2, 303, and 407.1 μM, Mg2+ was effective in every patch tested, which suggests that these channels still maintain the low affinity inhibitor site (see also Fig. 6 legend).
Increased Mg2+ sensitivity of the low affinity inhibitor site in inside-out patch (Fig. 6, A and B) can be explained by either lack of cytoskeleton or lower amounts of phospholipids in this configuration compared with whole cell. In cell-free patches, the cell geometry is destroyed and this may be a reason for change in channel sensitivity to Mg2+ (47, 54). It is well known from studies on potassium channels of Kir and Kv7 family that channel activity runs down in minutes in inside-out patches (e.g., Refs. 14, 15, 32), but this process is much slower in whole cell recording (21, 33). This has been attributed to loss of PI(4,5)P2 phospholipid in the membrane patches without ATP or other regenerating molecules being present (15). In this context, potentiation of the Mg2+ effect with repeated application, i.e., use dependence, can be explained by gradual loss of PI(4,5)P2 from the membrane and concomitant increased sensitivity of channel-PIP2 interactions to shielding by Mg2+.
It has been suggested that the kinase domain of TRPM7, by virtue of its MgATP regulation, may be responsible in part for Mg2+ and Mg-nucleotide sensitivity of the channel activity (51). We have shown, using site-directed mutagenesis, that phosphotransferase and channel activities are independent of each other. Point mutations destroying kinase activity failed to prevent Mg2+ inhibition of TRPM7 (35). Moreover, channel mutants with impaired autophosphorylation display Mg2+ dependence similar to wild type (35). These studies were performed in whole cell configuration, and it will be worthwhile to compare kinase-mutated channels to wild-type TRPM7 channels in inside-out mode, which can give more information on biophysical parameters affected by the kinase function.
Like several other TRP channels (43), TRPM7 is gated polymodally by Mg2+, alkalinization, and stretch/swelling. Studies in cell-free patches may shed light on whether osmoregulation of TRPM7 channels (37, 38) is affected by PIP2 levels and Mg2+. Thus a study (41) of native and recombinant Kv7 channels recently showed that osmoregulation of these channels results from the dilution of Mg2+ and polyamines in the cell leading to reduced screening of PIP2 negatively-charged head groups.
Generation of native Jurkat T-cell Mg2+ inhibition dose-response relations in whole cell and now in inside-out patch will allow detailed comparison of Mg2+ sensitivity of the native and over-expressed recombinant TRPM7 channels.
T-lymphocyte resting Mg2+ levels are estimated to be in the millimolar range (44) and at these concentrations most TRPM7 channels are expected to be inhibited. Interestingly, however, a significant proportion of Jurkat T cells exhibit preactivated macroscopic TRPM7 currents (6), suggesting that additional cytoplasmic signaling molecules regulate this channel and may be able to bypass tonic Mg2+ inhibition. One question in this regard is if Mg2+ sensitivity is static or can change when T cells are activated. Our results from multiple applications of the same Mg2+ concentration (Fig. 7) show that inhibition exhibits use dependence. Furthermore, the extent of use dependence varies depending on cell type examined (not shown). Future studies will elucidate the question of whether Mg2+ sensitivity is a dynamic property of this channel and if it varies depending on mitogenic activation of T cells.
GRANTS
This work was funded by a Scientist Development Grant from American Heart Association, National Center (10SDG2730001) and a grant from National Institute of Allergy and Infectious Diseases (1R15AI090613-01) (to J. A. Kozak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C. performed experiments; R.C. and J.A.K. analyzed data; R.C. and J.A.K. prepared figures; M.M. and J.A.K. conception and design of research; M.M. and J.A.K. interpreted results of experiments; J.A.K. drafted manuscript; J.A.K. edited and revised manuscript; J.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mark M. Rich for reading the manuscript and helpful comments.
REFERENCES
- 1. Ballester LY, Vanoye CG, George AL., Jr Exaggerated Mg2+ inhibition of Kir2.1 as a consequence of reduced PIP2 sensitivity in Andersen syndrome. Channels 1: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg(2+) inhibited channel and kinase. Adv Exp Med Biol 704: 173–183, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bell J. The sarcoplasmic reticulum potassium channel. Lipid effects. In: Ion Channel Reconstitution, edited by Miller C. New York: Plenum, 1986, p. 469–482 [Google Scholar]
- 4. Bell JE, Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J 45: 279–287, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev 231: 59–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chokshi RH, Matsushita M, Kozak JA. Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am J Physiol Cell Physiol 302: C1004–C1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clapham DE. Sorting out MIC, TRP, and CRAC ion channels. J Gen Physiol 120: 217–220, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357: 695–697, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA 103: 14883–14888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czystowska M, Szczepanski MJ, Szajnik M, Quadrini K, Brandwein H, Hadden JW, Whiteside TL. Mechanisms of T-cell protection from death by IRX-2: a new immunotherapeutic. Cancer Immunol Immunother 60: 495–506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dempster J. Analysis of single-channel currents. In: Computer Analysis of Electrophysiological Signals. London: Academic, 1993, p. 157–181 [Google Scholar]
- 12. Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Durand DB, Shaw JP, Bush MR, Replogle RE, Belagaje R, Crabtree GR. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol 8: 1715–1724, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron 13: 1413–1420, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem 272: 5388–5395, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hilgemann DW. Cytoplasmic ATP-dependent regulation of ion transporters and channels: mechanisms and messengers. Annu Rev Physiol 59: 193–220, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA 104: 20296–20301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84: 2293–2305, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kozak JA, Kerschbaum HH, Cahalan MD. Distinct properties of CRAC and MIC channels in RBL cells. J Gen Physiol 120: 221–235, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J Gen Physiol 126: 499–514, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kunami N, Yotsumoto F, Ishitsuka K, Fukami T, Odawara T, Manabe S, Ishikawa T, Tamura K, Kuroki M, Miyamoto S. Antitumor effects of CRM197, a specific inhibitor of HB-EGF, in T-cell acute lymphoblastic leukemia. Anticancer Res 31: 2483–2488, 2011 [PubMed] [Google Scholar]
- 23. Kuo CC, Hess P. Block of the L-type Ca2+ channel pore by external and internal Mg2+ in rat phaeochromocytoma cells. J Physiol 466: 683–706, 1993 [PMC free article] [PubMed] [Google Scholar]
- 24. Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26: 2673–2683, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Lee AG. Lipid-protein interactions. Biochem Soc Trans 39: 761–766, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem 275: 10182–10189, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol 19: 497–521, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol 101: 801–826, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA 96: 5820–5825, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci USA 105: 2011–2016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J 22: 5412–5421, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Macianskiene R, Gwanyanya A, Vereecke J, Mubagwa K. Inhibition of the magnesium-sensitive TRPM7-like channel in cardiac myocytes by nonhydrolysable GTP analogs: involvement of phosphoinositide metabolism. Cell Physiol Biochem 22: 109–118, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Marius P, Zagnoni M, Sandison ME, East JM, Morgan H, Lee AG. Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys J 94: 1689–1698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J Biol Chem 280: 20793–20803, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Montell C. Mg(2+) homeostasis: the Mg(2+)nificent TRPM chanzymes. Curr Biol 13: R799–801, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem 19: 1–8, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292: C460–C467, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem 269: 32327–32335, 1994 [PubMed] [Google Scholar]
- 40. Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Piron J, Choveau FS, Amarouch MY, Rodriguez N, Charpentier F, Merot J, Baro I, Loussouarn G. KCNE1-KCNQ1 osmoregulation by interaction of phosphatidylinositol-4,5-bisphosphate with Mg2+ and polyamines. J Physiol 588: 3471–3483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg(2+)-inhibited cation (MIC) channels. J Gen Physiol 119: 487–507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol 95: 189–196, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291: 1043–1047, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol 4: 329–336, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Sachs F. Stretch-activated ion channels: what are they? Physiology (Bethesda) 25: 50–56, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Schilling T, Gratopp A, DeCoursey TE, Eder C. Voltage-activated proton currents in human lymphocytes. J Physiol 545: 93–105, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114: 191–200, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed nonHodgkin lymphoma. Int J Cancer 19: 621–626, 1977 [DOI] [PubMed] [Google Scholar]
- 53. Selyanko AA, Sim JA. Ca2+-inhibited noninactivating K+ channels in cultured rat hippocampal pyramidal neurones. J Physiol 510: 71–91, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suchyna TM, Markin VS, Sachs F. Biophysics and structure of the patch and the gigaseal. Biophys J 97: 738–747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trezise DJ, John VH, Xie XM. Voltage- use-dependent inhibition of Na+ channels in rat sensory neurones by 4030W92, a new antihyperalgesic agent. Br J Pharmacol 124: 953–963, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weiss A, Samelson LE. T lymphocyte activation In: Fundamental Immunology (5th ed.), edited by Paul WE. Philadelphia, PA: Lippincott Williams & Wilkins, 2003, p. 321–364 [Google Scholar]
- 57. Wyllie DJA. Single-channel recording. In: Patch-Clamp Analysis: Advanced Rechniques, edited by Walz W, Boulton AA, Baker GB. Totowa, NJ: Humana, 2002, p. 69–109 [Google Scholar]
- 58. Xie LH, John SA, Ribalet B, Weiss JN. Phosphatidylinositol-4,5-bisphosphate (PIP2) regulation of strong inward rectifier Kir2.1 channels: multilevel positive cooperativity. J Physiol 586: 1833–1848, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell 7: 1047–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Yi J, Wu XS, Crites T, Hammer JA., III Actin retrograde flow and acto-myosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T-cells. Mol Biol Cell 23: 834–852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA 90: 6295–6299, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]