Abstract
The functional role of the voltage-gated K+ (KV) channels in human detrusor smooth muscle (DSM) is largely unexplored. Here, we provide molecular, electrophysiological, and functional evidence for the expression of KV2.1, KV2.2, and the electrically silent KV9.3 subunits in human DSM. Stromatoxin-1 (ScTx1), a selective inhibitor of KV2.1, KV2.2, and KV4.2 homotetrameric channels and of KV2.1/9.3 heterotetrameric channels, was used to examine the role of these channels in human DSM function. Human DSM tissues were obtained during open bladder surgeries from patients without a history of overactive bladder. Freshly isolated human DSM cells were studied using RT-PCR, immunocytochemistry, live-cell Ca2+ imaging, and the perforated whole cell patch-clamp technique. Isometric DSM tension recordings of human DSM isolated strips were conducted using tissue baths. RT-PCR experiments showed mRNA expression of KV2.1, KV2.2, and KV9.3 (but not KV4.2) channel subunits in human isolated DSM cells. KV2.1 and KV2.2 protein expression was confirmed by Western blot analysis and immunocytochemistry. Perforated whole cell patch-clamp experiments revealed that ScTx1 (100 nM) inhibited the amplitude of the voltage step-induced KV current in freshly isolated human DSM cells. ScTx1 (100 nM) significantly increased the intracellular Ca2+ level in DSM cells. In human DSM isolated strips, ScTx1 (100 nM) increased the spontaneous phasic contraction amplitude and muscle force, and enhanced the amplitude of the electrical field stimulation-induced contractions within the range of 3.5–30 Hz stimulation frequencies. These findings reveal that ScTx1-sensitive KV2-containing channels are key regulators of human DSM excitability and contractility and may represent new targets for pharmacological or genetic intervention for bladder dysfunction.
Keywords: detrusor, Western blot, immunocytochemistry, patch-clamp, voltage-gated K+ channels
increased detrusor smooth muscle (DSM) contractions are often associated with overactive bladder (OAB), a pathophysiological condition that affects millions of individuals by disturbing their sleep, work, quality of life, and social interactions (3, 4). The exact mechanism that underlies human DSM contractility still remains unclear. Potassium (K+) channels play a critical role in controlling DSM electrical activity, and therefore contractility (3, 7, 20–22, 29–32, 35, 36). Inhibition of certain types of K+ channels leads to membrane depolarization, activation of the L-type voltage-gated Ca2+ (CaV) channels, and DSM contraction. Activation of K+ channels leads to hyperpolarization of the cell membrane, inhibition of CaV-channels, a decrease in intracellular Ca2+ concentration, and DSM relaxation (30).
Among K+ channels that control cell excitability and contractility, the KV channel superfamily is the most diverse, but the least investigated in human DSM (30). In the human genome the KV channel superfamily is represented by more than 40 genes encoding the pore-forming α-subunits (15, 16, 30). KV channels are classified into 12 subfamilies based on their amino acid sequence homology. Each functional KV channel is composed of four α-subunits. The members of KV1–4, KV7, and KV10–12 channel subfamilies can form functional homotetrameric channels, whereas the members of KV5, KV6, KV8, and KV9 channel subfamilies do not form their own homotetramers, but rather form heterotetrameric channels with KV2 channel subunits (30). KV channels that contain KV2 (KV2.1 or KV2.2) subunits are referred to as “KV2-containing channels.” KV2-containing channels could form their own homotetramers or heterotetramers with electrically silent KV (KV5, KV6, KV8, and KV9) subunits.
At least several KV channels are expressed in DSM of mammals (8, 10, 14, 21, 28, 36). Expression of KV2.1, KV5.1, KV6.1, KV6.2, and KV6.3 channel subunits has been shown in mouse DSM (36). The expression of KV2.1 and KV2.2 channels has been shown in rat DSM (8), and the expression of KV2.1 and electrically silent KV channel subunits has been detected in guinea pig DSM (21). However, it is questionable whether these findings in animal models could be directly translated to humans, since species differences in human and rodent DSM function are well documented (1, 3, 5, 8, 17, 21, 30).
Knowledge about KV channels in human DSM is limited and only the molecular expression of some KV1 channels has been proven (10). There is a lack of information about expression, function, and regulation of each individual KV channel in human DSM. Microelectrode studies have shown that the nonselective KV channel inhibitor 4-aminopyridine (4-AP) affects the after-hyperpolarization phase of the action potential and increases the frequency of the action potentials in human DSM (17). However, because of the nonselectivity of 4-AP, these experiments do not provide any information about the physiological role of individual members of the KV channel family in human DSM. The lack of KV channel subtype selective inhibitors with high affinity and selectivity has hampered the study of these channels not only in human DSM but also in other tissues. Recently, highly specific KV channel inhibitors have emerged enabling researchers to examine individual KV channel subtypes and determine their functional roles in DSM (30).
Stromatoxin-1 (ScTx1), a peptide isolated from African tarantulas, has been shown to selectively inhibit KV2.1, KV2.2, and KV4.2 homotetramers, as well as KV2.1/6.3 and KV2.1/9.3 heterotetrameric channels (11, 12, 27). Recently, we showed that ScTx1 is a useful pharmacological tool to study the function of KV channels in DSM of animal species (8, 21). We demonstrated that KV2-containing channels sensitive to ScTx1 regulate rat and guinea pig DSM excitability and contractility (8, 21). However, the expression and function of the KV2 channels have never been studied in human DSM. Dr. Alison Brading, a world-renowned expert in ion channel DSM research, has emphasized that “It would seem particularly important for those studying the ion channels in a particular species to extend their studies to correlate channel properties with function, and there are still far too few basic studies on human material” (5). A recent review article further outlines the urgent need of studies on native human DSM (30).
The aim of this study was to examine the expression of ScTx1-sensitive KV2-containing channels in human DSM, and to reveal their functional role in human DSM excitability and contractility. To achieve this aim, we applied a multidisciplinary approach using molecular biology techniques, live-cell Ca2+ imaging, patch-clamp electrophysiology, and isometric DSM tension recordings of human DSM isolated strips. Because human is the target species of interest for therapeutic intervention, the present study on native human DSM is critical to validate animal models reflecting human bladder function in the development of novel therapeutic strategies for the treatment of bladder pathologies.
MATERIALS AND METHODS
Human DSM tissue collection.
All human studies were carried out in accordance with the reviewed and approved protocol HR 16918 by the Institutional Review Board of the Medical University of South Carolina (MUSC). All tissue specimens were obtained from patients who did not have a preoperative history of OAB and had an American Urological Association symptom score of less than 8. In this study we used tissues from 27 patients (21 Caucasians and 6 African-Americans), of which there were 16 males and 11 females aged 24 to 85 (average age 59.6 ± 3.2 yr). The clinical diagnoses of the patients were as follows: 22 patients had urothelial carcinoma, 1 had urethral adenocarcinoma, 1 had enterovesical fistula, and 3 patients had reconstructive lower urinary tract surgeries. Following surgery, the specimens were immediately transported from the operating room to the laboratory in ice-cold Ca2+-free dissection solution (DS) (see Solutions and Drugs). The mucosal layer of the specimen, including the urothelium, was removed and the human DSM tissue was collected for the study. For the molecular biology experiments, a small piece of human DSM tissue was separated during surgery and stored in RNAlater solution.
Fresh human DSM single cell isolation for RT-PCR, immunocytochemistry, live-cell Ca2+ imaging, and patch-clamp experiments.
Fresh human DSM single cells were isolated as previously described (20). Briefly, small human DSM strips (5–10 mm long; 2–4 mm wide) were excised from the DSM specimens. Two to three strips were placed in 2 ml of DS, supplemented with 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dl-dithiothreitol and incubated for 30 min at 37°C. Next, the human DSM strips were transferred to 2 ml of DS containing 1 mg/ml BSA, 1 mg/ml collagenase (type II from Sigma), and 100 μM CaCl2 for 7–14 min at 37°C. After this incubation, the human DSM strips were washed with fresh DS containing BSA. Individual DSM cells were released from the tissue by passing the enzyme-treated strips through the tip of a Pasteur pipette. The human DSM single cells obtained from these procedures were used for electrophysiological recording, live-cell Ca2+ imaging, and molecular biology studies (RT-PCR and immunocytochemistry). Under an Axiovert 40CFL microscope (Carl Zeiss, Göttingen, Germany) with Nomarski interference contrast, human DSM cells were characterized by a spindle-shape, in association with bright, and shiny contours. Furthermore, human DSM cells responded to mechanical or pharmacological stimulation with carbachol by contraction as evidenced by DSM cell shortening and becoming thicker with oval ends. This indicates functional contractile proteins and that the cells are physiologically active—and therefore ideal for patch-clamp, live-cell Ca2+ imaging, and “single-cell RT-PCR” experiments. We have further characterized the DSM cells by immunocytochemistry using phalloidin 488 which stains for F-actin.
RNA extraction/RT-PCR/sequencing.
For RT-PCR analysis, the human DSM was kept in RNAlater (Qiagen) and immediately used for RNA extraction. Total RNA was extracted from human DSM tissue as well as from freshly isolated human DSM cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Single human DSM cells were allowed to settle at the bottom of the chamber for 5 min, before individual selection by suction into a glass pipette. Single DSM cells were then expelled into a 1.5 ml centrifuge tube with RNAlater and then pelleted at 1,000 g for 3 min. The extracted RNA was reverse-transcribed into cDNA using M-MLV Reverse Transcriptase (Promega, Madison, WI) and oligo(dT) primers. All primers specific for KV2.1, KV2.2, KV4.2, and KV9.3 were designed on the basis of the cDNA complete sequences of human in GenBank. Specific primer pair sequences are listed in Table 1. The cDNA production was PCR amplified using GoTaq Green Master Mix (Promega) and specific primers for the KV channel subunits. The PCR annealing temperature for each primer pair was optimized using a mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich) and sequenced at the University of South Carolina Environmental Genomics Core Facility to confirm their identity.
Table 1.
RT-PCR primers for the subunits of the KV channels sensitive to ScTx1 and the silent KV9.3 channel subunit
| KV Channel | Sense | Antisense | Production, bp |
|---|---|---|---|
| KV2.1 | ATGCGAAGTATGTCAAGCA | CATGGTACATCCCTAGAACG | 334 |
| KV2.2 | ATGACCACTGTTGGCTATG | GTTTGGGTGAGAATGAGG | 537 |
| KV4.2 | ATAGATACGGTAAGAGCCA | TGTGATATTAGTCCCAGTCA | 417 |
| KV9.3 | GCACTCGGTAGGACTTCG | AGGATCGCTCACCACAAT | 481 |
KV, voltage-gated K+; ScTx1, stromatoxin-1.
Western blot analysis.
For Western blotting, fresh human DSM tissue was cut and used for membrane protein extraction. DSM protein was extracted as previously described (9, 20, 29). The protein sample was stored at −80°C until use for SDS-PAGE and Western blot analysis. Western blotting was performed as follows: the protein was mixed with 5× Laemmli buffer (1:4) and denatured for 5 min at 95°C. Subsequently, human DSM proteins (∼50 μg) were loaded into adjacent lanes, subjected to 4–20% pre-case SDS-PAGE for 2.5 h at 20 mA, and transferred onto a polyvinylidene difluoride membrane at 40 mA for 2 h using semidry blot. The membrane was then blocked with 3% BSA-Tris-buffered saline-Tween 20 buffer for 2 h at room temperature, and the blots were incubated with the affinity-purified rabbit polyclonal primary antibodies: anti-KV2.1 (DRK1 1:400), anti-KV2.2 (CDRK 1:2,000), anti-KV4.2 (Kcnd2 1:200) (Alomone Labs, Jerusalem, Israel) and anti-KV9.3 [KV9.3(K-12) 1:200] (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-KV9.3 (KCNS3 1:200) (Abcam) overnight at 4°C. Then, the membrane was washed with TBS four times and incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase diluted to 1:2,500 respectively in the blocking buffer for 1 h at room temperature. Bound antibodies were detected by ECL substrate kit (Amersham, Piscataway, NJ) according to the manufacturer's instructions. Staining specificity was verified by preincubation of the antibodies with a control peptide.
Immunocytochemistry.
Human DSM cells were enzymatically isolated from fresh human DSM tissue, and dropped on a glass coverslip to settle at room temperature, then processed for the following steps. Cells were fixed with prewarmed 4% paraformaldehyde for exactly 10 min. Cells were then washed twice in PBS, blocked, and permeabilized in PBS containing 10% normal donkey serum and 0.1% Triton X-100 for 30 min. Once again, cells were washed in PBS and incubated with rabbit polyclonal primary antibodies: anti-KV2.1, anti-KV2.2, and anti-KV4.2 (DRK1, CDRK and Kcnd2, 1:100, Alomone Labs) at 37°C for 1 h. After that, cells were washed two times with PBS and labeled with secondary antibodies Cy3-conjugated anti-rabbit IgG, at 1:200, PBS-3% normal donkey serum-0.01% Triton X-100 (Jackson ImmunoResearch, West Grove, PA) for 1 h in the dark. After labeling, cells were washed with PBS and incubated with phalloidin for 2 h in the dark. Cells were then washed two more times and incubated with 4′,6-diamidino-2-phenylindole for 15 min and washed again, then mounted onto slides with DABCO. Control treatments were carried out as follows: 1) omission of the primary antibody for confirming the specificity of the secondary antibody, and 2) absorption of the primary antibody by a competing peptide for confirming the specificity of the primary antibody. Confocal images were acquired with an LSM 510 META confocal microscope (Carl Zeiss).
Electrophysiological (patch-clamp) recording.
The amphotericin-B perforated patch-clamp technique was applied in all electrophysiological experiments to preserve the native physiological environment of the human DSM cells. Several drops (∼0.5 ml) of the DS containing the isolated single cells were placed in an experimental chamber. Cells were allowed to adhere to the glass bottom of the chamber for ∼20 min and after that were washed with extracellular solution. Most cells were elongated and had a bright, shiny appearance when examined with a phase-contrast microscope. Cells were used for patch-clamp recordings within 24 h after isolation. Axopatch 200B amplifier, Digidata 1440 A, and pCLAMP 10.2 software (Molecular Devices) were used for electrophysiological recordings of the ionic currents. The signal was filtered with eight-pole Bessel filter 900CT/9L8L (Frequency Devises, Ottawa, IL). The glass pipettes used for patch-clamp experiments were made from Borosilicate glass (Sutter Instruments, Novato, CA) and pulled using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan) to give a final tip resistance of 4–6 MΩ. The pipettes were polished with a Micro Forge MF-830 fire polisher (Narishige Group). All patch-clamp experiments were performed in voltage-clamp mode, in the presence of the large-conductance Ca2+-activated K+ (BK) channel selective inhibitor iberiotoxin (200 nM). The leak current was not subtracted during or after the experiments. The average value of the current amplitude during the last 50 ms of the 250-ms depolarization step was taken to evaluate the steady-state KV current. For the control current, at least five pulses within 10 min were recorded and averaged. Only cells with stable control currents for at least 10 min were used to examine the effect of ScTx1. All patch-clamp experiments were carried out at room temperature.
Ca2+ imaging experiments.
For intracellular Ca2+ imaging experiments, 250 μl poly-l-lysine was added to each 35 mm diameter glass-bottom dish, incubated at 37°C for 1 h, then dishes were aspirated and washed with 2 ml sterile water three times. DSM cell suspension was added into the dishes and kept for 30 min to allow cell adherence to the bottom of the dishes. Fura-2 AM solution was diluted with bath solution to a final concentration of 2 μM. Supernatant was then removed from the dishes, 250 μl fura-2 AM working solution was added into the dishes, and samples were stored in a dark room for 30 min. Fura-2 AM bath solution was then removed and cells were washed with bath solution three times. Ca2+ imaging recordings were acquired with an OLYMPUS IX81 motorized inverted research microscope, with 40 × oil objective, equipped with the MetaFluor 7.7.2.0 software. All Ca2+ imaging experiments were carried out at room temperature.
Isometric human DSM tension recordings.
Intact DSM isolated strips used for functional studies were prepared as previously described (20). Briefly, mucosa-free whole human DSM tissues were dissected into individual strips (5–10 mm long and 2–4 mm wide). DSM strips were then suspended on clips between a force displacement transducer and a stationary hook, then immersed in thermostatically controlled tissue baths (37°C) filled with Ca2+-containing physiological saline solution (PSS). After application of a mechanical stretch of 1.0 g, human DSM strips were washed every 15 min with fresh PSS for a period of 1 h. Next, human DSM strips that exhibited spontaneous phasic contraction amplitude >0.1 g after the initial stretch to 1 g of tension were treated with tetrodotoxin (TTX, 1 μM) to block neurotransmitter release and minimize nerve-mediated effects. Human DSM strips in which the spontaneous phasic contraction amplitude was <0.1 g were subjected to electrical field stimulation (EFS) and were not treated with TTX. Increasing EFS frequencies (3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, 50 Hz) were first applied to each human DSM strip as a control. Then, ScTx1 (100 nM) was added to the bath for 30 min followed by application of the same EFS protocol. EFS pulses were generated using a PHM-152I stimulator (MED Associates, Georgia, VT) and parameters were as follows: pulse amplitude was 20 V, pulse width was 0.75 ms, stimulus duration was 3 s, and polarity was reversed for alternating pulses.
Solutions and drugs.
The Ca2+-free DS had the following composition (in mM): 80 monosodium glutamate: 55 NaCl, 6 KCl, 10 glucose, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 2 MgCl2, pH 7.3, adjusted with NaOH. The Ca2+-containing PSS was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4 and 11 glucose, and was aerated with 95% O2-5% CO2 to obtain pH 7.4. The extracellular (bath) solution used in the electrophysiological and Ca2+-imaging experiments contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin-B. TTX was purchased from Sigma-Aldrich (St. Louis, MO). ScTx1 was purchased from Alomone Labs. TTX was dissolved in citrate buffer and ScTx1 was dissolved in distilled water.
Data analysis and statistics.
MiniAnalysis software (Synaptosoft, Decatur, GA) was used for data analyses of human DSM contractions. Clampfit version 10.2 software (Molecular Devices) was used for the patch-clamp electrophysiological data analyses. GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) was used for statistical analyses. All data and original recordings were illustrated using GraphPad Prism and CorelDraw Graphic Suite X3 (Corel, Ottawa, ON, Canada) software. The relative change in spontaneous phasic contraction parameters was assessed by normalizing spontaneous phasic contraction response to control conditions (100%) and is expressed in percentages. For the EFS-induced contractions, the contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%. Experimental results are summarized as means ± SE for n, the number of human DSM strips or cells, and N, the number of patients. Data were compared using two-way ANOVA or paired two-tailed Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
RT-PCR.
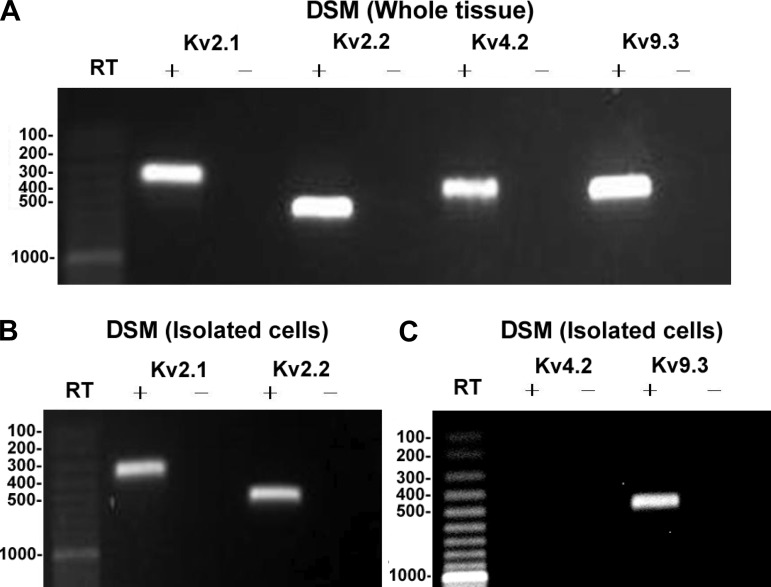
The expression of mRNA messages for KV2.1, KV2.2, KV4.2 and KV9.3 channel subunits was detected in human DSM whole tissue (Fig. 1). Other cell types such as neurons, fibroblasts, and vascular myocytes are also present within the DSM layer. Thus, it is plausible that KV2.1, KV2.2, KV4.2, and KV9.3 channel detection may come from cell types other than DSM cells. To eliminate the possible interference from non-DSM cell types, we applied single-cell RT-PCR experiments on freshly isolated human DSM cells. This experimental approach allows us to use selectively only the isolated DSM cells while avoiding any contamination from non-DSM cells (1, 8, 9, 20, 21, 29). DSM cells were characterized by their elongated, spindle shape, with a bright and shiny contour. Single-cell RT-PCR experiments confirmed the mRNA expression of KV2.1, KV2.2, and KV9.3 channel subunits in human DSM single cells (Fig. 1). mRNA expression of the KV4.2 channel subunit was not confirmed in single DSM cells, suggesting that this channel subunit is not expressed in human DSM cells. A lack of genomic DNA contamination was also confirmed by performing negative control experiments in the absence of the reverse transcriptase (−RT). All RT-PCR purified products from the intact human DSM tissue and isolated human DSM cells were sequenced to confirm their identity. PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich) and sequenced at the University of South Carolina Environmental Genomics Core Facility to confirm their identity. Results were verified in at least 30 different preparations obtained from 10 patients.
Fig. 1.
RT-PCR detection of mRNA messages for voltage-gated K+ channel subunits KV2.1, KV2.2, KV4.2, and KV9.3 in human detrusor smooth muscle (DSM) whole tissue (A) and freshly isolated single cells (B and C). KV2.1, 334 bp; KV2.2, 537 bp; KV4.2, 417 bp; and KV9.3, 481 bp. No products were observed in the negative controls (−RT) in which reverse transcriptase was left out of the reaction.
Western blot analysis.
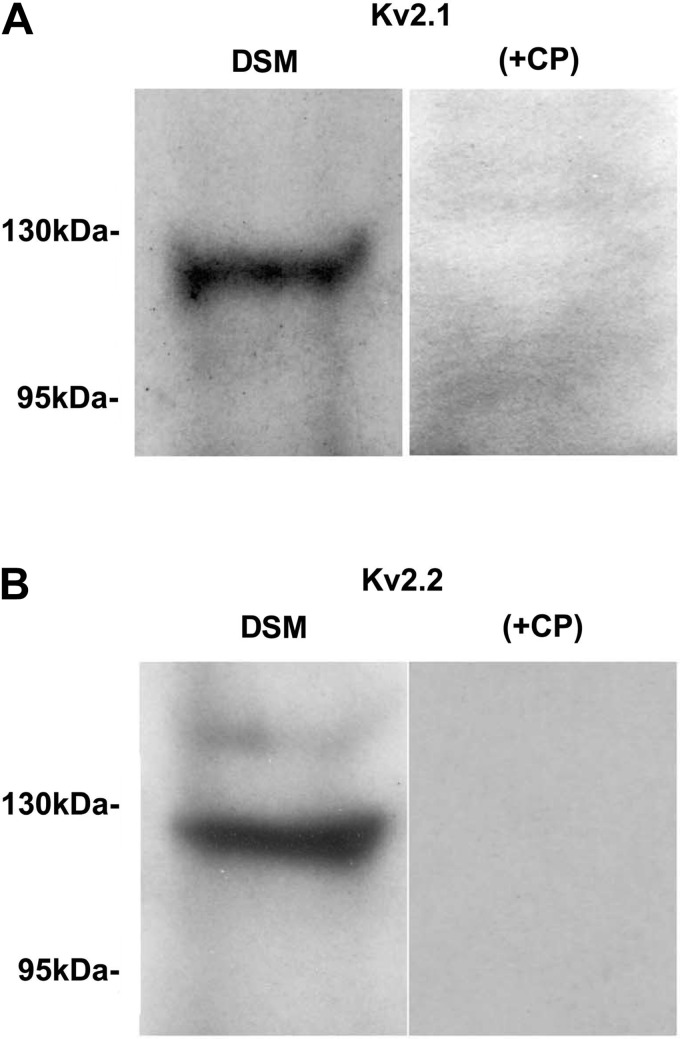
To confirm the expression of these KV2-containing channels at the protein level in human DSM tissue, we applied Western blot analysis. The presence of the KV2.1 and KV2.2 proteins in human DSM tissue was confirmed by using channel subunit-specific antibodies (Fig. 2). Consistent with our single cell RT-PCR data, no KV4.2 protein was detected in whole human DSM tissue. The specific antibodies for KV9.3 channel did not show any signal in whole human DSM (data not illustrated). Preabsorption of the primary antibody with its antigenic competing peptide indicated the specificity of the antibodies for their intended epitope (Fig. 2). The results were verified in three separate Western blot reactions using proteins isolated from three patients.
Fig. 2.
Western blot detection of KV2.1 and KV2.2 channel protein expression in human DSM whole tissue. A: KV2.1 channel. B: KV2.2 channel. The immunoreactive band was eliminated by a competing peptide (+CP).
Immunocytochemistry.
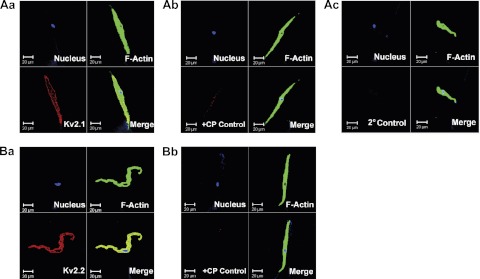
Immunocytochemical labeling was further applied to confirm the specific expression of KV2.1, and KV2.2 proteins at the level of single DSM cells and to demonstrate that the KV2 proteins were localized to the cell membrane (Fig. 3). The results were carefully controlled for specificity using the omission of the primary antibody or absorption of the primary antibody by a competing peptide (Fig. 3). The data were verified in 12 single DSM cells freshly isolated from three patients.
Fig. 3.
Immunocytochemical detection of KV2.1 (A) and KV2.2 (B) channel in freshly isolated single human DSM cells using KV2.1 and KV2.2 channel-specific antibody. Red staining (bottom left panels) indicates detection of KV2.1 channel (Aa) and KV2.2 channel (Ba); after absorption of the primary antibody with a competing peptide (Ab and Bb); and after the primary antibody was omitted and cells were incubated with the secondary antibody only (2°Control) (Ac). Cell nuclei are shown in blue (top left panels); F-actin is shown in green (top right panels). The merged images (bottom right) illustrate the overlap of all 3 images. Images were obtained via confocal microscope at ×63 and clearly demonstrate that the KV2 channel proteins were localized to the cell membrane.
ScTx1-sensitivity of the whole cell KV current in human DSM cells.
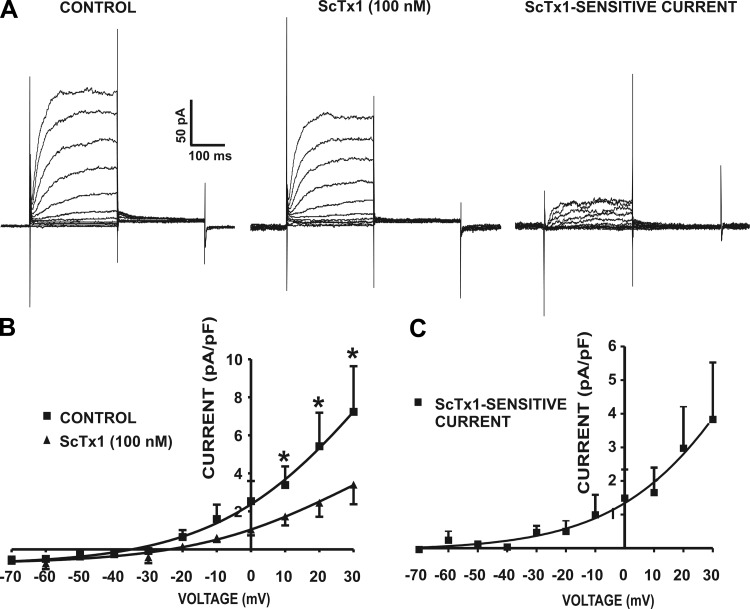
Our recent study revealed that KV2.1 and electrically silent KV channels sensitive to ScTx1 are likely important regulators of guinea pig DSM excitability (21). Here, we went one step further to evaluate the contribution of the KV2 channels sensitive to ScTx1 in human DSM excitability. Whole cell voltage-clamp experiments were performed in the presence of 200 nM iberiotoxin to eliminate the contribution of the BK current to the total whole cell current. The average capacitance of the human DSM cells was 26.5 ± 6.1 pF (n = 4, N = 4). The cells were held at −70 mV, and then a 250-ms-long series of potentials between −70 mV and +30 mV were applied in 10-mV increments, and then the cells were repolarized back to −40 mV for another 250 ms. With increasing depolarization of the membrane potential, the human DSM cells responded with gradual increases of the whole cell current amplitude. Tail currents were recorded upon repolarization back to −40 mV (Fig. 4). Under these experimental conditions, ScTx1 (100 nM) was applied to study the contribution of the ScTx1-sensitive current to the total KV current in human DSM. The results showed that ScTx1 (100 nM) caused significant inhibition of the whole cell current amplitude at positive voltages from +10 mV to +30 mV (n = 4, N = 4; P < 0.05; Fig. 4). The remaining whole cell current, after application of the ScTx1, was probably determined by other types of KV channels. Current-voltage relationships of the control current and ScTx1-insensitive current are illustrated in Fig. 4B. Figure 4C represents the current-voltage relationship of the ScTx1-sensitive current (n = 4, N = 4).
Fig. 4.
Stromatoxin-1 (ScTx1) sensitivity of voltage-dependent whole cell KV current in freshly isolated human DSM cells. A: representative recordings of whole cell outward KV currents elicited by a depolarizing voltage step protocol (−70 mV to +30 mV in 10-mV increments) depicting the effect of ScTx1. ScTx1 (100 nM) significantly decreased the voltage step-induced outward current in human DSM cells. ScTx1-sensitive current was obtained after subtraction of the remaining ScTx1-insensitive current from the control current. B: current-voltage relationship in the presence or absence of 100 nM ScTx1. ScTx1 (100 nM) significantly decreased the whole cell outward KV current in human DSM cells (n = 4, N = 4; *P < 0.05). C: current-voltage relationship of the subtracted ScTx1-sensitive current in human DSM cells (n = 4, N = 4). Values are means ± SE. All experiments were performed in the presence of the large-conductance Ca2+ activated K+ (BK) channel selective inhibitor iberiotoxin (200 nM).
Inhibition of ScTx1-sensitive KV2-containing channels increases intracellular Ca2+ levels.
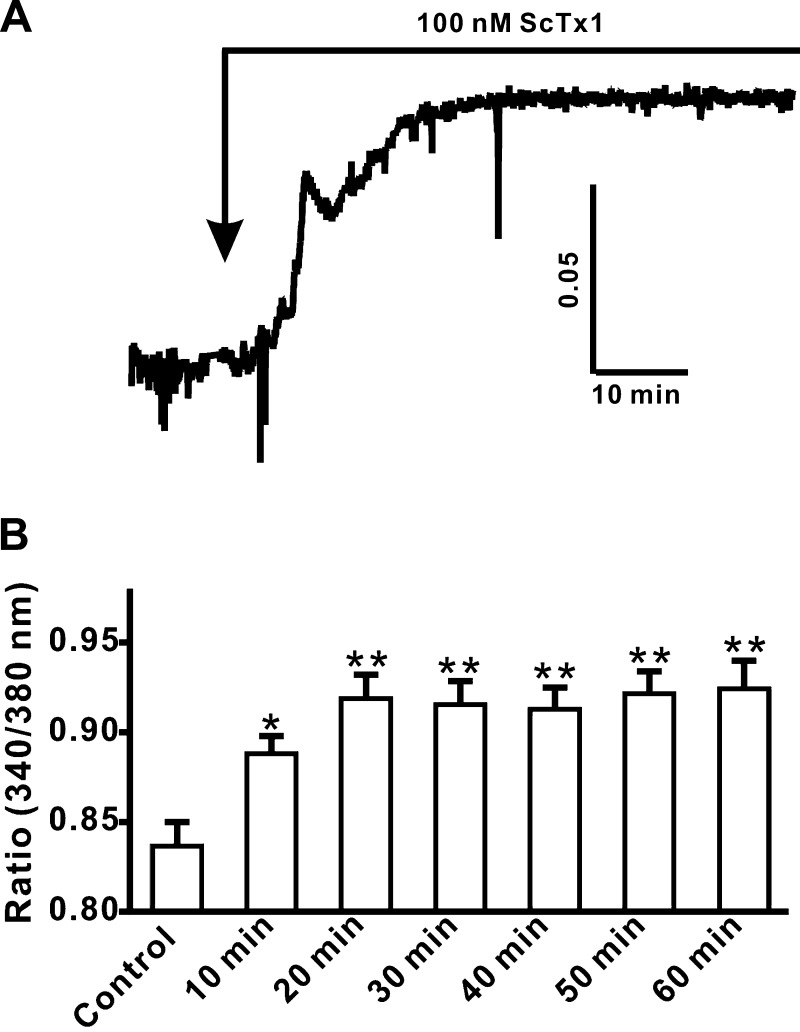
To determine the role of ScTx1-sensitive KV2-containing channels on the intracellular Ca2+ levels, live-cell real-time Ca2+ imaging was carried out with fura-2. As shown in Fig. 5, the intracellular Ca2+ level was significantly increased following application of 100 nM ScTx1 (Fig. 5A). Under control conditions, the fluorescence ratio (340/380 nM) was 0.84 ± 0.01 (n = 31, N = 7), and in the presence of 100 nM ScTx1, the fluorescence ratio increased to 0.92 ± 0.01 (n = 31, N = 7; Fig. 5B). Intracellular Ca2+ level reached a plateau within 20 min following 100 nM ScTx1 application (Fig. 5A). Thus, inhibition of ScTx1-sensitive KV2-containing channels increases intracellular Ca2+ levels in freshly isolated human DSM cells.
Fig. 5.
ScTx1 increases the intracellular free Ca2+ level in freshly isolated human DSM cells. A: original trace illustrating the increase in the intracellular free Ca2+ level following the application of 100 nM ScTx1. B: summary data showing a significant increase in the intracellular free Ca2+ level after application of 100 nM ScTx1. This increase in Ca2+ levels reached a plateau within the first 20 min following ScTx1 application. Values are means ± SE (n = 31, N = 7; *P < 0.05; **P < 0.01).
Role of ScTx1-sensitive KV2-containing channels in myogenic human DSM contractions.
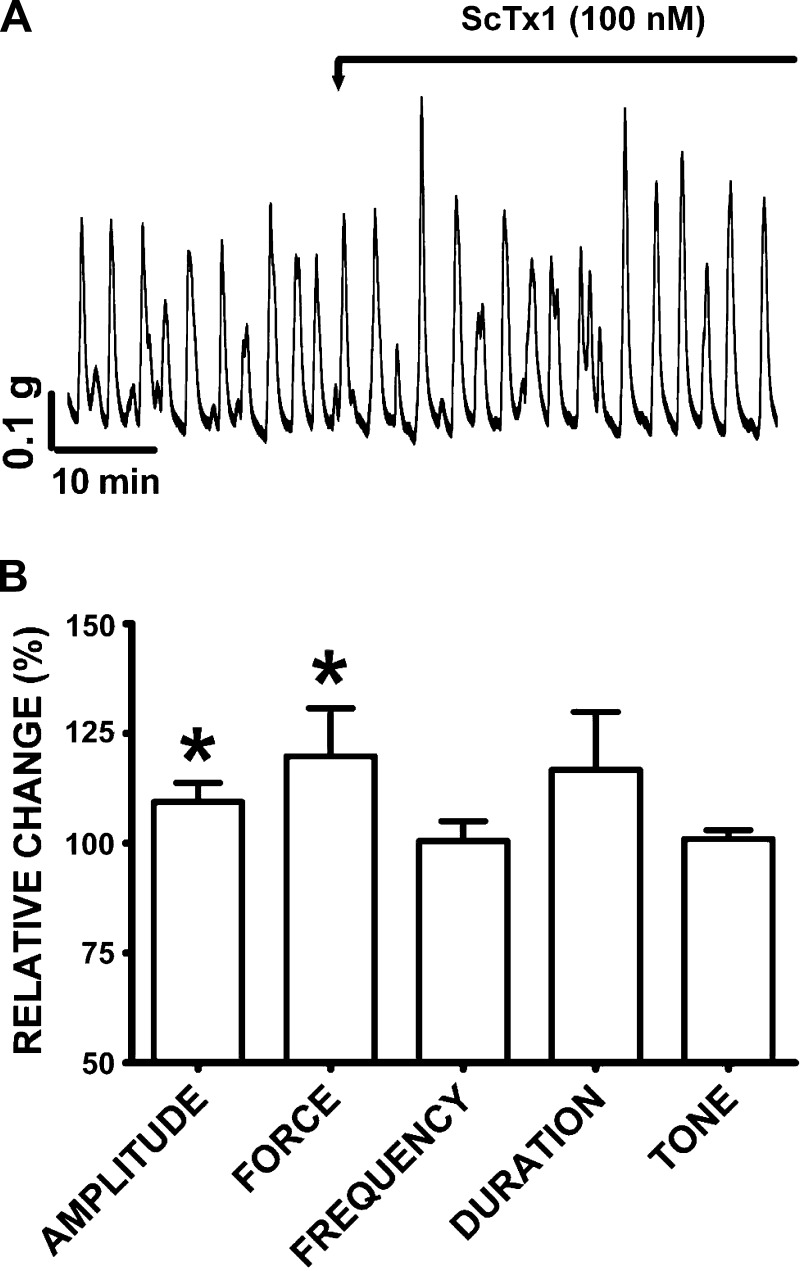
ScTx1 was employed to investigate the potential functional role of KV2.1, KV2.2, and KV2.1/9.3 channels in human DSM spontaneous phasic contractions. Blocking these KV channels with ScTx1 (100 nM) significantly increased the DSM spontaneous phasic contraction amplitude and force (Fig. 6). Within the first 10-min period after ScTx1 (100 nM) addition, the DSM spontaneous phasic contraction amplitude increased by 9.4 ± 4.3% and muscle force integral increased by 19.8 ± 10.9% (n = 16, N = 11; P < 0.05; Fig. 6). ScTx1 did not have any significant effects on the spontaneous phasic contraction frequency, phasic contraction duration, and muscle tone (n = 16; N = 11; P > 0.05; Fig. 6).
Fig. 6.
ScTx1 increases the amplitude and force of the spontaneous phasic contractions in human DSM isolated strips. A: original DSM tension recordings illustrating the effect of ScTx1 (100 nM) on the spontaneous phasic contractions of human DSM isolated strips. B: summary data showing significant increases in human DSM spontaneous phasic contraction amplitude and force upon ScTx1 (100 nM) application. A 10-min period before ScTx1 application was taken as a control. The spontaneous contractions under control conditions were taken to be 100% and data were normalized. The effect of ScTx1 was evaluated during the first 10 min following ScTx1 application. Values are means ± SE (n = 16; N = 11; *P < 0.05). TTX (1 μM) was present throughout the experiments.
Role of ScTx1-sensitive KV2 containing channels in nerve-evoked human DSM contractions.
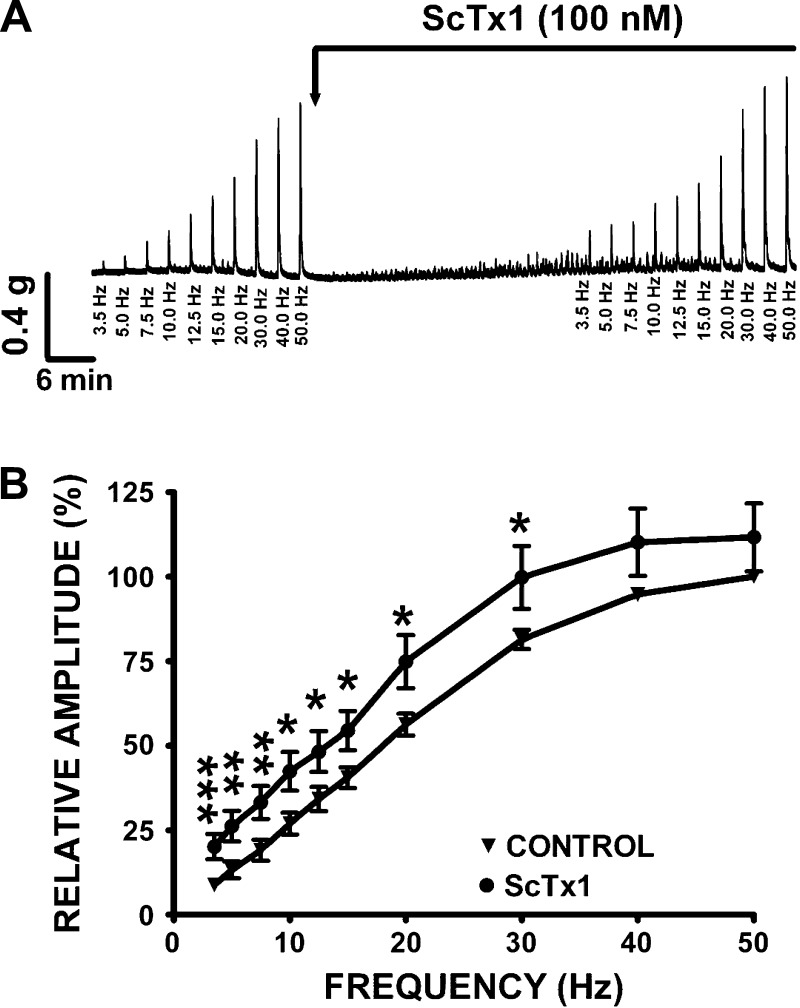
In this experimental series, ScTx1 (100 nM) was employed to investigate the functional implication of KV2.1, KV2.2, and KV2.1/9.3 channels inhibition on EFS-induced contractions of human DSM strips (Fig. 7). ScTx1 (100 nM) significantly increased the amplitude of the EFS-induced contractions at all stimulation frequencies ranging from 3.5 to 30 Hz (n = 24; N = 11; P < 0.05; Fig. 7). At 20 Hz EFS, ScTx1 (100 nM) increased the amplitude of the EFS-induced contraction by 18.6 ± 4.7% (n = 24, N = 11; P < 0.05; Fig. 7).
Fig. 7.
ScTx1 increases the amplitude of the electrical field stimulation (EFS)-induced contractions in human DSM isolated strips. A: original traces of EFS-induced contractions (3.5–50 Hz) in the absence or presence of 100 nM ScTx1 illustrating an increase of the amplitude of the contractions following 100 nM ScTx1 application. As illustrated, ScTx1 also induces spontaneous phasic contractions, consistent with the results illustrated in Fig. 6. B: frequency-response curves showing a significant increase in the amplitude of the EFS-induced contractions following application of ScTx1 (100 nM). The EFS-induced contraction amplitude at a stimulation frequency of 50 Hz under control conditions was taken to be 100%. Values are means ± SE (n = 24, N = 11; *P < 0.05, **P < 0.01, ***P < 0.005).
Collectively, these results suggest that KV2.1, KV2.2, and KV2.1/9.3 channels work to oppose human DSM myogenic and nerve-evoked contractions.
DISCUSSION
This study showed for the first time the expression and function of KV2-containing channels sensitive to ScTx1 in native human DSM. Specifically, we used a multidisciplinary experimental approach to demonstrate the expression of KV2.1 and KV2.2 channel subunits and the electrically silent KV9.3 channel subunit as well as their functional role in human DSM excitability and contractility.
Our RT-PCR experiments revealed mRNA expression of KV2.1, KV2.2, KV4.2, and KV9.3 channel subunits in whole human DSM tissue (Fig. 1). To avoid interference of mRNA signals from other cell types such as neurons, fibroblasts, and interstitial cells, we conducted single-cell RT-PCR experiments on freshly isolated human DSM cells. Unlike RT-PCR experiments on whole human DSM tissue, single-cell RT-PCR experiments revealed only the mRNA expression of KV2.1, KV2.2 and the electrically silent KV9.3 channel subunits (Fig. 1). These results indicated clearly that human DSM cells express the KV2.1, KV2.2 and the silent KV9.3 channel subunits, but not KV4.2 channel subunit. Furthermore, our Western blot experiments confirmed the expression of KV2.1 and KV2.2 channel subunits at the protein level (Fig. 2). Immunocytochemical experiments also demonstrated the protein expression of KV2.1 and KV2.2 channel in single human DSM cells and its cell membrane localization (Fig. 3). Although expressed at mRNA level, detection of the electrically silent KV9.3 subunit protein was not possible at this time because no selective KV9.3 antibody is commercially available to further confirm KV9.3 protein expression. Thus, we conclude that human DSM expresses KV2.1 and KV2.2 channel subunits, and at least mRNA of the electrically silent KV9.3 channel subunit.
The next step was to investigate the role of KV2-containing channels in human DSM excitability. Earlier studies conducted by conventional patch-clamp technique indicated the presence of Ca2+ independent K+ current in human DSM cells, which was inhibited by the nonselective KV channel blocker 3,4-diaminopyridine (10). Here, for the first time, we provide electrophysiological data on KV2-containing channels in freshly isolated human DSM cells, using the amphotericin-perforated patch-clamp technique in physiological conditions with an intact intracellular environment. The main focus of our patch-clamp experiments was to evaluate the contribution of KV2-containing channels sensitive to ScTx1 in human DSM excitability. These experiments were performed following inhibition of the BK channels with 200 nM iberiotoxin. Under such experimental conditions the inhibitory effect of ScTx1 was significant at positive voltages from +10 mV to +30 mV (Fig. 4B), corresponding to the peak of the action potential in human DSM (17, 26). The results showed that ScTx1 significantly decreased the amplitude of the whole cell KV current in human DSM cells (Fig. 4). At more negative voltages, which correspond to the resting membrane potential in human DSM, ScTx1 did not significantly inhibit the whole cell KV current. This observation supports the concept that in human DSM cells KV2-containing channels most likely contribute to the repolarization phase of the action potential, rather than the resting membrane potential. The assumption that KV2 channels contribute to the repolarization phase of the action potential is consistent with recent reports, which showed no effect of ScTx1 on the resting membrane potential in guinea pig and mouse DSM (19, 21). Collectively, the findings suggest that KV2-containing channels sensitive to ScTx1 contribute to human DSM excitability and are most likely involved in control of the repolarization phase of the action potential in human DSM.
In human DSM, CaV-channels are responsible for the initiation of the action potential (17, 26). Here, we demonstrated that inhibition of the KV2-channels with ScTx1 significantly increases the intracellular Ca2+ levels in freshly isolated human DSM cells (Fig. 5). This increase of the intracellular Ca2+ is caused by inhibition of the ScTx1-sensitive KV2 channels and most likely subsequent activation of CaV channels in human DSM cells. Our findings are supported by earlier studies showing that nonselective blockade of the KV channel with 4-AP causes an inhibition of the slow after-hyperpolarization, and increases the action potential frequency by activation of the CaV channels in human DSM (17). These results indicate a functional role of KV2-containing channels in providing a link between human DSM excitability, intracellular Ca2+ levels, and therefore contractility in human DSM.
An increase in intracellular Ca2+ concentration is a key process required for activation of DSM spontaneous contractions. Several studies have demonstrated the importance of Ca2+ influx via CaV channels in the DSM contraction induced by muscarinic and purinergic stimulation (3, 37, 38). DSM spontaneous phasic contractions are myogenic in origin, and TTX (1 μM) does not affect these spontaneous phasic contractions (3, 19, 35). Our data were consistent with these previous reports. For the purpose of the current study, we used TTX only as a precaution to block the transmission of the nerve impulses within the autonomic nerves. Our studies on human DSM contractility reveal that ScTx1 significantly increased the spontaneous phasic contraction amplitude and force in human DSM isolated strips, consistent with our previous findings in rat and guinea pig DSM (8, 21). However, ScTx1 did not cause any significant effect on spontaneous phasic contraction frequency, phasic contraction duration, and muscle tone. In experimental animals a burst of action potentials triggers a single phasic contraction (18). If a similar mechanism operates in human DSM, one should expect that blocking of the KV2-containing channel with ScTx1 would increase the frequency of the action potentials, and therefore the amplitude of the phasic contraction without any effect on the phasic contraction frequency.
Davies et al. (10) showed an increase in the amplitude of the spontaneous phasic contractions of human DSM strips, without any effect on the phasic contraction frequency or muscle tone following KV1 channel inhibition with correolide or agiotoxin-2. Collectively, our data indicate that by decreasing membrane excitability, KV2-containing channels also oppose human DSM spontaneous phasic contractions under physiological conditions. Interestingly, ScTx1 had more profound effects on the spontaneous phasic contractions in guinea pig as compared with human DSM (21). This could be explained by the differential expression of the KV2-containing channels. Human DSM expresses both KV2.1 and KV2.2 channels whereas guinea pig DSM expresses only KV2.1 but not KV2.2 channels (21).
We studied the nerve-evoked contractions by stimulating the DSM nerves with EFS (Fig. 7). In both animal and human DSM, TTX (1 μM) completely eliminates the EFS-induced contractions (4, 8, 17, 19). The TTX-sensitivity confirms the neuronal origin underlying the EFS-induced contractions. Our results also show that ScTx1 significantly increases the amplitude of EFS-induced contractions over a range of stimulation frequency from 3.5 to 30 Hz, indicating the functional role of these channels in human DSM nerve-evoked contractions. This is consistent with our earlier animal studies (8, 21), and revealed that KV2-containing channels also work to oppose human DSM contractility in response to excitatory neurotransmitters.
Phosphorylation of the KV2-containing channels is now recognized as a major means of regulating their functional activity (23, 24). Our previous studies indicate a major role for protein kinase A in DSM function (7, 22, 32). It is likely that phosphorylation of the KV2-containing channels by protein kinase A also plays a role in regulating human DSM function, but this topic requires further investigation.
The pathophysiology of OAB associated with detrusor overactivity is poorly understood, and the current treatment is limited to antimuscarinics that have many adverse effects (4). Therefore, a better understanding of the basic physiology of DSM is critical for developing novel therapeutic approaches against OAB. Compelling lines of evidence by our group and others have led us to now consider the KV2-containing channels as promising pharmacological targets for some types of bladder dysfunctions. In rat and guinea pig DSM, our laboratory has shown that pharmacological blockade of KV2-containing channels with ScTx1 increased DSM contractility (8, 21). Other studies have also shown that rat detrusor hyperreflexia was associated with a significant decrease in KV2.1 channel mRNA levels (14). Therefore, mutations in KV2-containing channels would be expected to cause bladder dysfunction.
The clinical significance of modulating the KV2-containing channels is twofold. Targeting the KV2-containing channels with channel opening agents will reduce detrusor overactivity and alleviate OAB. Alternatively, targeting KV2-containing channels with selective inhibitors may have the potential for increasing DSM contractility, and thus have clinical application for the treatment of some types of urinary retention due to detrusor underactivity. Moreover, increasing KV2 channels expression by genetic manipulation can potentially reduce detrusor overactivity. To facilitate these novel therapeutic approaches, we first need to better understand KV2-containing channel expression, function, and regulation in human DSM. The present study has validated the family of KV2-containing channels as important physiological regulators of human DSM function. Importantly, such channels are potential pharmacological targets for the treatment of some highly prevalent urological disorders such as OAB and/or some types of urinary retention. Current pharmacological therapy for OAB consists of primarily antimuscarinic therapy which has limited efficacy and is associated with considerable side effects. Cholinergic agonists, the primary therapy for detrusor underactivity, have almost no efficacy. The development of KV2-channel modulators would have potential favorable effects on both of these conditions. However, development of such agents would require some degree of selectivity for the lower urinary tract as KV2-containing channel expression has been reported in some other smooth muscle tissues (2, 25, 34) as well as some other human tissues such as pulmonary artery (13, 33), neurons (23), and pancreatic delta cells (6). Thus, application of such agents for lower urinary tract conditions may result in collateral effects elsewhere. Clinical trials of KV2-containing channel modulators would need to be performed to validate such assumption and assess the efficacy and therapeutic safety window.
In conclusion, ScTx1-sensitive KV2-containing channels are expressed in human DSM. They control human DSM excitability, intracellular Ca2+ levels, and myogenic and nerve-evoked contractions. The KV2-containing channels may represent new targets for pharmacological or genetic manipulation of human DSM. This study is a fundamental basis for future investigation on human DSM tissues from patients with bladder pathologies which may help reveal the role of KV2-containing channels in the etiology of these bladder pathologies.
GRANTS
This study was supported by National Institutes of Health Grant DK-084284 (to G. V. Petkov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.L.H., M.C., S.A.A., Q.C., and G.V.P. performed the experiments; K.L.H., S.A.A., Q.C., and G.V.P. analyzed the data; K.L.H., E.S.R., and G.V.P. interpreted the results of the experiments; K.L.H., M.C., S.A.A., Q.C., and G.V.P. prepared the figures; K.L.H., S.A.A., and G.V.P. drafted the manuscript; K.L.H., M.C., S.A.A., Q.C., E.S.R., and G.V.P. edited and revised the manuscript; K.L.H., M.C., S.A.A., Q.C., E.S.R., and G.V.P. approved the final version of the manuscript; E.S.R. and G.V.P. conception and design of the research.
ACKNOWLEDGMENTS
We thank Medical University of South Carolina (MUSC) Urology staff surgeons Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, and Jonathan Picard; as well as the MUSC Urology Residents Drs. Lydia Labocetta, Elizabeth Peacock, Matthew Young, Vaughn Taylor, Erin Burns, Samuel Nickles, Ahmed M. El-Zawahry, Avi C. Weiss, Gary W. Bong, Kelly Doyle, Matthew McIntyre, Matt Eskridge, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, and Vinh Q. Trang for help with human tissue collection; and Drs. John Malysz, Wenkuan Xin, Shankar Parajuli, Rupal Soder, and Ms. Amy Smith for the critical evaluation of the manuscript.
Present address of M. Chen: Ocean University of China, Qingdao, PR China 266003.
REFERENCES
- 1. Afeli SA, Hristov KL, Petkov GV. Do β-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am J Physiol Renal Physiol 302: F251–F263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 291: C348–C356, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun M, Ramracheya R, Amisten S, Bengtsson M, Moritoh Y, Zhang Q, Johnson PR, Rorsman P. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia 52: 1566–1578, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies AM, Batchelor TJ, Eardley I, Beech DJ. Potassium channel KV alpha1 subunit expression and function in human detrusor muscle. J Urol 167: 1881–1886, 2002 [PubMed] [Google Scholar]
- 11. Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol 62: 48–57, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon 43: 555–574, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Firth AL, Remillard CV, Platoshyn O, Fantozzi I, Ko EA, Yuan JX. Functional ion channels in human pulmonary artery smooth muscle cells: voltage-dependent cation channels. Pulm Circ 1: 48–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gan XG, An RH, Bai YF, Zong DB. Expressions of voltage-gated K+ channel 2.1 and 2.2 in rat bladder with detrusor hyperreflexia. Chin Med J (Engl) 121: 1574–1577, 2008 [PubMed] [Google Scholar]
- 15. Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev 55: 583–586, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayase M, Hashitani H, Kohri K, Suzuki H. Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181: 2355–2365, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360–C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikematsu N, Dallas ML, Ross FA, Lewis RW, Rafferty JN, David JA, Suman R, Peers C, Hardie DG, Evans AM. Phosphorylation of the voltage-gated potassium channel Kv2.1 by AMP-activated protein kinase regulates membrane excitability. Proc Natl Acad Sci USA 108: 18132–18137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol 103: 95–101, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Kyle B, Bradley E, Ohya S, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. Contribution of Kv2.1 channels to the delayed rectifier current in freshly dispersed smooth muscle cells from rabbit urethra. Am J Physiol Cell Physiol 301: C1186–C1200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montgomery BS, Thomas PJ, Fry CH. The actions of extracellular magnesium on isolated human detrusor muscle function. Br J Urol 70: 262–268, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol 587: 625–640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohya S, Tanaka M, Watanabe M, Maizumi Y. Diverse expression of delayed rectifier K+ channel subtype transcripts in several types of smooth muscles of the rat. J Smooth Muscle Res 36: 101–115, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Platoshyn O, Remillard CV, Fantozzi I, Mandegar M, Sison TT, Zhang S, Burg E, Yuan JX. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287: L226–L238, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uchida W, Masuda N, Shirai Y, Shibasaki K, Satoh N, Takenada T. The role of extracellular Ca2+ in carbachol-induced tonic contraction of the pig detrusor smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 350: 398–402, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Wu C, Sui G, Fry CH. The role of the L-type Ca(2+) channel in refilling functional intracellular Ca(2+) stores in guinea-pig detrusor smooth muscle. J Physiol 538: 357–369, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]