Abstract
RTS,S/AS01, a vaccine targeting pre-erythrocytic stages of Plasmodium falciparum, is undergoing clinical trials. We report an analysis of cellular immune response to component antigens of RTS,S – hepatitis B surface antigen (HBs) and P. falciparum circumsporozoite (CS) protein – among Tanzanian children in a Phase IIb RTS,S/AS01E trial. RTS,S/AS01 E vaccinees make stronger T cell IFN-γ, CD69 and CD25 responses to HBs peptides than do controls, indicating that RTS,S boosts pre-existing HBs responses. T cell CD69 and CD25 responses to CS and CS-specific secreted IL-2, were augmented by RTS,S vaccination. Importantly, more than 50% of peptide-induced IFN-γ+ lymphocytes were NK cells and the magnitude of the NK cell CD69 response to HBs peptides correlated with secreted IL-2 concentration. CD69 and CD25 expression and IL-2 secretion may represent sensitive markers of RTS,S-induced, CS-specific T cells. The potential for T cell-derived IL-2 to augment NK cell activation in RTS,S-vaccinated individuals, and the relevance of this for protection, needs to be explored further.
Keywords: Malaria, vaccine, NK cell, IL-2
Introduction
Malaria remains a major cause of human disease in the developing world (1). Despite reports of declining malaria morbidity in several African countries (2), a safe and effective malaria vaccine would make a substantial contribution to malaria control programmes. The most advanced vaccine, RTS,S/AS, has consistently been found to confer partial protection against malaria infection and clinical malaria episodes in phase II trials (3, 4). RTS,S, a chimaeric recombinant protein expressed in Saccharomyces cerevisiae, comprises the central repeat and C terminal portions of the Plasmodium falciparum circumsporozoite (CS) protein fused to Hepatitis B virus surface antigen (HBs) combined with excess HBs into virus-like particles. RTS,S administered with the GSK proprietary adjuvant AS01 (containing liposomes, MPL and QS21) to Tanzanian and Kenyan children aged 5-17 months was safe (5) and reduced the risk of clinical P. falciparum malaria by approximately 50% in the first 8 months after vaccination (6) and approximately 45% over a period of 15 months (7).
Identifying immunological correlates of protection for RTS,S would facilitate evaluation of future vaccines and may allow vaccines to be specifically engineered to induce protective responses. Anti-CS antibody titres in excess of 20μg/ml of CS-specific IgG seem to be required for protection against infection (8) whilst titres of approx 40EU/ml are associated with a significant step-change in the risk of clinical malaria (7). Preclinical data are suggestive of protective roles for IFN-γ-producing CD8+ T cells (reviewed in (8)) and there has been a consistent trend for vaccinated individuals who were protected against challenge infection to make stronger CD4+ T cell cytokine responses than individuals who were not protected (9-11).
Here we report an analysis of post-vaccination cellular immune responses of children enrolled, between the 5-17 months of age, in a Phase IIb trial of RTS,S/AS01E in Korogwe, Tanzania. We have identified additional, sensitive markers of CS-specific T cell recall responses and a previously unrecognised response by natural killer (NK) cells. These newly identified markers of cellular immunity to RTS,S reveal a potential new pathway of vaccine-induced pre-erythrocytic immunity, namely rapid activation of NK cells by antigen-specific IL-2-secreting CD4+ T cells.
Materials and methods
Study subjects and vaccinations
Four hundred and forty seven, 5- to 17-month old Tanzanian children were enrolled in a Phase IIb, randomised, double blind, controlled trial of RTS,S/AS01E (ClinicalTrials.gov registration number NCT00380393) and represent the Tanzanian cohort of a two site study also involving children from Kenya (total enrolment = 894) (6). All children had previously received routine Hepatitis B vaccinations as part of the expanded programme on immunisations. Children received 3 doses of RTS,S/AS01E or 3 doses of human diploid cell rabies vaccine (Sanofi-Pasteur) at 4 week intervals (acceptable range 20-60 days). Venous blood was collected from 354 children between 4.5 and 10.5 (mean 7.9) months after the third vaccination. A full blood count was made and Giemsa-stained thick blood films were examined for malaria parasitaemia. CS and HBs antibodies were measured by validated ELISA as previously described (7) (12).
Ethical approval for the trial, including this immunological assessment, was obtained from the Tanzanian Medical Research Coordinating Committee, the London School of Hygiene and Tropical Medicine Ethics Committee and the Western Institutional Review Board, Seattle, USA. Written informed consent was provided by study participants and/or their legal guardians. An independent Data Safety Monitoring Board and local safety monitors were appointed.
PBMC preparation and culture
PBMC were isolated from heparinised blood by density gradient centrifugation (13), resuspended at 2 × 106 cells/ml in growth medium (GM: RPMI-1640 containing heat-inactivated normal human AB serum (10%), L-Glutamine (2mM), penicillin and streptomycin (each at 100 IU/ml)) in U-bottom 96-well tissue culture plates (total volume = 200μl) and incubated at 37°C in 5% CO2 for 24 hours. Brefeldin A (3 μg/ml) was added 3 hours prior to harvesting. PBMC were stimulated with peptide pools (15-mers, overlapping by 11 amino acids, each peptide at 1 μg/ml, purity > 85%; Eurogentec) representing the vaccine sequences of the hepatitis B surface antigen (HBs) (53 peptides) or the CS protein (31 peptides) or with recombinant human (rh)IL-12+IL-18 (each at 100ng/ml) as a positive control. Negative controls contained GM alone.
Culture supernatants were analysed by Luminex® for IL-2, IL-10, IFN-γ, sCD40-L, IL-12p70, IL-17, IL-15, and IFNα-R2.
Cell surface and intracellular staining for flow cytometry
Cell staining was performed as described (13) using mouse monoclonal antibodies: anti-CD3 PerCP (SK7; BD Biosciences); anti-CD56 APC (N901; Beckman Coulter); anti-CD69 PE (CH-4; Serotec); anti-IFN-γ FITC (D9D10; Serotec); anti-CD4 FITC (RPA-T4; eBioscience) and anti-CD25 APC (BC96; eBioscience). Data were acquired on a Becton Dickinson FACSCalibur and analysed with FlowJo (TreeStar).
Given the small blood volumes available and the limitation of 4 colour flow cytometry, CD69 was chosen as a marker of activation as it is upregulated within 24 hours in both T cells and NK cells. It was not possible, within this 4 colour assay, to include CD69 and CD25 in the same staining panel.
Statistical analysis
Only data from children who were fully vaccinated according to protocol were presented for analysis. Due to insufficient PBMCs being obtained from some children, numbers of samples tested vary among the assays performed. Data acquisition was performed on blinded samples. Data were imported into Stata 11.0 (Stata Statistical Software) or GraphPad Prism v5.0c for analysis. For flow cytometry, responders are defined as those with a peptide-specific response ≥ twice their own negative control value. For cytokine secretion (Luminex) assays, responders are defined as those with a peptide-specific cytokine response ≥ the mean + 2SD of their own negative control value. Differences in the proportions of responding individuals were determined using Fisher’s exact Test. Paired comparisons within groups were made using Wilcoxon’s matched-pair signed rank test. Unpaired comparisons were made using Mann-Whitney tests. Correlations between different cellular parameters were assessed using Spearman’s correlation coefficient. For comparison of antibody titres with cellular immune parameters, all data were log10 transformed, resulting in a normal distribution. Pearson correlation coefficients with p-values were calculated.
Results
After exclusion of clotted (n = 4), small (n = 104) and low viability (n = 61) samples, 185 samples (81 RTS,S, 104 control) were available for analysis (Table I). Of these, 178 samples (80 RTS,S, 98 control) generated data on CD69 expression and IFN-γ production and 116 samples (55 RTS,S, 61 control) generated data on CD25 expression.
Table I.
Baseline characteristics of subjects
|
RTS,S/AS01E n= 81 |
Control n=104 |
||
|---|---|---|---|
| Age (months)* | 11.7 (± 0.4) | 11.9 (± 0.3) | |
| Sex (% Female) | 52.5 | 48.1 | |
| [Hb] (g/dL)* | 10.6 (± 1.1) | 10.7 (± 1.0) | |
| WBC(10−9/L)* | 9.9 (± 3.3) | 9.6 (± 3.4) | |
|
P. falciparum positive
(n) ** |
0 | 1 | |
|
Anti-
HBSAg Ab titre (U/ml) *** |
Pre-vacc | 148 (43-382) | 258 (87-643) |
| Month 3 | 48489 (4708 -132314) | 213 (77-475)† | |
| Month 12 | 9357 (3937-16269) | 148 (33-436)† | |
|
Anti-CS
Ab titre (U/ml) *** |
Pre-vacc | 0.25 (0.25 – 0.25) | 0.25 (0.25 – 0.25) |
| Month 3 | 506 (314-814) | 0.25 (0.25 – 0.25)† | |
| Month 12 | 37 (23-58) | 0.25 (0.25 – 0.25)† | |
Mean (SD)
Microscopic analysis of Giemsa-stained blood smears
Median values plus interquartile ranges (25%-75%)
Titres are significantly higher in RTS,S/AS01 vaccinees than controls (p ≤ 0.01)
Sixteen of these children (3 in the RTS,S group and 13 in the control group) developed a clinical episode of malaria during the follow up period for this study.
Vaccination with RTS,S/AS01E induces CS-specific T cell responses and boosts T cell responses to HBs
PBMC were cultured for 24 hours without stimulation (GM), with rhIL-12+IL-18 or with HBs or CS peptides to quantify effector memory T cell responses. Cell surface expression of the early activation marker CD69 and intracellular expression of IFN-γ were analysed in all CD3+ T cells and cell surface expression of the high affinity IL-2 receptor α chain (CD25) was analysed in all CD4+ lymphocytes. Representative data from one RTS,S vaccinee are shown in Fig. 1A. Aggregated data are shown in Fig. 1B - E.
Figure 1. T cell responses to HBs and CS peptides in vaccinees and controls.
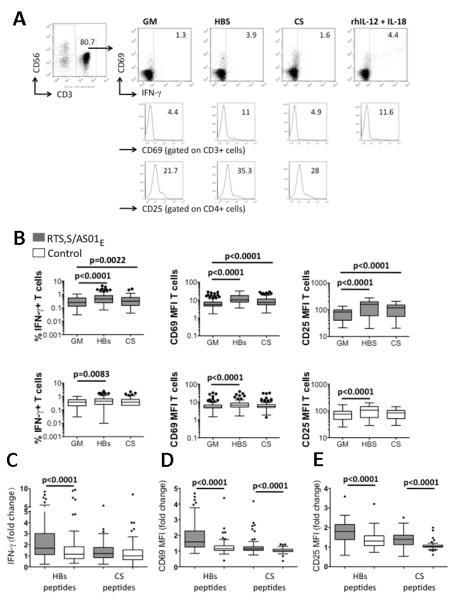
PBMCs were cultured in growth medium alone (GM), with HBs or CS peptides or with rIL-12/IL-18 for 24 h and analysed by flow cytometry. (A) An example of the gating strategy for CD3+ and CD4+ T cells, CD69, IFN-γ and CD25 expression for one RTS,S/AS01E vaccinated subject.
(B) Percentage of T cells expressing IFN-γ (left plots) and mean MFI for CD69 (middle plots) and CD25 (right plots) expression on T cells in RTS,S/AS01E vaccinees (upper plots) and controls (lower plots). Fold change in T cell IFN-γ % (C), T cell CD69 MFI (D) and CD4+ T cell CD25 MFI (E). The horizontal line within each box represents the median; the top and bottom of each box represent the 25th and 75th percentiles, respectively, and the whiskers represent the upper and lower limits of values within 1.5 times the interquartile range. Circles denote outliers. Data from 80 RTS,S/AS01E vaccinees and 98 controls is presented for IFN-γ and CD69; data from 55 RTS,S/AS01E vaccinees and 61 controls is presented for CD25. P values are derived from paired Wilcoxon (B) and unpaired Mann Whitney (C,D,E) tests.
T cells from both RTS,S-vaccinated and control (but previously HBs vaccinated) children made significant T cell IFN-γ responses to HBs peptides (Figure 1B) but responses were significantly higher among RTS,S vaccinees than among controls (Fig. 1C). The proportion of RTS,S vaccinees responding to HBs was significantly higher than the proportion of controls who responded (Table II) indicating that, even in this recently HBs-vaccinated cohort, RTS,S/AS01E effectively boosts HBs-specific T cell responses. By contrast, although a T cell IFN-γ response to CS peptides was observed in the RTS,S recipients (Figure 1B), neither the magnitude (Figure 1C) nor the frequency (Table II) of T cell IFN-γ responses to CS peptides differed significantly between RTS,S-vaccinated and control children.
Table II.
Proportion of individuals with T cell or NK cell CD69, IFN-γ or CD25 responses1 to HBs or CS peptides
| Antigen | Group | CD692 | IFN-γ2 | CD253 | |||
|---|---|---|---|---|---|---|---|
| T cells | NK cells | T cells | NK cells | T cells | |||
| HBs |
RTS,S/AS01
E |
n (%) |
29 (36.2) |
51 (63.8) |
30 (37.5) |
37 (46.3) |
27 (49.1) |
| Control | n (%) |
4 (4.1) |
7 (7.1) |
22 (22.5) |
18 (17.5) |
5 (8.2) |
|
| P4 | <0.0001 | <0.0001 | 0.021 | <0.0001 | <0.0001 | ||
| CS |
RTS,S/AS01
E |
n (%) |
6 (7.5) |
11 (13.8) |
13 (16.3) |
21 (26.2) |
5 (9.1) |
| Control | n (%) |
0 (0) |
3 (3.1) |
16 (16.3) |
20 (20.4) |
0 (0) |
|
| P4 | 0.006 | 0.01 | 1.000 | 0.38 | 0.02 | ||
Responders are defined as those with a peptide-specific response greater than or equal to twice the value of their own negative control (GM) value
CD69 and IFN-γ data are based on 80 RTS,S/AS01E vaccinees and 98 Controls.
CD25 data are based on 55 RTS,S/AS01E vaccinees and 61 Controls.
Fisher’s exact test
To determine whether other markers of antigen-specific memory T cells might be more sensitive than IFN-γ secretion, T cell expression of CD69 and CD25 was examined. Expression of both CD69 and CD25 - assessed as mean fluorescence intensity (MFI) (Figure 1) or as the percentage of positive cells (data not shown) - was significantly upregulated on T cells from both RTS,S-vaccinees and controls after re-stimulation with HBs peptides and on T cells from RTS,S-vaccinees, but not controls, after CS peptide re-stimulation (Fig. 1B). The fold change in both CD69 (Fig. 1D) and CD25 (Fig. 1E) expression was significantly greater among RTS,S-vaccinees than among controls after restimulation with either HBs or CS peptides, and the proportion of RTS,S-vaccinees with positive CD69 and CD25 responses was significantly higher than the proportion of controls making such responses (Table II), indicating that CD69 and CD25 expression identify T cell memory responses to CS peptides that are not evident by IFN-γ secretion.
IL-2 is a sensitive marker of CS-specific responses in RTS,S/AS01E vaccinated individuals
Culture supernatants of 92 randomly selected individuals (38 of whom were subsequently revealed to be RTS,S recipients and 54 to be controls) were analysed by Luminex®. Concentrations of IFN-γ, IL-2 and IL-10 differed significantly between RTS,S/AS01E vaccinees and controls (Fig. 2).
Figure 2. Cytokine secretion from HBs and CS peptide stimulated PBMCs in vaccinees and controls.
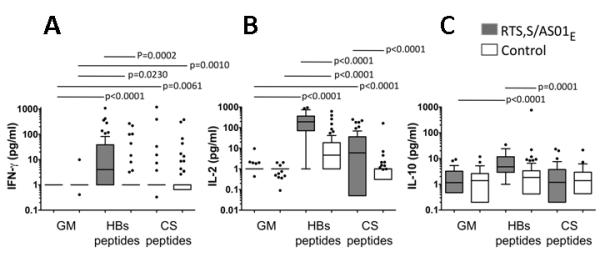
PBMCs were cultured in growth medium alone (GM), with HBs or CS peptides for 24 h and culture supernatants from 38 RTS,S/AS01E vaccinees and 54 controls were collected and analyzed by Luminex bead array for (A) IFN-γ, (B) IL-2 and (C) IL-10. The horizontal line within each box represents the median; the top and bottom of each box represent the 25th and 75th percentiles, respectively, and the whiskers represent the upper and lower limits of values within 1.5 times the interquartile range. Circles denote outliers. P values are derived from paired Mann Whitney tests.
Responses to HBs peptides
Concentrations of IFN-γ (Fig. 2A) and IL-2 (Fig. 2B) were significantly higher in HBs culture supernatants than in control (GM) supernatants among both RTS,S/AS01E recipients and controls, indicating that HBs vaccination in infancy had induced a durable effector memory cell response. However HBs-induced IFN-γ and IL-2 concentrations, and the proportion of subjects with a detectable IFN-γ or IL-2 response, were significantly higher among RTS,S/AS01E vaccinees than among controls (Table III), confirming that RTS,S/AS01E vaccination efficiently boosts HBs responses. IL-10 concentrations were significantly higher in HBs-stimulated cultures than in control cultures of RTS,S/AS01E recipients (Fig. 2C) but there was no significant IL-10 response among the controls and significantly more vaccinees than controls made a detectable IL-10 response (Table III), suggesting either that HBs-specific IL-10 is induced by RTS,S/AS01E but not by routine HBs vaccination or that IL-10 responses are short-lived and had been boosted in RTS,S/AS01E vaccinees but had waned to undetectable levels in control subjects.
Table III.
Proportion of individuals with a secreted cytokine response1 to HBs or CS peptides.
| Antigen | Group | IFN-γ | IL-2 | IL-10 | |
|---|---|---|---|---|---|
| HBs |
RTS,S/AS01E N = 38 |
n (%) |
20 (52.6) |
37 (97.4) |
18 (47.4) |
|
Control N = 54 |
9 (16.7) |
32 (59.3) |
8 (14.8) |
||
| p | 0.0005 | <0.0001 | 0.0009 | ||
| CS |
RTS,S/AS01E N = 38 |
n (%) |
6 (15.8) |
26 (68.4) |
4 (10.5) |
|
Control N = 54 |
13 (24.1) |
3 (5.6) |
3 (5.6) |
||
| p | 0.44 | <0.0001 | 0.44 |
Responders are defined as those with a peptide-specific cytokine response greater than or equal to the mean + 2SD of their own negative control (GM) values
Responses to CS peptides
CS-induced IFN-γ concentrations were slightly higher than in control cultures but neither the concentration (Fig 2A) nor the proportion of responders (Table III) differed between RTS,S vaccinees and controls, suggesting that this response may result from natural exposure to malaria sporozoites. CS-induced IL-10 secretion did not differ significantly from background among RTS,S/AS01E vaccinees or controls (Fig. 2C, Table III). Robust CS-specific IL-2 responses were observed among RTS,S/AS01E vaccinees (CS versus GM, P < 0.0001) but not among controls (CS versus GM, P = 0.07), CS-specific IL-2 concentrations were significantly higher among vaccinees than controls (P < 0.0001) (Fig. 2B) and significantly more vaccinees than controls had a detectable CS-specific IL-2 response (P < 0.0001) (Table III). Since antigen-specific T cells are the primary source of IL-2, these data suggest that CS-specific IL-2 may be a more sensitive marker of T cell responses to RTS,S than CS-specific IFN-γ production.
Natural Killer cells of RTS,S/AS01E vaccinees are activated in HBs- and CS-stimulated cultures
We have reported that T cell IL-2 responses to malaria (14) and viral vaccine antigens (15) are associated with markedly enhanced NK cell responses to the same organisms. To explore whether RTS,S/AS01E vaccination enhances NK cell responses to HBs and/or CS, we compared IFN-γ and CD69 expression in CD3− CD56+ NK cells between the two groups of children. Representative data for one RTS,S vaccinee are shown in Fig. 3A; aggregate data are shown in Fig. 3B,C,D and Table II.
Figure 3. NK cell responses among HBs and CS peptide stimulated PBMCs in vaccinees and controls.
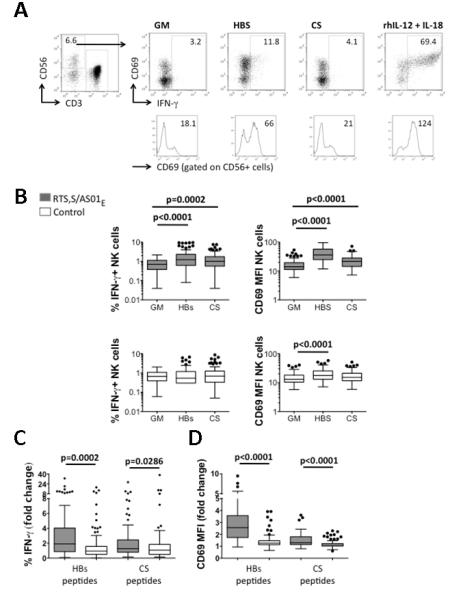
PBMCs from RTS,S/AS01E vaccinees (n = 80) and controls (n = 98) were cultured in growth medium alone (GM), with HBs or CS peptides or with rIL-12/IL-18 for 24 h and analysed by flow cytometry. (A) An example of the gating strategy for NK cells (CD3-CD56+), CD69 and IFN-γ expression for one RTS,S/AS01E vaccinated subject.
(B) Percentage of NK cells expressing IFN-γ (left plots) and mean MFI for CD69 expression on NK cells (right plots) in RTS,S/AS01E vaccinees (upper plots) and controls (lower plots). Fold change in NK cell IFN-γ % (C) and NK cell CD69 MFI (D). The horizontal line within each box represents the median; the top and bottom of each box represent the 25th and 75th percentiles, respectively, and the whiskers represent the upper and lower limits of values within 1.5 times the interquartile range. Circles denote outliers. P values are derived from paired Wilcoxon (B) and unpaired Mann Whitney (C,D) tests.
Responses to HBs peptides
Both IFN-γ secretion and CD69 expression were significantly upregulated in NK cells of RTS,S/AS01E-vaccinees after restimulation of PBMCs with HBs peptides; among the controls, NK cell CD69 expression was significantly upregulated but IFN-γ secretion was not. Both the magnitude and the frequency of NK cell IFN-γ and CD69 responses to HBs restimulation were higher among RTS,S vaccinees than among controls (Fig. 3C; Table II), indicating that NK cell responses may be indicative of an antigen-specific memory response and can be boosted by revaccination.
Responses to CS peptides
Statistically significant NK cell IFN-γ and CD69 responses were observed among CS-stimulated PBMCs of RTS,S-vaccinated children (CS vs. GM, p = 0.0002 and p < 0.0001, for IFN-γ and CD69 respectively) but not control children (Fig 3B). NK cell IFN-γ and CD69 responses in CS-stimulated cultures were significantly higher among RTS,S vaccinees than controls (Fig. 3C,D) and the frequency (Table II) of NK cell CD69 upregulation was higher among RTS,S-vaccinees than among controls.
NK cell activation correlates with IL-2 secretion
Since we have previously observed that NK cell “recall” responses are mediated by IL-2 from memory T cells (14, 15) we looked for associations between IL-2 secretion and NK cell responses to HBs or CS peptides (Fig. 4). We observed statistically significant associations between IL-2 concentration and NK cell CD69 expression in response to both HBs (p < 0.0001; Fig. 4B) and CS (p = 0.035; Fig. 4F) among RTS,S/AS01E-vaccinees, and in response to HBs (p = 0.02 Fig 4D) but not CS among controls (Fig 4H), indicating that IL-2 may be sufficient to upregulate NK CD69 expression. However, NK cell IFN-γ responses were not significantly correlated with IL-2 concentration for either HBs- or CS-stimulated cells in either vaccinated or control subjects (Fig 4A,C,E,G), consistent with the notion that other signals - in addition to IL-2 - are required for activation of NK cells to full effector status (14, 15). (NB: Although it may appear that NK cell IFN-γ and IL-2 responses to CS peptides in the RTS,S vaccinees are mutually exclusive (Fig 4E) this relationship was not statistically significant (χ2 = 0.73, p = 0.39)).
Figure 4. Correlation between IL-2 secretion and NK cell IFN-γ or CD69 responses to HBs and CS peptides.
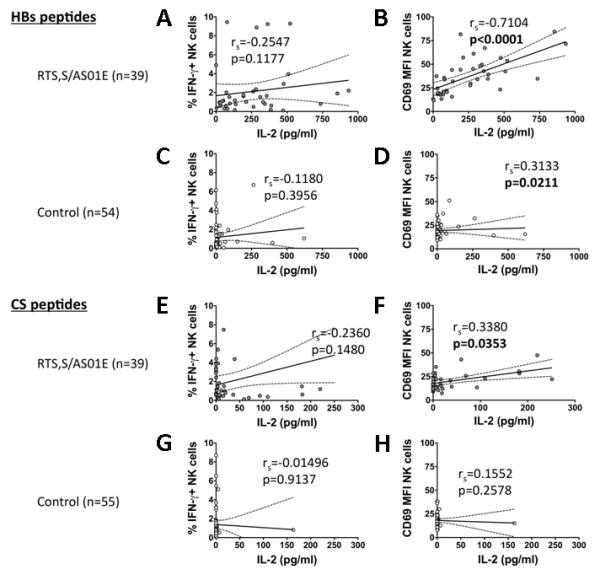
NK cell IFN-γ % (A,C,E,G) and CD69 MFI (B,D,F,H) responses were compared with IL-2 secretion (pg/ml) for PBMC cultured with (A-D) HBs or (E-H) CS peptides for 24 h, from RTS,S/AS01E vaccinees (n = 39, A,B,E,F) and controls (n = 55, C,D,G,H). Each circle represents data for one individual child. Solid lines = the goodness of fit (r2) as determined by Spearman’s correlation analysis; dotted lines = 95% confidence band.
NK cells contribute substantially to post-vaccination IFN-γ responses
Although the percentage of NK cells secreting IFN-γ after RTS,S/AS01E vaccination was greater (HBs: median 1.23%, range 0.08-16.55%; CS: median 1.00%, range 0.04 – 7.48%) than the percentage of T cells making IFN-γ (HBs: median 0.31%, range 0.04-2.56%; CS: median 0.46%, range 0.07 – 4.39), T cells are far more numerous in peripheral blood than are NK cells. To determine which population was the major contributor to the early IFN-γ response, the data were re-analysed by gating on all IFN-γ positive cells and analysing them for expression of CD3 and CD56 (Figure 5A). Among RTS,S/AS01E-vaccinees, NK cells were the largest population of cells making IFN-γ in response to both HBs (51.8%; SEM 2.3%) and CS (52.4%; SEM 2.6%) peptides (Figure 5B).
Figure 5. Relative contribution of T cells and NK cells to the total IFN-γ response to HBs and CS peptides in RTS,S/AS01E vaccinated subjects.
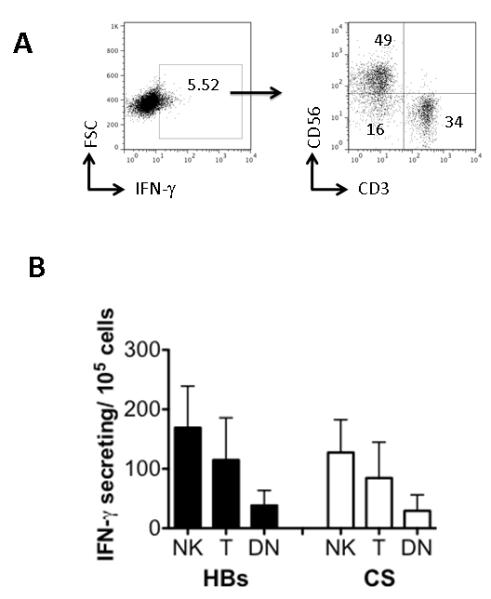
PBMCs from RTS,S/AS01E vaccinees (n = 80) were cultured with HBs or CS peptides for 24 h and analysed by flow cytometry. (A) An example of the gating strategy to identify IFN-γ+ cells and then to determine the proportion of these cells that are either T cells (CD3+), NK cells (CD3−, CD56+) or CD3−CD56− cells for HBs-stimulated cells from one vaccinated subject. (B) Bar charts indicate the absolute number (mean/SD per 105 PBMCs) of IFN-γ+ cells that are NK cells, T cells or CD3−CD56− (DN, double negative) lymphocytes among HBs-stimulated (filled bars) or CS-stimulated (open bars) PBMC.
Cell-mediated immune responses correlate with humoral responses to RTS,S
Finally, we looked for associations between anti-HBs and anti-CS antibody titres (measured at the 3 month time point, i.e. 4 weeks after the third dose of vaccine) (Table 1) and cellular immune parameters. In the control group, there were no significant correlations between anti-HBs titres and any of the cellular parameters. The number of individuals in the control group with a positive anti-CS titre was too small (n = 2) to allow meaningful comparisons with cellular parameters in this group.
By contrast, in the RTS,S vaccinated group, anti-HBs titres were significantly correlated with percentages of both T cells and NK cells secreting IFN-γ (Pearson’s correlation: r = 0.29, p = 0.01; r = 0.26, p = 0.02, respectively) whereas anti-CS titres were not correlated with T cell or IL-2 responses (r ≤ 0.13, p ≥ 0.27 in all cases) but showed a tendency to correlate with NK cell IFN-γ and NK cell CD69 expression, although this was of borderline significance (Pearson’s correlation: r = 0.21, p = 0.08, in both cases).
Discussion
RTS,S/AS01 is the first malaria vaccine to enter phase III clinical trials (16). Associations have been observed between resistance to infection and CS-specific antibody titres (17-20) and between resistance to clinical malaria, CS-specific IFN-γ (10, 11, 21) and secreted IL-2 (21) responses, but these associations are not correlates of protection at the individual level. Although the number of children in this study who experienced at least one episode of clinical malaria was too small to draw any legitimate conclusions regarding individual correlates of protection, our primary purpose was to look for additional markers of RTS,S-induced cellular immune responses that could be evaluated as correlates of immunity in future trials.
This study demonstrates that RTS,S/AS01 very effectively boosts cellular responses induced by prior hepatitis B virus vaccination; this can be detected as upregulation of CD69, CD25 and IFN-γ in CD4+ T cells, upregulation of CD69 and IFN-γ among NK cells and enhanced PBMC secretion of IL-2, IFN-γ and IL-10. These results are consistent with our previous observations (14, 15) that IL-2 emanating from memory T cells can activate NK cells, although we were surprised that restimulation of PBMCs with peptide alone was sufficient for NK cell activation since we have previously observed that optimal NK cell responses also require cytokine-mediated (22, 23) and contact-dependent (24) signals from accessory cells and that this depends on preserving the pattern recognition pathways initiated by whole pathogens (15). However, in a separate study, we have observed that recombinant HBs antigen is sufficient to activate NK cells in individuals whose cells produced very large amounts of IL-2 (A. Horowitz, S.E. Moore and E.M. Riley, unpublished) suggesting that the need for accessory cell signalling for NK activation may be (partially) overridden in the presence of high levels of IL-2. If so, then T cell-derived IL-2 may synergise with accessory cell-derived signals induced by whole pathogens, leading to even more potent NK cell responses than we have observed here, but further experiments are required to test this hypothesis. Importantly, the frequency and magnitude of the NK cell IFN-γ response was such that NK cells were found to contribute more than half of the HBs-specific IFN-γ response, raising the distinct possibility that NK cells may be important effectors of the immediate response to HBV after vaccination.
RTS,S vaccination also induces CD4+ T cell and NK cell responses to CS peptides. Although we did not observe significant CS-specific T cell IFN-γ responses among RTS,S/AS01 recipients when compared to controls, we did observe very clear induction of IFN-γ from NK cells, enhancement of CD69 expression in T cells and NK cells, upregulation of CD25 on CD4+ T cells and secretion of IL-2 (but not IFN-γ or IL-10) after in vitro restimulation of PBMCs with CS peptides. Since CD8+ T cells have been shown to inhibit parasite development within hepatocytes in an IFN-γ-dependent manner (25), the observation that NK cells can secrete IFN-γ within 24 hours of re-stimulation with CS antigen raises the possibility that NK cells may also be effective against malaria-infected liver cells. It would also be of interest to explore whether NK cell cytotoxicity – which can also be enhanced by vaccination (15) -contributes to killing of malaria liver stages.
Overall, cellular responses to CS peptides were lower in magnitude and prevalence than those to HBs peptides. This may, in part, be explained by the fact that RTS,S recipients had received six doses of HBs (three doses of HBV vaccine in infancy plus three doses of RTS,S) but only three doses of CS. It might also suggest that the circulating CS-specific T cells differ qualitatively from HBs-specific T cells, secreting predominantly IL-2. This conclusion is supported by recent studies of RTS,S in the USA (26), Gabon (27), Ghana (28) and Kenya (Olotu, Bejon et al, manuscript submitted for publication): in each of these studies CS-responsive IL-2+ CD4+ T cells were significantly more frequent after vaccination whereas CS-specific IFN-γ- or TNF-producing T cells were less frequently observed. A marginally significant association between CS-specific IL-2 secretion and protection from infection has been observed in Mozambiquan infants immunised with RTS,S/AS02 (21), and in the Kenyan (Olotu et al) and American studies (26) CS-responsive IL-2+ CD4+ T cells and TNF+ CD4+ T cells were associated with protection against clinical malaria and malaria infection, respectively. Thus, a picture is emerging that RTS,S induces a CS-specific CD4+ T cell response that is characterised by production of IL-2 and TNF but not IFN-γ, and that IL-2 and/or TNF production may be causally associated with vaccine efficacy. It remains to be determined whether IL-2 is a reliable correlate of protection and, if so, whether it is mediating its protective function through NK cell activation (as suggested from this study), by promoting longevity and renewal of memory T cells, or by acting as a growth factor for B cells or follicular Th cells. Although an association between the frequency of IL-2+ CD4+ T cells and anti-CS antibody titers has been described in RTS,S vaccinees (26) we did not observe any correlation between secreted IL-2 concentration - or T cell activation of IFN-γ secretion - and anti-CS titre in the current study. Rather, we observed a tendency for NK cell responses to correlate with antibody titres. Whether this is a genuine causal association, or whether NK cells are simply a very sensitive marker of antigen-presenting cell or T cell functions that were not directly measured, remains to be determined.
The discrepancy between the very low levels of CS-specific IFN-γ-producing T cells detected here, and in other recent studies (26-28), and the higher numbers of such cells detected previously (10, 11, 21) is likely explained by differences in experimental protocol. Exogenous T cell co-stimulation with anti-CD28 and anti-CD49d (11, 21) or recombinant IL-2 and IL-7 (10), detection of IFN-γ-producing cells by ELISPOT (9, 11) (which does not differentiate T cells from NK cells) or restimulation of cells with peptide and exogenous IL-2 for 14 days (29) are all likely to amplify estimates of T cell responsiveness. Nevertheless, it would be interesting to explore whether additional doses of RTS,S or repeated exposure to sporozoites would change the T cell response from an IL-2/TNF dominant to an IFN-γ-dominant profile but - if so - it should not be automatically assumed that this would be clinically beneficial. T cell IL-2 production declines progressively with each episode of TCR stimulation whilst IFN-γ production is maintained or increases (30, 31). IL-2 secretory capacity endows T cells with long term memory potential (30, 31) but the possibility that IL-2 is an effector molecule in its own right is rarely considered (32). Our data suggest that maintaining IL-2 secretion may sustain NK cell responses and markedly increase the pool of early effector cells available to control pathogen colonisation, invasion or replication.
The observation that a substantial proportion of the IFN-γ produced by PBMCs in vaccinated individuals is produced not by T cells but by NK cells suggests that some data on correlates of protection may need to be reappraised. ELISPOT analysis of mixed PBMCs (9, 29, 33) or quantification of secreted cytokines from PBMC or whole blood (21) has typically been interpreted as evidence for antigen-specific T cell responses. Flow cytometry with intracellular cytokine staining suggested (appropriately) that CD4+ and CD8+ T cells make IFN-γ after 24-48h restimulation with CS peptides (10, 11) but conclusions regarding the phenotype of IFN-γ-producing cells in mixed PBMC ELISPOTS based on T cell depletion (9, 33) are less robust since we now know that CD4+ T cell-derived IL-2 is essential for NK cell IFN-γ production (14, 15). The distinct possibility that some IFN-γ-producing cells in these ELISPOT assays were NK cells, should be considered.
This study also raises questions about the current focus on using numbers of IFN-γ-producing T cells as a primary measure of vaccine immunogenicity (34); it is possible that optimising numbers of IL-2-producing T cells - in order to generate an immediate and potent NK response as well as long term memory - may be a better strategy. Although further studies are required to determine whether T cell IL-2 production might be a reliable indicator of vaccine-induced immunity, IL-2 production is the most consistently observed function of RTS,S induced CS-specific CD4+ T cells (26-28) and may underlie correlations between the induction of multifunctional (IFN-γ/TNF/ IL-2/ CD154+) CD4+ T cells and protection in murine vaccination models of leishmaniasis (35) and tuberculosis (36) and in human malaria vaccine trials (37, 38). The data from this study reveal a small number of easily testable hypotheses that may explain why IL-2 is emerging as a correlate of protection against these very diverse infections.
Acknowledgements
We would like to thank Carolynne Stanley, Raimos Olomi, Salum Msham, Jayne Gould, Anangisye Malabeja, Omar Abdul, Samwel Gesase, Lincoln Malle, Sadiki Ismael, Neema Mturi and Martha M. Lemnge and the project team at the PATH Malaria Vaccine Initiative (MVI) for their assistance with this study. Teun Bousema provided statistical advice and Robbert van der Most made very helpful comments on the manuscript.
Footnotes
Funding: This study was funded by the PATH/MVI and GlaxoSmithKline Biologicals. JCRH was in receipt of a Royal Society Incoming Fellowship (ref LJC/USA/2005/Hafalla) and a Wellcome Trust Visiting Fellowship (ref 079920) and CJD held a Wellcome Trust Research Fellowship in Tropical Medicine (ref 063516).
References
- 1.Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith DL, Moyeed RA, Snow RW. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow RW, Marsh K. Malaria in Africa: progress and prospects in the decade since the Abuja Declaration. Lancet. 2010;376:137–139. doi: 10.1016/S0140-6736(10)60577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballou WR. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 2009;31:492–500. doi: 10.1111/j.1365-3024.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 4.Casares S, Brumeanu TD, Richie TL. The RTS,S malaria vaccine. Vaccine. 28:4880–4894. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Olivier A, Benns S, Olomi R, Msham S, Lang T, Gould J, Hallez K, Guerra Y, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Dekker D, Malle L, Ismael S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, Bejon P, von Seidlein L. Safety of the malaria vaccine candidate, RTS,S/AS01E in 5 to 17 month old Kenyan and Tanzanian Children. PLoS One. 5:e14090. doi: 10.1371/journal.pone.0014090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, Demoitie MA, Stallaert JF, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Ballou WR, Cohen J, Riley EM, Lemnge MM, Marsh K, von Seidlein L. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, Vansadia P, Carter T, Njuguna P, Awuondo KO, Malabeja A, Abdul O, Gesase S, Mturi N, Drakeley CJ, Savarese B, Villafana T, Lapierre D, Ballou WR, Cohen J, Lemnge MM, Peshu N, Marsh K, Riley EM, von Seidlein L, Bejon P. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalvani A, Moris P, Voss G, Pathan A, Kester K, Brookes R, Lee E, Koutsoukos M, Plebanski M, Delchambre M, Flanagan K, Carton C, Slaoui M, Van Hoecke C, Ballou W, Hill A, Cohen J. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180:1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 10.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4+ and CD8+ T cells producing IFN-gamma. J Immunol. 2003;171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 11.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG., Jr Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 12.Cambron P, Jacquet JM, Hoet B, Lievens M. Development and technical and clinical validation of a quantitative enzyme-linked immunosorbent assay for the detection of human antibodies to hepatitis B surface antigen in recipients of recombinant hepatitis B virus vaccine. Clin Vaccine Immunol. 2009;16:1236–1246. doi: 10.1128/CVI.00431-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz A, Riley EM. Activation of human NK cells by malaria-infected red blood cells. Methods Mol Biol. 2010;612:429–446. doi: 10.1007/978-1-60761-362-6_29. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 16.Vekemans J, Leach A, Cohen J. Development of the RTS,S/AS malaria candidate vaccine. Vaccine. 2009;27(Suppl 6):G67–71. doi: 10.1016/j.vaccine.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Bojang K, Milligan P, Pinder M, Vigneron L, Alloueche A, Kester K, Ballou W, Conway D, Reece W, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood B, Hill A, McAdam K, Tornieporth N, Cohen J, T. D. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 18.Polhemus ME, Remich SA, Ogutu BR, Waitumbi JN, Otieno L, Apollo S, Cummings JF, Kester KE, Ockenhouse CF, Stewart A, Ofori-Anyinam O, Ramboer I, Cahill CP, Lievens M, Dubois MC, Demoitie MA, Leach A, Cohen J, Ballou WR, Heppner DG., Jr Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One. 2009;4:e6465. doi: 10.1371/journal.pone.0006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, Salim N, Shomari M, Aebi T, Schellenberg DM, Carter T, Villafana T, Demoitie MA, Dubois MC, Leach A, Lievens M, Vekemans J, Cohen J, Ballou WR, Tanner M. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–2544. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 20.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, Lievens M, Vekemans J, Sigauque B, Dubois MC, Demoitie MA, Sillman M, Savarese B, McNeil JG, Macete E, Ballou WR, Cohen J, Alonso PL. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa A, Naniche D, Aponte JJ, Manaca MN, Mandomando I, Aide P, Sacarlal J, Renom M, Lafuente S, Ballou WR, Alonso PL. Plasmodium falciparum-specific cellular immune responses after immunization with the RTS,S/AS02D candidate malaria vaccine in infants living in an area of high endemicity in Mozambique. Infect Immun. 2009;77:4502–4509. doi: 10.1128/IAI.00442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169:2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 23.Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, Riley EM. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol. 2003;171:5396–5405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- 24.Newman KC, Korbel DS, Hafalla JC, Riley EM. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2006;2:e118. doi: 10.1371/journal.ppat.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumsden JM, Schwenk RJ, Rein LE, Moris P, Janssens M, Ofori-Anyinam O, Cohen J, Kester KE, Heppner DG, Krzych U. Protective Immunity Induced with the RTS,S/AS Vaccine Is Associated with IL-2 and TNF-alpha Producing Effector and Central Memory CD4 T Cells. PLoS One. 6:e20775. doi: 10.1371/journal.pone.0020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnandji ST, Fendel R, Mestre M, Janssens M, Vekemans J, Held J, Gnansounou F, Haertle S, von Glasenapp I, Oyakhirome S, Mewono L, Moris P, Lievens M, Demoitie MA, Dubois PM, Villafana T, Jongert E, Olivier A, Cohen J, Esen M, Kremsner PG, Lell B, Mordmuller B. Induction of Plasmodium falciparum-specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D) PLoS One. 6:e18559. doi: 10.1371/journal.pone.0018559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansong D, Asante KP, Vekemans J, Owusu SK, Owusu R, Brobby NA, Dosoo D, Osei-Akoto A, Osei-Kwakye K, Asafo-Adjei E, Boahen KO, Sylverken J, Adjei G, Sambian D, Apanga S, Kayan K, Janssens MH, Lievens MJ, Olivier AC, Jongert E, Dubois P, Savarese BM, Cohen J, Antwi S, Greenwood BM, Evans JA, Agbenyega T, Moris PJ, Owusu-Agyei S. T cell responses to the RTS,S/AS01(E) and RTS,S/AS02(D) malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS One. 6:e18891. doi: 10.1371/journal.pone.0018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinder M, Reece WH, Plebanski M, Akinwunmi P, Flanagan KL, Lee EA, Doherty T, Milligan P, Jaye A, Tornieporth N, Ballou R, McAdam KP, Cohen J, Hill AV. Cellular immunity induced by the recombinant Plasmodium falciparum malaria vaccine, RTS,S/AS02, in semi-immune adults in The Gambia. Clin Exp Immunol. 2004;135:286–293. doi: 10.1111/j.1365-2249.2004.02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, Watkins K, Sinden RE, Leach A, Moris P, Tornieporth N, Schneider J, Dubovsky F, Tierney E, Williams J, Heppner DG, Jr., Gilbert SC, Cohen J, Hill AV. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Hill AV, Reyes-Sandoval A, O’Hara G, Ewer K, Lawrie A, Goodman A, Nicosia A, Folgori A, Colloca S, Cortese R, Gilbert SC, Draper SJ. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 35.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 36.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 38.Berthoud TK, Fletcher H, Porter D, Thompson F, Hill AV, Todryk SM. Comparing human T cell and NK cell responses in viral-based malaria vaccine trials. Vaccine. 2009;28:21–27. doi: 10.1016/j.vaccine.2009.09.132. [DOI] [PubMed] [Google Scholar]


