Abstract
BACKGROUND
A small subset of acute myeloid leukemia (AML) cases has cuplike nuclei. Others have shown that these neoplasms have distinctive clinicopathologic and molecular features.
METHODS
We searched for AML cases with cuplike nuclei at our institution over a 10-year interval. We used a strict definition for cuplike nuclei, ≥10% blasts with nuclear invaginations ≥25% of the nuclear area. We reviewed relevant data and compared the results with a control group of AML without cuplike nuclei.
RESULTS
We identified 22 cases of AML with cuplike nuclei, classified as AML without maturation (FAB AML M1). Compared with a control group AML M1cases, AMLs with cuplike nuclei were significantly associated with FLT3-ITD mutations (86 vs. 38%, p=0.002), NPM1 mutations (86 vs. 19%, p<0.0001), both mutations (77% vs. 14%, p<0.0001), normal karyotype (86 vs. 40%, p=0.003), bone marrow blast count (90% vs. 84%, p=0.016), myeloperoxidase positivity (95% vs. 30% blasts, p=0.001), higher D-dimer levels (>5000 vs. 569 ng/mL, p=0.001), and absence of CD7 (91% vs. 52%, p=0.007), CD34 (82% vs. 5%, p<0.0001), and HLA-DR (59% vs. 10%, p=0.001). There were no differences in age, sex, or peripheral blood counts. The positive predictive value of recognizing AML with cuplike nuclei for FLT3-ITD, NPM1, and both mutations was 81%, 86%, and 77%, respectively.
CONCLUSIONS
Cuplike nuclei in AML are highly associated with the presence of NPM1 and FLT3-ITD mutations and a number of clinicopathologic and immunophenotypic features. The recognition of AML with cuplike nuclei may be helpful in streamlining the workup of these neoplasms.
Keywords: acute myeloid leukemia, cup-like nuclear morphology, NPM1 mutations, FLT3 mutations, immunophenotype, cytogenetics
Introduction
Acute myeloid leukemia (AML) is a molecularly heterogeneous group of monoclonal hematopoietic stem cell disorders. The current 2008 World Health Organization (WHO) classification of AML specifically recognizes the importance of recurrent genetic abnormalities that are crucial for correct diagnosis and proper patient management. In addition, molecular targets for which therapeutic modalities exist, such as inhibitors of a mutated fms-like tyrosine kinase-3 gene (FLT3)1 are emerging in clinical practice, which mandates evaluation for these abnormalities as part of the diagnostic workup.
While the detection of recurrent genetic abnormalities requires conventional cytogenetics and special molecular techniques, some types of AML with recurrent genetic abnormalities, such as cases with the t(8;21), t(15;17), and inv(16), have distinctive morphologic findings that allow one to predict the presence of these abnormalities with a high degree of certainty. In routine clinical practice, in settings where testing for all genetic abnormalities or rapid, real-time testing is not feasible, recognizing distinctive morphologic findings is helpful by allowing the pathologist to streamline or prioritize appropriate molecular tests.
Cases of AML in which the blasts have cuplike nuclei (i.e. prominent nuclear invaginations and also referred to as “fishmouth” nuclei) have distinctive morphologic findings. We and others have shown a high frequency of FLT3 mutations of internal tandem duplication (ITD) in the juxtamembrane domain (referred to as FLT3-ITD) and nucleophosmin gene (NPM1) mutations in these neoplasms. 2-4 Recently, we encountered two cases of AML with cuplike nuclei and predicted the presence of FLT3-ITD and NPM1 mutations at the time of initial morphologic evaluation.
In this study, we review the clinicopathologic, immunophenotypic, cytogenetic, and molecular features of cases of AML with cuplike nuclei seen at our institution during the past 10 years. We show a number of clinicopathologic and immunophenotypic correlates with these morphologic findings, including some not previously described, and we report the predictive value of identify cuplike nuclei in AML for FLT3-ITD and NPM1 mutations.
Material and Methods
Case Identification
Patient information was obtained from a search of case files from 1998 to 2008 at The University Texas M.D. Anderson Cancer Center. The criteria for recognizing a case of AML as having cuplike nuclei included 10% or more blasts with at least one prominent nuclear invagination (or “cup”) spanning at least 25% of the nuclear diameter. Available Wright-Giemsa-stained peripheral blood and bone marrow aspirate smears, and hematoxylin-eosin-stained bone marrow aspirate clot and trephine biopsy specimens were reviewed. An age-matched control group of AML without maturation (AML M1) without cuplike nuclei was used for comparison. Clinical information was obtained from review of medical records with the last update in January, 2009. The Institutional Review Board of The University of Texas M. D. Anderson Cancer Center approved this study.
Cytochemistry, Flow Cytometry Immunophenotyping, and Conventional Cytogenetics
Bone marrow (BM) aspirate smears were assessed by cytochemical analysis for myeloperoxidase and α-naphthyl butyrate esterase using methods described previously.5 Three- or 4-color flow cytometry immunophenotypic analysis was performed on bone marrow aspirate specimens (except one case on peripheral blood) using a FACScan or FACSCalibur instrument, as described previously.5 Antibodies specific for the following antigens were used: CD3, CD7, CD10, CD13, CD19, CD20, CD33, CD34, CD45, CD56, CD64, CD117, HLA-DR, myeloperoxidase and terminal deoxynucleotidyl transferase (TdT). Blasts were gated for analysis using CD45 expression and light side scatter characteristics. An antigen was considered positive using an arbitrary but standard cutoff of at least 20% blasts expressing the antigen as compared with an isotype control. Conventional cytogenetic analysis was performed on BM aspirate specimens using standard Giemsa trypsin G-banding procedures as described previously.6
Detection of FLT3 and NPM1 Mutations
Genomic DNA was extracted from BM aspirates or archival paraffin blocks of BM biopsy or clot specimens. FLT3 was assessed for FLT3-ITD and codon 835-836 point mutations in the activation loop of tyrosine kinase domain by polymerase chain reaction (PCR) assays followed by capillary electrophoresis.5, 7 The forward primers were labeled with 6-carboxyfluorescein. For codon 835-836 point mutation analysis, PCR products were digested with EcoRV. In unmutated cases, digestion creates two fragments of which the 80 bp fragment is labeled. In contrast, a 129 bp fragment is detected in mutated cases.5 To detect NPM1 mutations, exon 12 was amplified by PCR using the following primers: GATGTTGAACTATGCAAAGAGACA (forward) and AACCAAGCAAAGGGTGGAGTT (reverse). The PCR products were purified using the MinElute TM PCR purification Kit (QIAGEN, Valencia, California) and directly sequenced using a GGCATTTTGGACAACACA (reverse) primer and the fluorescence dye chain-terminator chemistry method (Sanger sequencing) on ABI Prism 3100 or 3130 Genetic Analyzer (Applied Biosystems, Foster City, Calif.). ABI Gene Mapper (Applied Biosystems) software was used to analyze the raw data.
Immunohistochemical Analysis for Detection of NPM1 Mutations
Immunohistochemical studies were performed on a subset of cases using previously described methods.8 Briefly, routinely processed BM trephine biopsy tissue sections were subjected to antigen retrieval and immunostained with an anti-NPM monoclonal antibody, clone 376 generated by one of the authors (BF) using an alkaline phosphatase monoclonal anti-alkaline phosphatase (APAAP) technique. In addition, the BM tissue sections were stained in parallel with a mouse monoclonal antibody directed against nucleolin (C23) (Santa Cruz, Biotechnology, Santa Cruz, California), another nucleolar protein, which served as a nuclear staining control. In NPM1 mutated AML cases, NPM protein is abnormally localized in the cytoplasm of most leukemic cells whereas nucleolin/C23 expression is restricted to the nucleus.8 In NPM1 unmutated cases, NPM protein is restricted to the nucleus.
Statistical Analysis
The χ2 and Fisher's exact tests were used to compare various parameters between the study and control groups. The t-test was used to compare continuous variables. The Kaplan-Meier method was used to estimate overall survival (OS) and event-free survival (EFS). All patients were included in assessment of EFS. All analyses were performed using Statistica 6 software (StatSoft Inc., Tulsa, OK).
Results
We identified 35 patients of AML with cuplike nuclei. All of these cases fit in the category of acute myeloid leukemia without maturation in the 2001 WHO classification and AML M1 using the French-American-British (FAB) system. These cases represented 21% of all new cases of AML without maturation and 1% of all new AML cases. Of 35 patients of AML with cuplike nuclei, only 22 cases had a complete molecular work-up, and these 22 cases constitute our study group. An age-matched control group of 21 AML without maturation cases without cuplike nuclei was established for comparison.
Clinical and Laboratory Findings
The clinical and laboratory findings are summarized in Table 1. The study group had 9 men and 13 women. Their age ranged from 18 to 81 years with a median of 59 years. At presentation, the leukocyte count ranged from 1.4 to 249.0 × 109/L (normal range, 4-11 × 109/L) with a median of 59.3 × 109/L; hemoglobin level ranged from 61 to 114 g/L, with a median of 89 g/L (normal range, 120-160 g/L for women and 140-180 g/L for men); and the platelet count ranged from 10 to 197 × 109/L with a median of 47 × 109/L (normal range, 140-440 × 109/L). The D-dimer level measured at time of diagnosis in 13 patients ranged from 393 to >5000 ng/ml (normal range, 0-230 ng/ml), being >5000 ng/ml in 8 of 13 patients tested. Compared with the control group, patients with AML with cuplike nuclei had a higher D-dimer level (p=0.001). There were no significant differences in age, sex distribution, leukocyte count, hemoglobin level and platelet count.
Table I.
Characteristics in AML with prominent nuclear invagination (study group) and in control group.
| AML | Age (y) range, mean | Sex M: F | BM blast % range, median | WBC (×109/L) range, median | Hemoglobin (g/L) range, median | Platelet (×109/L) range, median | D-dimer (ng/mL) range, median | Myeloperoxidase (%) range, median |
|---|---|---|---|---|---|---|---|---|
| Study group (n=22) | ||||||||
| 18-81 | 67-99 | 1.4-249.0 | 61-114 | 10-197 | 383->5000 | 3-100 | ||
| 59 | 9 : 13 | 90 | 59.3 | 89 | 47 | >5000 | 95 | |
| Control group (n=21) | ||||||||
| 20-83 | 32-93 | 1.1-199.5 | 66-121 | 6-328 | 131->5000 | 3-98 | ||
| 63 | 12 : 9 | 84 | 23.0 | 100 | 43 | 569 | 30 | |
| p value | 0.996 | 0.366 | 0.016 | 0.133 | 0.160 | 0.997 | 0.001 | 0.001 |
There were no significant differences in age, sex, or peripheral blood counts between the study and control groups. There was a significant difference in bone marrow (BM) blast percentage, myeloperoxidase positivity, and the level of D-dimers at diagnosis.
All patients received cytarabine-based intensive chemotherapy. As cases of AML with cuplike nuclei were accumulated over the long period of time, different combinations of cytotoxic drugs were used, but there were no differences in therapy between the cuplike nuclei and control groups. Seventeen patients received fludarabine, cytarabine, and G-CSF (cuplike nuclei, n=9; control group, n=8). Sixteen patients received idarubicin and cytarabine (cuplike nuclei, n=7; control group, n=9). Four patients received fludarabine, cytarabine and idarubicin (cuplike nuclei, n=3; control group, n=1). Three patients received clofarabine and cytarabine (cuplike nuclei, n=1; control group, n=2). One patient in the cuplike nuclei group received mitoxantrone, etoposide, and cytarabine; another patient in the cuplike nuclei group received cyclophosphamide, cytarabine, and topotecan; one control patient received daunorubicin, cytarabine, etoposide, thioguanine, and dexamethasone.
With a median follow-up period of 10 months (range, <1-88 months), 11 of 22 (50%) patients with AML with cuplike nuclei achieved a complete remission, which was similar to the 43% complete remission rate in the control group, p=0.763. The 3-year overall survival was 22% (CI, 11-33%) in patients with AML with cuplike nuclei and 19% (CI, 10-28%) in the control group. There was no significant difference in the overall or event-free survival between the cuplike nuclei and control groups, p=0.637 and p=0.186, respectively.
Morphologic Findings
Representative examples of the morphologic findings in the cases of AML with cuplike nuclei are shown in Images 1A and 1B. The percentage of BM blasts in the cuplike nuclei group ranged from 67 to 99%, with a median of 90%. Their blast percentage in BM was statistically higher compared with the control group, 84% (p=0.016). The median percentage of myeloid cells maturing beyond immature cells was 3% (range, 0-8%), fulfilling the diagnostic criteria of the FAB classification. The blasts were often medium-sized with scant cytoplasm containing a few fine azurophilic granules and infrequent Auer rods. Prominent nuclear invaginations were identified in at least 10% of the blasts in each case. In some blasts, invaginations occurred at the periphery of the nucleus, imparting a cuplike appearance. In other blasts, invaginations occurred over the nucleus and resembled a large, pale-staining nucleolus.
Cytochemical studies showed that the blasts in all cases analyzed were positive for myeloperoxidase. Myeloperoxidase reactivity was strongly positive and was detected in >70% of blasts in 19 of 22 cases of AML with cuplike nuclei. Three cases had many fewer myeloperoxidase positive blasts, 3% in two neoplasms and 18% in one neoplasm. The median number of myeloperoxidase positive blasts was 95%, significantly higher in the cuplike nuclei group compared with the control group, 30% (p=0.001). Although usually strongly positive, the myeloperoxidase reactivity in the blasts of AML with cuplike nuclei did not have intense, needle-like positivity that is characteristic of acute promyelocytic leukemia (Image 1C). Butyrate esterase was negative in all but 3 cuplike nuclei cases; the three positive cases had 2, 6 and 14% positive cells, respectively. There was no statistical difference in butyrate esterase activity compared with the control group.
Immunophenotypic Findings
Flow cytometric immunophenotypic analysis of cases of AML with cuplike nuclei showed that the blasts were uniformly positive for CD45 and myeloperoxidase. CD13, CD33 and CD117 were also usually expressed. The blasts were negative for CD3, CD10, CD19, and CD20. Compared with the control group, the blasts in AML with cuplike nuclei were more likely to be negative for CD7 (91% versus 52%, p=0.007), CD34 (82% versus 5%, p<0.0001) and HLA-DR (59% versus 10%, p=0.001) (Table 2). The expression of other antigens was similar between the cases of AML with cuplike nuclei and the control groups. In the AML with cuplike nuclei groups, there were no significant differences in immunophenotype between cases with FLT3-ITD, NPM1, or both mutations and those patients who did not mutations.
Table 2.
Characteristics in AML with prominent nuclear invagination (study group) and in controlgroup.
| Lack of CD34 | Lack of HLA-DR | Lack of CD7 | Normal karyotype* | FLT3-ITD | NPM1 mutations | |
|---|---|---|---|---|---|---|
| Study group (n=22) | ||||||
| 18/22 (82%) | 13/22 (59%) | 20/22 (91%) | 19/22 (86%) | 19/22 (86%) | 19/22 (86%) | |
| Control group (n=21) | ||||||
| 1/21 (5%) | 2/21 (10%) | 11/21 (52%) | 8/20 (40%) | 8/21 (38%) | 4/21 (19%) | |
| p value | <0.0001 | 0.001 | 0.007 | 0.003 | 0.002 | <0.0001 |
Cases of AML with prominent nuclear invaginations (so-called cuplike nuclei) were significantly associated with high frequency of NPM1 and FLT3 mutations, normal cytogenetics, and lack of expression of CD34, HLA-DR and CD7.
The cytogenetic abnormalities in various cases:
1) Study group (with cuplike nuclei) subdivided into:
a) Cases with mutated NPM1 and FLT3-ITD: (n=17):
15 normal cytogenetics
47,XX,+8[15]/ 46,XX[5]
46,XX,t(2;5)(p15;q35)[17]/46,XX[3]
b) Cases with mutated NPM1, wild type FLT3, and normal cytogenetics (n=2)
c) Cases with unmutated NPM1 and FLT3-ITD: (n=2) :
1 normal cytogenetics
46,XX,-16,add(21)(q22),+der(21)t(1;21)(q21;q22)[20]
d)1 case with unmutated NPM1, wild type FLT3, and normal cytogenetics
2) Control group subdivided into:
a) Cases with mutated NPM1 (n=4):
3 normal cytogenetics
1 case with 47,XX,add(2)(q33),+4[20]
b) Cases with unmutated NPM1 (n=17):
5 cases with normal cytogenetics
1 case with no analyzable metaphases
11 cases with abnormal cytogenetics as listed below:
41-45,XX,del(1)(p13p31),t(3;5)(q25;q13),-7,add(11)(p15),-12,der(16), t(16;17)(q14;q21), -18,+21,-22,+1 -2mar[cp19]/ 46,XX[1]
44,XY,add(3)(p11),-5,add(8)(p11),-11,-17,-21,+2mar[14]/42-44,idem,del(17)(p11.2)[cp6]
45,XX,del(5)(q23q32),-7[23]/45,XX,del(5)(q23q32),del(6)(q21),-7[3]
47,XY,+8[4]/49,XY,+8,+10,+22[16]
48,XX,add(6)(q27),+add(6)(q27),+21[20]
45,XY,+2,-5,del(7)(q11.2),add(8)(p21),idic(11)(p11.2),add(15)(p11),-17[4]/46,XY,+2, -5,del(7)(q11.2),add(8)(p21),idic(11)(p11.2),add(15)(p11),-17,+mar[8]/46,XY[8]
47,XY,+8[20]
47,XX,+8[19]
49-52,XX,-3,-4,-5,-9,+19,-20,+6-9 amr[CP19]/46XX[1]
48,XX,+4,+14[19]
46,XY,del(2)(p14p23),del(3)(p21p22)[9]
Cytogenetic and Molecular Findings
Nineteen of 22 (86%) cases of AML with cuplike nuclei had normal conventional cytogenetic results (Table 2). In the 3 abnormal cases, the abnormalities included trisomy 8 (one case), t(2;5)(p15;q35) (one case), and the combination of t(1;21)(q21;q22) with monosomy 16 (one case). Of note, there was no evidence of recurrent cytogenetic abnormalities such as t(8;21), t(15;17), or inv(16). Compared with the control group, cases of AML with cuplike nuclei had a higher frequency of normal cytogenetics (86% versus 40%; p=0.003).
FLT3-ITD mutations were identified in 19 cases of AML with cuplike nuclei. In the control group, FLT3-ITD was identified in 7 cases, a FLT3 codon 835 point mutation were detected in 1 case, and both FLT3-ITD and FLT3 codon 835 point mutation were shown in 1 case. Compared with the control group, cases of AML with cuplike nuclei had a higher frequency of FLT3-ITD, 86% versus 38%; p=0.002).
NPM1 mutations were identified in 19 of 22 (86%) cases of AML with cuplike nuclei, a frequency significantly higher than in the control group, 4 of 21 (19%) cases (p<0.0001). Sixteen of 21 (76%) cases of AML with cuplike nuclei had both FLT3-ITD and NPM1 mutation.
Twenty cases (9 study and 11 control group cases) were also studied by immunohistochemical staining for NPM. The anti-NPM antibody used recognizes both mutant and wild-type NPM. Abnormal cytoplasmic localization of NPM protein was observed in all of 9 cases carrying NPM1 mutations. Interestingly, 2 of 11 cases lacking NPM1 mutations also expressed cytoplasmic NPM.
We calculated the positive and negative predictive values of cuplike nuclei for predicting FLT3-ITD and NPM1 mutations in AML. The positive predictive value (PPV) of cuplike nuclei for the presence of FLT3-ITD is 86% and for NPM1mutation, also 86%. The negative predictive value (NPV) of the absence of cuplike nuclei for FLT3-ITD is 57%, and for NPM1 81%. The PPV of cuplike nuclei in AML for predicting the presence of both FLT3-ITD and NPM1 mutation is 77%.
Discussion
The current 2008 WHO classification of AML recognizes the importance of recurrent genetic abnormalities for diagnosis and proper patient management and uses the umbrella term AML with recurrent genetic abnormalities for these neoplasms. Compared with the 2001 version of the WHO classification, the number recurrent genetic abnormalities specifically recognized has grown substantially. For example, in recent years the poor prognostic impact of FLT3-ITD 9, 10 and the associations of mutations in the transcription factor CCAAT/enhancer binding protein alpha (CEBPα) and NPM1 genes with more favorable prognosis have been recognized.11 As a result, a group of experts has recommended an approach to patient management that incorporates FLT3/ITD, CEBPα, and NPM1 data: patients with FLT3-ITD are assigned to allogeneic stem cell transplantation, whereas patients with either CEBPα or NPM1 mutations in the absence of FLT3-ITD are assigned to less aggressive standard chemotherapy.12 In addition, it seems inevitable that additional molecular targets and target-specific therapeutic agents will emerge in clinical practice that will increase the demand for real-time molecular evaluation of AML cases. The ideal panel of molecular markers to be tested routinely in AML cases is currently being debated in the clinical and pathology communities. However, it seems obvious that universal testing for all known recurrent genetic abnormalities may not be practicable, particularly for those tests that are time-consuming (e.g. Sanger sequencing). Morphologic findings that reliably predict or are highly associated with molecular abnormalities are helpful for prioritizing or streamlining the molecular workup of AML cases.
One important result of this study is that cases of AML with cuplike nuclei are highly associated with FLT3-ITD or NPM1 mutation or both. Over 80% of AML cases with cuplike nuclei have either FLT3-ITD or NPM1 mutation and 17 of 22 (77%) cases in this study had both FLT3-ITD and NPM1 mutation. According to the literature, NPM1 mutations occur in 27-35% of adult patients with AML13, 14; FLT3-ITD is found in 15-35% of adult patients with AML. 7, 15, 16 Therefore, the frequency of FLT3-ITD or NPM1 mutations in patients with cuplike nuclei significantly exceeds the reported frequency in general adult patients with AML. Although the prognosis of patients with AML with cuplike nuclei did not differ from control group we assessed, we suspect that the prognosis of patients AML with cuplike nuclei may improve when mutation-specific therapeutic modalities become available.
The recognition of cuplike nuclei in AML also has a high positive predictive value for both FLT3-ITD and NPM1 mutation suggesting that confirmatory testing for FLT3-ITD could be performed first, followed by NPM1 and CEBPα mutations in cases in which the FLT3 gene is wild type. However, the lack of cuplike nuclei has a relatively low negative predictive value for FLT3-ITD mutations and does not justify omitting testing for FLT3-ITD.
There is some controversy in the literature regarding the morphologic features of AML with cuplike nuclei. In this study, all cases of AML with cuplike nuclei were classified using the 2001 WHO classification as AML without maturation or in the FAB system as AML M1. A recent study by Kroschinsky and colleagues suggested that cuplike nuclei can be found in both non-monocytic and monocytic cases of AML.4 It is well known that nuclear clefts and folding are common features in AML with monocytic differentiation, which can be highly reminiscent of AML with cuplike nuclei. However, when we examined 80 cases of AML with monocytic differentiation (with either FAB M4 or M5 morphologic features), no cases with cuplike nuclei were detected. A likely explanation for the different frequencies of cuplike nuclei in our study compared with the study by Kroschinsky and colleagues rests in the criteria used for cuplike nuclei. We used a relatively strict threshold of 10% compared with a 5% threshold in the study by Kroschinsky and colleagues.4 Importantly, in that study the correlation of nuclear morphology with a high frequency of FLT3-ITD and NPM1 mutation was observed only in cases of AML without monocytic differentiation, similar to our own results.
It should be noted that a limitation the data we present is limited number of patients and lack of information regarding blasts with cup-like morphology in other hematologic neoplasms such as high grade myelodysplastic syndromes, blast phase of myeloproliferative neoplasms and acute lymphoblastic leukemia. In our experience, cuplike nuclei morphology is uncommon in these disorders but a larger, more systematic analysis is needed. It is also unclear if cup-like morphology in these other settings would have clinicopathologic and molecular similar to de novo AML with cuplike nuclei.
Others have shown that immunohistochemical analysis for NPM can be used as a surrogate for the presence of NPM1 mutations in AML cases. As a result of mutations in exon 12, the carboxy terminus of NPM loses nucleolar localization motifs and gains a nuclear export signal, resulting in cytoplasmic NPM, distinct from its purely nuclear location in unmutated cases.8 In general, our results in 20 cases support this application of NPM immunohistochemistry. All 9 NPM1 mutated cases of AML expressed cytoplasmic NPM. Of interest, however, 2 AML cases (1 with cuplike nuclei and 1 control case) lacked NPM1 mutation but demonstrated strong cytoplasmic NPM. The phenomenon has been observed rarely by one of us (BF) previously.17 One possible explanation is that NPM1 mutations other than those occurring at exon 12 may disrupt nuclear cytoplasmic shuttling of NPM. Albiero and colleagues have reported an AML patient carrying an exon 11 mutation that encoded a truncated form of nucleophosmin with aberrant cytoplasmic localization.18, 19 Another possibility, however, is that cytoplasmic localization of NPM is not entirely specific for the presence of NPM1 mutations.
As described by others,2, 20, 21 AML cases with cuplike nuclei have distinctive clinicopathologic and immunophenotypic features and the cases in this study showed similar findings. The blasts of AML with cuplike nuclei often do not express CD7, CD34 and HLA-DR. Patients with AML with cuplike nuclei in this study had a significantly higher BM blast count, a greater number of blasts positive for myeloperoxidase shown by cytochemistry, and higher levels of D-dimers. In addition to their potential biologic relevance, these findings create problems in differential diagnosis with acute promyelocytic leukemia (APL), particularly the microgranular variant, in which neoplastic cells with prominent bilobed nuclei can resemble, in part, blasts with cuplike nuclei. The differential diagnosis of AML with cuplike nuclei and APL is further complicated by the strong myeloperoxidase reactivity and frequent absence of CD34 and HLA-DR. Although the problems inherent in the morphological differential diagnosis between AML with cuplike nuclei and APL are not stressed in the literature, this may can be truly difficult for a practicing pathologist, because of the clinical consequence of missing a case of APL. The difficulties of the differential diagnosis between AML with cuplike nuclei and APL are confirmed by the fact that Kussick and colleagues had to perform rapid molecular analysis to rule out APL in 13 of 19 (68%) cases of AML with cuplike nuclei in their study.2 At our institution, we routinely perform an immunofluorescence-based test for promyelocytic leukemia protein oncogenic domain (POD), to exclude APL with t(15;17). The POD test was performed in 14 of 22 (64%) cases of AML with cuplike nuclei in this study, stressing the complexity of the differential diagnosis between AML with cuplike nuclei and APL.
In conclusion, AML cases with cuplike nuclei represent an uncommon morphological variant of AML highly associated with FLT3-ITD and NPM1 mutation. If strict criteria are applied as in this study, those being 10% of blasts with prominent nuclear invaginations representing at least 25% of the nuclear area, the presence of cuplike nuclei in AML represents a distinctive morphologic finding that can be used to prioritize the molecular workup of AML cases.
Image 1. Morphologic features of blasts with prominent nuclear invaginations (so-called cuplike nuclei).
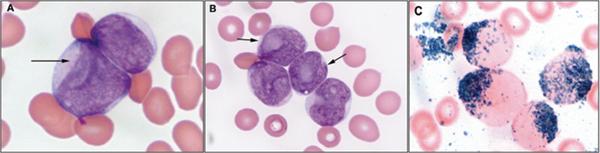
(A and B). Blasts are medium-sized with prominent nuclear invagination [resembling a cup or the mouth of a fish (arrow)], scant to moderate cytoplasm containing a few azurophilic granules (Wright-Giemsa bone marrow aspirate smear, Olympus, × 1,000); (C). Blasts are myeloperoxidase positive (Olympus, × 1,000);
Acknowledgements
This study was supported, in part, by a grant from Leukemia Specialized Programs of Research Excellence (grant P50 CA 100632-04) and by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) to BF. We like to thank Martin Nguyen for performing the immunohistochemical analysis. We also thank Lori Heydon and Mark Routbort for analytic support. We also thank LaKisha Rodgers and Geneva Williams for invaluable help in manuscript preparation.
Footnotes
Disclosure/Conflict of Interest
There are no conflicts of interest to declare.
References
- 1.Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leuk Lymphoma. 2008 May;49(5):852–863. doi: 10.1080/10428190801895352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kussick SJ, Stirewalt DL, Yi HS, et al. A distinctive nuclear morphology in acute myeloid leukemia is strongly associated with loss of HLA-DR expression and FLT3 internal tandem duplication. Leukemia. 2004 Oct;18(10):1591–1598. doi: 10.1038/sj.leu.2403458. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Rassidakis GZ, Li J, et al. High frequency of NPM1 gene mutations in acute myeloid leukemia with prominent nuclear invaginations (“cuplike” nuclei). Blood. 2006 Sep 1;108(5):1783–1784. doi: 10.1182/blood-2006-03-014340. [DOI] [PubMed] [Google Scholar]
- 4.Kroschinsky FP, Schakel U, Fischer R, et al. Cup-like acute myeloid leukemia: new disease or artificial phenomenon? Haematologica. 2008 Feb;93(2):283–286. doi: 10.3324/haematol.11669. [DOI] [PubMed] [Google Scholar]
- 5.Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations. Am J Clin Pathol. 2004 Sep;122(3):348–358. doi: 10.1309/5DGB-59KQ-A527-PD47. [DOI] [PubMed] [Google Scholar]
- 6.Hao S, Sanger W, Onciu M, Lai R, Schlette EJ, Medeiros LJ. Mantle cell lymphoma with 8q24 chromosomal abnormalities: a report of 5 cases with blastoid features. Mod Pathol. 2002 Dec;15(12):1266–1272. doi: 10.1097/01.MP.0000037310.82136.99. [DOI] [PubMed] [Google Scholar]
- 7.Kiyoi H, Naoe T. FLT3 in human hematologic malignancies. Leuk Lymphoma. 2002 Aug;43(8):1541–1547. doi: 10.1080/1042819021000002866. [DOI] [PubMed] [Google Scholar]
- 8.Falini B, Martelli MP, Bolli N, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood. 2006 Sep 15;108(6):1999–2005. doi: 10.1182/blood-2006-03-007013. [DOI] [PubMed] [Google Scholar]
- 9.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002 Jun 15;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 10.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002 Jul 1;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Preudhomme C, Sagot C, Boissel N, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA). Blood. 2002 Oct 15;100(8):2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 12.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007 Jan 15;109(2):431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood. 2006 May 15;107(10):4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 14.Falini B, Mecucci C, Saglio G, et al. NPM1 mutations and cytoplasmic nucleophosmin are mutually exclusive of recurrent genetic abnormalities: a comparative analysis of 2562 patients with acute myeloid leukemia. Haematologica. 2008 Mar;93(3):439–442. doi: 10.3324/haematol.12153. [DOI] [PubMed] [Google Scholar]
- 15.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003 Sep;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 16.Kiyoi H, Yanada M, Ozekia K. Clinical significance of FLT3 in leukemia. Int J Hematol. 2005 Aug;82(2):85–92. doi: 10.1532/IJH97.05066. [DOI] [PubMed] [Google Scholar]
- 17.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005 Jan 20;352(3):254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 18.Albiero E, Madeo D, Bolli N, et al. Identification and functional characterization of a cytoplasmic nucleophosmin leukaemic mutant generated by a novel exon-11 NPM1 mutation. Leukemia. 2007 May;21(5):1099–1103. doi: 10.1038/sj.leu.2404597. [DOI] [PubMed] [Google Scholar]
- 19.Falini B, Albiero E, Bolli N, et al. Aberrant cytoplasmic expression of C-terminal-truncated NPM leukaemic mutant is dictated by tryptophans loss and a new NES motif. Leukemia. 2007 Sep;21(9):2052–2054. doi: 10.1038/sj.leu.2404839. author reply 2054; discussion 2055-2056. [DOI] [PubMed] [Google Scholar]
- 20.Wetzler M, McElwain BK, Stewart CC, et al. HLA-DR antigen-negative acute myeloid leukemia. Leukemia. 2003 Apr;17(4):707–715. doi: 10.1038/sj.leu.2402865. [DOI] [PubMed] [Google Scholar]
- 21.Scott AA, Head DR, Kopecky KJ, et al. HLA-DR-, CD33+, CD56+, CD16- myeloid/natural killer cell acute leukemia: a previously unrecognized form of acute leukemia potentially misdiagnosed as French-American-British acute myeloid leukemia-M3. Blood. 1994 Jul 1;84(1):244–255. [PubMed] [Google Scholar]


