Abstract
Epilepsy is the third most common chronic brain disorder, and is characterized by an enduring predisposition to generate seizures. Despite progress in pharmacological and surgical treatments of epilepsy, relatively little is known about the processes leading to the generation of individual seizures, and about the mechanisms whereby a healthy brain is rendered epileptic. These gaps in our knowledge hamper the development of better preventive treatments and cures for the ≈30% of epilepsy cases that prove resistant to current therapies. Here, we focus on the rapidly growing body of evidence that supports the involvement of inflammatory mediators—released by brain cells and peripheral immune cells—in both the origin of individual seizures and the epileptogenic process. We first describe aspects of brain inflammation and immunity, before exploring the evidence from clinical and experimental studies for a relationship between inflammation and epilepsy. Subsequently, we discuss how seizures cause inflammation, and whether such inflammation, in turn, influences the occurrence and severity of seizures, and seizure-related neuronal death. Further insight into the complex role of inflammation in the generation and exacerbation of epilepsy should yield new molecular targets for the design of antiepileptic drugs, which might not only inhibit the symptoms of this disorder, but also prevent or abrogate disease pathogenesis.
Introduction
Epilepsy is a brain disorder characterized by an enduring predisposition to generate seizures, and by emotional and cognitive dysfunction.1 This disorder affects ≈50 million people worldwide and, hence, is one of the most common neurological disorders. Despite the availability of a wide range of antiepileptic drugs (AEDs), about one-third of individuals with epilepsy still experience seizures that do not respond to medication.2 Thus, an urgent need exists for effective therapies to be developed. This need is further increased by the fact that currently available AEDs are mainly symptomatic:3 they block seizures but do not affect the underlying pathology or the progression of the disorder.4 Understanding the mechanisms that are involved in the generation of epilepsy should aid the development of novel drugs that modify the epileptic process.2
Over the past 10 years an increasing body of clinical and experimental evidence has provided strong support to the hypothesis that inflammatory processes within the brain might constitute a common and crucial mechanism in the pathophysiology of seizures and epilepsy.5–8 The first insights into the potential role of inflammation in human epilepsy were derived from clinical evidence indicating that steroids and other anti-inflammatory treatments displayed anticonvulsant activity in some drug-resistant epilepsies.9–11 Additional evidence came from febrile seizures,12 which always coincide with—and are often caused by—a rise in the levels of pro inflammatory agents.13
Chronic brain inflammation—comprising activation of microglia, astrocytes, endothelial cells of the blood–brain barrier (BBB), and peripheral immune cells, and the concomitant production of inflammatory mediators—was first observed in patients with Rasmussen encephalitis.14 Evidence of immune system activation in some patients with seizure disorders, the high incidence of seizures in autoimmune diseases, and the discovery of limbic encephalitis as a cause of epilepsy15–17 led to the suggestion that immune and inflammatory mechanisms have roles in some forms of epilepsy.5,18
Evidence is emerging that inflammation might be a consequence as well as a cause of epilepsy. Several inflammatory mediators have been detected in surgically resected brain tissue from patients with refractory epilepsies, including temporal lobe epilepsy (TLE) and cortical dysplasia-related epilepsy (Supplementary Table 1 online).5,8 The finding that brain inflammation occurred in epilepsies that were not classically linked to immunological dysfunction highlighted the possibility that chronic inflammation might be intrinsic to some epilepsies, irrespective of the initial insult or cause, rather than being only a consequence of a specific underlying inflammatory or autoimmune etiology. The mounting evidence for a role for inflammatory processes in human epilepsy has led to the use of experimental rodent models to identify putative triggers of brain inflammation in epilepsy, and to provide mechanistic insights into the reciprocal causal links between inflammation and seizures (Supplementary Table 2 online).5,7,19 Experimental studies have shown that seizure activity per se can induce brain inflammation, and that recurrent seizures perpetuate chronic inflammation. Seizure-associated cell loss can contribute to inflammation but is not a pre-requisite for inflammation to occur. In addition, models of systemic or CNS infections suggested that pre- existing brain inflammation increases the predisposition to seizures, associated with alterations in neuronal excitability and enhanced seizure-induced neuropathology. Additional mechanistic insights into the role of inflammation in seizures and the development of epilepsy have been gained through use of pharmacological approaches that interfere with specific inflammatory mediators (Supplementary Table 3 online), and from changes in seizure susceptibility in genetically modified mice with perturbed inflammatory pathways.20–27
In this article, we will review the clinical and experimental evidence supporting a role for brain inflammation in epilepsy. We will critically analyze whether brain inflammation is a cause or a consequence of seizures, and whether cell loss is related to such brain inflammation. We will also examine the contributions of neurons, astrocytes, microglia and peripheral immune cells to brain inflammation. Finally, we will address whether inflammation contributes to the mechanisms involved in the generation of individual seizures and/or the transformation of a normal brain into one that generates spontaneous seizures (that is, epileptogenesis). The latter scenario predicts that pharmacological interventions targeting inflammation should ameliorate seizures and epilepsy. Such approaches to overcome pharmacoresistant epilepsies will be discussed.
Inflammation and immunity in the CNS
Inflammation consists of the production of a cascade of inflammatory mediators (a dynamic process), as well as anti-inflammatory molecules and other molecules induced to resolve inflammation, as a response to noxious stimuli (such as infection or injury), or immune stimulation, and is designed to defend the host against pathogenic threats. Inflammation is characterized by the production of an array of inflammatory mediators from tissue-resident or blood-circulating immunocompetent cells, and involves activation of innate and adaptive immunity (Box 1). Both innate and adaptive immunity have been implicated in epilepsy, and microglia, astrocytes and neurons are believed to contribute to the innate immunity-type processes that cause inflammation of the brain.
Box 1 | Innate and adaptive immunity in activation of inflammation.
Innate immunity
Innate immunity represents a nonspecific immediate host response against invading pathogens. Leukocytes—including natural killer cells, granulocytes (neutrophils, eosinophils and basophils–mast cells)—cells of the monomyelocytic lineage (monocytes, macrophages and microglia), dendritic cells, and Toll-like receptors (TLRs) are involved in the activation of innate immunity. TLRs, which are transmembrane proteins expressed by immunocompetent cells such as antigen-presenting cells (APCs), share common cytoplasmic domains with the interleukin (IL)-1 receptor family and use partly overlapping signaling molecules with IL-1 receptor type 1. TLRs have a key role in recognizing conserved motifs broadly shared by pathogens as well as endogenous molecules termed ‘danger signals’ released from damaged or stressed cells. TLR activation initiates innate immune responses and inflammation during infection, or in response to tissue injury.34,177 TLR signaling involves recruitment of cytoplasmatic adaptor proteins and subsequent induction of the protein kinase cascades, leading to activation of the nuclear factor kappa B (NFκB)-inducible or interferon-γ-inducible genes that orchestrate the inflammatory response. Stimulation of TLRs by pathogens leads to release of cytokines such as IL-12, which are involved in the transition between innate and adaptive immunity.31
Adaptive immunity
The adaptive immune system is activated in response to innate immunity and enables the host to recognize and remember specific non-self antigens to mount humoral (production of antibodies) or cell-mediated immune responses by B and T lymphocytes, respectively. APCs—dendritic cells, macrophages and B cells, and brain-resident microglia—stimulate naive T cells to become effector cells. On antigen presentation, clonal selection and expansion of lymphocytes occurs. Dysregulation of adaptive immunity and loss of tolerance to self-antigens could result in the development of autoimmunity. A subpopulation of T cells called regulatory T cells (CD4+CD25+) restrict autoimmune activity, thereby helping to maintain immune system homeostasis, and tolerance to self-antigens.
The brain has traditionally been considered an immunoprivileged site because of the presence of the BBB, the lack of a conventional lymphatic system, and the limited trafficking of peripheral immune cells. Nevertheless, both the innate and adaptive immune responses are readily evoked within the CNS in response to pathogens, self-antigens, or tissue injury of several etiologies. Microglia, astrocytes, neurons, BBB endothelial cells, and peripheral immune cells extravasating into brain parenchyma can all produce proinflammatory and anti-inflammatory molecules.28,29 The contribution of each cell population to brain inflammation depends on the origin (for example, CNS versus systemic) and the type (for example, infectious versus sterile) of the initial precipitating event.5,7,30 The BBB represents a key regulatory element of the communication between intrinsic brain cells and peripheral immunocompetent cells (Box 2).
Box 2 | The blood–brain barrier.
Under physiological conditions, the blood–brain barrier (BBB) strictly controls the entry of blood-borne cells and molecules (including serum proteins) into the brain. Brain injury resulting from infection, stroke, trauma and/or prolonged seizures can alter the BBB,98,178,179 thereby permitting blood-to-brain extravasation of peripheral immune cells or molecules that would otherwise be excluded. Brain inflammation (for example, in perivascular astrocytes) can affect the permeability properties of the BBB directly via cytokine-mediated activation of metalloproteinases or tight junction disruption, or indirectly by promoting transmigration of leukocytes.180,181 Systemic or CNS inflammation leads to cytokine and chemokine production in blood or within the CNS, and to receptor-mediated upregulation of selectins and tight adhesion molecules (intercellular adhesion molecule 1 [ICAM-1], intercellular adhesion molecule 2 [ICAM-2], vascular cell adhesion molecule 1 [VCAM-1], and platelet endothelial cell adhesion molecule [PECAM]) on endothelial cells in postcapillary venules. Cytokines and chemokines also activate integrins (lymphocyte function-associated antigen 1 [LFA-1], MAC-1 [CD11b/CD18], very late antigen-4 [VLA-4]) enabling tight adhesion of leukocytes to the endothelium, and their transmigration and chemoattraction towards the site of infection or injury
As noted above, an inflammatory response in the CNS can be induced in the absence of infection. Brain inflammation has been reported following ischemic stroke or traumatic brain injury (TBI), and during chronic neurodegenerative diseases. In all these conditions, pronounced activation of microglia and astrocytes takes place in brain regions affected by the specific disease, and these cells act as major sources of inflammatory mediators. Recruitment of peripheral immune cells might also occur.30–33
The activation of innate immunity and the transition to adaptive immunity are mediated by a large variety of inflammatory mediators, among which cytokines—polypeptides that act as soluble mediators of inflammation—have a pivotal role.31,34 These molecules include interleukins (ILs), interferons (IFNs), tumor necrosis factors (TNFs) and growth factors (for example, transforming growth factor [TGF]-β). Cytokines are released by immunocompetent and endothelial cells, as well as by glia and neurons in the CNS, thereby enabling communication between effector and target cells during an immune challenge or tissue injury. Following their release, cytokines interact with one or more cognate receptors. The most extensively studied prototypical inflammatory cytokines in the CNS are IL-1β, TNF and IL-6.35–37 Cytokine activity can be regulated at multiple levels, including gene transcription, cleavage of cytokine precursors (for example, pro-IL-1β, pro-TNF) by specific proteolytic enzymes, and cellular release, as well as through receptor signaling (discussed below). All cell types in the brain seem capable of expressing cytokines and their receptors, with low basal expression of these molecules being rapidly upregulated following CNS insults. Chemokines comprise a specific class of cytokines that act as chemoattractants to guide the migration of leukocytes from blood through the endothelial barrier into sites of infection or injury.38 These cytokines also regulate microglial motility and neural stem cell migration, provide axon guidance during brain development, and promote angiogenesis, neurogenesis and synaptogenesis.39,40 the release of chemokines is often stimulated by proinflammatory cytokines such as IL-1β.
Several mechanisms have been identified that attenuate the inflammatory response, indicating the importance of such strict control for homeostasis and prevention of injury. Regulatory mechanisms include production of proteins that compete with cytokines to bind their receptors, such as IL-1 receptor antagonist protein (IL-1ra),41 and decoy receptors that bind cytokines and chemokines but are incapable of signaling, thereby acting as molecular traps to prevent such ligands from interacting with biologically active receptors.42 Proteins that inhibit cytokine-induced signal transduction (for example, suppressor of cytokine signaling proteins)43 or transcription (for example, Nurr1–CoREST or activity transcription factor 3),44,45 as well as an array of soluble mediators with anti-inflammatory activities (such as IL-10 and TGF-β),46 are produced concomitantly with proinflammatory molecules to resolve inflammation. For example, glucocorticoids, via activation of glucocorticoid receptors and, consequently, downregulation of nuclear factor-κB (NFκB) and activator protein 1 activity, inhibit innate immune responses and, hence, act as an endogenous anti-inflammatory feedback system. Proinflammatory cytokines are powerful enhancers of glucocorticoid levels in adrenal glands via corticotropin-releasing hormone47,48 and adrenocorticotropic hormone (ACTH). Glucocorticoids also elicit immunosuppressive effects through inhibition of leukocyte extravasation from the vasculature, and through regulation of T helper cell differentiation.49 The CNS can also negatively regulate the inflammatory response in a reflexive manner, using the efferent activity of the vagus nerve to inhibit release of proinflammatory molecules from tissue macrophages.50
Immunity and inflammation in epilepsy
Clinical evidence
Clinical evidence for an important causal role for autoimmune disorders as triggers for seizures and epilepsy has emerged in several contexts. Identifiable autoimmune disorders such as systemic lupus, vasculitis, multiple sclerosis, and paraneoplastic syndromes can all cause recurrent seizures.51 Furthermore, catastrophic epilepsy can result from autoimmune brain processes; for example, Rasmussen encephalitis—a devastating catastrophic epilepsy of childhood that ultimately leads to hemibrain atrophy, hemiparesis and progressively severe seizures—has been linked to the presence of autoantibodies, including glutamate receptor 3 antibodies, although these antibodies are not present in all cases.52–54 Brains of individuals affected by Rasmussen encephalitis contain reactive astrocytosis, activated microglial cells and proinflammatory mediators, and are infiltrated by lymphocytes.8,55–59 Catastrophic epilepsy has also been associated with other disorders in which auto antibodies attack brain tissue. These disorders include paraneoplastic limbic encephalitis, and the more recently discovered nonparaneoplastic limbic encephalitis associated with antibodies against N-methyl-d-aspartate (NMDA) receptors and glutamic acid decarboxylase, or against voltage-gated potassium channels.15–17 These disorders often present with status epilepticus and psychiatric disturbances, and often are followed by aggressive, treatment-resistant epilepsy. In patients with such diseases, immune therapies are often more successful than standard AED treatment at disease onset.60
Clinical evidence suggests that inflammation is also an important factor in the onset and perpetuation of epilepsy not caused by an autoimmune process. Proinflammatory precipitants, such as fever, lead to and exacerbate seizures in patients with epilepsy.12 In addition, evidence of brain inflammation has been found to be associated with diverse pathological etiologies in patients with treatment-resistant epilepsy who underwent surgical resection to remove the seizure focus. Proinflammatory molecules, reactive astrocytosis, activated microglia, and other indicators of inflammation have been found in the resected hippocampi of patients with TLE,61–64 in and around epileptic tubers in patients with tuberous sclerosis,65–68 and in association with epileptic cortical dysplastic lesions.69,70 These inflammatory markers were not, however, found in specimens obtained from healthy control patients.8, 61–68
Experimental evidence
Over the past decade, research using in vivo and in vitro experimental models has focused on how inflammation is generated in the brain in the context of epilepsy, how inflammation modulates epilepsy, and whether inflammation is always detrimental to cell survival or if it can be neuroprotective. Such research has also sought to determine how inflammatory mechanisms might be harnessed to develop therapies for epilepsy. Here, we discuss the outcomes of this experimental work.
Do seizures cause inflammation?
In adult rats and mice, induction of recurrent short seizures or single prolonged seizures (status epilepticus; defined as a seizure lasting >30 min) by chemoconvulsants or electrical stimulation triggers rapid induction of inflammatory mediators in brain regions of seizure activity onset and propagation (Supplementary Table 2 online).19,21,62,71–87 Immunohistochemical studies on rodent brains after induction of status epilepticus demonstrated subsequent waves of inflammation during the epileptogenic process (that is, the process underlying the onset and chronic recurrence of spontaneous seizures after an initial precipitating event), involving various cell populations. Findings from these and other studies show that proinflammatory cytokines (IL-1β, TNF and IL-6) are first expressed in activated microglia and astrocytes, and cytokine receptor expression is upregulated in microglia, astrocytes and neurons.5 These initial events are followed by the induction of cyclooxygenase-2 (COX-2) and, hence, prostaglandins, and upregulation of components of the complement system in microglia, astrocytes and neurons.62,83–85,88 In addition to the molecules mentioned above, chemokines and their receptors are produced—predominantly in neurons and in activated astrocytes—days to weeks after status epilepticus.87–91
An ensuing wave of inflammation is induced in brain endothelial cells by seizures, and includes upregulation of IL-1β and its receptor IL-1R1,63 the complement system,62 and adhesion molecules (P-selectin, E-selectin, intercellular adhesion molecule 1 [ICAM] and vascular cell adhesion molecule 1).92,93 The presumed cascade of events leading to this vascular inflammation involves seizure-induced activation of perivascular glia, which produce and release cytokines and prostaglandins. Importantly, no peripheral immune cells or blood-derived inflammatory molecules are required for vascular inflammation, as such events have been replicated in vitro in isolated guinea pig brain undergoing seizure activity.94
The presence of inflammation originating from the brain might promote the recruitment of peripheral inflammatory cells. Indeed, chemokines expressed by neurons and glia and in the cerebrovasculature following seizures might direct blood leukocytes into the brain,92 which would be consistent with the reported emergence of granulocytes during epileptogenesis,63,92 and sparse T lymphocytes in chronic epileptic tissue from TLE models and humans.63,92
As in human epileptic brain specimens, brain tissue from rodents with experimental chronic TLE contains both activated astrocytes and microglia expressing inflammatory mediators.61,63,95 Evidence for brain vessel inflammation associated with BBB breakdown is also prevalent.63,92,94,96–98
The findings discussed above show that brain inflammation induced by status epilepticus develops further during epileptogenesis and, together with the human data, demonstrate that this phenomenon persists in chronic epileptic tissue, thereby supporting the idea that inflammation might be intrinsic to—and perhaps a biomarker of—the epileptogenic process.79,95,99–101
Does inflammation cause seizures?
Three lines of evidence from rodent models suggest that brain inflammation promotes neuronal hyper-excitability and seizures. First, although the functions of many inflammatory mediators remain unresolved, clear evidence exists for an active role for IL-1β, TNF, IL-6, prostaglandin E2 (PGE2; Supplementary Table 3 online) and the complement cascade102 in seizure generation and exacerbation. Seizure activity leads to the production of inflammatory molecules that, in turn, affect seizure severity and recurrence, and this action takes place through mechanisms distinct from the transcriptional events traditionally known to be activated during systemic inflammation (discussed below; Figure 1).
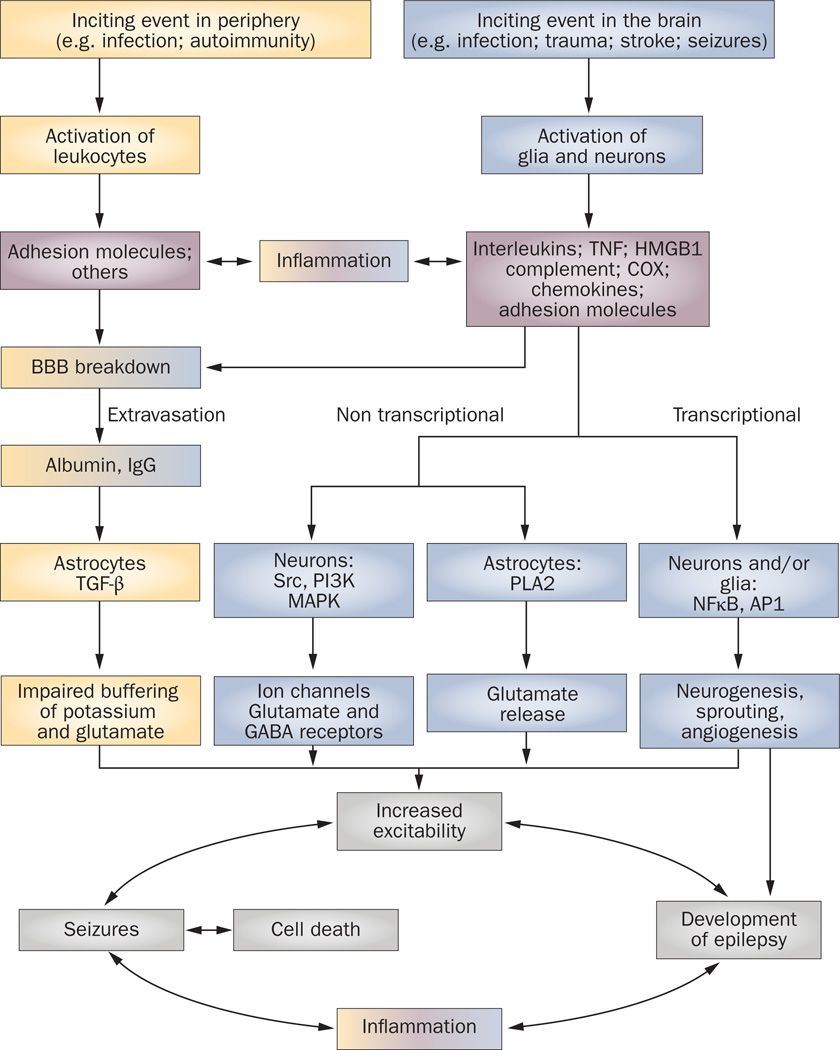
Figure 1. Pathophysiological cascade of inflammatory events in epilepsy.
Pathological events intiated in the CNS by local injuries, or peripherally following infections or as a result of autoimmune disorder, can lead to activation of brain cells or leukocytes, respectively. These cells release inflammatory mediators into the brain or blood, thereby eliciting a cascade of inflammatory events that cause a spectrum of physiopathological outcomes. The effects of brain inflammation contribute to the generation of individual seizures and cell death, which, in turn, activates further inflammation, thereby establishing a vicious circle of events that contributes to the development of epilepsy. The peripheral pathway is shown in yellow, the CNS pathway is shown in blue, and the inflammatory molecules are shown in pink. The merged colors indicate the contribution of each pathway to inflammation and BBB damage. Abbreviations: AP1, activator protein 1; BBB, blood–brain barrier; COX, cyclooxygenase; GABA, γ-aminobutyric acid; HMGB1, high-mobility group box 1; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa B; PI3K, phosphoinositide 3-kinase; PLA2, phospholipases A2; TGF-β, transforming growth factor β; TNF, tumor necrosis factor.
Second, fever is the most frequent cause of seizures in children worldwide.12,103 Fever denotes an elevation of core temperature resulting from an increase in set point for body temperature within specific cells of the hypothalamus,104 and is generated in the setting of a systemic inflammatory response involving inflammatory mediators such as cytokines and prostaglandins.105 Research has also shown that fever involves release of cytokines within the brain;106 remarkably, elevating brain temperature per se seems to result in the release of IL-1β within the hippocampus.107 Cerebrospinal fluid studies in children108–111 and animal models107,112 have implicated the release of endogenous cytokines, especially IL-1β, in the generation of febrile seizures6,12 and, possibly, in the development of epilepsy after febrile seizures.95,113–115
Third, systemic injection of lipopolysaccharide, a prototypical inducer of inflammation both in the periphery and in the brain, lowers seizure threshold in the short112,116 and long term,117–119 and increases spike-and-wave discharges in a rat model of absence seizures.120 Lipopolysaccharide-induced changes in seizures threshold involve brain cytokines—namely, IL-1β or TNF—and COX-2 activation.
Remarkably, while most of the observed effects of lipopolysaccharide in the adult brain have been transient,116 exposure to lipopolysaccharide during specific developmental ages in rats (postnatal day 7 or 14) can result in enduring changes in neuronal excitability117–119,121 that are associated with lasting augmentation of stress-related gene expression.121 Increased intrinsic hippocampal excitability and alterations in glutamate receptor subunit expression were found in adult rats exposed to lipopolysaccharide during infancy.117,122 Clues have recently emerged to the mechanism whereby lipopolysaccharide—an activator of Toll-like receptor 4 (TLR4)—augments seizures.123 The probable scenerio is that lipopolysaccharide mimics the actions of an endogenously released ‘danger signal’ produced by stressed or injured neurons, in the form of a protein called high mobility group box 1 (HMGB1). On release from neurons, this protein interacts with TLR4 to promote seizures, which, in turn, induce an additional wave of HMGB1 release from activated astrocytes and micro-glia, leading to a positive feedback cycle of seizures and inflammation. This novel pathway could provide a crucial mechanism underlying recurrent seizures (Figure 1).
Does inflammation cause cell loss?
Available studies suggest that seizure-related or injury-related inflammation might contribute to cell loss and synaptic reorganization, which are important mediators of the development of hyperexcitable circuits that lead to epilepsy after insults such as status epilepticus or TBI in the adult rodent brain.4,35,124 Inflammation is induced rapidly following such insults, preceding neurodegeneration in lesional models of seizures.73,125,126 This finding is consistent with the idea that inflammation augments cell death, which is further supported by data from studies involving injection of inflammatory mediators together with excitotoxic stimuli.33
Activation of microglia and astrocytes and production of cytokines and PGE2 can occur in seizure models where cell loss is not detected in immature95,125 or adult rodents.21,72,120,127,128 Such observations suggest that rather than being a consequence of cell loss, seizure-induced brain inflammation can contribute to cell death.6 Additional interactions between inflammation and cell death in the context of epilepsy have been observed. Brain injury, such as TBI, causes tissue inflammation that seems to contribute to both cell death and long-term hyperexcitability.129–131
In the context of CNS injury (for example, in chronic neurodegenerative diseases or acute stroke), inflammation can have a neuroprotective role.132,133 Indeed, whether microglia, macrophages and/or T cells are destructive or neuroprotective seems to depend on their activation status, which is orchestrated by the specific inflammatory environment.49,132 This balance, together with the specific brain regions in which inflammation develops (for example, white matter in multiple sclerosis), might account for the relatively low incidence of seizures in other neurological disorders associated with brain inflammation.134
Mechanistic insights
Several established and novel mechanisms could mediate the effects of inflammatory mediators on neuronal excitability and epilepsy (Figure 1). Some of these mechanisms could be involved in the precipitation and recurrence of seizures, while others are implicated in the development of epileptogenesis.6 These mechanisms constitute potential molecular targets for drug design, and are briefly summarized here.
As discussed above, IL-1β and HMGB1 activate convergent signaling cascade34,135,136 through binding to IL-1R1 and TLR4, respectively. The downstream pathways activated by these ligands converge with the TNF pathways at the transcription factor NFκB, which regulates the synthesis of chemokines, cytokines, enzymes (for example, COX-2) and receptors (for example, TLRs, IL-1R1, and TNF p55 and p75 receptors).137 This transcriptional pathway modulates the expression of genes involved in neurogenesis, cell death and survival, and in synaptic molecular reorganization and plasticity138—processes that occur concomitantly with epileptogenesis in experimental models.124,139
Interestingly, in addition to NFκB activation and gene expression changes, occupancy of IL-1R1 or TLRs leads to the simultaneous activation of a second rapid, non transcriptional pathway involving two kinase systems, namely ceramide-mediated activation of the tyrosine kinase Src,140–144 and activation of the mitogen-activated protein kinases (extracellular signal-regulated kinases).41,136,145 These two pathways result in phosphorylation of voltage-dependent and receptor-coupled ion channels, thereby directly affecting neuronal excitability and seizure threshold.146 For example, the proconvulsant activity of IL-1β depends on IL-1R1-mediated phosphorylation of the NMDA receptor 2B subunit via Src, and, hence, neuronal calcium influx.140,141 Since the ceramide-activated Src system is a major modulator of ion channel activity, inhibitors of this system (acting at the cytosolic adaptor MyD88, the biosynthetic steps of ceramide, or the level of Src activity) should arrest inflammation-mediated hyperexcitability. Of note, as the hippocampus is the second-richest brain area in IL-1R1 (after the hypothalamus), IL-1β-mediated signaling might markedly influence neuronal excitability and seizure threshold.
Additional mechanisms of hyperexcitability to those already discussed include cytokine-mediated glutamate release from astrocytes,147 inhibition of glial glutamate reuptake,148 and changes in glutamate and γ-aminobutyric acid receptor trafficking and subunit compositions.149,150 Prostaglandins might also be candidate molecular targets to reduce inflammation-mediated hyperexcitability, because PGE2 increases neuronal firing and excitatory postsynaptic potentials, probably by reducing potassium currents in CA1 neurons.151,152
Inflammatory mediators can increase vascular permeability to serum albumin, which promotes excitability in surrounding neurons by compromising ion buffering and the glutamate reuptake capacity of astrocytes.153 In this context, albumin-mediated activation of TGF-β1 receptor signaling induces the transcription of various proinflammatory genes in astrocytes, which may markedly contribute both to astrocyte dysfunction and to persistent brain inflammation.153,154
The role of inflammation in the comorbidities of epilepsy, including depression and cognitive impairment, is under investigation. Chronic activation of cytokine-dependent inflammatory signaling might precipitate the development of depressive behaviors,155 and could, conceivably, contribute to neuronal dysfunction manifesting as cognitive deficits.156
Immune and anti-inflammatory therapies
If immune mechanisms and inflammation do indeed have a role in the generation of seizures, immune-modulating and anti-inflammatory therapies might be effective treatments for some or all forms of epilepsy. Therapies such as ACTH, corticosteroids, plasmapheresis and intravenous immunoglobulin (IVIg) have been employed to treat seizures and/or epilepsy, with varying success. These therapies have all been employed in patients with presumed autoimmune limbic encephalitis, where early and aggressive treatment often seems to be useful,60 and in patients with Rasmussen encephalitis, in whom therapy success rates are much more variable and hemispherectomy remains the treatment of choice.157 The presumed mechanism of action of the therapeutic agents listed above is suppression of inflammation; how ever, other modes of action might also be involved, including direct effects on brain excitability,158 and suppression of endogenous proconvulsant brain agents.159,160 The use of steroids in various forms is common for more severe, treatment-resistant forms of childhood epilepsy. The successful use of ACTH—a peptide that releases endogenous steroids in the patient—as a treatment for infantile spasms, which represent a severe form of childhood epilepsy that is resistant to conventional AEDs, was initially shown empirically, then confirmed in randomized controlled trials. Consequently, ACTH remains a mainstay of therapy for this condition.161 The mechanism of spasm suppression by ACTH has been speculated to be at least partly driven by direct effects of steroids on cortical excitability158 and through melanocortin receptor-mediated ACTH suppression of endogenous convulsants,160,162 rather than through steroid-related immune modulation.
ACTH, steroids and IVIg have all been employed to treat AED-unresponsive pediatric epilepsies, including Lennox–Gastaut syndrome,163,164 Landau–Kleffner syndrome,165,166 difficult partial epilepsies, and myoclonic–astatic epilepsies.167 Unfortunately, determination of whether patients received benefit from these treatments is problematic, since most of these epilepsies are extremely heterogeneous in etiology and severity, and exhibit notoriously variable courses. In addition, most of the clinical studies are retrospective case series, with occasional prospective case series that lack controls.168,169 Follow-up duration in these case series was also often variable. A recent review of investigations of IVIg in intractable childhood epilepsy found no randomized or controlled studies and, in fact, only two case series employed statistics in assessing outcome.164 One series showed a statistically significant reduction in seizures with IVIg treatments, while the other revealed a nonsignificant trend with such therapy.164 Well-controlled, blinded studies have been published for the use of ACTH and steroids for infantile spasms;161,170–172 however, a Cochrane Collaboration review on the use of ACTH for other childhood epilepsies, published in 2007, found only a single randomized controlled trial, which only included five patients.173 The authors of this review concluded that, at present, no evidence exists to support either the safety or the efficacy of ACTH for general pediatric epilepsies.173
Conclusions
Clearly, preclinical data support further attempts to modulate seizures and epilepsy by influencing inflammation. As noted above, therapeutic interventions with anti-inflammatory therapies have, to date, consisted only of immune modulators such as IVIg, plasmapheresis, corticosteroids and ACTH, and the mechanistic actions of these therapies have not been well studied.
In cases of epilepsy where an immune etiology is suspected, and particularly where circulating auto-antibodies might have a role, a reasonable approach is to address direct removal of such antibodies, as is currently done using plasmapheresis, or to reduce the autoimmune attack through use of IVIg. Notably, IVIgs have also been shown to induce IL-1ra release from peripheral blood cells, suggesting an additional anti-inflammatory mechanism of action for this therapy.174
ACTH has been proven to be an effective therapy for infantile spasms (although the mechanism of action is unclear), whereas the use of plasmapheresis, IVIg or steroids in other epilepsy syndromes is controversial. Considerations when employing such treatments include absence of controlled clinical data, the substantial cost of these therapies, and the risk of known adverse events, which, although uncommon, can be life-threatening. Such events include increased risk of infection, cardiomyopathy, coagulation disorder, and hypersensitivity. As a result of the positive response of the patients selected for such treatments, the results of controlled trials are eagerly anticipated.
A suggestion has been made that some of the anticonvulsant effects of the ketogenic diet—used for the management of refractory epilepsies in children and adolescents—might be mediated by anti-inflammatory actions. Specifically, fatty acids induce activation of the nuclear hormone receptor peroxisome proliferator-activated receptor α, a transcription factor that down-regulates NFκB-activated proinflammatory genes.175
Importantly, preclinical data suggest that direct targeting of conditions such as IL-1β-mediated hyperexcitability, might be warranted, because this mechanism could contribute to different types of seizures. Several important questions remain, such as whether inflammatory mechanisms are important at all stages of epileptogenesis and epilepsy, whether patients all have a similar degree of inflammation,69 and whether various epilepsy etiologies are associated with inflammation that can be targeted therapeutically. Imaging techniques such as PET or MRI spectroscopy are undergoing development to evaluate brain inflammation in epilepsy, which could help to identify people who would benefit from anti-inflammatory treatments. A clinical trial of an IL-1β synthesis inhibitor was initiated in 2010.176 If this and other trials are successful, assessment of which patients are responding to anti-inflammatory therapy might become possible and, hence, the underlying epilepsy pathologies in which inflammation is important might be determined. Working forward from animal models, and simultaneously backward from patients on the basis of successful intervention, could ultimately provide us with the best understanding of those epilepsies in which inflammatory mechanisms are most critical.
Key points.
-
▪
Epilepsies of various etiologies not classically linked to immunological dysfunction can be associated with inflammation resulting from increased levels of inflammatory mediators in the brain
-
▪
Inflammatory mediators can be produced by glia, neurons, endothelial cells of the blood–brain barrier, and peripheral immune cells
-
▪
Brain inflammation might contribute to the onset and perpetuation of seizures in a variety of epilepsies
-
▪
Experimental and clinical research is required to generate novel therapeutic anti-inflammatory approaches that ameliorate seizures and modify the underlying pathophysiology of epilepsy
Review criteria.
Articles were selected for this Review by searching the PubMed database with the following terms: “brain inflammation” or “inflammation” or “cytokines and/or chemokines and/or COX and/or complement” in combination with “epilepsy” or “seizures”; “inflammation” in combination with “cell death/neurodegeneration”; “innate and/or adaptive immunity” in combination with “epilepsy and/or seizures”; “anti-inflammatory treatments” in combination with “epilepsy or neurological disorders”; and “blood–brain barrier” in combination with “epilepsy and/or seizures and/or inflammation”. Only English language articles were considered. No restrictions related to publication date were set.
Acknowledgments
This work was supported by contributions to A. Vezzani from Fondazione Cariplo, Fondazione Monzino and Parents Against Childhood Epilepsy (P.A.C.E.), the NIH R37 NS35439 and American Epilepsy Society Research Initiative award given to T. Z. Baram, and a Shaw Family Initiative On Inflammation award given to J. French. We apologize to the many authors whose work was not cited because of space limitations.
Footnotes
Competing interests
A. Vezzani and J. French declare an association with the following company: Vertex Pharmaceuticals. T. Z. Baram declares associations with the following companies: Pfizer, Questcor Pharmaceuticals. See the article online for full details of the relationships. T. Bartfai declares no competing interests.
Author contributions
A. Vezzani, J. French, T. Bartfai and T. Z. Baram contributed equally to researching data for the article, discussion of content, writing, and reviewing and/or editing of the manuscript before submission.
Supplementary information
Supplementary information is linked to the online version of the paper at www.nature.com/nrneurol
Contributor Information
Annamaria Vezzani, Department of Neuroscience, Mario Negri Institute for Pharmacological Research, Via Giuseppe La Masa 19, 20156 Milan, Italy.
Jacqueline French, NYU Comprehensive Epilepsy Center, 223 East 34th Street, New York, NY 10016, USA.
Tamas Bartfai, Molecular and Integrative Neurosciences Department, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Tallie Z. Baram, Pediatrics, Anatomy and Neurobiology, and Neurology, UC Irvine, ZOT 4475, Irvine, CA 4475, USA
References
- 1.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 2.Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6:793–804. doi: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 3.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 4.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 5.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Vezzani A, Baram TZ. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riazi K, Galic MA, Pittman QJ. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 2010;89:34–42. doi: 10.1016/j.eplepsyres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Choi J, et al. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J. Neuroinflammation. 2009;6:38. doi: 10.1186/1742-2094-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riikonen R. Infantile spasms: therapy and outcome. J. Child Neurol. 2004;19:401–404. doi: 10.1177/088307380401900601. [DOI] [PubMed] [Google Scholar]
- 10.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9:353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
- 11.Wirrell E, Farrell K, Whiting S. The epileptic encephalopathies of infancy and childhood. Can. J. Neurol. Sci. 2005;32:409–418. doi: 10.1017/s0317167100004388. [DOI] [PubMed] [Google Scholar]
- 12.Dubé CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007;30:490–496. doi: 10.1016/j.tins.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen T, Olsewski J, Lloyd-Smith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/wnl.8.6.435. [DOI] [PubMed] [Google Scholar]
- 15.Bien CG, et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- 16.Dalmau J, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent A, Bien CG. Anti-NMDA-receptor encephalitis: a cause of psychiatric, seizure, and movement disorders in young adults. Lancet Neurol. 2008;7:1074–1075. doi: 10.1016/S1474-4422(08)70225-4. [DOI] [PubMed] [Google Scholar]
- 18.Aarli JA. Epilepsy and the immune system. Arch. Neurol. 2000;57:1689–1692. doi: 10.1001/archneur.57.12.1689. [DOI] [PubMed] [Google Scholar]
- 19.Ravizza T, Balosso S, Aronica E, Vezzani A. In: Epilepsy: Mechanisms, Models, and Translational Perpsectives. Rho JM, editor. Ch. 4. Boca Raton: CRC Press; 2010. pp. 45–59. [Google Scholar]
- 20.Campbell IL, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl Acad. Sci. USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vezzani A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl Acad. Sci. USA. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balosso S, et al. Tumor necrosis factor-α inhibits seizures in mice via p75 receptors. Ann. Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- 23.Ravizza T, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47:1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 24.Samland H, et al. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J. Neurosci. Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- 25.Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 1997;158:438–445. [PubMed] [Google Scholar]
- 26.Kelley KA, et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Sarro G, et al. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol. Biochem. Behav. 2004;77:761–766. doi: 10.1016/j.pbb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 32.Appel SH, Beers DR, Henkel JS. T cell–microglial dialog in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2009;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 34.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 35.Bartfai T, et al. Interleukin-1 system in CNS stress: seizures, fever, and neurotrauma. Ann. N.Y. Acad. Sci. 2007;1113:173–177. doi: 10.1196/annals.1391.022. [DOI] [PubMed] [Google Scholar]
- 36.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 37.Bartfai T, Schultzberg M. Cytokines in neuronal cell types. Neurochem. Int. 1993;22:435–444. doi: 10.1016/0197-0186(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 38.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szekanecz Z, Koch AE. Chemokines and angiogenesis. Curr. Opin. Rheumatol. 2001;13:202–208. doi: 10.1097/00002281-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–336. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 43.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khuu CH, Barrozo RM, Hai T, Weinstein SL. Activating transcription factor 3 (ATF3) represses the expression of CCL4 in murine macrophages. Mol. Immunol. 2007;44:1598–1605. doi: 10.1016/j.molimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Ho HH, Antoniv TT, Ji JD, Ivashkiv LB. Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-γ via superinduction of ATF-3 and suppression of AP-1. J. Immunol. 2008;181:5089–5097. doi: 10.4049/jimmunol.181.7.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 48.Rothwell NJ. CRF is involved in the pyrogenic and thermogenic effects of interleukin 1 beta in the rat. Am. J. Physiol. 1989;256:E111–E115. doi: 10.1152/ajpendo.1989.256.1.E111. [DOI] [PubMed] [Google Scholar]
- 49.Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N.Y. Acad. Sci. 1999;876:1–13. doi: 10.1111/j.1749-6632.1999.tb07618.x. [DOI] [PubMed] [Google Scholar]
- 50.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 51.Najjar S, Bernbaum M, Lai G, Devinsky O. Immunology and epilepsy. Rev. Neurol. Dis. 2008;5:109–116. [PubMed] [Google Scholar]
- 52.Rogers SW, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science. 1994;265:648–651. doi: 10.1126/science.8036512. [DOI] [PubMed] [Google Scholar]
- 53.Mantegazza R, et al. Antibodies against GluR3 peptides are not specific for Rasmussen’s encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J. Neuroimmunol. 2002;131:179–185. doi: 10.1016/s0165-5728(02)00261-8. [DOI] [PubMed] [Google Scholar]
- 54.Watson R, et al. Absence of antibodies to glutamate receptor type 3 (GluR3) in Rasmussen encephalitis. Neurology. 2004;63:43–50. doi: 10.1212/01.wnl.0000132651.66689.0f. [DOI] [PubMed] [Google Scholar]
- 55.Bien CG, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen’s encephalitis. Ann. Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 56.Baranzini SE, Laxer K, Bollen A, Oksenberg JR. Gene expression analysis reveals altered brain transcription of glutamate receptors and inflammatory genes in a patient with chronic focal (Rasmussen’s) encephalitis. J. Neuroimmunol. 2002;128:9–15. doi: 10.1016/s0165-5728(02)00109-1. [DOI] [PubMed] [Google Scholar]
- 57.Pardo CA, et al. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45:516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- 58.Wirenfeldt M, et al. Increased activation of Iba1+ microglia in pediatric epilepsy patients with Rasmussen’s encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiol. Dis. 2009;34:432–440. doi: 10.1016/j.nbd.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi Y, Mine J, Kubota Y, Yamazaki E, Fujiwara T. A substantial number of Rasmussen syndrome patients have increased IgG CD4+ T cells, TNFα, and granzyme B in CSF. Epilepsia. 2009;50:1419–1431. doi: 10.1111/j.1528-1167.2008.01977.x. [DOI] [PubMed] [Google Scholar]
- 60.Vincent A, Irani SR, Lang B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr. Opin. Neurol. 2010;23:144–150. doi: 10.1097/WCO.0b013e32833735fe. [DOI] [PubMed] [Google Scholar]
- 61.Crespel A, et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 2002;952:159–169. doi: 10.1016/s0006-8993(02)03050-0. [DOI] [PubMed] [Google Scholar]
- 62.Aronica E, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 2007;26:497–511. doi: 10.1016/j.nbd.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 63.Ravizza T, et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 64.van Gassen KL, et al. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia. 2008;49:1055–1065. doi: 10.1111/j.1528-1167.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 65.Maldonado M, et al. Expression of ICAM-1, TNF-α, NFκB, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol. Dis. 2003;14:279–290. doi: 10.1016/s0969-9961(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 66.Boer K, et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. doi: 10.1016/j.eplepsyres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Boer K, et al. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 2010;20:704–719. doi: 10.1111/j.1750-3639.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iyer AM, et al. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience. 2010;19:929–945. doi: 10.1016/j.neuroscience.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 69.Iyer A, et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51:1763–1773. doi: 10.1111/j.1528-1167.2010.02547.x. [DOI] [PubMed] [Google Scholar]
- 70.Ravizza T, et al. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 2006;24:128–143. doi: 10.1016/j.nbd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Minami M, Kuraishi Y, Satoh M. Effects of kainic acid on messenger RNA levels of IL-1β, IL-6, TNF-α and LIF in the rat brain. Biochem. Biophys. Res. Commun. 1991;176:593–598. doi: 10.1016/s0006-291x(05)80225-6. [DOI] [PubMed] [Google Scholar]
- 72.Vezzani A, et al. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Simoni MG, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur. J. Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 74.Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol. Dis. 2004;16:321–334. doi: 10.1016/j.nbd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Eriksson C, Tehranian R, Iverfeldt K, Winblad B, Schultzberg M. Increased expression of mRNA encoding interleukin-1β and caspase-1, and the secreted isoform of interleukin-1 receptor antagonist in the rat brain following systemic kainic acid administration. J. Neurosci. Res. 2000;60:266–279. doi: 10.1002/(SICI)1097-4547(20000415)60:2<266::AID-JNR16>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 76.Voutsinos-Porche B, et al. Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol. Dis. 2004;17:385–402. doi: 10.1016/j.nbd.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 77.Jung KH, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol. Dis. 2006;23:237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Lee B, Dziema H, Lee KH, Choi YS, Obrietan K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol. Dis. 2007;25:80–91. doi: 10.1016/j.nbd.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorter JA, et al. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J. Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polascheck N, Bankstahl M, Loscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp. Neurol. 2010;224:219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Holtman L, et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84:56–66. doi: 10.1016/j.eplepsyres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 82.Dhote F, et al. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology. 2007;238:166–176. doi: 10.1016/j.tox.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 83.Yoshikawa K, Kita Y, Kishimoto K, Shimizu T. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J. Biol. Chem. 2006;281:14663–14669. doi: 10.1074/jbc.M511089200. [DOI] [PubMed] [Google Scholar]
- 84.Rozovsky I, et al. Selective expression of clusterin (SGP-2) and complement C1qB and C4 during responses to neurotoxins in vivo and in vitro . Neuroscience. 1994;62:741–758. doi: 10.1016/0306-4522(94)90473-1. [DOI] [PubMed] [Google Scholar]
- 85.Kulkarni SK, Dhir A. Cyclooxygenase in epilepsy: from perception to application. Drugs Today (Barc.) 2009;45:135–154. doi: 10.1358/dot.2009.45.2.1322481. [DOI] [PubMed] [Google Scholar]
- 86.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 87.Fabene PF, Bramanti P, Constantin G. The emerging role for chemokines in epilepsy. J. Neuroimmunol. 2010;224:22–27. doi: 10.1016/j.jneuroim.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 88.Xu JH, et al. CCR3, CCR2A and macrophage inflammatory protein (MIP)-1a, monocyte chemotactic protein-1 (MCP-1) in the mouse hippocampus during and after pilocarpine-induced status epilepticus (PISE) Neuropathol. Appl. Neurobiol. 2009;35:496–514. doi: 10.1111/j.1365-2990.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- 89.Foresti ML, et al. Chemokine CCL2 and its receptor CCR2 are increased in the hippocampus following pilocarpine-induced status epilepticus. J. Neuroinflammation. 2009;6:40. doi: 10.1186/1742-2094-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manley NC, Bertrand AA, Kinney KS, Hing TC, Sapolsky RM. Characterization of monocyte chemoattractant protein-1 expression following a kainate model of status epilepticus. Brain Res. 2007;1182:138–143. doi: 10.1016/j.brainres.2007.08.092. [DOI] [PubMed] [Google Scholar]
- 91.Wu Y, et al. Expression of monocyte chemoattractant protein-1 in brain tissue of patients with intractable epilepsy. Clin. Neuropathol. 2008;27:55–63. doi: 10.5414/npp27055. [DOI] [PubMed] [Google Scholar]
- 92.Fabene PF, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008;14:1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Librizzi L, et al. Expression of adhesion factors induced by epileptiform activity in the endothelium of the isolated guinea pig brain in vitro. Epilepsia. 2007;48:743–751. doi: 10.1111/j.1528-1167.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 94.Librizzi L, Ravizza T, Vezzani A, de Curtis M. Expression of IL-1β induced by epileptiform activity in the isolated guinea pig brain in vitro. Presented at the 9th European Congress on Epileptology; Rhodes. 2010. [Google Scholar]
- 95.Dubé CM, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J. Neurosci. 2010;30:7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Vliet EA, et al. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 97.Marchi N, et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 99.Marcon J, et al. Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis. Neurobiol. Dis. 2009;34:121–132. doi: 10.1016/j.nbd.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 100.Majores M, Eils J, Wiestler OD, Becker AJ. Molecular profiling of temporal lobe epilepsy: comparison of data from human tissue samples and animal models. Epilepsy Res. 2004;60:173–178. doi: 10.1016/j.eplepsyres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Lukasiuk K, Dabrowski M, Adach A, Pitkanen A. Epileptogenesis-related genes revisited. Prog. Brain Res. 2006;158:223–241. doi: 10.1016/S0079-6123(06)58011-2. [DOI] [PubMed] [Google Scholar]
- 102.Xiong ZQ, Qian W, Suzuki K, McNamara JO. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J. Neurosci. 2003;23:955–960. doi: 10.1523/JNEUROSCI.23-03-00955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berg AT, Darefsky AS, Holford TR, Shinnar S. Seizures with fever after unprovoked seizures: an analysis in children followed from the time of a first febrile seizure. Epilepsia. 1998;39:77–80. doi: 10.1111/j.1528-1157.1998.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 104.Zetterstrom M, Sundgren-Andersson AK, Ostlund P, Bartfai T. Delineation of the proinflammatory cytokine cascade in fever induction. Ann. N.Y. Acad. Sci. 1998;856:48–52. doi: 10.1111/j.1749-6632.1998.tb08311.x. [DOI] [PubMed] [Google Scholar]
- 105.Gatti S, Vezzani A, Bartfai T. In: Febrile Seizures. Baram TZ, Shinnar S, editors. Ch. 12. San Diego: Academic Press; 2002. pp. 169–184. [Google Scholar]
- 106.Cartmell T, Luheshi GN, Rothwell NJ. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J. Physiol. 1999;518:585–594. doi: 10.1111/j.1469-7793.1999.0585p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1β contributes to the generation of experimental febrile seizures. Ann. Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002;43:920–923. doi: 10.1046/j.1528-1157.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 109.Haspolat S, et al. Interleukin-1β, tumor necrosis factor-α, and nitrite levels in febrile seizures. J. Child Neurol. 2002;17:749–751. doi: 10.1177/08830738020170101501. [DOI] [PubMed] [Google Scholar]
- 110.Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50:407–411. doi: 10.1212/wnl.50.2.407. [DOI] [PubMed] [Google Scholar]
- 111.Lahat E, Livne M, Barr J, Katz Y. Interleukin-1β levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr. Neurol. 1997;17:34–36. doi: 10.1016/s0887-8994(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 112.Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia. 2005;46:1906–1913. doi: 10.1111/j.1528-1167.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 113.French JA, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 114.Scantlebury MH, Heida JG. Febrile seizures and temporal lobe epileptogenesis. Epilepsy Res. 2010;89:27–33. doi: 10.1016/j.eplepsyres.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 115.Dubé C, et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sayyah M, Javad-Pour M, Ghazi-Khansari M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: involvement of proinflammatory factors: nitric oxide and prostaglandins. Neuroscience. 2003;122:1073–1080. doi: 10.1016/j.neuroscience.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 117.Galic MA, et al. Postnatal inflammation increases seizure susceptibility in adult rats. J. Neurosci. 2008;28:6904–6913. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Auvin S, et al. Inflammation in rat pups subjected to short hyperthermic seizures enhances brain long-term excitability. Epilepsy Res. 2009;86:124–130. doi: 10.1016/j.eplepsyres.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 119.Auvin S, Mazarati A, Shin D, Sankar R. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol. Dis. 2010;40:303–310. doi: 10.1016/j.nbd.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kovacs Z, et al. Facilitation of spike–wave discharge activity by lipopolysaccharides in Wistar Albino Glaxo/Rijswijk rats. Neuroscience. 2006;140:731–742. doi: 10.1016/j.neuroscience.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 121.Mouihate A, et al. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J. Neurosci. 2010;30:7975–7983. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioral deficits and alters N-methyl-d-aspartate receptor subunit mRNA in the adult rat brain. Eur. J. Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maroso M, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 124.Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 1997;77:2685–2696. doi: 10.1152/jn.1997.77.5.2685. [DOI] [PubMed] [Google Scholar]
- 125.Rizzi M, et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol. Dis. 2003;14:494–503. doi: 10.1016/j.nbd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 126.Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience. 2006;137:301–308. doi: 10.1016/j.neuroscience.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 127.Plata-Salaman CR, et al. Kindling modulates the IL-1β system, TNF-α, TGF-β1, and neuropeptide mRNAs in specific brain regions. Brain Res. Mol. Brain Res. 2000;75:248–258. doi: 10.1016/s0169-328x(99)00306-x. [DOI] [PubMed] [Google Scholar]
- 128.Ravizza T, et al. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol. Dis. 2008;31:327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 129.Longhi L, et al. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit. Care Med. 2009;37:659–665. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- 130.Clausen F, et al. Neutralization of interleukin-1β modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2009;30:385–396. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- 131.Lloyd E, Somera-Molina K, Van Eldik LJ, Watterson DM, Wainwright MS. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation. 2008;5:28. doi: 10.1186/1742-2094-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 133.Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 134.Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: epidemiology, pathophysiology and management. CNS Drugs. 2009;23:805–815. doi: 10.2165/11310900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perkins ND. Integrating cell-signaling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell. Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 136.Hoebe K, Beutler B. Forward genetic analysis of TLR-signaling pathways: an evaluation. Adv. Drug Deliv. Rev. 2008;60:824–829. doi: 10.1016/j.addr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 137.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 138.O’Neill LA, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 139.Pitkanen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14(Suppl. 1):16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 140.Balosso S, et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1β. Brain. 2008;131:3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Viviani B, et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1β induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J. Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1β. Proc. Natl Acad. Sci. USA. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tabarean IV, Korn H, Bartfai T. Interleukin-1β induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 145.Zhang R, et al. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1β. Neurobiol. Dis. 2010;38:68–77. doi: 10.1016/j.nbd.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 146.Viviani B, Gardoni F, Marinovich M. Cytokines and neuronal ion channels in health and disease. Int. Rev. Neurobiol. 2007;82:247–263. doi: 10.1016/S0074-7742(07)82013-7. [DOI] [PubMed] [Google Scholar]
- 147.Bezzi P, et al. CXCR4-activated astrocyte glutamate release via TNF-α: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 148.Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- 149.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ferguson AR, et al. Cell death after spinal cord injury is exacerbated by rapid TNFα-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 152.Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 153.David Y, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J. Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cacheaux LP, et al. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J. Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dantzer R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1β on learning and memory. Brain Behav. Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bien CG, Schramm J. Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res. 2009;86:101–112. doi: 10.1016/j.eplepsyres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 158.Joels M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ. Corticotropin (ACTH) acts directly on amygdala neurons to downregulate corticotropin-releasing hormone gene expression. Ann. Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 160.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pellock JM, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 162.Brunson KL, Avishai-Eliner S, Baram TZ. ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability. Int. Rev. Neurobiol. 2002;49:185–197. doi: 10.1016/s0074-7742(02)49013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sinclair DB. Prednisone therapy in pediatric epilepsy. Pediatr. Neurol. 2003;28:194–198. doi: 10.1016/s0887-8994(02)00513-1. [DOI] [PubMed] [Google Scholar]
- 164.Mikati MA, Kurdi R, El-Khoury Z, Rahi A, Raad W. Intravenous immunoglobulin therapy in intractable childhood epilepsy: open-label study and review of the literature. Epilepsy Behav. 2010;17:90–94. doi: 10.1016/j.yebeh.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 165.Marescaux C, et al. Landau–Kleffner syndrome: a pharmacologic study of five cases. Epilepsia. 1990;31:768–777. doi: 10.1111/j.1528-1157.1990.tb05518.x. [DOI] [PubMed] [Google Scholar]
- 166.Villani F, Avanzini G. The use of immunoglobulins in the treatment of human epilepsy. Neurol. Sci. 2002;23(Suppl. 1):S33–S37. doi: 10.1007/s100720200013. [DOI] [PubMed] [Google Scholar]
- 167.You SJ, Jung DE, Kim HD, Lee HS, Kang HC. Efficacy and prognosis of a short course of prednisolone therapy for pediatric epilepsy. Eur. J. Paediatr. Neurol. 2008;12:314–320. doi: 10.1016/j.ejpn.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 168.Mikati MA, Saab R, Fayad MN, Choueiri RN. Efficacy of intravenous immunoglobulin in Landau–Kleffner syndrome. Pediatr. Neurol. 2002;26:298–300. doi: 10.1016/s0887-8994(01)00402-7. [DOI] [PubMed] [Google Scholar]
- 169.Verhelst H, et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure. 2005;14:412–421. doi: 10.1016/j.seizure.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 170.Baram TZ, et al. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 171.Mackay MT, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lux AL, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicenter randomised trial. Lancet Neurol. 2005;4:712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- 173.Gayatri NA, Ferrie CD, Cross H. Corticosteroids including ACTH for childhood epilepsy other than epileptic spasms. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD005222.pub2. Art. No.: CD005222. [DOI] [PubMed] [Google Scholar]
- 174.Crow AR, Song S, Semple JW, Freedman J, Lazarus AH. A role for IL-1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg? Blood. 2007;109:155–158. doi: 10.1182/blood-2006-05-023796. [DOI] [PubMed] [Google Scholar]
- 175.Cullingford T. Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia. 2008;49(Suppl. 8):70–72. doi: 10.1111/j.1528-1167.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- 176.ClinicalTrials.gov. Study of VX-765 in subjects with treatment-resistant partial epilepsy. 2010 [online], http://clinicaltrials.gov/ct2/show/NCT01048255.
- 177.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 178.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 180.Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010;2010:484987. doi: 10.1155/2010/484987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gloor SM, et al. Molecular and cellular permeability control at the blood–brain barrier. Brain Res. Brain Res. Rev. 2001;36:258–264. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]