Abstract
Background
The suggestion that the neurohormone oxytocin may have clinical application in the treatment of schizophrenia was first published in 1972. Since then, a considerable body of research on a variety of fronts--including several recent double-blind treatment trials—has buttressed these early reports, providing support for the assertion that the oxytocin system is a promising and novel therapeutic target for this devastating malady. Herein, we review the diverse, convergent lines of evidence supporting the therapeutic potential of oxytocin in psychotic illness.
Methods
We performed a systematic review of preclinical and clinical literature pertaining to oxytocin’s role in schizophrenia.
Results
Multiple lines of evidence converge to support the antipsychotic potential of oxytocin. These include several animal models of schizophrenia, pharmacological studies examining the impact of antipsychotics on the oxytocin system, human trials in patients examining aspects of the oxytocin system, and several double-blind, placebo-controlled clinical treatment trials.
Conclusions
There exists considerable, convergent evidence that oxytocin has potential as a novel antipsychotic with a unique mechanism of action. Auspiciously, based on the few chronic trials to date, its safety profile and tolerability appear very good. That said, several critical clinical questions await investigation before widespread use is clinically warranted.
Keywords: Intranasal, Brain/physiology, Double-Blind Method, Humans, Schizophrenia, Social Perception, Oxytocin/administration and dosage
1. Introduction
Schizophrenia is one of the most disabling disorders in psychiatry, with a disturbingly small proportion of patients afflicted with this disorder able to maintain independent function (van Os & Kapur, 2009). Despite decades of intensive research, a precise, comprehensive, systems-level understanding of its etiology and pathophysiology remains elusive (Keshavan, Nasrallah, & Tandon, 2011; Meyer-Lindenberg, 2010). In terms of the treatment of schizophrenia, though psychosocial interventions add significant value (Guo et al., 2010), psychopharmacological treatment remains the backbone of care (Leucht et al., 2011; van Os & Kapur, 2009). Regarding antipsychotic psychopharmacology, despite a long program of intense drug discovery, all established medications to date rely on D2 receptor antagonism--either exclusively or in conjunction with antagonism of 5-HT2A receptors--as the mechanism of action (Agid et al., 2007; van Os & Kapur, 2009). This with the possible exception of clozapine (Dziedzicka-Wasylewska, Faron-Gorecka, Gorecki, & Kusemider, 2008; Nord & Farde, 2011), an antipsychotic of note in that data reviewed below indicate that clozapine may have a unique relationship with the subject of this review: the oxytocin system. Recent years have seen a groundswell of clinically oriented research on this system, opening the possibility for a genuinely novel treatment for psychotic illness.
In the context of the current fervent interest in oxytocin as a therapy in psychiatry, it is interesting to note that it was first proposed as a potential treatment for schizophrenia in the early seventies (Bujanow, 1972), 20 years before the structure of its receptor was discovered (Kimura, Tanizawa, Mori, Brownstein, & Okayama, 1992), and 10 years before the sequencing of its gene (Ivell & Richter, 1984). Almost forty years since that initial report, following several decades of basic science and animal research, two recent double-blind placebo-controlled trials have confirmed and bolstered this initial finding, pointing toward oxytocin as having genuine therapeutic potential (Feifel, Macdonald, et al., 2010; C. A. Pedersen et al., 2011). In the following review, after a brief summary of the oxytocin system, we will summarize the data that supports the therapeutic use of oxytocin in schizophrenia.
2. Oxytocin Fundamentals
Evolutionarily, the nine-amino acid (nonapeptide) family of which oxytocin is part is ancient, with slight variants found in species from mollusks to meerkats (Donaldson & Young, 2008; Gimpl & Fahrenholz, 2001; Madden & Clutton-Brock, 2010). Demonstrating a surprising homology of behavioral effects, oxytocin and its homologs have been linked to social and reproductive behaviors in a truly remarkable diversity of species (Goodson, Schrock, Klatt, Kabelik, & Kingsbury, 2009; Oldfield & Hofmann, 2010; Wagenaar, Hamilton, Huang, Kristan, & French). Structurally, oxytocin is very similar to arginine vasopressin (AVP), and together these nonapeptides share four phylogenically-related receptors (AVPR1a, AVPR1b, AVPR2, OTR) (Gimpl & Fahrenholz, 2001). This structural homology is important, as some of oxytocin’s central effects (Sala et al., 2011; Schorscher-Petcu et al., 2010), as well as potential side effects (i.e. hyponatremia) (Seifer, Sandberg, Ueland, & Sladen, 1985; Stratton, Stronge, & Boylan, 1995) may be mediated by binding to AVP receptors (Li et al., 2008; Liggins, 1963).
When considering the therapeutic use of oxytocin in psychiatric illness in general- and schizophrenia in particular--several facets of its central neurophysiology warrant brief mention. These include: its synthesis and release, its receptor system, its modes of central action, and its regulation by and interaction with other important systems of interest, including gonadal hormones, dopamine and glutamate. Given other comprehensive reviews on these topics (Carter, Grippo, Pournajafi-Nazarloo, Ruscio, & Porges, 2008; Gimpl & Fahrenholz, 2001; Insel, 1992, 2010; Landgraf & Neumann, 2004; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011), these issues are addressed only briefly here
In humans, oxytocin acts as both a hormone and a neurotransmitter, and has been used therapeutically for decades based on its effects on the smooth muscle of the uterus (in the context of delivery) and breast (in the context of lactation) (Sogolow, 1966; Uvnas-Moberg & Eriksson, 1996). These peripheral actions are effected when endogenous oxytocin is synthesized in magnocellular neurons in the paraventricular and supraoptic nuclei of the hypothalamus, moved by axonal transport to the posterior pituitary, and released into the systemic circulation (Fig 1). Worth noting in this context is that there is some controversy regarding the extent to which peripheral and central release of oxytocin are coordinated, and thus how much peripheral OT levels can be considered a surrogate marker for central activity. In brief, though there is evidence that the activity of the central and peripheral oxytocin systems can be dissociated (Amico, Challinor, & Cameron, 1990; Amico, Tenicela, Johnston, & Robinson, 1983; Neumann, 2007), there is also accumulating evidence in humans that its central activity and peripheral release are often correlated (Burri, Heinrichs, Schedlowski, & Kruger, 2008; Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010; Light et al., 2000; Strathearn, Fonagy, Amico, & Montague, 2009b) (see (Veenema & Neumann, 2008; Veening, de Jong, & Barendregt, 2010) for reviews of this topic). Of interest here, correlations between peripheral OT levels and neurobehavioral outcomes include interesting associations in patients with schizophrenia (discussed below) (M. Goldman, Marlow-O'Connor, Torres, & Carter, 2008; Rubin et al., 2011; Rubin et al., 2010).
Figure 1. Oxytocin: Central Synthesis and Peripheral Effects.

Though there is evidence for oxytocin synthesis outside the brain (Gutkowska & Jankowski, 2008), oxytocin is predominantly synthesized by specialized neurons in two nuclei in the hypothalamus: the paraventricular (PVN) and supraoptic (SON). Once synthesized, oxytocin serves dual roles as both a central neurotransmitter/neuromodulator (see fig 2) as well as a peripheral hormone. In point of fact, there are receptors for oxytocin throughout the body, though its primary therapeutic use to date have been in the gravid uterus and lactating breast, where it stimulates contractions and milk ejection, respectively. Of note, the correlation and coordination of peripheral and central oxytocin release (and therefore the relevance of peripheral oxytocin levels to brain-based illness) is complex and a matter of active research (see (Feldman, Gordon, & Zagoory-Sharon, 2011; Ludwig & Leng, 2006; Veening, et al., 2010) for contemporary perspectives).
**figures reprinted with permission from Oxford University Press.
Besides its vital role as a hormone, oxytocin also acts in an important central network as a peptide neurotransmitter (Buijs & Van Heerikhuize, 1982; Sofroniew, Weindl, Schrell, & Wetzstein, 1981)(fig 2). These central actions, have been elaborated in a growing variety of human MRI studies (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Domes et al., 2007; Domes et al., 2010; Gamer, Zurowski, & Buchel, 2010; Kirsch et al., 2005; Petrovic, Kalisch, Singer, & Dolan, 2008; Riem et al., 2011; Rilling et al., 2011; Strathearn, Fonagy, Amico, & Montague, 2009a), occur via mechanisms that differ significantly from classical neurotransmitters (i.e. GABA) (Landgraf & Neumann, 2004) (figure 2). That is, unlike classic neurotransmitters, neuropeptide neurotransmitters such as oxytocin have both direct effects via axonal release from parvocellular neurons in the PVN, as well as diffuse “volume” effects due to somatodendritic release from magnocellular neurons (Ludwig & Leng, 2006; Neumann, 2007) (fig 2). These latter effects uniquely occur with neuropeptides because unlike classical neurotransmitters, neuropeptides have no reuptake system and a longer extracellular half life. As such, they are able to produce a range of effects in areas at some distances from their release due to short-range diffusion in the extracellular fluid and CSF (Landgraf & Neumann, 2004; Veening, et al., 2010).
Figure 2. Oxytocin: Central effects.
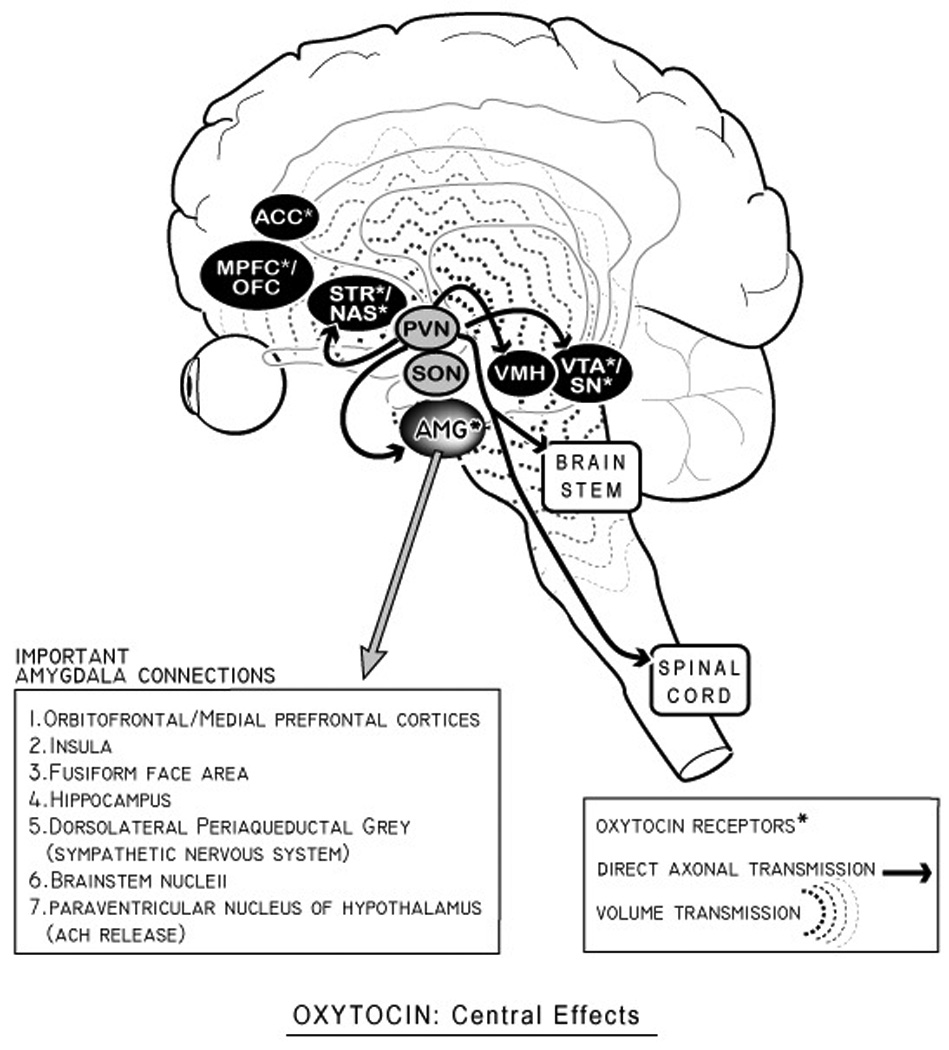
Oxytocin has at least two modes of central activity: 1) direct axonal transmission from parvocellular neurons in the paraventricular nucleus (PVN: solid arrows above); and 2) volume diffusion effects (i.e ‘volume transmission’) which has effects in areas with oxytocin receptors. This latter set of “volume effects” are the result of somatodendritic release of oxytocin from both supraoptic nuclei (SON and PVN), and may partially occur via short-range diffusion in the CSF (Ludwig & Leng, 2006; McGregor, Callaghan, & Hunt, 2008; Veening, et al., 2010). As discussed in the text, the amygdala (with important connections in pull-out box) is a critical node of oxytocin’s central activity in humans, and plays a role in some theories about oxytocin’s role in schizophrenia (Rosenfeld, Lieberman, & Jarskog, 2010). Other areas of overlap between schizophrenia and oxytocinergic function include the hippocampus and the dopaminergic ventral tegmental area/striatum. Only selected oxytocin-receptor containing brain areas are shown here; for a more complete list, see reviews in (de Bono, 2003; Gimpl & Fahrenholz, 2001; Skuse & Gallagher, 2009). Abbreviations: ACC (anterior cingulate cortex), MPFC (medial prefrontal cortex), OFC (orbitofrontal cortex), STR (striatum), NAS (nucleus accumbens), AMG (amygdala), VMH (ventromedial hypothalamus), VTA (ventral tegmental area), SN (substantia nigra).
As noted in figure 2, OT receptors have been located in a number of brain areas relevant to schizophrenia, including the substantia nigra, the nucleus of the solitary tract, the central nucleus of the amygdala, the lateral septal nucleus, and parts of the basal ganglia (Broad et al., 1999; Gimpl & Fahrenholz, 2001; Loup, Tribollet, Dubois-Dauphin, & Dreifuss, 1991; Loup, Tribollet, Dubois-Dauphin, Pizzolato, & Dreifuss, 1989; Tribollet, Dubois-Dauphin, Dreifuss, Barberis, & Jard, 1992) (figure 2). Several factors about OTRs warrant mention here. First, though only one oxytocin receptor (OTR) has been identified and cloned, recent research has identified polymorphisms of the OTR in the human population, and the role of genetic variations in human OTRs is an area of intense interest, with several studies showing these variations have associations with important central functions in humans (Inoue et al., 2011; X. Liu et al., 2011; Montag, Fiebach, Kirsch, & Reuter, 2011; Rodrigues, Saslow, Garcia, John, & Keltner, 2009; Thompson, Parker, Hallmayer, Waugh, & Gotlib, 2011). Besides polymorphisms in the OTR, basic science research by Insel and others has also demonstrated that a second factor--the spatial distribution and density of OTRs in different brain areas--is a critical parameter determining OTs species-specific central effects (Insel, 1997), as well as dynamic effects based on developmental stages (i.e. parturition) (Bale, Davis, Auger, Dorsa, & McCarthy, 2001; Meddle, Bishop, Gkoumassi, van Leeuwen, & Douglas, 2007). Lasty, epigenetic variations in the OTR system can occur as the result of environmental influences like early caregiving, part of the way nurture is transformed into nature (F. Champagne, Diorio, Sharma, & Meaney, 2001; F. A. Champagne et al., 2004), and part of the complex role OT plays in the translation of social factors (isolation and social defeat) into neurobiology (Norman et al., 2010; Timmer, Cordero, Sevelinges, & Sandi, 2011). All told, a greater understanding of variations in the plastic, socially-sensitive OT system may be illuminative in understanding the considerable role different social and environmental stressors (i.e. prenatal stress, childhood trauma, social defeat) play in the development of schizophrenia (Lieberman, Sheitman, & Kinon, 1997; Pruessner, Champagne, Meaney, & Dagher, 2004; Selten & Cantor-Graae, 2005).
When considering treatment of humans with intranasal oxytocin, it is worth noting that although the precise mechanism wherein intranasal oxytocin exerts its central effects is underspecified (Born et al., 2002; Illum, 2004), the central oxytocin system is regulated as feed-forward system, with synchronous bursting of neural networks leading to spikes of OT release (Rossoni et al., 2008). As such, small amounts of exogenous oxytocin delivered to the brain may prime and trigger sustained, endogenous release from the central system. This may account for the interesting fact that in some experiments, intranasal oxytocin increases peripheral oxytocin levels for an extended time (> 1 hour) (Burri, et al., 2008), well beyond OT’s short plasma half-life, which is on the order of 2–12 minutes (Mens, Witter, & van Wimersma Greidanus, 1983; Robinson & Coombes, 1993; Robinson & Jones, 1982). Furthermore, due to its evolutionary role in plastic and long-lasting developmental brain processes (Carter, 2003), the time frame of therapeutic effects of exogenous oxytocin may differ from that of treatments based on classical neurotransmission.
Finally, regarding oxytocin and schizophrenia, it is important to note that oxytocin has significant interactions with other systems of interest in the illness, including estrogen (which regulates synthesis of both OT and OTRs (Choleris, Devidze, Kavaliers, & Pfaff, 2008; Patisaul, Scordalakes, Young, & Rissman, 2003)), serotonin (Emiliano, Cruz, Pannoni, & Fudge, 2007), dopamine (Baskerville & Douglas, 2010; Shahrokh, Zhang, Diorio, Gratton, & Meaney, 2010; Succu et al., 2007), and glutamate (Hrabovszky & Liposits, 2008; Ninan, 2011). These interactions, explored in the experiments reviewed below, are helpful to keep in mind when considering the “final common pathway” by which oxytocin may exert its therapeutic effects.
3. Preclinical models of schizophrenia and oxytocin
Though all animal models of schizophrenia contain theoretical and translational challenges, they provide an invaluable way to discern putative antipsychotic effects and allow the used of techniques (intracerebral microdialysis, gene knockout, maternal deprivation) not available in humans (Feifel & Shilling, 2010). In the case of oxytocin, several animal models predictive of antipsychotic efficacy both support its antipsychotic-like effects and point toward putative mechanisms of action (Table 1). These models include a variety of pharmacologic, environmental and genetic manipulations, all of which induce different aspects of the syndrome of schizophrenia.
Table 1.
Preclinical Studies supporting antipsychotic effects of oxytocin
| Authors | Model /Parameter | Main Findings | Notes |
|---|---|---|---|
| Sarnyai et al. (1990) [1] | Cocaine-induced hyperactivity | Both pimozide (dopamine receptor antagonist) and oxytocin blocked cocaine-induced hyperactivity, the latter in a “U” shaped, dose-response manner. Effects of oxytocin on dopamine were centered in nucleus accumbens. |
Along with [2–8], supports dopaminergic MOA for OT. |
| Uvnas-Moberg et al.(1992) [9] | APD effect on pOT levels | Clozaril and amperozide (clozaril-like antipsychotic) increased pOT levels, whereas haloperidol did not. | Along with [10, 11], supports unique relationship between oxytocin and clozapine |
| Feifel et al. (1999) [12] | PPI model | SC OT restored PPI that had been reduced by dizocilpine (NMDA antagonist), and amphetamine (indirect DA agonist), but not apomorphine (direct DA agonist). | Along with [13, 14], supports glutamatergic/NMDA receptor MOA. |
| Lee et al. (2005) [14] | PCP model | PCP-induced social dysfunction caused decreases in hypothalamic oxytocin mRNA expression, and increases in OTR binding in the central amygdala (ceA). Infusion of OT into ceA restored social behavior in PCP-treated rats. |
PCP (a noncompetitive NMDA receptor antagonist) induces social deficits in animals [15] and psychosis in humans [16]. Along with [12, 13], supports a glutamatergic/NMDA receptor MOA for OT. |
| Lee et al (2007) [17] | Prental stress model | Prenatal-stressed rats, that show decreased social interaction, demonstrated less OT mRNA in the PVN, and increased OTR binding in ceA. As in [14], OT injected into ceA reversed social deficits. |
A variety of prenatal and early childhood stressors may play a role in the development of schizophrenia [18, 19]. |
| Qi, et al. (2008) [8] | Methamphetamine (MAP)-induced hyperactivity | ICV OT inhibited MAP hyperactivity in a dose-dependant manner. Atosiban, a selective OTR antagonist, attenuated this effect. OT inhibited the MAP-induced reduction of DA turnover in the striatum and accumbens. |
Along with [1]--and see other references in that note--supports a dopaminergic MOA for OT. |
| Caldwell et al. (2009) [13] | PPI model | Mice with disruption of the OT gene (OTKO mice) showed unique PPI deficits with PCP, but not amphetamine or apomorphine. | Along with [12, 14], supports glutamatergic/NMDA receptor MOA. |
| Qi, et al (2009) [20] | Methamphetamine (MAP)-induced conditioned place preference (CPP) | OT inhibited the acquisition and facilitated the extinction and stress-induced reinstatement of MAP-induced CPP. OT blocked stress-induced elevations in glutamate in the medial prefrontal cortex mPFC, and effect blocked by atosiban (an OTR antagonist). |
This study joins [12–14] in pointing to a gluatmatergic MOA for OT, and, like [21] locates glutamatergic effects in the mPFC. Human studies note PFC dysfunction linked to DA dysregulation [22], part of the “functional dysconnection” hypothesis of SCZ [23]. |
| Kiss et al. (2010) [10] | APD effect on oxytocin-producing cells | Clozapine and olanzapine, but not risperidone or haloperidol, activated OT and AVP cells, as measured by early gene activation (c-Fos) in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of hypothalamus. | Along with [9, 11], supports unique relationship between oxytocin and clozapine. |
| Feifel et al (2011) [24] | PPI model | Oxytocin, but not carbetocin (a longer-acting structural analog of oxytocin) increased PPI and decreased acoustic startle in Brown Norway rats, a strain with natural PPI deficits. | These same Brown Norway rats demonstrate facilitation of PPI to clozapine but not haloperidol [25], another tie between these compounds. |
Abbreviations: APD antipsychotic drug, AVP arginine vasopressin, DA dopamine, ICV intracerebral, MOA mechanism of action, NMDA N-methyl-D-aspartate, OT oxytocin, OTR oxytocin receptor, PCP phencyclidine, pOT plasma oxytocin level, PPI prepulse inhibition, PVN paraventricular nucleus, SC subcutaneous
A first model, building on the dopaminergic theory of schizophrenia (Carlsson, 1977; Carlsson & Carlsson, 2006), uses stimulants (amphetamine, cocaine) to model the hyperactivity and hyperdopaminergic state presumed to be associated with psychosis. Established antipsychotics consistently reverse stimulant-induced hyperactivity, and thus this model serves as a predictive screen for potential antipsychotic drugs with anti-dopamine mechanisms. Using such a model, in 1990 Sarnyai et al demonstrated that subcutaneous oxytocin decreased cocaine-induced hyperactivity in a “U-shaped” dose-response manner, similar to the antipsychotic pimozide (Sarnyai, Szabo, Kovacs, & Telegdy, 1990). Histological analysis validated that these findings were related to dopaminergic neurotransmission in the nucleus accumbens, part of the mesolimbic dopamine system implicated in schizophrenia (Sarter, Nelson, & Bruno, 2005; Weinberger & Lipska, 1995). In a similar set of experiments using methamphetamine (MAP), Qi et al demonstrated that intracerebral oxytocin inhibited MAP-induced hyperactivity in a dose-dependent manner; atosiban (an OTR antagonist) attenuated these effects (Qi et al., 2008). As in the study by Sarnyai above, these dopaminergic effects were localized to the striatum and accumbens. In a later experiment, this same group showed that the acquisition of MAP-induced conditioned place preference (CPP)—an animal model of addiction--was inhibited by OT, which also facilitated the extinction of this preference and blocked its stress-induced reinstatement (Qi et al., 2009). In this experiment, microdialysis in the prefrontal cortex showed that OT inhibited restraint-stress induced extracellular glutamate levels. This finding is of interest given the interaction proposed between prefrontal cortical dysfunction and aberrant dopaminergic transmission in schizophrenia (Meyer-Lindenberg et al., 2002), and given that the abovementioned stimulant models implicate a dopaminergic mechanism of action for OT. As discussed above, and in a variety of other experiments ((Baskerville & Douglas, 2010; Drago et al., 1986; Drago, Contarino, & Busa, 1999; Kovacs & Telegdy, 1983; Y. Liu & Wang, 2003; Qi, et al., 2008; Shahrokh, et al., 2010), and see (Skuse & Gallagher, 2009) for recent review), there are extensive interactions between oxytocin and central dopaminergic systems. As such, and given that the excessive dopamine transmission in the mesolimbic system is strongly implicated in the positive psychotic symptoms of schizophrenia, the above series of experiments support that oxytocin’s inhibitory regulation of mesolimbic dopamine may be a part of its mechanism of antipsychotic action.
A second pharmacological animal model of schizophrenia utilizes antagonists for the excitatory glutamate/NMDA receptor such as phencyclidine (PCP) or ketamine to induce behavioral syndromes that mimic negative symptoms of schizophrenia. For example, chronic PCP administration in rodents causes them to display social withdrawal, a clinical hallmark of schizophrenia (Qiao et al., 2001; Sams-Dodd, 1999). Importantly, clozapine--considered the most efficacious antipsychotic, especially for negative symptoms (Azorin et al., 2001; Davis, Chen, & Glick, 2003; Lewis et al., 2006)--reverses PCP-induced social withdrawal more effectively than other antipsychotics (Qiao, et al., 2001) lending validity to this animal model. As noted above, oxytocin has been linked with clozapine treatment in several pharmacological (Kiss, Bundzikova, Pirnik, & Mikkelsen, 2010; Uvnas-Moberg, Alster, & Svensson, 1992) and a genetic study (Souza, de Luca, Meltzer, Lieberman, & Kennedy, 2010).
Using a PCP model in rats, Lee et al demonstrated that chronic PCP treatment decreased hypothalamic OT mRNA expression and increased OTR binding in the central nucleus of the amygdala (CeA) (Lee, Brady, Shapiro, Dorsa, & Koenig, 2005). Notably, direct OT infusion into the CeA selectively restored the normal quality and quantity of social behavior (Lee, et al., 2005). This latter finding—reversal of experimentally-induced social deficits with direct injection of OT into the ceA—has been replicated in a prenatal stress model of schizophrenia (Lee, Brady, Shapiro, Dorsa, & Koenig, 2007). As background, rats from mothers exposed to unpredictable prenatal stress show neuroendocrine imbalances, behavioral deficits (reduced social drive), as well as decreased PPI, indicating a resemblance to aspects of schizophrenia (Koenig et al., 2005; Lee, et al., 2007). Examining a group of these rats, Lee et al found they had less OT mRNA in the PVN, increased OTR binding in the ceA, as well as the aforementioned reversal of social incompetence by direct administration of OT to the ceA (Lee, et al., 2007). This group of findings are noteworthy given the presence of OT-responsive neuronal populations in the central nucleus of the amygdala (Huber, Veinante, & Stoop, 2005; Viviani et al., 2011), the role of the amygdala in social cognition (Adolphs, 2006), the stress-diathesis models of schizophrenia (Brown, 2011; van Os, Kenis, & Rutten, 2010), as well as broader-scope theories tying oxytocin’s activity in the amygdala to its antipsychotic properties (Rosenfeld, et al., 2010).
A third animal model used in schizophrenia research utilizes a neurophysiological measure of sensorimotor gating called prepulse inhibition of the startle reflex (PPI) (Braff, Geyer, & Swerdlow, 2001; Geyer, Krebs-Thomson, Braff, & Swerdlow, 2001). Anatomically, PPI is mediated by a cortico-striato-pallido-pontine circuitry, which includes the nucleus accumbens, the hippocampus, and the basolateral amygdala (Koch & Schnitzler, 1997; Swerdlow, Geyer, & Braff, 2001), all areas implicated in the pathophysiology of schizophrenia. Moreover, the PPI disruptions found in patients with schizophrenia can be restored with established antipsychotics, particularly second generation antipsychotics (Braff, et al., 2001), and schizophrenia-like deficits in PPI can be induced in animals using psychomimetic drugs acting on different neurotransmitter systems of relevance to schizophrenia including dopamine enhancers (amphetamine), and NMDA antagonists such as PCP, ketamine and dizocilpine (MK801) (Caldwell, Stephens, & Young, 2009; Geyer & Ellenbroek, 2003; Geyer, et al., 2001). Finally, and pertaining to the experiments discussed below, PPI deficits can also be used to distinguish between first and second-generation antipsychotics: though both families of antipsychotic are effective at reversing PPI deficits induced by dopamine enhancers, only second generation antipsychotics are effective at reversing PPI deficits induced by NMDA antagonists (Geyer, et al., 2001).
Using a PPI model, Feifel and Reza demonstrated that subcutaneous OT restored PPI that had been disrupted by both a dopamine enhancer (amphetamine) and an NMDA antagonist (diziclopine) suggesting a second-generation antipsychotic-like profile. Interestingly, though, OT did not by reverse PPI deficits induced by the direct dopamine agonist apomorphine (Feifel & Reza, 1999). More recently, Feifel et al extended these findings by investigating the effects of OT in a non-pharmacological model of PPI deficits using the Brown Norway rat. This strain of rat has naturally low PPI compared to other strains, and thus may represent a more appropriate model of the inherent PPI deficits in schizophrenia (Palmer et al., 2000). Moreover, the antipsychotics clozapine, but not haloperidol, restored PPI in Brown Norway rats to normal levels, indicating that PPI deficits in these rats may serve as a valid predictive screen for second-generation antipsychotics (Feifel, Shilling, & Melendez, 2011). Most recently, when Feifel et al tested the activity of a longer-acting OT analog (carbetocin) and OT in this Brown Norway strain, they found that subcutaneous administration of OT (but not carbetocin) significantly enhanced PPI, similar to the effects of clozapine (Feifel, Shilling, & Belcher, 2011).
Another group to use the PPI model in a unique strain of mice did so with animals that were genetically-engineered without the gene for oxytocin (oxytocin knock-out (OTKO) mice) (Caldwell, et al., 2009). OTKO mice, it has been demonstrated, have impaired social memory (Ferguson, Aldag, Insel, & Young, 2001), show more anxiety-like behaviors than normal mice (Mantella, Vollmer, Li, & Amico, 2003), and exhibit inflexibility of change in learned behavior (Sala, et al., 2011). In this particular experiment, Caldwell et al demonstrated that homogeneous (OTKO) mice (OT −/−), were significantly more susceptible to PCP-induced PPI deficits than mice expressing the normal OT gene (OT +/+), suggesting that endogenous OT may play an antipsychotic role by protecting against the disruption of glutaminergic circuits. Notably, and similar in some respects to the abovementioned findings from Feifel, et al (Feifel & Reza, 1999), this group did not find a similar genotype-by-treatment effect when the dopaminergic compounds amphetamine and apomorphine were used to disrupt PPI, again suggesting that endogenous OT’s antipsychotic-like effects may be specific for glutaminergic--but not dopaminergic--perturbations.
Besides these animal models, another line of evidence supporting oxytocin’s antipsychotic activity comes from evidence suggesting that the stimulation of endogenous OT may contribute to the therapeutic effects of certain antipsychotics. For example, Uvnas-Moberg demonstrated that both clozaril and amperozide (a clozaril-like compound) produced elevations of plasma oxytocin levels, whereas haloperidol did not (Uvnas-M 1992). More recently, Kiss et al demonstrated that that several antipsychotic drugs induced c-Fos activity in hypothalamic magnocellular OT neurons, with clozaril and olanzapine showing more robust effects than risperidone and haloperidol (Kiss 2010). Additional evidence that the endogenous OT system mediates at least some of clozapine’s therapeutic effects comes from a human study that found that that a variant of the oxytocin gene (rs2740204) was significantly associated with clozaril treatment response, and was nominally associated with negative symptoms (Souza, et al., 2010). On the topic of potential genetic links between oxytocin and schizophrenia, a just-published report from a unique Arab-Israeli schizophrenia cohort suggested involvement of four candidate genes including genes for both OT and AVP (Teltsh et al., 2011).
Notwithstanding challenges in translating from animal models to the complex, heterogeneous disease that is schizophrenia (Feifel & Shilling, 2010), multiple converging lines of evidence from animal research support that oxytocin may have central actions highly relevant to the phenomenology and treatment of schizophrenia, and point to potential mechanisms, brain regions, and circuits that may mediate these effects. Furthermore, these experiments implicate that certain antipsychotics--clozaril in particular--may have a unique relationship with the central oxytocin system.
4. Human studies of the oxytocin system in schizophrenia
A variety of studies, dating back several decades, have examined different aspects of the oxytocin system in patients with schizophrenia. Aspects of the system that have been studied include: 1) levels of oxytocin and oxytocin-related molecules (i.e. the oxytocin carrier protein neurophysin) in the CSF and blood; 2) levels of cellular activity in the brain areas (PVN, SON) where oxytocin is synthesized; and 3) the relationship of oxytocin levels to clinical symptoms or abilities (See Table 2). Each of these experiments should be seen in the light of abovementioned controversies regarding the relationship and function of the central and peripheral oxytocin systems (vide supra section (2)), as well as the heterogeny of symptoms in patients with the illness. Though each of the parameters these researchers have studied is an indirect measure of central oxytocin activity, it should be remembered that until the widespread use of functional imaging, there was no way to measure the central activity of oxytocin in humans.
Table 2.
Studies of the oxytocin system in patients with schizophrenia
| Authors | N | OT parameter | Main Findings | Notes |
|---|---|---|---|---|
| Linkowski et al. (1984) | 12 SCZ (9 M, 3 F) 12 controls | CSF OT-neurophysin (hNpII) levels | Apomorphine-stimulated CSF hNpII levels were significantly lower in SCZ patients than controls | Neurophysin II is a carrier protein for oxytocin. See also notes for Legros (1992) |
| Beckmann et al. (1985) [26] | 28 M with SPT; 15 controls | cOT | cOT increased in patients with SCZ, and were higher in patients on APDs (butyrophenones and phenothiazines, not clozaril). cOT increased after three weeks of treatment with APDs. |
Relevance of cOT levels to central function debated (see text and [27]). |
| Legros et al. (1992) [28] | 9 M SCZ 14 M controls | Plasma OT-neurophysin (hNpII) levels | Basal hNpII levels were increased in SCZ patients compared to controls, and were significantly higher in the paranoid than in the non-paranoid SCZ group. Apomorphine caused elevation in plasma nNpII levels in normals; this response was blunted in SCZ patients. |
Apomorphine, a DA agonist, stimulates OT release in humans [28, 29], consistent with bidirectional DA-OT interactions [30, 31] |
| Mai et al. (1993) [32] | 11 SCZ (gender not reported) 10 controls | Neurophysin staining in post-mortem brain samples | Abnormal neurophysin staining in PVN, globus pallidus, substantia nigra in brains of patients with SCZ. | Patients were essentially untreated, making effects of medication unlikely. |
| Glovinsky et al. (1994) [33] | 40 SCZ (31 M, 9 F); 15 controls | cOT | cOT levels did not differ within subject based on APD status (treated or withdrawn), nor between SCZ patients and controls. | |
| Goldman et al. (2008) [34] | 15 SCZ (6 PHS, 4 PNS, 5 NNS), 7 controls | pOT | pOT levels increased in PHS patients compared to PNS or NNS or controls, and higher pOT associated with greater accuracy of rating facial emotions. pOT levels were inversely correlated with anterior hippocampal volume. |
Along with [35] indicates potential of differential role of OT in various SCZ subtypes, especially PHS patients, who also show impaired hippocampal function [36], and structural pathology in the amygdala and anterior lateral hippocampus [37] |
| Keri et al. (2009) [38] | 50 SCZ (16 M, 34 F) 50 controls | pOT | Controls showed elevated pOT levels after trust-related interactions, whereas patients with SCZ d not. Low pOT levels after trust-related interactions were associated with negative symptoms. No relationship found between pOT and APD. | Notable, given social sensitivity of OT effects [39], role of interpersonal stressors in SCZ [40], and oxytocin’s augmentation of facial affect recognition in SCZ [41]. |
| Rubin et al. (2010) [42] | 50 SCZ (27 M, 23 F) 58 controls | pOT | Female patients with higher pOT had less severe positive symptoms and overall psychopathology. In both sexes, patients with higher pOT levels showed more prosocial behaviors (a subset of the Positive and Negative Symptom Score (PANSS)). Clinical symptoms improved during menstrual phases characterized by high levels of estrogen and progesterone. |
Gender differences noted in clinical course of SCZ [43, 44]. Estrogen and prolactin regulate OT and OTR expression [45–48]. |
| Souza et al. (2010) [49] | 140 M and F SCZ pts | OT and OTR genes | The OT gene was significantly associated with clozaril treatment response and nominally associated with negative symptoms. Variants in the OTR rs237887) were nominally associated with BPRS severity and improvement in positive symptoms. |
Clozaril and oxytocin also associated in [9, 10]. |
| Rubin et al. (2011) [50] | 48 SCZ (26 M, 22 F) 57 controls | pOT | Higher pOT levels related to perceiving faces as happier in both female patients and controls, but not in men. Women demonstrated menstrual-cycle dependent fluctuations in emotional cue responses. | Gender differences noted in other studies of pOT [51, 52], and in some [53] but not other studies of IN OT (for review [39]). |
Abbreviations: APD antipsychotic drug, BPRS brief psychotic rating scale, cOT cerebrospinal fluid oxytocin level, DA dopamine, NNS normonatremic nonpolydipsic schizophrenia, OT oxytocin, OTR oxytocin receptor, PHS polydipsic hyponatremic schizophrenia, PNS polydipsic normonatremic schizophrenia, pOT plasma oxytocin level, PPI prepulse inhibition, PVN paraventricular nucleus, SCZ schizophrenia
Most early studies of central oxytocin levels and their relation to schizophrenia found evidence for a perturbation of the oxytocin system in this disorder, although the specific direction of change has not always been consistent. For example, though Beckman et al (1985) found increased baseline levels of CSF OT in patients with schizophrenia (levels were further increased with haloperidol treatment) (Beckmann, Lang, & Gattaz, 1985), and Linkowski et al found higher CSF levels of the oxytocin carrier protein neurophysin in schizophrenia patients (Linkowski, Geenen, Kerkhofs, Mendlewicz, & Legros, 1984), a later study showed no change in CSF OT based on illness or treatment (Glovinsky, Kalogeras, Kirch, Suddath, & Wyatt, 1994). Postmortem studies of oxytocin-rich areas in the brains of unmedicated schizophrenia patients have demonstrated morphometric differences in the PVN, internal palladium, and substantia nigra (Mai, Berger, & Sofroniew, 1993), and studies of hypothalamic regions associated with oxytocin release (PVN) have shown reduced cell density in patients with schizophrenia (Bernstein et al., 1998). Most recently, as part of a multifaceted set of experiments in patients with schizophrenia and polydipsia, Goldman, et al, found deformations in brain areas involved with the modulation of neuroendocrine responses (anterior lateral hippocampus, amygdala) in certain subsets of such patients (M. B. Goldman et al., 2011).
Aside from these assays of the central OT system, early researchers have also examined peripheral oxytocin levels in patients with schizophrenia, finding lower baseline levels of oxytocin carrier proteins (neurophysins) compared to normal controls, with levels in the schizophrenia group inversely associated with the level of paranoia (Legros et al., 1992). A group of subsequent studies by Goldman, et al, have demonstrated lower levels of plasma OT in patients with polydipsia and schizophrenia (M. Goldman, et al., 2008), and found these levels to be inversely correlated with anterior hippocampal volume (M. B. Goldman, Torres, et al., 2007), positively correlated with hippocampal-mediated HPA feedback (M. B. Goldman, Wood, et al., 2007), and predictive of patients’ ability to correctly identify facial emotions (M. Goldman, et al., 2008). This latter finding of a correlation between social cognition and plasma oxytocin in patients with schizophrenia has since been replicated (Rubin, et al., 2011), and extended in treatment trials showing oxytocin enhancement of social cognition ((Averbeck, Bobin, Evans, & Shergill, 2011; C. Pedersen et al., 2010), vide infra). Additionally, the aforementioned findings around the role of OT and the HPA axis in schizophrenia again points to the role of OT in the stress-diathesis aspect of the illness (van Os, et al., 2010), and raises the possibility that OT function may distinguish between some of the clinical subtypes (i.e. polydipsia) within the broad schizophrenia heading.
Further studies have also found correlations between pOT and schizophrenia. Following seminal studies in normal subjects indicating oxytocin’s role in trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), and a correlation of pOT levels with trustworthiness (Zak, Kurzban, & Matzner, 2005), Keri et all examined whether OT played a role in trust in patients with schizophrenia. In this study, patients with schizophrenia had lower pOT levels following a trust-related social interaction than normal controls, who demonstrated increased levels following these interactions (Keri, Kiss, & Kelemen, 2009). Non-trust related OT levels were also lower in patients, (though not significantly so) and low trust-related pOT levels predicted the negative symptoms of schizophrenia (Keri, et al., 2009). These findings relating to trust are of interest in regards to the schizophrenia symptom of paranoia, and highlight the role of OT in the amygdala, a brain structure shown in normals to be important in interpersonal trust assessments (Engell, Haxby, & Todorov, 2007; Koscik & Tranel, 2011), and responsive to OT in trust-related situations (Baumgartner, et al., 2008).
Biological sex (chromosomal maleness or femaleness) is a critical variable influencing oxytocin’s function, and an awareness of the sex-specificity of many of its actions informs a review of the oxytocin literature.* Shedding light on a potential sex-specific role of oxytocin in schizophrenia, Rubin et al described that female patients with higher pOT levels had attenuated positive symptoms and psychopathology, and that in both males and females, higher oxytocin levels were associated with more prosocial behaviors (Rubin, et al., 2010). In a follow up study, this group also reported that although OT levels did not fluctuate across menstrual phase, higher pOT levels in women were associated with increased sensitivity to happy facial emotion, less severe positive symptoms and less overall psychopathology (Rubin, et al., 2011). On the topic of sex and OT, it is worth noting that several studies have shown that oxytocin levels can fluctuate throughout the menstrual cycle ((Liedman et al., 2008; Salonia et al., 2005) but see (Shukovski, Healy, & Findlay, 1989; Stock, Bremme, & Uvnas-Moberg, 1991)), and that oral contraceptive use (which was exclusionary in (Rubin, et al., 2011; Rubin, et al., 2010)) may increase oxytocin levels in healthy women ((Silber, Almkvist, Larsson, Stock, & Uvnas-Moberg, 1987; Stock, Karlsson, & von Schoultz, 1994), but see (Salonia, et al., 2005)). Furthermore, the issues around OT and sex raised by these experiments are especially salient given that: 1) estrogen upregulates oxytocin receptors (Patisaul, et al., 2003), plays a role in oxytocin’s activity in the amygdala (Choleris, et al., 2008), and appears to influence the course of psychotic symptoms in women (Bergemann, Parzer, Runnebaum, Resch, & Mundt, 2007) (vide supra section 1); 2) other sex-specific hormones (i.e. testosterone) may moderate OT’s effects (van Anders, Goldey, & Kuo, 2011); 3) sex is an important factor in the natural history of schizophrenia (Tandon, Keshavan, & Nasrallah, 2008); and 3) studies indicate that sex moderates OT levels (Holt-Lunstad, Birmingham, & Light, 2011; Salonia, et al., 2005), stress-based (Grewen, Girdler, Amico, & Light, 2005; Taylor et al., 2000) and behavioral aspects of OT (Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010), the effect of OT on the brain in face processing tasks (Domes, et al., 2007; Domes, et al., 2010), as well as some of the effects of treatment with exogenous OT (Bartz, Zaki, Bolger, & Ochsner, 2011). To whit, the role of sex in oxytocin’s baseline and dynamic activity--as well as the impact of sex and gonadal hormones on OT treatment of patients with schizophrenia--requires much further study.
5. Clinical trials using oxytocin in patients with schizophrenia
Surprisingly, perhaps, the first documented report of the use of oxytocin in schizophrenia predates the animal research discussed above by more than a decade (Bujanow, 1972). In several early reports, investigators in the USSR reported that 6–10 treatments of between 10–25 IU oxytocin given IV or IM induced rapid therapeutic effects and prevented hospitalization in patients with schizophrenia (Bujanow, 1972), potentially by acting as a “psychic energizer” reversing energy, apathy, and depression (Bakharev, Tikhomirov, & Lozhkina, 1986). In spite of their prescience, these early reports describe case series (Bujanow, 1972), or did not use standardized diagnostic or outcome scales (Bakharev, et al., 1986). Only recently, more than thirty years later, have rigorously designed and executed clinical trials of exogenous OT been conducted in patients with schizophrenia.
In the first of these, Feifel et al treated 15 outpatients with residual symptoms of schizophrenia with 3 weeks of adjunctive intranasal oxytocin (40 IU twice daily) in a randomized, double-blind crossover study (Feifel, Macdonald, et al., 2010). In this experiment, oxytocin produced significantly greater therapeutic effects across a broad-spectrum of symptoms including both positive and negative symptom clusters based upon changes in the Positive and Negative Syndrome Scale (PANSS), though improvement in positive symptoms appeared more robust. Supporting the clinical salience of these effects, Clinical Global Impression (CGI) scores also improved significantly with oxytocin. Importantly, given the paucity of chronic treatment trials, is that oxytocin was well tolerated from the perspective of reported side effects, vital signs and blood chemistry studies. Finally, it was found that in contrast to single administration studies of OT in normal subjects, which tended to produce amnestic effects on verbal memory (Bruins, Hijman, & Van Ree, 1992; Fehm-Wolfsdorf, Born, Voigt, & Fehm, 1984), schizophrenia patients exhibited improved verbal memory after 3 weeks of daily intranasal oxytocin (Feifel, Cobb, et al., 2010).
Replicating and extending these findings, Pedersen et al recently conducted a randomized, placebo-controlled, two-week trial in which outpatients and inpatients with schizophrenia received either intranasal placebo or 24 IU twice daily. Similar to Feifel et al (Feifel, Macdonald, et al., 2010), this study reported significant, global decreases in PANSS scores with oxytocin compared to placebo. In addition, this group found significant improvements in several social cognition measures (theory of mind, facial trustworthiness) in the oxytocin group compared to the placebo group, extending oxytocin’s well-documented prosocial effects in normal subjects (K. Macdonald & Macdonald, 2010) into a clinical population with impairments in this arena (Averbeck, et al., 2011; Tremeau, 2006), and providing support for oxytocin’s ability to ameliorate some of the social cognitive deficits in schizophrenia. These social deficits, which have more association with the community function of schizophrenia patients than neurocognition (Fett et al., 2011), are poorly treated with current medications (Penn 2009). Interestingly, Pedersen et al found significant separation between oxytocin and placebo in PANSS scores after the second week of treatment, whereas the study by Feifel et al (Feifel, Macdonald, et al., 2010) did not observe an oxytocin advantage until the third week of treatment. In considering this difference, it is notable that though magnitude of symptoms was similar between these studies, there were differences in dosing, setting (inpatient vs. outpatient), and patient characteristics (average age, years of illness).
Besides these chronic treatment trials, several recent, single-dose studies of interest have extended oxytocin’s well-documented ability to alter responses to socially salient stimuli in normals (K. Macdonald & Macdonald, 2010; Riem, et al., 2011) to patients with schizophrenia, replicating the abovementioned prosocial findings by Pedersen, et al (C. A. Pedersen, et al., 2011). In one such study, Averbeck et al first demonstrated that patients with schizophrenia performed worse than normals on a specific emotion recognition task, and subsequently showed--using the same task--that double-blind oxytocin treatment improved patient’s recognition of emotions (Averbeck, et al., 2011). A second, similar study by Goldman, et al examined the effect of two doses of IN OT (10 and 20 IU) and placebo on emotion recognition in three groups: schizophrenia patients with (PS) and without polydipsia (NPS), and normals (M. B. Goldman, Gomes, Carter, & Lee, 2011). Besides demonstrating a differential effect of these two doses in patients (10 IU worsened performance, 20 IU improved performance), this experiment showed a differential benefit of the 20 IU dose in PS vs. NPS. Besides demonstrating a dose effect of OT, these findings raise the potential of oxytocin to have differential effects in subsets of patients with schizophrenia. Together, these single-application experiments highlight the unique potential of oxytocin to target the social cognitive and emotion-processing impairments that are such a disabling component of schizophrenia (Fett, et al., 2011; Ursu et al., 2011).
In reviewing the extant clinical research using IN OT in patients with schizophrenia, several practical issues deserve special mention. On the positive side, the extant research around the safety and tolerability of oxytocin indicates that both short term ((E. Macdonald et al., 2011) for recent review) and chronic use are both safe and tolerable in the populations and doses studied to date. Chronic trials supporting oxytocin’s safety and tolerability include the two and three-week schizophrenia trials cited above (Feifel, Macdonald, et al., 2010; C. A. Pedersen, et al., 2011), as well as a 13-week study of 40 IU BID in women with constipation (Ohlsson et al., 2005). These findings are encouraging, given the often morbid side effects of current pharmacologic treatments for schizophrenia (Rummel-Kluge et al., 2010). Until more research accrues in vulnerable populations (i.e. patients with polydipsia) and with different dosing, however, we recommend ongoing vigalence, given OT’s potential cross-reactivity with vasopressin (Li, et al., 2008), and the subsequent possibility of hyponatremia and water intoxication (Liggins, 1963; Seifer, et al., 1985; Stratton, et al., 1995), but see (Rasmussen, Simonsen, Sandgaard, Hoilund-Carlsen, & Bie, 2004).
Though intranasal oxytocin appears quite safe and tolerable, there are several practical barriers to its therapeutic drug development in humans. These include the lack of intellectual property ownership of the actual hormone, lack of US FDA approval for any psychiatric indication, and challenges around the actual availability of the drug (Kubzansky, Mendes, Appleton, Block, & Adler, 2009). These practical challenges to the use of oxytocin exist in addition to the many outstanding scientific questions around its therapeutic profile (box 1), and require novel solutions (i.e. oxytocin analogues, new delivery systems).
Box 1: Pending questions around the use of oxytocin in schizophrenia.
DOSING AND DELIVERY: What are the optimal dose and frequency of treatment, in the context of several studies indicating a dose effect of oxytocin (M. B. Goldman, Gomes, et al., 2011; Uvnas-Moberg, et al., 1992), as well as the finding of sustained levels after a single treatment (Burri, et al., 2008). Is the intranasal route the optimal one, or might less onerous routes (i.e. patch) be more practical or effective ?
ADJUNCTIVE OR PRIMARY: Does oxytocin have a clinical impact as monotherapy ? How does adjunctive oxytocin impact the effect of currently prescribed antipsychotics ? In women, do oral contraceptives or hormonal status impact these effects ? Would co-administration of estrogen with OT augment its effects ?
SUBPOPULATIONS AND SYMPTOM CLUSTERS: Given the clinical heterogeny of schizophrenia, are there subpopulations (i.e. females, patients with negative symptoms) that are more prone to benefit from oxytocin? Might some patient groups experience additional side effects from oxytocin (i.e. patients with polydipsia)? Are there symptom clusters (positive, negative, social cognition) that show particular benefit ?
BIOMARKERS: Noting that several studies show single-dose effects of oxytocin (Averbeck, et al., 2011; M. B. Goldman, Gomes, et al., 2011), are there clinical biomarkers which may be early predictors of a therapeutic response (i.e. social cognition measures, EEG, eye tracking) ?
RECEPTORS: What role do the known, consequential oxytocin receptor polymorphisms (Inoue, et al., 2011; X. Liu, et al., 2011; Montag, et al., 2011; Rodrigues, et al., 2009; Thompson, et al., 2011) or variations in regional oxytocin receptor density have in oxytocin’s therapeutic effects or in syndromic variations of schizophrenia ?
LEVELS: Does an individual’s oxytocin level (salivary, plasma, urinary, cerebrospinal fluid) have any predictive value in terms of a patient’s therapeutic response to oxytocin ? Which is more important, baseline or dynamic levels (i.e. in response to stressors, social stimuli or treatment)?
CONTEXT: Given the context-dependent effects of oxytocin (Bartz, et al., 2011), and the suggestion of OT augmentation of aspects of a helping relationship (Bryant, Hung, Guastella, & Mitchell, 2011), are there particular treatment settings or psychosocial interventions (social skills training (Roberts & Penn, 2009)) that may work synergistically with OT ?
NEURODEVELOPMENT/PLASTICITY: Noting the neurodevelopmental and stress-diathesis aspects of schizophrenia (van Os, et al., 2010), and given the suggestion that oxytocin may serve a plasticity-enhancing or neuroprotective function (Ceanga, Spataru, & Zagrean, 2010; Cohen et al., 2010; Leuner, Caponiti, & Gould, 2011; Tomizawa et al., 2003; Windle et al., 2004) might oxytocin have a particular role in “psychosis prone” individuals ?
6. Conclusions, Questions and Future Directions
En toto, the above set of convergent findings--from preclinical work with oxytocin, from studies of the oxytocin system in patients with schizophrenia, and from several randomized, placebo-controlled trials--strongly support that oxytocin holds promise as a novel treatment for schizophrenia. That said, our knowledge around the clinical use of oxytocin is limited, and considerable work needs to be done to address a host of outstanding questions regarding the use of oxytocin in psychotic illness (box 1). Fortunately for the field--and for the patients who may benefit from oxytocin therapy--ongoing study of the therapeutic potential of this ancient system will likely continue at its current feverish pace, and several large trials studying oxytocin in schizophrenia are in process. We anticipate these trials will furnish critical data to address some of these important questions.
Table 3.
Oxytocin treatment of patients with schizophrenia
| Authors | N | Parameter studied | Main Findings | Notes |
|---|---|---|---|---|
| Bunjanow et al. (1972) [54] | Not mentioned | General psychopathology (underspecified) | OT induced “rapid therapeutic effects” and “hospitalizations were prevented”. | Case reports in letter to the editor. |
| Bakharev (1984) [55] | 46 inpatients 27 M (OT), 19 M (conventional APDs) | Subsets of SCZ symptoms (not standardized) | Improvements in self and clinician-rated “asthenodepressive, apathodepressive, hypochondriac symptoms” compared with conventional ADPs. | Double-blind, placebo-controlled, between-subjects design. |
| Feifel, et al. (2010) [56] | 15 outpatients (12 M, 3 F) | PANSS, CGI, side effects | OT improved PANSS and CGI at 3 week time point. OT was well-tolerated based on patient reports and labs. |
Double-blind, placebo-controlled, within-subjects design of adjunctive OT. |
| Averbeck, et al (2011) [41] | Experiment 1) 30 patients (24 M, 6F), 29 controls Experiment 2) 21 patients (21 M) | Emotion recognition (hexagon emotion discrimination test [57]) | Patients had deficit in emotion recognition compared to controls. OT improved ability of patients to recognize most emotions. |
Experiment 1: between-subjects, Experiment 2: within-subjects, crossover |
| Goldman, et al (2011) [35] | 13 patients 5 polydipsic (PS: 3 M, 2F), 8 nonpolydipsic (NPS: 4M, 4F) 11 controls | Presence and intensity of facial emotions. | 10 IU dose caused decreased emotion recognition in patients due to emotion overidentification. 20 IU dose improved emotion recognition in PS vs NPS, specifically around fear recognition |
Within-subjects, one-time treatment with multiple doses. |
| Pederson et al. (2011) [58] | 20 inpatients and outpatients (17 M, 3 F) | PANSS, social cognition | OT improved both PANSS scores and several measures of social cognition after 2 weeks of treatment. | Double-blind, placebo-controlled, between-subjects design of adjunctive OT. |
Abbreviations: APD antipsychotic drug, CGI clinical global impression scale, F female, M male, NPS nonpolydipsic schizophrenia, OT oxytocin, PS polydipsic schizophrenia, SCZ schizophrenia
Acknowledgments
Thanks to Melissa Fleischauer and Tina MacDonald for editorial support. The research in this review and Dr. Feifel’s lab is supported by the NIMH (R34 MH091285).
Footnotes
Although the term “gender” is still often used in the oxytocin literature to refer to this important factor, following the National Academy of Sciences recommendation (Wizemann, T. M., & Pardue, M. L. (Eds.). (2001). Exploring biological contributions to human health: Does sex matter ? Washington DC: National Academy Press.), we use the term “sex” throughout to refer to biological maleness or femaleness.
References
- Adolphs R. How do we know the minds of others? Domain-specificity, simulation, and enactive social cognition. Brain Res. 2006;1079(1):25–35. doi: 10.1016/j.brainres.2005.12.127. [DOI] [PubMed] [Google Scholar]
- Agid O, Mamo D, Ginovart N, Vitcu I, Wilson AA, Zipursky RB, et al. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response--a double-blind PET study in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(6):1209–1215. doi: 10.1038/sj.npp.1301242. [DOI] [PubMed] [Google Scholar]
- Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab. 1990;71(6):1531–1535. doi: 10.1210/jcem-71-6-1531. [DOI] [PubMed] [Google Scholar]
- Amico JA, Tenicela R, Johnston J, Robinson AG. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. J Clin Endocrinol Metab. 1983;57(5):947–951. doi: 10.1210/jcem-57-5-947. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychological medicine. 2011:1–8. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorin JM, Spiegel R, Remington G, Vanelle JM, Pere JJ, Giguere M, et al. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. The American journal of psychiatry. 2001;158(8):1305–1313. doi: 10.1176/appi.ajp.158.8.1305. [DOI] [PubMed] [Google Scholar]
- Bakharev VD, Tikhomirov SM, Lozhkina TK. Psychotropic properties of oxytocin. Neurosci Behav Physiol. 1986;16(2):160–164. doi: 10.1007/BF01186517. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(7):2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in cognitive sciences. 2011 doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16(3):e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10(2):187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C. Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychological medicine. 2007;37(10):1427–1436. doi: 10.1017/S0033291707000578. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, et al. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83(3):867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Broad KD, Levy F, Evans G, Kimura T, Keverne EB, Kendrick KM. Previous maternal experience potentiates the effect of parturition on oxytocin receptor mRNA expression in the paraventricular nucleus. Eur J Neurosci. 1999;11(10):3725–3737. doi: 10.1046/j.1460-9568.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Progress in neurobiology. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins J, Hijman R, Van Ree JM. Effect of a single dose of des-glycinamide-[Arg8]vasopressin or oxytocin on cognitive processes in young healthy subjects. Peptides. 1992;13(3):461–468. doi: 10.1016/0196-9781(92)90075-e. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Hung L, Guastella AJ, Mitchell PB. Oxytocin as a moderator of hypnotizability. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Van Heerikhuize JJ. Vasopressin and oxytocin release in the brain--a synaptic event. Brain research. 1982;252(1):71–76. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- Bujanow W. Hormones in the treatment of psychoses. Br Med J. 1972;4(5835):298. doi: 10.1136/bmj.4.5835.298-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33(5):591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14(2):190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Does dopamine play a role in schizophrenia? Psychological medicine. 1977;7(4):583–597. doi: 10.1017/s003329170000622x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Carlsson ML. A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues in clinical neuroscience. 2006;8(1):137–142. doi: 10.31887/DCNS.2006.8.1/acarlsson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79(3):383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Ceanga M, Spataru A, Zagrean AM. Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neuroscience letters. 2010;477(1):15–18. doi: 10.1016/j.neulet.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22(8):889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Archives of general psychiatry. 2003;60(6):553–564. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]
- de Bono M. Molecular approaches to aggregation behavior and social attachment. J Neurobiol. 2003;54(1):78–92. doi: 10.1002/neu.10162. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Drago F, Caldwell JD, Pedersen CA, Continella G, Scapagnini U, Prange AJ., Jr Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav. 1986;24(5):1185–1188. doi: 10.1016/0091-3057(86)90168-1. [DOI] [PubMed] [Google Scholar]
- Drago F, Contarino A, Busa L. The expression of neuropeptide-induced excessive grooming behavior in dopamine D1 and D2 receptor-deficient mice. European journal of pharmacology. 1999;365(2–3):125–131. doi: 10.1016/s0014-2999(98)00877-2. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Gorecka A, Gorecki A, Kusemider M. Mechanism of action of clozapine in the context of dopamine D1–D2 receptor hetero-dimerization--a working hypothesis. Pharmacological reports : PR. 2008;60(5):581–587. [PubMed] [Google Scholar]
- Emiliano AB, Cruz T, Pannoni V, Fudge JL. The interface of oxytocin-labeled cells and serotonin transporter-containing fibers in the primate hypothalamus: a substrate for SSRIs therapeutic effects? Neuropsychopharmacology. 2007;32(5):977–988. doi: 10.1038/sj.npp.1301206. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J Cogn Neurosci. 2007;19(9):1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Born J, Voigt KH, Fehm HL. Human memory and neurohypophyseal hormones: opposite effects of vasopressin and oxytocin. Psychoneuroendocrinology. 1984;9(3):285–292. doi: 10.1016/0306-4530(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Feifel D, Cobb P, MacDonald K, Nguyen A, Minassian A, Perry W. Daily Intranasal Oxytocin Improves Verbal Memory In Schizophrenia Patients (poster); Paper presented at the American College of Neuropsychopharmacology; 2010. [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology. 1999;141(1):93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD. Promise and pitfalls of animal models of schizophrenia. Curr Psychiatry Rep. 2010;12(4):327–334. doi: 10.1007/s11920-010-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Belcher A. The Effects of Oxytocin and its Analog, Carbetocin, on Genetic Deficits in Sensorimotor Gating. European Neuropsychopharmacology. 2011 doi: 10.1016/j.euroneuro.2011.09.004. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Melendez G. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behavioral neuroscience. 2011;125(2):268–272. doi: 10.1037/a0022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental science. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and biobehavioral reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156(2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11(3):273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia research. 2008;98(1–3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Gomes AM, Carter CS, Lee R. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology. 2011;216(1):101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Torres IJ, Keedy S, Marlow-O'Connor M, Beenken B, Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007;17(7):554–562. doi: 10.1002/hipo.20292. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Wang L, Wachi C, Daudi S, Csernansky J, Marlow-O'Connor M, et al. Structural pathology underlying neuroendocrine dysfunction in schizophrenia. Behavioural brain research. 2011;218(1):106–113. doi: 10.1016/j.bbr.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Wood G, Goldman MB, Gavin M, Paul S, Zaheer S, et al. Diminished glucocorticoid negative feedback in polydipsic hyponatremic schizophrenic patients. J Clin Endocrinol Metab. 2007;92(2):698–704. doi: 10.1210/jc.2006-1131. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325(5942):862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhai J, Liu Z, Fang M, Wang B, Wang C, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: A randomized, 1-year study. Arch Gen Psychiatry. 2010;67(9):895–904. doi: 10.1001/archgenpsychiatry.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. Oxytocin revisited: It is also a cardiovascular hormone. Journal of the American Society of Hypertension : JASH. 2008;2(5):318–325. doi: 10.1016/j.jash.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011;36(8):1249–1256. doi: 10.1016/j.psyneuen.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Liposits Z. Novel aspects of glutamatergic signalling in the neuroendocrine system. J Neuroendocrinol. 2008;20(6):743–751. doi: 10.1111/j.1365-2826.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yamasue H, Tochigi M, Abe O, Liu X, Kawamura Y, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. 2011;68(11):1066–1072. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154(6):726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, "Just the Facts" 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophrenia research. 2011;127(1–3):3–13. doi: 10.1016/j.schres.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Tanizawa O, Mori K, Brownstein MJ, Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J Neurosci Res. 2010;88(3):677–685. doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behavioural brain research. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, et al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural brain research. 2005;156(2):251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia. 2011;49(4):602–611. doi: 10.1016/j.neuropsychologia.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Telegdy G. Effects of oxytocin, des-glycinamide-oxytocin and anti-oxytocin serum on the alpha-MPT-induced disappearance of catecholamines in the rat brain. Brain research. 1983;268(2):307–314. doi: 10.1016/0006-8993(83)90497-3. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton A, Block J, Adler GK. Protocol for an experimental investigation of the roles of oxytocin and social support in neuroendocrine, cardiovascular, and subjective responses to stress across age and gender. BMC Public Health. 2009;9(1):481. doi: 10.1186/1471-2458-9-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3–4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30(10):1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain research. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ, Gazzotti C, Carvelli T, Franchimont P, Timsit-Berthier M, von Frenckell R, et al. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17(6):611–617. doi: 10.1016/0306-4530(92)90019-4. [DOI] [PubMed] [Google Scholar]
- Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia--a critical systematic review and meta-analysis of randomised long-term trials. Schizophrenia research. 2011;127(1–3):83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2011 doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SW, Barnes TR, Davies L, Murray RM, Dunn G, Hayhurst KP, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophrenia bulletin. 2006;32(4):715–723. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, et al. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol. 2008;19(2):225–232. doi: 10.1681/ASN.2007010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;17(4):205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Liedman R, Hansson SR, Howe D, Igidbashian S, McLeod A, Russell RJ, et al. Reproductive hormones in plasma over the menstrual cycle in primary dysmenorrhea compared with healthy subjects. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2008;24(9):508–513. doi: 10.1080/09513590802306218. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Antidiuretic effects of oxytocin, morphine and pethidine in pregnancy and labour. Aust N Z J Obstet Gynaecol. 1963;3:81–83. doi: 10.1111/j.1479-828x.1963.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19(6):560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234(3):162–165. doi: 10.1007/BF00461555. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2011;55(3):137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555(2):220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500(1–2):223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Macdonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Madden JR, Clutton-Brock TH. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc Biol Sci. 2010 doi: 10.1098/rspb.2010.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Berger K, Sofroniew MV. Morphometric evaluation of neurophysin-immunoreactivity in the human brain: pronounced inter-individual variability and evidence for altered staining patterns in schizophrenia. Journal fur Hirnforschung. 1993;34(2):133–154. [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144(6):2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154(2):358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148(10):5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262(1):143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468(7321):194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature reviews. Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5(3):267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Montag C, Fiebach CJ, Kirsch P, Reuter M. Interaction of 5-HTTLPR and a variation on the oxytocin receptor gene influences negative emotionality. Biological Psychiatry. 2011;69(6):601–603. doi: 10.1016/j.biopsych.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35(Pt 5):1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. Journal of neurochemistry. 2011 doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS neuroscience & therapeutics. 2011;17(2):97–103. doi: 10.1111/j.1755-5949.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosomatic medicine. 2010;72(6):519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Truedsson M, Bengtsson M, Torstenson R, Sjolund K, Bjornsson ES, et al. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol Motil. 2005;17(5):697–704. doi: 10.1111/j.1365-2982.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RG, Hofmann HA. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse startle deficit in the Brown Norway rat: a potential genetic model. Behavioral neuroscience. 2000;114(2):374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(8):787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Rau S, Salimi K, Gibson C, Leserman J, Penn D. Oxytocin Treatment Improves Social Cognition and Reduces Psychotic Symptoms in Schizophrenia (poster); Paper presented at the American College of Neuropsychopharmacology; 2010. [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia research. 2011 doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(6):441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56(5):856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Qiao H, Noda Y, Kamei H, Nagai T, Furukawa H, Miura H, et al. Clozapine, but not haloperidol, reverses social behavior deficit in mice during withdrawal from chronic phencyclidine treatment. Neuroreport. 2001;12(1):11–15. doi: 10.1097/00001756-200101220-00010. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS, Simonsen JA, Sandgaard NC, Hoilund-Carlsen PF, Bie P. Effects of oxytocin in normal man during low and high sodium diets. Acta Physiol Scand. 2004;181(2):247–257. doi: 10.1111/j.1365-201X.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, et al. Oxytocin Modulates Amygdala, Insula, and Inferior Frontal Gyrus Responses to Infant Crying: A Randomized Controlled Trial. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DL, Penn DL. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: a preliminary study. Psychiatry Res. 2009;166(2–3):141–147. doi: 10.1016/j.psychres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Robinson IC, Coombes JE. Neurohypophysial peptides in cerebrospinal fluid: an update. Ann N Y Acad Sci. 1993;689:269–284. doi: 10.1111/j.1749-6632.1993.tb55553.x. [DOI] [PubMed] [Google Scholar]
- Robinson IC, Jones PM. Oxytocin and neurophysin in plasma and CSF during suckling in the guinea-pig. Neuroendocrinology. 1982;34(1):59–63. doi: 10.1159/000123278. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld AJ, Lieberman JA, Jarskog LF. Oxytocin, Dopamine, and the Amygdala: A Neurofunctional Model of Social Cognitive Deficits in Schizophrenia. Schizophrenia bulletin. 2010 doi: 10.1093/schbul/sbq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol. 2008;4(7) doi: 10.1371/journal.pcbi.1000123. e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, et al. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophrenia research. 2011 doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia research. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophrenia research. 2010;123(2–3):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biological Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47(2):164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Reviews in the neurosciences. 1999;10(1):59–90. doi: 10.1515/revneuro.1999.10.1.59. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Szabo G, Kovacs GL, Telegdy G. Oxytocin attenuates the cocaine-induced exploratory hyperactivity in mice. Neuroreport. 1990;1(3–4):200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophrenia bulletin. 2005;31(1):117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(24):8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer DB, Sandberg EC, Ueland K, Sladen RN. Water intoxication and hyponatremic encephalopathy from the use of an oxytocin nasal spray. A case report. J Reprod Med. 1985;30(3):225–228. [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? The British journal of psychiatry : the journal of mental science. 2005;187:101–102. doi: 10.1192/bjp.187.2.101. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukovski L, Healy DL, Findlay JK. Circulating immunoreactive oxytocin during the human menstrual cycle comes from the pituitary and is estradiol dependent. The Journal of clinical endocrinology and metabolism. 1989;68(2):455–460. doi: 10.1210/jcem-68-2-455. [DOI] [PubMed] [Google Scholar]
- Silber M, Almkvist O, Larsson B, Stock S, Uvnas-Moberg K. The effect of oral contraceptive pills on levels of oxytocin in plasma and on cognitive functions. Contraception. 1987;36(6):641–650. doi: 10.1016/0010-7824(87)90037-0. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13(1):27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Weindl A, Schrell U, Wetzstein R. Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta histochemica. Supplementband. 1981;24:79–95. [PubMed] [Google Scholar]
- Sogolow SR. An historical review of the use of oxytocin prior to delivery. Obstetrical & gynecological survey. 1966;21(2):155–172. doi: 10.1097/00006254-196604000-00001. [DOI] [PubMed] [Google Scholar]
- Souza RP, de Luca V, Meltzer HY, Lieberman JA, Kennedy JL. Schizophrenia severity and clozapine treatment outcome association with oxytocinergic genes. Int J Neuropsychopharmacol. 2010:1–6. doi: 10.1017/S1461145710000167. [DOI] [PubMed] [Google Scholar]
- Stock S, Bremme K, Uvnas-Moberg K. Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Human reproduction. 1991;6(8):1056–1062. doi: 10.1093/oxfordjournals.humrep.a137484. [DOI] [PubMed] [Google Scholar]
- Stock S, Karlsson R, von Schoultz B. Serum profiles of oxytocin during oral contraceptive treatment. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 1994;8(2):121–126. doi: 10.3109/09513599409058033. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009a;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult Attachment Predicts Maternal Brain and Oxytocin Response to Infant Cues. Neuropsychopharmacology. 2009b doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton JF, Stronge J, Boylan PC. Hyponatraemia and non-electrolyte solutions in labouring primigravida. Eur J Obstet Gynecol Reprod Biol. 1995;59(2):149–151. doi: 10.1016/0028-2243(95)02042-q. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology. 2007;52(3):1034–1043. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophrenia research. 2008;102(1–3):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Teltsh O, Kanyas-Sarner K, Rigbi A, Greenbaum L, Lerer B, Kohn Y. Oxytocin and vasopressin genes are significantly associated with schizophrenia in a large Arab-Israeli pedigree. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–11. [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer M, Cordero MI, Sevelinges Y, Sandi C. Evidence for a Role of Oxytocin Receptors in the Long-Term Establishment of Dominance Hierarchies. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6(4):384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8(1):59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann N Y Acad Sci. 1992;652:29–38. doi: 10.1111/j.1749-6632.1992.tb34343.x. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, et al. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. The American journal of psychiatry. 2011;168(3):276–285. doi: 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109(4):473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Eriksson M. Breastfeeding: physiological, endocrine and behavioural adaptations caused by oxytocin and local neurogenic activity in the nipple and mammary gland. Acta Paediatr. 1996;85(5):525–530. doi: 10.1111/j.1651-2227.1996.tb14078.x. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The Steroid/Peptide Theory of Social Bonds: Integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36(9):1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiology & behavior. 2010;101(2):193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Hamilton MS, Huang T, Kristan WB, French KAA. Hormone-Activated Central Pattern Generator for Courtship. Curr Biol. doi: 10.1016/j.cub.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophrenia research. 1995;16(2):87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24(12):2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizemann TM, Pardue ML, editors. Exploring biological contributions to human health: Does sex matter ? Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48(5):522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
References
- 1.Sarnyai Z, et al. Oxytocin attenuates the cocaine-induced exploratory hyperactivity in mice. Neuroreport. 1990;1(3–4):200–202. doi: 10.1097/00001756-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Drago F, et al. Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav. 1986;24(5):1185–1188. doi: 10.1016/0091-3057(86)90168-1. [DOI] [PubMed] [Google Scholar]
- 3.Drago F, Contarino A, Busa L. The expression of neuropeptide-induced excessive grooming behavior in dopamine D1 and D2 receptor-deficient mice. European journal of pharmacology. 1999;365(2–3):125–131. doi: 10.1016/s0014-2999(98)00877-2. [DOI] [PubMed] [Google Scholar]
- 4.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16(3):e92–e123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121(3):537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 6.Shahrokh DK, et al. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs G, Telegdy G. Effects of oxytocin, des-glycinamide-oxytocin and anti-oxytocin serum on the alpha-MPT-induced disappearance of catecholamines in the rat brain. Brain research. 1983;268(2):307–314. doi: 10.1016/0006-8993(83)90497-3. [DOI] [PubMed] [Google Scholar]
- 8.Qi J, et al. Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(6):441–448. doi: 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- 9.Uvnas-Moberg K, Alster P, Svensson TH. Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology (Berl) 1992;109(4):473–476. doi: 10.1007/BF02247726. [DOI] [PubMed] [Google Scholar]
- 10.Kiss A, et al. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. J Neurosci Res. 2010;88(3):677–685. doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- 11.Souza RP, et al. Variants in the oxytocin gene and risk for schizophrenia. Schizophr Res. 2010;121(1–3):279–280. doi: 10.1016/j.schres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Feifel D, Reza T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology. 1999;141(1):93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell HK, Stephens SL, Young WS., 3rd Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol Psychiatry. 2009;14(2):190–196. doi: 10.1038/sj.mp.4002150. [DOI] [PubMed] [Google Scholar]
- 14.Lee PR, et al. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30(10):1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- 15.Sams-Dodd F. Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Reviews in the neurosciences. 1999;10(1):59–90. doi: 10.1515/revneuro.1999.10.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Allen RM, Young SJ. Phencyclidine-induced psychosis. The American journal of psychiatry. 1978;135(9):1081–1084. doi: 10.1176/ajp.135.9.1081. [DOI] [PubMed] [Google Scholar]
- 17.Lee PR, et al. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AS. The environment and susceptibility to schizophrenia. Progress in neurobiology. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 20.Qi J, et al. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56(5):856–865. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Ninan I. Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. Journal of neurochemistry. 2011 doi: 10.1111/j.1471-4159.2011.07430.x. [DOI] [PubMed] [Google Scholar]
- 22.Meyer-Lindenberg A, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5(3):267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophrenia research. 1995;16(2):87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- 24.Feifel D, Shilling PD, Belcher A. The Effects of Oxytocin and its Analog, Carbetocin, on Genetic Deficits in Sensorimotor Gating. European Neuropsychopharmacology. 2011 doi: 10.1016/j.euroneuro.2011.09.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feifel D, Shilling PD, Melendez G. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behavioral neuroscience. 2011;125(2):268–272. doi: 10.1037/a0022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckmann H, Lang RE, Gattaz WF. Vasopressin--oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10(2):187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- 27.Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiology & behavior. 2010;101(2):193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Legros JJ, et al. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17(6):611–617. doi: 10.1016/0306-4530(92)90019-4. [DOI] [PubMed] [Google Scholar]
- 29.Nussey SS, et al. Responses of plasma oxytocin and arginine vasopressin to nausea induced by apomorphine and ipecacuanha. Clinical endocrinology. 1988;28(3):297–304. doi: 10.1111/j.1365-2265.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 30.Baskerville TA, et al. Dopamine-oxytocin interactions in penile erection. The European journal of neuroscience. 2009;30(11):2151–2164. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- 31.Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neuroscience and biobehavioral reviews. 2011;35(3):939–955. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Mai JK, Berger K, Sofroniew MV. Morphometric evaluation of neurophysin-immunoreactivity in the human brain: pronounced inter-individual variability and evidence for altered staining patterns in schizophrenia. Journal fur Hirnforschung. 1993;34(2):133–154. [PubMed] [Google Scholar]
- 33.Glovinsky D, et al. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11(3):273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 34.Goldman M, et al. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia research. 2008;98(1–3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman MB, et al. Divergent effects of two different doses of intranasal oxytocin on facial affect discrimination in schizophrenic patients with and without polydipsia. Psychopharmacology. 2011;216(1):101–110. doi: 10.1007/s00213-011-2193-8. [DOI] [PubMed] [Google Scholar]
- 36.Goldman MB, et al. Diminished glucocorticoid negative feedback in polydipsic hyponatremic schizophrenic patients. J Clin Endocrinol Metab. 2007;92(2):698–704. doi: 10.1210/jc.2006-1131. [DOI] [PubMed] [Google Scholar]
- 37.Goldman MB, et al. Structural pathology underlying neuroendocrine dysfunction in schizophrenia. Behavioural brain research. 2011;218(1):106–113. doi: 10.1016/j.bbr.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Soc Neurosci. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- 39.Bartz JA, et al. Social effects of oxytocin in humans: context and person matter. Trends in cognitive sciences. 2011 doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual review of clinical psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 41.Averbeck BB, et al. Emotion recognition and oxytocin in patients with schizophrenia. Psychological medicine. 2011:1–8. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LH, et al. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophrenia research. 2010;124(1–3):13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendrek A, Stip E. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert review of neurotherapeutics. 2011;11(7):951–959. doi: 10.1586/ern.11.78. [DOI] [PubMed] [Google Scholar]
- 44.Figueira ML, Ouakinin S. Gender-related endocrinological dysfunction and mental disorders. Current opinion in psychiatry. 2010;23(4):369–372. doi: 10.1097/YCO.0b013e3283399b86. [DOI] [PubMed] [Google Scholar]
- 45.Murakami G, et al. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. The European journal of neuroscience. 2011;34(3):469–477. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- 46.Popeski N, et al. Prolactin and oxytocin interaction in the paraventricular and supraoptic nuclei: effects on oxytocin mRNA and nitric oxide synthase. J Neuroendocrinol. 2003;15(7):687–696. doi: 10.1046/j.1365-2826.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- 47.Patisaul HB, et al. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(8):787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 48.Champagne F, et al. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souza RP, et al. Schizophrenia severity and clozapine treatment outcome association with oxytocinergic genes. Int J Neuropsychopharmacol. 2010:1–6. doi: 10.1017/S1461145710000167. [DOI] [PubMed] [Google Scholar]
- 50.Rubin LH, et al. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophrenia research. 2011 doi: 10.1016/j.schres.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon I, et al. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Domes G, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Bujanow W. Hormones in the treatment of psychoses. Br Med J. 1972;4(5835):298. doi: 10.1136/bmj.4.5835.298-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakharev VD, Tikhomirov SM, TK L. Psychotropic properties of oxytocin. Probl Endokrinol (Mosk) 1984;30(2):37–41. [PubMed] [Google Scholar]
- 56.Feifel D, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 57.Calder A, et al. Facial emotion recognition after bilateral amygdala damage: differentially severe impairment of fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- 58.Pedersen CA, et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia research. 2011 doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]


