Abstract
Target protein - magnetic nanoparticle (MNP) conjugates, i.e. α-glucosidase–MNP and protein tyrosine phosphatase 1B (PTP1B)–MNP, were prepared and evaluated for the first time for affinity extraction of the enzyme inhibitors from herbal extracts. Four ligands extracted from granati pericarpium were identified by ESI-MS analysis. In vitro tests indicated that they inhibited both α-glucosidase and PTP1B, two important target proteins for diabetic treatment.
Screening for ligands binding to therapeutic target proteins is an effective approach to discover drug leads.1–3 High throughput screening (HTS) based on colorimetric or fluorometric measurements on multiwell microplates is the most commonly used method for screening of enzyme inhibitors.4–6 HTS works perfectly with large libraries of synthetic compounds, but not applicable sometimes to assay complex samples such as herbal extracts that may contain many UV-absorbing or fluorescent compounds.7–8 In addition, since herbal medicines are characteristic of being complexes of many active constituents, identification of the therapeutic agents becomes even more challenging.9–10 Studies on biological fingerprinting of herbal medicines by using deliberately devised chromatographic procedures were reported.11–12 Over the past years, search for enzyme inhibitors present in medicinal plants has been one of the major interests in drug discovery and development.13–14 To study these complex sample matrices, methods based on affinity chromatography (LC), mass spectrometry (MS), and capillary electrophoresis (CE) are used.15–17 However, these methods are normally either tedious/time-consuming or require dedicated skills for sample pre-treatments.
Use of nanometer sized materials in biological studies provides new avenues to probe biomolecular interactions.18 Functionalized magnetic nanoparticles (MNPs), in particular, find wide applications not only in areas such as magnetic resonance imaging, drug delivery, and biosensing, but also in biological and chemical separations due to the convenience of magnetic solid-liquid separation.19–21 Based on the theory of ligand – protein interaction, protein-MNP conjugates promise to be very useful for extraction of ligands from complex sample matrices. Antibody conjugated MNPs were used for separation of target biomarkers from human plasma.22 Human serum albumin (HSA) functionalized MNPs (HSA-MNPs) were evaluated for ligand fishing from pharmaceutical formulations.23 Extraction of low levels of manose from serum samples by using Con-A –MNP conjugates was demonstrated.24 We recently developed a facile protocol for isolation and identification of active ingredients from herbal extracts by using HSA – MNP conjugates and electrospray ionization-tandem MS (ESI-MS/MS).25–26 Although HSA, the most abundant protein in blood plasma, plays a major role in transport and deposition of endogenous and exogenous ligands, it’s not a therapeutic target protein.27 Therefore, the ligands identified using HSA-MNP conjugates may have only limited significance in terms of therapeutic drug discovery.
Herein we demonstrate an effective strategy to identify enzyme inhibitors present in complex matrices. It is based on affinity extraction of ligands using target protein – MNP conjugates and ESI-MS analysis. Since the magnetic solid-liquid separation involved in the extraction is very quick and easy to do, the identification process is simple and quick. Two target proteins for anti-diabetic agents, α-glucosidase and protein tyrosine phosphatase 1B (PTP1B) were studied as model proteins in this work. It’s well known that α-glucosidase inhibitors such as acarbose, miglitol, and voglibose delay the absorption of carbohydrates from the small intestine and thus lower the postprandial blood glucose and insulin levels.28–29 PTP1B has been studied as another target for treating diabetes. It was reported that inhibition on PTP1B specifically addressed insulin resistance, likely causing weight loss.30–31 We prepared α-glucosidase – MNP and PTP1B – MNP conjugates. Affinity extraction with the use of these conjugates coupled with ESI-MS analysis were evaluated for screening inhibitors in the extract of granati pericarpium (GP, also named pomegranate peel, pomegranate rind and pomegranate husk.). It’s documented that pomegranate’s health benefits include anticancer,32 antibacterial,33 antidiarrhoeal,34 antifungal 35 and antioxidant.36–37 It was also shown that pomegranate extract inhibited α-glucosidase activity in vitro 38 and administration of 200 mg/Kg of pomegranate extract normalized all the diabetes mellitus changes induced by alloxan in vivo. 39 However, identities of the anti-diabetic agents in pomegranate remain so far unknown. Since herbal medicines as usual are complexes of many active constituents, identification of the therapeutic agents has been difficult. From ligand – protein interaction theory, affinity extraction with target protein – MNPs promises a convenient and selective isolation of these agents from complex matrices. To the best of our knowledge, there has been no report on screening and identification of natural enzyme inhibitors using a target protein-MNP conjugate based affinity extraction and ESI-MS/MS approach.
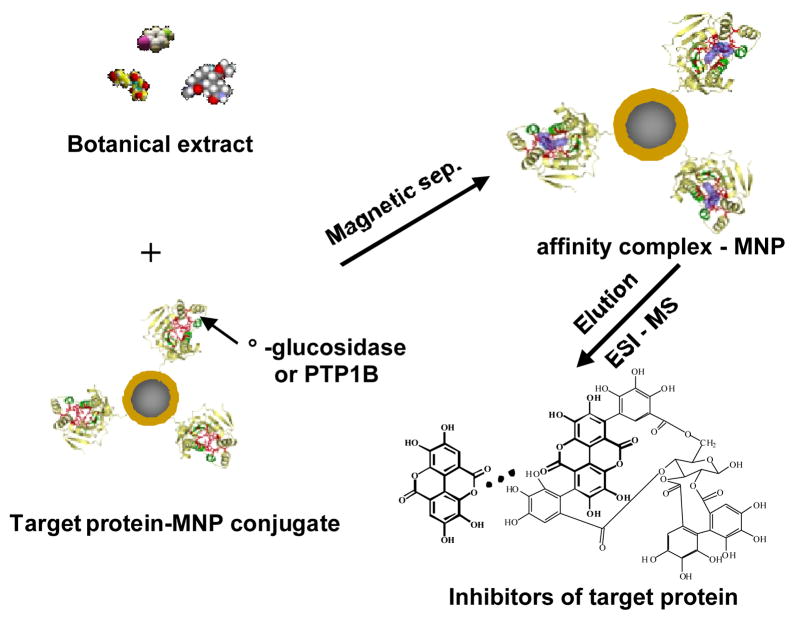
In order to prepare highly stable and active target protein-MNP conjugates, covalent bonding between a protein molecule and a MNP via a coupling agent was investigated. First, a conventional alcohol-based silanization protocol with APTMS was carried out to prepare amine-terminated MNPs. These MNPs were then activated with glutaraldehyde that served as the cross linker and spacer. A protein solution (5 mg/mL) was mixed with the activated MNPs. The solution was incubated for 24 hrs to accomplish protein-MNP conjugation. The coupling yield was estimated to be ~90% by comparing the UV absorption of the solution before and after the coupling reaction. Enzymatic activity of the prepared α-glucosidase – MNP conjugate was assessed by investigating its binding capability towards voglibose, a known inhibitor of α-glucosidase. The experimental procedure is illustrated in Fig. 1. Affinity extraction was carried out by mixing 1 mL voglibose solution (20 μg/mL) with 100 μL α-glucosidase – MNP suspension. After 5 min incubation on a shaker, the MNPs were collected by magnetic separation and washed three times with the ammonium acetate buffer to avoid non-specific adsorption. Ligands bound to α-glucosidase – MNPs were eluted with buffer containing 50% ACN. The eluent was then analyzed by ESI-MS to determine voglibose. It was found that extraction of voglibose with α-glucosidase-MNPs was effective. The extraction recovery of voglibose was estimated to be 82 ± 2.7% from the peak heights in ESI-MS spectra (n = 5). These results indicated the enzyme remained active after conjugation with MNP. Since the magnetic separation was very quick and easy to perform, it took about 10 min to complete the identification procedure. α-Glucosidase – MNPs were stored in a storing buffer at 4 °C. After 2 months in storage, the enzymatic activity of the conjugate remained >90% as assessed from the extraction efficiency.
Fig. 1.
Schematic illustration of target protein-MNP based affinity extraction coupled with ESI-MS/MS for identification of enzyme inhibitors in complex matrices.
Isolation and identification of enzyme inhibitors from GP extract were carried out using both α-glucosidase – MNP and PTP1B – MNP conjugates coupled with ESI-MS/MS analysis.
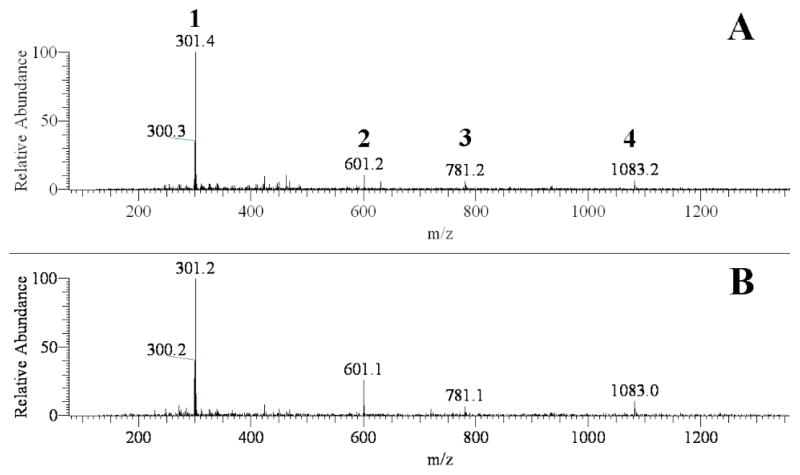
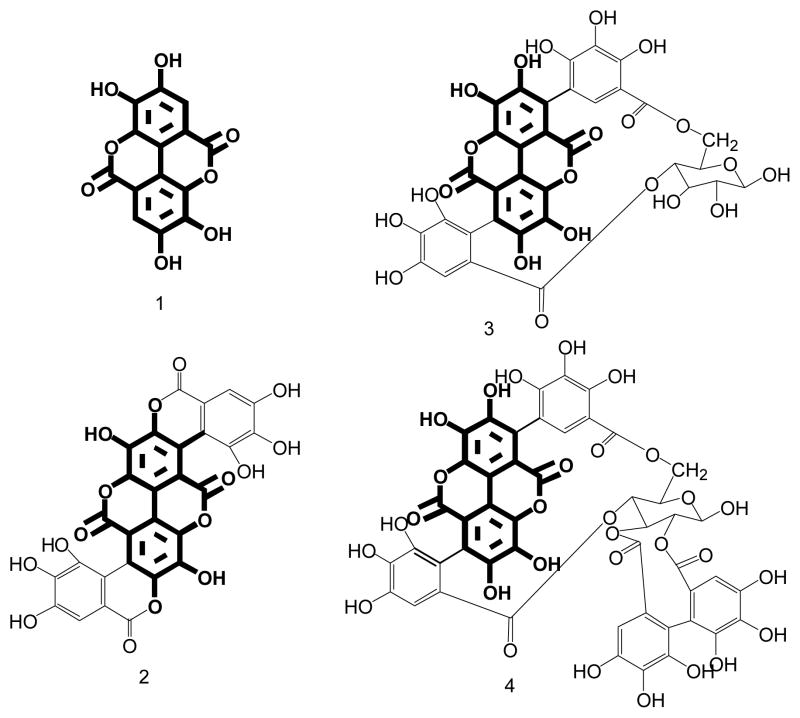
Although anti-diabetic health benefits of pomegranate extract is documented,38–39 identification of the therapeutic agents has been a challenge because many bioactive constituents are present in the extract. However, from the ligand-receptor theory, affinity extraction with the use of target protein–MNP conjugates may offer a convenient and effective approach to tackle this problem. The ESI-MS spectra from analysis of GP extract with α-glucosidase – MNPs and PTP1B – MNPs are shown in Fig. 2A and 2B, respectively. It was noticed that the spectra were very similar for three GP extracts separately prepared on different days. In the extract many ingredients were found to bind to α-glucosidase – MNPs. These compounds were, therefore, isolated from the extract matrix by affinity extraction and detected by ESI-MS, resulting in a complex MS spectrum (Fig. 2A). Among the numerous ions appearing in the spectrum, four ions, i.e. m/z 301, 601, 781, and 1083 matched the molecular masses of [M-H]-for ellagic acid, gallagic acid, punicalin, and punicalagin, respectively (see Fig. 3 for the chemical structures of these compounds). The chemical structures were further verified by MS/MS (the MS/MS spectra are shown in Fig. S2). Affinity extraction-based screening assay of GP extract was also carried out with PTP1B-MNPs. The ESI-MS spectrum obtained is shown in Fig. 2B. To our surprise, the four compounds were again extracted out from the matrix. It can be seen by comparing Fig 2A with 2B that the fewer ligands binding to PTP1B –MNPs compared with those binding to α-glucosidase – MNPs. It’s worth noting that all the four ligands have a common structural unit (marked bold in Fig. 3). These results indicated that the binding cavities of α-glucosidase and PTP1B possess similar structural binding features, which is very informative for design of potential inhibitors for these enzymes. It’s also worth noting that according to the results obtained from this work this group of active ingredients present in GP extract act on both of the target proteins for treating diabetes, i.e. α-glucosidase and PTP1B, which might produce combined anti-diabetic health effects.
Fig. 2.
ESI-MS spectra (in negative ion mode) of 50% ACN eluent from α-glucosidase-MNPs (A) and PTP1B-MNPs (B) after extraction. Peak identifications: 1, ellagic acid; 2, gallagic acid; 3, punicalin; 4, punicalagin.
Fig. 3.
Chemical structures of ellagic acid (compound 1), gallagic acid (compound 2), punicalin (compound 3), and punicalagin (compound 4).
Enzyme inhibitory activity of the ligands identified was investigated using in vitro incubation models. Since ellagic acid was the most abundant ligand binding to both α-glucosidase and PTP1B in GP extract, it was chosen for the test. Although the inhibitory effects of ellagic acid on PTP1B were studied, 40 there has been no report on its effects on α-glucosidase. The maltose-α-glucosidase model was used to study the inhibitory effects of ellagic acid on α-glucosidase, and its effects on PTP1B were investigated using the 4-nitrophenyl phosphate disodium salt (PNPP)-PTP1B model. Voglibose and sodium orthovanadate were used as positive controls for α-glucosidase and PTP1B models, respectively. It was found that ellagic acid at 10 μg/mL showed 28 ± 3.1% inhibition versus 63 ± 1.4% by voglibose at 100 μg/mL on α-glucosidase (Table 1). These results were comparable to those reported previously for derivatives of ellagic acid.41 The inhibitory effect of ellagic acid on PTP1B was also found very strong. At a concentration of 10 μg/mL it showed an inhibition of 66 ± 2.2% which was much stronger than that from sodium orthovanadate at a similar concentration.
Table 1.
In vitro tests on inhibitory activity
| Model | Test Compound | Concentration (μg/mL) | Inhibition (%) |
|---|---|---|---|
| α-glucosidase | Ellagic acid | 10 | 27.83 |
| α-glucosidase | Voglibose[a] | 100 | 62.69 |
| PTP1B | Ellagic acid | 10 | 66.21 |
| PTP1B | Soduim orthovanadate[a] | 100 | 100.52 |
Positive control
In summary, it is herein shown that screening assay based on affinity extraction with the use of therapeutic target protein-MNP conjugates and ESI-MS/MS analysis serves as a rapid and reliable platform for discovering enzyme inhibitors in natural products. Two target protein-MNP conjugates, i.e. α-glucosidase-MNP and PTP1B-MNP, were tested for identify anti-diabetic active compounds in granati pericarpium extract. Four ligands binding to both α-glucosidase-MNP and PTP1B-MNP, were identified as ellagic acid, gallagic acid, punicalin, and punicalagin by ESI-MS/MS. In vitro incubation tests revealed that ellagic acid exhibited strong inhibition not only on α-glucosidase, but also on PTP1B. Since GP extract contains multiple ligands that act on multiple therapeutic target proteins, i.e. α-glucosidase and PTP1B, it might have combined anti-diabetic health effects, which obviously needs further investigation.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (20872137/B020402 to XL), China Postdoctoral Science Foundation (2011M501312 to LSQ) and US National Institutes of Health (GM089557) to YML.
Contributor Information
Yi-Ming Liu, Email: yiming.liu@jsums.edu.
Xun Liao, Email: liaoxun@cib.ac.cn.
References
- 1.Archbold JK, Flanagan JU, Watkins HA, Gingell JJ, Hay DL. Trends Pharmacol Sci. 2011;32:591–600. doi: 10.1016/j.tips.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Hage DS, Anguizola JA, Jackson AJ, Matsuda R, Papastavros E, Pfaunmiller E, Tong Z, Vargas-Badilla J, Yoo MJ, Zheng X. Anal Met. 2011;3:1449–1454. doi: 10.1039/C1AY05068K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble MEM, Endicott JA, Johnson LN. Science. 2004;303:1800–1805. doi: 10.1126/science.1095920. [DOI] [PubMed] [Google Scholar]
- 4.Biardi JE, Nguyen KT, Lander S, Whitley M, Nambiar KP. Toxicon. 2011;57:342–347. doi: 10.1016/j.toxicon.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Ahsen O, Bömer U. Chem Bio Chem. 2005;6:481–490. doi: 10.1002/cbic.200400211. [DOI] [PubMed] [Google Scholar]
- 6.Ma H, Horiuchi KY. Drug Discov Today. 2006;11:661–668. doi: 10.1016/j.drudis.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kool J, Lingeman H, Niessen W, Irth H. Comb Chem High T Scr. 2010;13:548–561. doi: 10.2174/138620710791515815. [DOI] [PubMed] [Google Scholar]
- 8.Goddard J-P, Reymond J-L. Curr Opin Biotech. 2004;15:314–322. doi: 10.1016/j.copbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Xue T, Roy R. Science. 2003;300:740–741. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 10.Qiu J. Nature. 2007;448:126–128. doi: 10.1038/448126a. [DOI] [PubMed] [Google Scholar]
- 11.Lei X, Kong L, Zou H, Ma H. J Chromatogr A. 2009;1216:2179–2184. doi: 10.1016/j.chroma.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 12.Su X, Hu L, Kong L, Lei X, Zou H. J Chromatogr A. 2007;1154:132–137. doi: 10.1016/j.chroma.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 14.Cao H, Yu R, Choi Y, Ma Z-Z, Zhang H, Xiang W, Lee DY-W, Berman BM, Moudgil KD, Fong HHS, van Breemen RB. Pharmacol Res. 2010;61:519–524. doi: 10.1016/j.phrs.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou H, Zhang Q, Guo Z, Guo B, Chen X. Angew Chem Int Edit. 2002;41:646–648. [Google Scholar]
- 16.Greis KD. Mass Spectrom Reviews. 2007;26:324–328. doi: 10.1002/mas.20127. [DOI] [PubMed] [Google Scholar]
- 17.Glatz Z. J Chromatogr B. 2006;841:23–28. doi: 10.1016/j.jchromb.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Ji X, Lin P, Liu Y-M. Anal Biochem. 2011;411:88–93. doi: 10.1016/j.ab.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz E, Willner I. Angew Chem Int Edit. 2004;43:6042–6045. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Bao J, Wang L, Zhang F, Li Y. Chemistry - A Eur J. 2006;12:6341–6347. doi: 10.1002/chem.200501334. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Xu Y, Yao H, Xie L, Yao J, Lu H, Yang P. Chemistry - A Eur J. 2009;15:10158–10166. doi: 10.1002/chem.200901347. [DOI] [PubMed] [Google Scholar]
- 22.Lin P-C, Chou P-H, Chen S-H, Liao H-K, Wang K-Y, Chen Y-J, Lin C-C. Small. 2006;2:485–489. doi: 10.1002/smll.200500387. [DOI] [PubMed] [Google Scholar]
- 23.Moaddel R, Marszałł MP, Bighi F, Yang Q, Duan X, Wainer IW. Anal Chem. 2007;79:5414–5417. doi: 10.1021/ac070268+. [DOI] [PubMed] [Google Scholar]
- 24.Lin P-C, Tseng M-C, Su A-K, Chen Y-J, Lin C-C. Anal Chem. 2007;79:3401–3408. doi: 10.1021/ac070195u. [DOI] [PubMed] [Google Scholar]
- 25.Qing L-S, Xue Y, Zheng Y, Xiong J, Liao X, Ding L-S, Li B-G, Liu Y-M. J Chromatogr A. 2010;1217:4663–4668. doi: 10.1016/j.chroma.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qing L-S, Xue Y, Deng W-L, Liao X, Xu X-M, Li B-G, Liu Y-M. Anal Bioanal Chem. 2011;399:1223–1231. doi: 10.1007/s00216-010-4399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter DC, Ho JX. Adv Protein Chem. 1994;45:153–160. doi: 10.1016/s0065-3233(08)60640-3. [DOI] [PubMed] [Google Scholar]
- 28.van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Diabetes Care. 2005;28:154–162. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 29.Lebovitz HE. Endocrin Metab Clin. 1997;26:539–551. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 30.Johnson TO, Ermolieff J, Jirousek MR. Nat Rev Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- 31.Montalibet J, Kennedy BP. Drug Discov Today. 2005;2:129–135. [Google Scholar]
- 32.Das AK, Mandal SC, Banerjee SK, Sinha S, Das J, Saha B, Pal M. J Ethnopharmacol. 1999;68:205–212. doi: 10.1016/s0378-8741(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 33.César de Souza Vasconcelos L, Sampaio MCC, Sampaio FC, Higino JS. Mycoses. 2003;46:192–196. doi: 10.1046/j.1439-0507.2003.00884.x. [DOI] [PubMed] [Google Scholar]
- 34.Guo S, Deng Q, Xiao J, Xie B, Sun Z. J Agr Food Chem. 2007;55:3134–3139. doi: 10.1021/jf063443g. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni AP, Aradhya SM, Divakar S. Food Chem. 2004;87:551–557. [Google Scholar]
- 36.Pharmacopoeia of the People’s Republic of China. Vol. 1. Chemical Industry Press; Beijing, China: 2010. p. 87. [Google Scholar]
- 37.González-Molina E, Moreno DA, García-Viguera C. Food Chem. 2009;115:1364–1372. [Google Scholar]
- 38.Xie Zhen-jian, Fan Yu, Tang Peng-Cheng, Jiao Shi-rong. Chin J Anhui Agri Sci. 2009;37:2829–2834. [Google Scholar]
- 39.Parmar HS, Kar A. Biofactors. 2007;31:17–24. doi: 10.1002/biof.5520310102. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y, Kang I, Won M, Lee J, Kim J, Lim S. Nat Pro Commun. 2010;5:1927–1933. [PubMed] [Google Scholar]
- 41.Tabopda TK, Ngoupayo J, Liu J, Ali MS, Khan SN, Ngadjui BT, Luu B. Chem Pharm Bull. 2008;56:847–850. doi: 10.1248/cpb.56.847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.