Abstract
Hypothalamic proopiomelanocortin (POMC) neurons are controlled by many central signals, including serotonin. Serotonin increases POMC activity and reduces feeding behavior via serotonion [5-hydroxytryptamine (5-HT)] receptors by modulating K+ currents. A potential K+ current is the M-current, a noninactivating, subthreshold outward K+ current. Previously, we found that M-current activity was highly reduced in fasted vs. fed states in neuropeptide Y neurons. Because POMC neurons also respond to energy states, we hypothesized that fasting may alter the M-current and/or its modulation by serotonergic input to POMC neurons. Using visualized-patch recording in neurons from fed male enhanced green fluorescent protein-POMC transgenic mice, we established that POMC neurons expressed a robust M-current (102.1 ± 6.7 pA) that was antagonized by the selective KCNQ channel blocker XE-991 (40 μM). However, the XE-991-sensitive current in POMC neurons did not differ between fed and fasted states. To determine if serotonin suppresses the M-current via the 5-HT2C receptor, we examined the effects of the 5-HT2A/5-HT2C receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) on the M-current. Indeed, DOI attenuated the M-current by 34.5 ± 6.9% and 42.0 ± 5.3% in POMC neurons from fed and fasted male mice, respectively. In addition, the 5-HT1B/5-HT2C receptor agonist m-chlorophenylpiperazine attenuated the M-current by 42.4 ± 5.4% in POMC neurons from fed male mice. Moreover, the selective 5-HT2C receptor antagonist RS-102221 abrogated the actions of DOI in suppressing the M-current. Collectively, these data suggest that although M-current expression does not differ between fed and fasted states in POMC neurons, serotonin inhibits the M-current via activation of 5-HT2C receptors to increase POMC neuronal excitability and, subsequently, reduce food intake.
Keywords: feeding behavior, KCNQ channels, hypothalamus
the hypothalamic melanocortin [proopiomelanocortin (POMC)] system is critical neural circuitry in the control of numerous homeostatic functions, including reproduction, stress response, and energy homeostasis (3, 5). POMC neurons are a target for numerous central and peripheral signals, including serotonin, to control energy balance (3, 11). In fact, serotonergic neurons project from the dorsal and median raphe nuclei to the arcuate nucleus, where the Gq-coupled serotonin [5-hydroxytryptamine (5-HT)] type 2C (5-HT2C) receptors are highly expressed (7). These receptors are also highly expressed in POMC neurons (10, 26) and are necessary for serotonin's control of POMC function (41). The 5-HT reuptake inhibitor/5-HT releaser d-fenfluramine and agonists of the 5-HT2C receptor activate POMC neurons (10, 11), and 5-HT2C receptor agonists also increase POMC gene expression in the arcuate nucleus (16, 42). Furthermore, 5-HT2C receptors modulate the activity of at least two channels [G protein inwardly rectifying K+ (GIRK) and nonselective cation channels] to control POMC neuronal excitability (26, 36).
Another potential cellular target of 5-HT2C receptors is the M-current, a subthreshold, noninactivating, voltage-dependent K+ current. The K+ channels that underlie the M-current are the KCNQ channel subunits (KCNQ2, KCNQ3, and KCNQ5). The subunits are expressed throughout the central nervous system (CNS), and the M-current is ubiquitous in most neuronal cell types (28). The coexpression of KCNQ2 and KCNQ3 is thought to be the primary heteromultimer that functions as the neuronal M-current. When coexpressed in heterologous cells, KCNQ2 and KCNQ3 produce a robust current with properties similar to those of the native neuronal M-current. KCNQ5 also produces a robust current similar to the native M-current when coexpressed with the KCNQ3 subunit (17, 28).
The M-current is modulated by Gαq protein-coupled receptors (4, 28). Gαq protein-coupled receptors can modulate the M-current through the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by PLC (39), similar to actions of the 5-HT2C receptor on GIRK channel activity in POMC neurons (26, 25). Since 5-HT2C receptors are coupled with Gαq protein and highly expressed in the arcuate nucleus and in POMC neurons and serotonin is known to inhibit the M-current in human cortical neurons (20), we hypothesized that 5-HT2C receptor agonists will inhibit the activity of the M-current in POMC neurons to control neuronal excitability and reduce food intake. Furthermore, we have also shown that fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y (NPY) neurons in the arcuate nucleus (30). Because NPY neuronal activity opposes POMC activity in the control-of-feeding behavior, we hypothesized that the control-of-feeding behavior in POMC neurons may also involve modulation of the M-current in POMC neurons during fasting. Therefore, we used whole cell patch recording and selective pharmacological reagents to examine the effects of fasting and 5-HT2C receptor agonists on the activity of the M-current in POMC neurons.
MATERIALS AND METHODS
Animal care and experimental treatments.
All animal treatments are in accordance with institutional guidelines based on National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at the Oregon Health and Science University. Male enhanced green fluorescent protein (EGFP)-POMC transgenic mice [originally provided by Dr. Malcolm Low (formerly affiliated with the Oregon Health and Science University and currently with the University of Michigan)] were selectively bred in-house and maintained under controlled temperature (25°C) and photoperiod conditions (12:12-h light-dark cycle) with food and water available ad libitum. Adult male mice (10–12 wk old) were fed or fasted for 24 h prior to experimentation. Average weight loss after a 24-h fast was 3.8 ± 0.2 g (n = 15) for male mice. Fasting had no effect on animal activity, fur condition, and response to handling.
Drugs.
The selective KCNQ channel blocker 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride (XE-991) and the 5-HT2C receptor antagonist 8-(5-[2,4-dimethoxy-5)-(4-trifluoromethylphenylsulfonamido)phenyl-5-oxopentyl]1,3,8-triazaspiro[4,5]decane-2,4-dione hydrochloride (RS-102221) were purchased from Tocris (Ellisville, MO) and dissolved in DMSO at a concentration of 40 mM and further dissolved in artificial cerebrospinal fluid (CSF) to a final concentration of <0.1%. The 5-HT2A/5-HT2C receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) and the 5-HT1B/5-HT2C agonist m-chlorophenylpiperazine (mCPP) were purchased from Sigma-Aldrich and dissolved in H2O. The transient receptor potential channel (TRPC) blocker 2-aminoethoxydiphenyl borate (2-APB) was purchased from Sigma-Aldrich and dissolved in DMSO at a concentration of 100 mM and further dissolved in artificial CSF to a final concentration of <0.1%. Tetrodotoxin (TTX) was purchased from Alomone Laboratories (Jerusalem, Israel) and dissolved in H2O. All drugs were bath-perfused until a steady-state response was obtained.
Preparation of basal hypothalamic slices.
Slices were prepared as described previously (24, 25, 30). Transgenic EGFP-POMC mice were killed quickly by decapitation at 1000–1100. The brain was rapidly removed from the skull, and a block containing the basal hypothalamus (BH) was immediately dissected. The BH block was submerged in cold (4°C) oxygenated (95% O2-5% CO2) high-sucrose CSF (in mM: 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 CaCl2, and 10 HEPES, pH 7.3, 300 mOsm). While the slices were bathed in high-sucrose CSF at 4°C for 10 min, coronal slices (250 μm) from the BH were cut on a Vibratome. The slices were then transferred to an auxiliary chamber, in which they were kept at room temperature (25°C) in artificial CSF (in mM: 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 glucose, pH 7.3, 310 mOsm) until recording (recovery for 2 h).
Visualized whole cell patch recording.
A single slice was transferred to the recording chamber mounted on an upright microscope (model BX51W1, Olympus) equipped with a video-enhanced digital camera (Rol-X-FM-12, Q Imaging, Surrey, BC, Canada) with infrared-differential interference contrast and a fluorescent light source (X-Cite 120 series, Exfo, Mississauga, ON, Canada). The slice was continually perfused with warm (35°C) oxygenated artificial CSF at 1.5 ml/min. Targeted neurons were viewed using infrared-differential interference contrast and blue excitation light with an ×40 water-immersion lens (Olympus). Normal artificial CSF and pipette solutions were used in electrophysiological recording (14, 24, 25). Standard whole cell patch recording procedures and pharmacological testing were carried out as previously described (30, 40). Whole cell voltage- and current-clamp recordings were performed using pipettes made of borosilicate glass and pulled using a micropipette puller (P-97 Flaming/Brown, Sutter Instrument, Novato, CA). Pipettes were filled with normal internal solution (in mM: 10 NaCl, 128 K-gluconate, 1 MgCl2, 10 HEPES, 1 ATP, 11 EGTA, and 0.25 GTP, pH 7.3, 300 mosM) with a 3- to 5-MΩ resistance. An Axopatch 200A amplifier, Digidata 1322A data acquisition system, and pCLAMP software (version 9.2, Molecular Devices) were used for data acquisition and analysis. Input resistance, series resistance, and membrane capacitance were monitored throughout the experiments. Only cells with stable series resistance (<30 MΩ, <20% change) and >500-MΩ input resistance were used for analysis. The access resistance was 80% compensated, and the calculated liquid junction potential (∼10 mV) was corrected.
To display reversal potential and rectification characteristics of the ligand-activated currents, current-voltage (I-V) plots were constructed by voltage steps from −50 to −140 mV at 10-mV increments applied at 1-s intervals from a holding potential of −60 mV. From this protocol, the slope conductance was determined from the I-V plot as the ratio of the current (pA) to the voltage (−50 to −60 mV). In voltage clamp, a deactivation protocol was used to measure the deactivation or relaxation current. This protocol was used to measure the M-current elicited during 500-ms voltage steps from −30 to −75 mV after a 300-ms prepulse to −20 mV, which included the membrane potential at which the maximal M-current could be obtained (30, 33). The amplitude of M-current relaxation or deactivation was measured as the difference between the initial (<10 ms) and sustained (>475 ms) current of the current trace in the control condition (TTX only, 0.5 μM, 5 min) minus the difference in the XE-991 condition (20 or 40 μM, +TTX, 10 min). The deactivation protocol was repeated twice for each bath solution and averaged for analysis. Perfusion with 5-HT2C receptor agonists was performed after control periods of 10-min each and, in some experiments, was followed by perfusion of 20 μM XE-991. The selective 5-HT2C receptor antagonist RS-102221 (20 μM) (2) was perfused for 30 min prior to the control protocols to ensure effective blocking of DOI, which was perfused for another 10 min. The effects of DOI (20 μM) with or without XE-991 (40 μM) or 2-APB (100 μM), a blocker of TRPCs, were also studied in current clamp to ascertain the effects on neuronal excitability.
Data analysis.
Statistical comparisons of the I-V plots between different feeding states and 5-HT receptor agonist and antagonist treatments were performed using a one- or two-way ANOVA with the Bonferroni-Dunn multiple comparison tests. Differences were considered statistically significant if the probability of error was <5%. All data are means ± SE.
RESULTS
Activity of the M-current in POMC neurons in fed and fasted male mice.
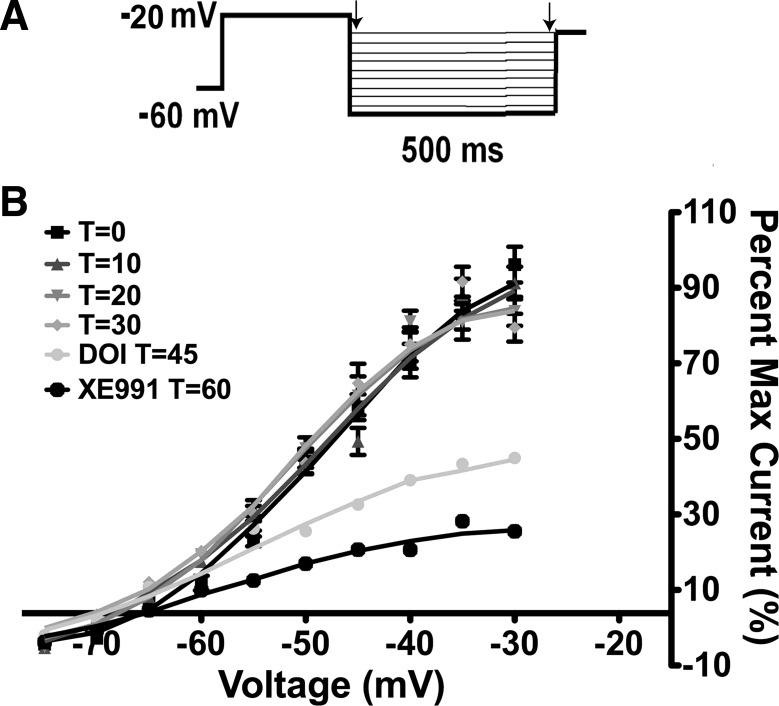
To measure the M-current in voltage clamp, we measured the deactivation of the whole cell K+ currents elicited by an established protocol (30) before and after application of drug treatment in the presence of TTX (0.5 μM). The deactivation protocol examines the voltage range (−30 to −75 mV) where the M-current has its most profound effect on excitability. The K+ current was calculated by subtraction of the current relaxation (difference between instantaneous and steady state; arrows in Fig. 1A). Because the M-current has been shown to decrease, or “run down,” after cell dialysis in whole cell recordings in other CNS neurons (35), the whole cell K+ currents evoked by the deactivation protocol were monitored over 30 min to determine the change in the relaxation currents (Fig. 1B). In POMC neurons from fed male mice, the outward K+ currents evoked by the protocol did not significantly decrease over 30 min, which is well within the experimental time frame. The current evoked by the protocol was also suppressed by perfusion with the 5-HT2A/5-HT2C receptor agonist DOI (20 μM) and the KCNQ channel blocker XE-991 (20 μM) (Fig. 1B). The effects of XE-991 (20 μM) washed out after 45 min (data not shown).
Fig. 1.
The M-current does not run down in proopiomelanocortin (POMC) neurons. A: whole cell patch-clamp recording of deactivation of the M-current in POMC neurons. From a holding potential of −60 mV, a voltage jump to −20 mV (300 ms) was followed by steps from −30 to −75 mV in 5-mV increments (500 ms) (31). B: K+ currents evoked by the deactivation protocol do not run down over the 30-min recording period in 3 cells. T = 0, 3 min after whole cell access. In 1 cell, 20 μM 2,5-dimethoxy-4-iodoamphetamine (DOI) was perfused for 15 min; then 20 μM XE-991 was perfused for 15 min to demonstrate the efficacy of DOI and the activity of the M-current.
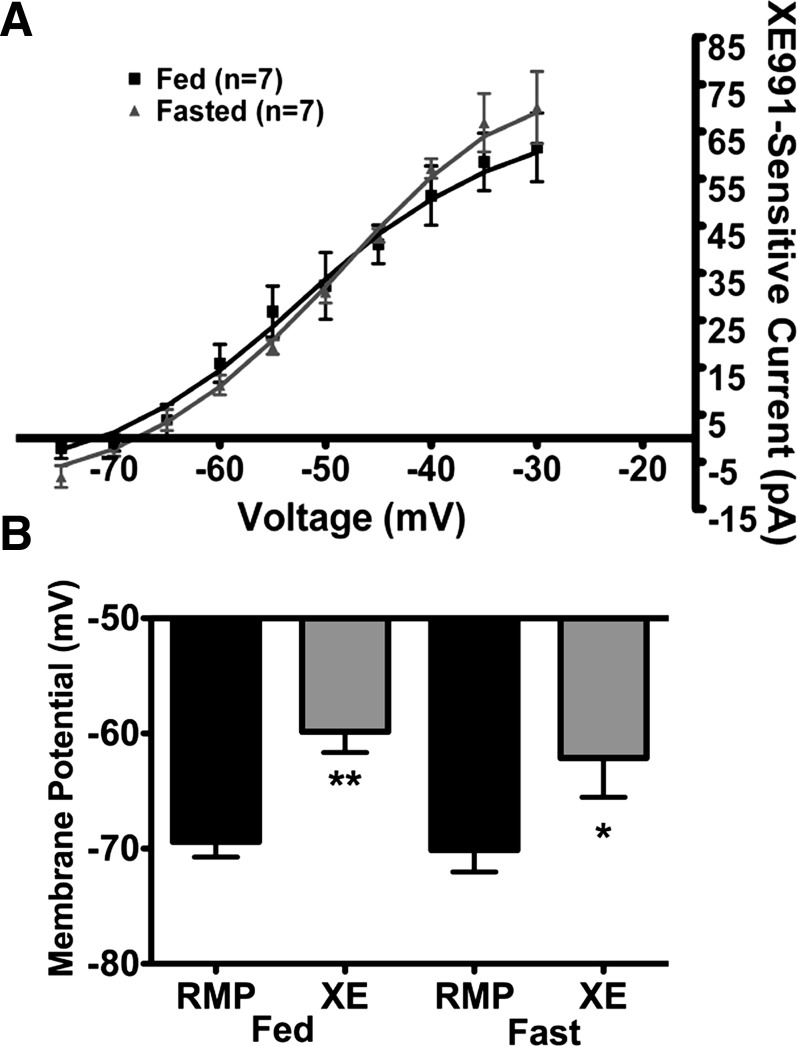
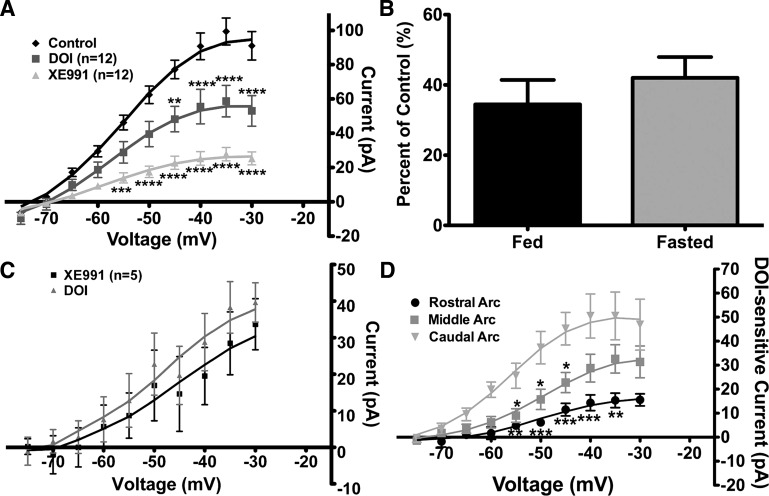
Recently, we showed that the XE-991 (40 μM)-sensitive M-current in NPY neurons differed between fed and fasted male mice (30). Therefore, we repeated these fed vs. fasted experiments in POMC neurons with 40 μM XE-991 for comparison. Interestingly, the M-current activity in POMC neurons was not significantly different between fed and fasted male mice (Fig. 2A). There was also no significant difference in resting membrane potential (−68.4 ± 1.3 and −70.1 ± 1.9 mV in fed and fasted male mice, respectively, n = 7), the depolarizing effects of XE-991 (9.6 ± 1.1 and 8.0 ± 2.0 mV in fed and fasted male mice, respectively; Fig. 2B), or the whole cell capacitance (16.4 ± 1.0 and 19.3 ± 2.2 pF in fed and fasted male mice, respectively).
Fig. 2.
XE-991 (40 μM)-sensitive M-current in POMC neurons is not affected by fasting. A: currents were recorded under control conditions and after 10 min of perfusion of 40 μM XE-991 in the presence of 0.5 μM TTX in fed and 24-h-fasted male mice. B: XE-991 (XE) depolarized POMC neurons significantly in fed and fasted male mice. RMP, resting membrane potential. *P < 0.05; **P < 0.01 (by Student's t-test).
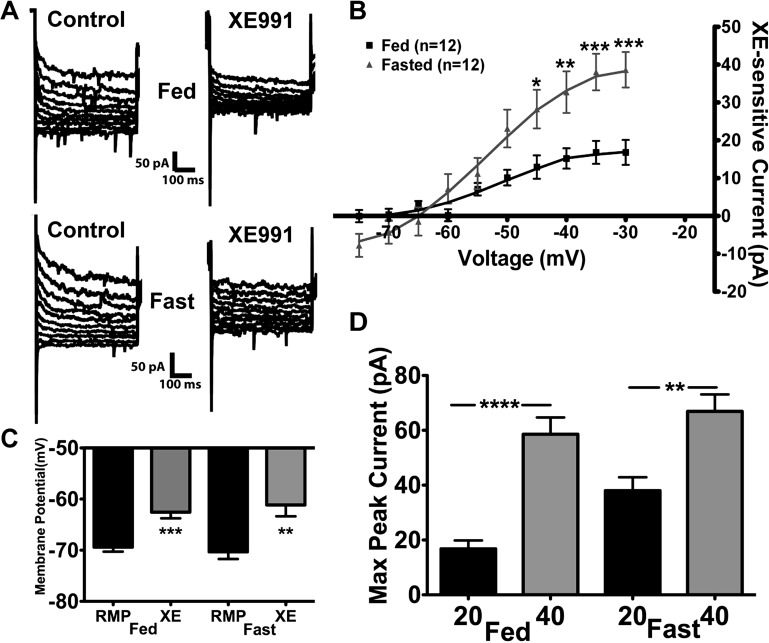
However, the XE-991 (20 μM)-sensitive current was significantly increased in 24-h-fasted (P < 0.05; Fig. 3, A and B) compared with fed male mice. Representative traces of the deactivation protocol illustrate the reduction in the relaxation current by 20 μM XE-991 in POMC neurons from fed and fasted male mice (Fig. 3A). The maximum XE-991-sensitive current measured at −35 mV was 16.8 ± 3.0 and 38.0 ± 4.8 pA in the fed and fasted male mice, respectively (n = 12 in each group; Fig. 3B). Moreover, XE-991 (20 μM) significantly depolarized POMC neurons in the fed and fasted states by 6.8 ± 1.0 and 9.2 ± 1.4 mV, respectively (Fig. 3C). It is interesting that the maximal current at −35 mV blocked by XE-991 (i.e., XE-991-sensitive current) was significantly higher at 40 than 20 μM in POMC neurons from fed and fasted male mice (Fig. 3D). The average difference at the maximum peak current between the XE-991 treatments (40 vs. 20 μM) was significantly greater in the fed than the fasted male mice (46.7 ± 4.0 vs. 28.9 ± 4.2 pA, P < 0.05), which may indicate a difference in KCNQ subunit expression between the two states (see discussion).
Fig. 3.
Lower concentrations of XE-991 illustrate a potential difference in KCNQ subunit composition in POMC neurons between fed and fasted male mice. A: representative traces of deactivation protocol in POMC neurons from fed and fasted male mice before and after perfusion of 20 μM XE-991. B: as in Fig. 2A, currents were recorded under control conditions and after 10 min of perfusion of 20 μM XE-991 in fed and 24-h-fasted male mice. Current-voltage plots were analyzed by 2-way ANOVA (P < 0.05, F = 4.5, df = 1) followed by Bonferroni-Dunn multiple comparison tests: *P < 0.05; **P < 0.01; ***P < 0.001 vs. fed. C: 20 μM XE-991 depolarized POMC neurons significantly in fed and fasted male mice. **P < 0.01; ***P < 0.001 (by Student's t-test). D: maximum XE-991-sensitive peak current at −35 mV in fed and fasted male mice perfused with 20 or 40 μM XE-991. Data were analyzed by 1-way ANOVA (P < 0.0001, F = 21.1, df = 3) followed by Bonferroni-Dunn multiple comparison tests: **P < 0.01; ****P < 0.0001.
Agonists of 5-HT2C receptors inhibit the M-current in POMC neurons.
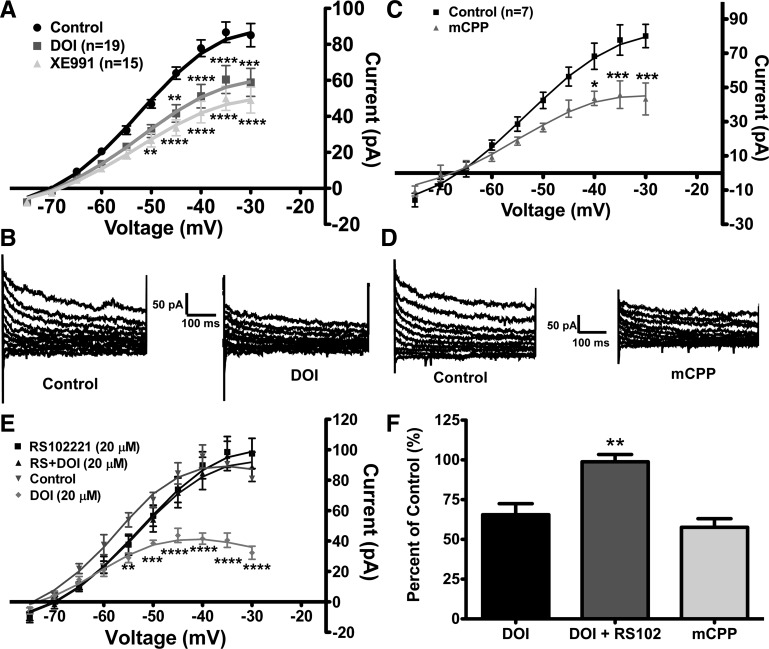
Initially, we used the 5-HT2A/5-HT2C receptor agonist DOI (20 μM) (1) to determine if serotonin modulates the M-current in POMC neurons. Deactivation protocols were run after the control and drug treatment periods to determine the change in the activity of the M-current. In a subsample of POMC neurons, DOI was followed by XE-991 (20 μM, 10 min) to ascertain how much of the M-current was attenuated by activation of the 5-HT2A/5-HT2C receptor by DOI. In 19 of 22 POMC neurons from fed male mice, DOI suppressed the peak M-current by 34.5 ± 6.9% or 26.3 ± 5.3 pA (n = 19, measured at −35 mV; Fig. 4, A and B). Next, XE-991 was perfused following DOI to determine how much of the M-current was suppressed by DOI, and, on the average, XE-991 suppressed the maximal M-current activity (at −35 mV) by an additional 19.1 ± 3.3%, for a total of 44.3 ± 6.4 pA (n = 15). Therefore, DOI may not suppress the entire M-current activity in POMC neurons from fed male mice. DOI also significantly depolarized POMC neurons by 7.5 ± 1.0 mV (P < 0.01), decreased the slope conductance from 2.6 ± 0.2 to 1.8 ± 0.2 nS (P < 0.01), and induced an inward current of 9.0 ± 1.5 pA (Fig. 4A).
Fig. 4.
Agonists of serotonin [5-hydroxytryptamine (5-HT)] type 2C (5-HT2C) receptors suppress activity of the M-current in POMC neurons from fed male mice. A: DOI suppressed activity of the M-current in 19 of 22 POMC neurons from fed male mice. Currents were recorded in control conditions and after 10 min of perfusion with 20 μM DOI. A subsample of DOI-inhibited cells was perfused for 10 min with 20 μM XE-991. Current-voltage plots were analyzed by 2-way ANOVA (P < 0.001, F = 9.8, df = 2) followed by Bonferroni-Dunn multiple comparison tests: **P < 0.01; ***P < 0.001; ****P < 0.0001 vs. control. B: representative traces of deactivation protocol in POMC neurons from fed male mice before and after perfusion with 20 μM DOI. C: perfusion for 10 min with another 5-HT2C receptor agonist, m-chlorophenylpiperazine (mCPP, 20 μM), suppressed the M-current in 7 of 8 POMC neurons from fed male mice. Plots were analyzed by 2-way ANOVA (P < 0.05, F = 6.9, df = 1) followed by Bonferroni-Dunn multiple comparison tests: *P < 0.05; ***P < 0.001. D: representative traces of deactivation protocol in POMC neurons from fed male mice before and after perfusion with 20 μM mCPP. E: to determine if the 5-HT2C receptor is mediating the effects of DOI, slices were incubated for 30 min with a selective antagonist to the 5-HT2C receptor, RS-102221 (RS, 20 μM), prior to 10 min of coperfusion with 20 μM DOI. In the presence of RS-102221, DOI did not suppress the M-current in POMC neurons (n = 8 cells). Data (control vs. DOI) were analyzed by 2-way ANOVA (P < 0.01, F = 31.7, df = 1) followed by Bonferroni-Dunn multiple comparison tests: **P < 0.01; ***P < 0.001; ****P < 0.0001 vs. control. F: change in maximum peak current (−35 mV) after perfusion of DOI (n = 19 cells), DOI + RS-102221 (RS102, n = 8 cells), or mCPP (n = 7 cells) in fed male mice. Data were analyzed by 1-way ANOVA (P < 0.01, F = 6.6, df = 2) followed by Bonferroni-Dunn multiple comparison tests: **P < 0.01 vs. DOI and mCPP.
To determine which serotonin receptor subtype is involved in the suppression of the M-current in POMC neurons, the selective 5-HT1B/5-HT2C receptor agonist mCPP (20 μM) (1) was perfused. In seven of eight cells, mCPP suppressed the M-current activity at −35 mV by 42.4 ± 5.4% in POMC neurons from fed male mice (Fig. 4, C and D). The mCPP-sensitive current was 32.0 ± 3.9 pA at −35 mV (P < 0.001). Similar to DOI, mCPP depolarized POMC neurons by 8.1 ± 2.3 mV, decreased the slope conductance from 3.3 ± 0.2 to 2.1 ± 0.3 nS (P < 0.01), and induced an inward current of 7.6 ± 3.3 pA. Because DOI and mCPP have agonist activity for 5-HT2C receptors in common, these data suggest that 5-HT2C receptor activation is involved in the serotonergic inhibition of the M-current in POMC neurons.
To confirm the above supposition, the selective 5-HT2C receptor antagonist RS-102221 (20 μM) was perfused for 30 min prior to whole cell access and continuously perfused during the control and DOI treatment periods. In the presence of the antagonist, DOI did not suppress the M-current activity in any of the POMC neurons (n = 8; Fig. 4E). As a positive control, three POMC neurons from different slices from the same animals were not treated with RS-102221, and DOI significantly suppressed the M-current in these neurons (ANOVA: P < 0.01, df = 1, F = 31.7). Compared with DOI alone, the 5-HT2C receptor antagonist clearly blocked the effects of DOI in suppressing M-current activity in fed male mice (Fig. 4F).
Because serotonin reduces food intake via POMC neuronal activity in fed animals, the effects of DOI on the M-current were examined in POMC neurons from 24-h-fasted male mice for comparison with the fed state. Indeed, in the fasted male mice, the DOI-sensitive current at −35 mV was 40.7 ± 7.1 pA (Fig. 5A; n = 12, P < 0.0001 vs. control), and DOI perfusion suppressed the activity of the M-current by a greater, although nonsignificant, amount compared with the fed male mice (42.0 ± 6.0% vs. 34.5 ± 6.9%; Fig. 5B). DOI also significantly depolarized POMC neurons by 7.1 ± 1.4 mV (P < 0.01) and decreased the slope conductance from 2.6 ± 0.3 to 1.7 ± 0.1 nS (P < 0.05). Furthermore, DOI induced an inward current in POMC neurons in fed and fasted male mice (11.4 ± 2.1 and 10.7 ± 3.2 pA, respectively).
Fig. 5.
DOI suppresses the M-current in fasted male mice similar to fed male mice, and expression of DOI-sensitive M-current is regionally dependent in arcuate POMC neurons. A: in fasted male mice, 20 μM DOI suppressed M-current activity. After DOI treatment, 20 μM XE-991 was perfused for 10 min. Current-voltage plots were analyzed by 2-way ANOVA (P < 0.0001, F = 17.5, df = 2) followed by Bonferroni-Dunn multiple comparison tests: **P < 0.01; ***P < 0.001; ****P < 0.0001 vs. control. B: percentage of control current suppressed by DOI at −35 mV in POMC neurons from fed and fasted male mice. C: DOI did not significantly suppress more M-current after XE-991 perfusion in POMC neurons from fed male mice. D: amount of M-current suppressed by DOI (DOI-sensitive current) is dependent on the region of the arcuate (Arc) nucleus. Suppression of the M-current was more robust in the caudal (n = 7 cells) and middle (n = 16 cells) regions than in the rostral region (n = 8 cells). Current-voltage plots were analyzed by 2-way ANOVA comparing arcuate regions (P < 0.01, F = 7.3, df = 2) followed by Bonferroni-Dunn multiple comparison tests: *P < 0.05; **P < 0.01; ***P < 0.001 vs. caudal.
In the fasted male mice, XE-991 perfusion after DOI suppressed significantly more M-current activity (71.3 ± 4.0% compared with control conditions), and the mean difference was −71.6 ± 10.1 pA at −35 mV (P < 0.0001). As in the fed male mice, XE-991 (20 μM) inhibited more of the M-current than DOI; however, the XE-991-sensitive current was significantly different (P < 0.01) from the DOI-sensitive current in the fasted, but not the fed, male mice (Fig. 5A vs. Fig. 4A). These data indicate that DOI does not inhibit all the M-current activity in POMC neurons, regardless of feeding state. If 5-HT2C receptor signaling inhibits a subset of KCNQ channels, then XE-991 should occlude any further actions of DOI. Therefore, POMC neurons (n = 5) were perfused with XE-991 prior to DOI (Fig. 5C), and as predicted, XE-991 occluded any further actions of DOI on the M-current. Also, after XE-991 (40 μM) blockade of the M-current, DOI (20 μM) depolarized POMC neurons by a small amount (3.2 ± 1.0 mV, n = 6, P > 0.05), which may reflect its effects on other channels (see discussion).
Sohn et al. (36) recently reported a regional response in arcuate POMC neurons to the 5-HT1B/5-HT2C receptor agonist mCPP. To determine if the suppression of the M-current in POMC neurons by DOI was regionally dependent, the neurons from fed and fasted male mice were sorted into three regions, the rostral arcuate nucleus (Figs. 40–43 in Ref. 22), the middle arcuate nucleus (Figs. 44–48 in Ref. 22), and the caudal arcuate nucleus (Figs. 49–52 in Ref. 22). Interestingly, the suppression of the M-current was significantly more robust in the caudal than the rostral region of the arcuate nucleus (Fig. 5D; P < 0.01). The suppression of the M-current in the middle region was only significantly different in the −40- to −50-mV range compared with the caudal region. At −35 mV, the average DOI-sensitive current was 15.3 ± 3.0 pA in the rostral region (n = 8), 32.6 ± 5.9 pA in the middle region (n = 16), and 49.8 ± 10.6 pA (n = 7) in the caudal region (P < 0.001 vs. rostral). Another difference was found in a very small subset (n = 3) of POMC neurons in the middle region, where DOI augmented the M-current activity at −35 mV by +57.3 ± 5.1% (data not shown). Although there was not a significant difference in the change in slope conductance after DOI between the arcuate regions, the input resistance was significantly different (P < 0.05) between the rostral and caudal regions: 1.6 ± 1.1, 1.1 ± 0.1, and 0.8 ± 0.1 GΩ in rostral, middle, and caudal regions, respectively.
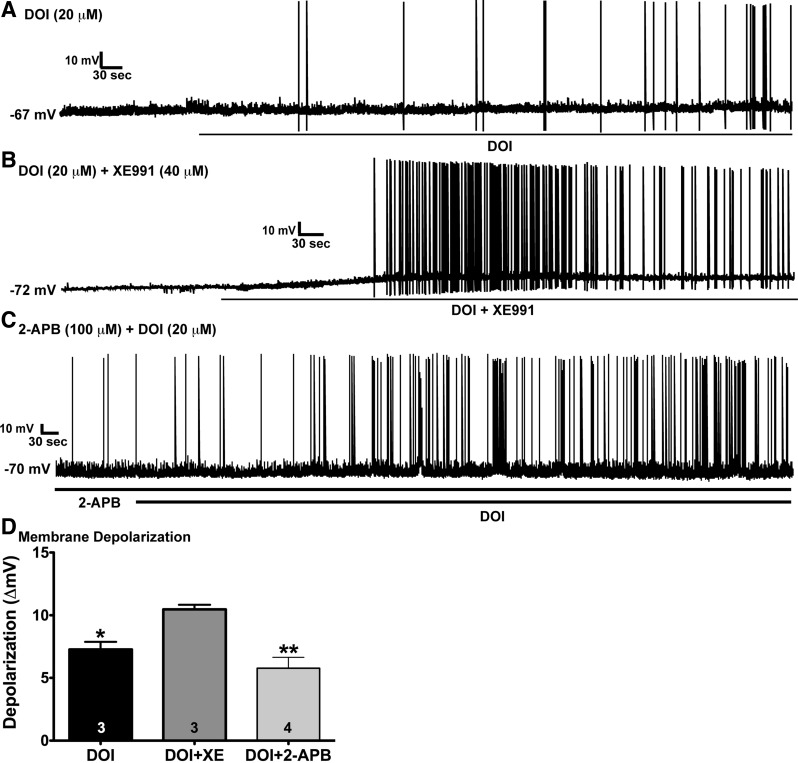
As predicted from our voltage-clamp data, DOI (20 μM) significantly depolarized POMC neurons by 7.3 ± 0.6 mV (n = 3; Fig. 6A) and induced firing. The depolarization in the current-clamp experiments was similar to the DOI-induced depolarization in the voltage-clamp experiments (7.5 ± 1.0 mV). The addition of XE-991 (40 μM), which fully inhibited the M-current, caused further depolarization (total = 10.5 ± 0.4 mV, n = 3) and robustly increased firing (Fig. 6B). However, in the presence of 2-APB (100 μM), which in our hands fully antagonizes transient receptor potential channel (TRPC) 1, TRPC4, and TRPC5 activity in POMC neurons (27), DOI still depolarized POMC neurons by 5.8 ± 0.9 mV (n = 4) and increased firing (Fig. 6C). DOI + XE-991 perfusion significantly depolarized the membrane potential compared with DOI alone and DOI + 2-APB (Fig. 6D). Therefore, besides hydrolyzing PIP2 and uncoupling GIRK channels from Gi,o protein-coupled (μ-opioid and GABAB) receptors, a major effect of 5-HT2C receptor activation is to inhibit the standing M-current in POMC neurons.
Fig. 6.
Effects of XE-991 and the transient receptor potential channel (TRPC) blocker 2-aminoethoxydiphenyl borate (2-APB) on DOI-induced depolarization and firing in POMC neurons. A: in current clamp, perfusion with 20 μM DOI significantly depolarized POMC neurons from fed male mice after 10 min by 7.3 ± 0.6 mV (n = 3) and induced firing. B: coperfusion of DOI and 40 μM XE-991, which fully inhibited the M-current, caused a depolarization of 10.5 ± 0.4 mV (n = 3) and robustly increased firing. C: DOI also depolarized POMC neurons (n = 4) in the presence of 100 μM 2-APB by 5.8 ± 0.9 mV and increased firing. D: DOI + XE-991 perfusion significantly depolarized membrane potential compared with DOI alone and DOI + 2-APB. Data were analyzed by 1-way ANOVA (P < 0.01, F = 11.2, df = 2) followed by Bonferroni-Dunn multiple comparison tests: *P < 0.05; **P < 0.01.
DISCUSSION
For the first time, we show that 5-HT2C receptor agonists inhibit the M-current in POMC neurons in fed and fasted states. In contrast to NPY neurons, in which the M-current (and KCNQ2 and KCNQ3 mRNA subunits) is downregulated in fasted states (30), in POMC neurons there was no change in M-current activity or the efficacy of the 5-HT2C receptor to inhibit the activity with fasting. Although fasting may have less of an effect on M-current activity, the strong serotonergic (5-HT2C) receptor-mediated inhibition of the M-current in POMC neurons plays a critical role in governing POMC neuronal excitability in fed and fasted states, and modulation of the M-current in arcuate neurons may be a critical target for the control of energy homeostasis.
Many neurotransmitters modulate the M-current to control neuronal excitability, including acetylcholine via its Gαq protein-coupled M1/M3/M5 receptors (6, 9) and histamine via H1 receptors (8). Numerous peptidergic neurotransmitters also target the M-current to control neuronal activity in the CNS. Gonadotropin-releasing hormone, substance P, angiotensin II, and bradykinin modulate the M-current via their cognate Gαq protein-coupled receptors (15, 18, 23, 34). Endogenous cannabinoids also suppress the M-current through the Gαq protein-coupled CB1 receptor (32). Although McCormick and Williamson (20) showed that serotonin could inhibit the M-current in cortical neurons, they did not ascertain the receptor subtype mediating this inhibition. Therefore, this is the first study to show that the Gαq protein-coupled 5-HT2C receptor can inhibit the M-current in CNS (POMC) neurons.
The common signaling pathway activated by these Gαq protein-coupled receptors to impinge on the M-current activity is initiated by the activation of PLC and the subsequent hydrolysis of PIP2. The hydrolysis of PIP2 alone can modulate the M-current activity, as reported for M1 receptors (39). Other neurotransmitters and hormones activate pathways involving products of PIP2 hydrolysis, diacylglycerol and inositol 1,4,5-trisphosphate, which in turn activate PKC and Ca2+-mediated signaling pathways, respectively (4). PKC modulates the M-current by phosphorylation of the KCNQ subunits through its association with an A-kinase anchoring protein (AKAP150/79) (13). Interestingly, serotonin, via the 5-HT2C receptor, activates a PLC-PKC pathway in POMC neurons (26), which generates two potential mechanisms for the suppression of KCNQ channel activity, PIP2 depletion and phosphorylation by PKC.
The 5-HT2C receptors modulate at least two other conductances to control POMC neuronal excitability. 5-HT2C receptor agonists attenuate the GABAB activation of GIRK channels by decreasing the concentration of PIP2 associated with the channels in the plasma membrane (26). This effect greatly diminishes any inhibitory synaptic input that is coupled to GIRK channels. Recently, the 5-HT1B/5-HT2C receptor agonist mCPP was shown to depolarize (∼2 mV) a small subpopulation (25%) of POMC neurons in the arcuate nucleus via an inward cation current that is antagonized by TRPC blockers (36). However, we found that DOI and mCPP induced a more robust depolarization (∼7 mV) and decreased the slope conductance as a result of the inhibition of the M-current in a majority of POMC neurons. Most importantly, by employing two 5-HT receptor agonists that overlap in their respective selectivity (DOI for 5-HT2A/5-HT2C and mCPP for 5-HT1B/5-HT2C) and a selective 5-HT2C receptor antagonist, RS-102221 (1), we are confident that the 5-HT2C receptor mediates the serotonin effects on the M-current. Moreover, in the presence of a TRPC blocker, DOI was effective in depolarizing POMC neurons and inducing firing. Therefore, 5-HT2C receptor activation has multiple effects on POMC neurons via inhibition of KCNQ channels (present results), via uncoupling of GIRK channels (26), and in a minority of neurons activating TRPCs (36).
Interestingly, the 5-HT2C receptor agonist DOI suppressed more of the M-current in POMC neurons from the caudal than the more rostral regions of the arcuate nucleus. The regionally dependent differences in M-current sensitivity may be due to diversity in POMC neuronal subpopulations similar to the coexpression of neurotransmitters and peptides (GABA, glutamate, acetylcholine, and dynorphin) (12, 19, 21) and response to peripheral (insulin and leptin) (38) and central (serotonin) signals (36). In addition, POMC neuronal diversity may also include expression of KCNQ channel subunits in composition and gene expression. Therefore, POMC neuronal diversity may be a result of the numerous homeostatic functions mediated by these critical neurons throughout the CNS, including reproduction, stress response, natural reward, and energy homeostasis.
Recently, we showed that fasting reduced the activity of the M current in NPY neurons due, in part, to a reduction in the expression of KCNQ2 and KCNQ3 subunits. NPY neurons are orexigenic and act in opposition to POMC neurons in the control of feeding behavior; thus we hypothesized that fasting would also alter the expression of the M-current in POMC neurons. Interestingly, the effects of fasting in POMC neurons on the M-current were dependent on the concentration of the selective KCNQ channel blocker XE-991. Specifically, using 20 μM XE-991, we found that the XE-991-sensitive current was significantly greater in the fasted male mice (Fig. 3). On the basis of an IC50 of 0.6 μM (37), 20 μM XE-991 should block KCNQ2 and KCNQ3 channels. Indeed, on the basis of our previous single-cell analysis in the guinea pig (29), KCNQ2 and KCNQ3 channel subunits are the predominant transcripts in POMC neurons. In slice preparations, 40 μM XE-991 should sufficiently block all KCNQ2/3 homomultimers and heteromultimers, as well as KCNQ5 hetero/homomultimers, which have lower sensitivity to XE-991 (IC50 = 65 μM for KCNQ5 homomultimers) (31). The difference in the amount of current blocked between the two concentrations suggests an alteration in the KCNQ channel subunit composition between the fed and the fasted state (perhaps more KCNQ5 in the fed state), although we cannot determine differences in subunit composition with any of the available pharmacological tools.
In conclusion, our data provide a new cellular target (KCNQ channels) for serotonin to activate POMC neurons and reduce feeding. By inhibiting the standing M-current, serotonin will depolarize the anorectic POMC neurons and induce firing. Moreover, by uncoupling inhibitory synaptic inputs linked to GIRK channels, serotonin will generate robust action potential firing and, subsequently, increase the secretion of POMC peptides involved in suppressing feeding behavior. The inhibition of the M-current by serotonin via the 5-HT2C receptor would increase POMC neuronal activity and activate the melanocortin neuron circuitry to reduce feeding behavior. The regional differences in M-current suppression by DOI suggest subtle changes in serotonergic inputs across the arcuate nucleus in POMC neurons. Along with our recent data on the regulation of the M-current by fasting and 17β-estradiol in NPY neurons (30), this new study further positions the M-current as a critical component in control of NPY and POMC neuronal activity by central and peripheral signals to control energy homeostasis.
GRANTS
This research was supported by National Institutes of Health Grants DK-68098 (M. J. Kelly and O. K. Rønnekleiv), NS-43330 (O. K. Rønnekleiv), NS-38809 (M. J. Kelly), and K99 DK-83457 (T. A. Roepke).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.A.R., O.K.R., and M.J.K. are responsible for conception and design of the research; T.A.R. and A.W.S. performed the experiments; T.A.R. and A.W.S. analyzed the data; T.A.R. and M.J.K. interpreted the results of the experiments; T.A.R. prepared the figures; T.A.R. drafted the manuscript; T.A.R., O.K.R., and M.J.K. edited and revised the manuscript; T.A.R., O.K.R., and M.J.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Ali Yasrebi for excellent technical assistance and Dr. Jian Qiu for helpful comments during these studies.
Present address of T. A. Roepke: School of Environmental and Biological Sciences, Department of Animal Sciences, Rutgers University, 84 Lipman Dr., New Brunswick, NJ 08901.
REFERENCES
- 1. Bickerdike MJ. 5-HT2C receptor agonists as potential drugs for the treatment of obesity. Curr Top Med Chem 3: 885–897, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective, 5-HT2c receptor antagonist. Neuropharmacology 36: 621–629, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221–232, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Fukuda K, Higashida H, Kubo T, Maeda A, Akiba I, Bujo H, Mishina M, Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature 355: 355–358, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Mol Brain Res 63: 325–339, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Guo J, Schofield GG. Activation of a PTX-insensitive G protein is involved in histamine-induced recombinant M-channel modulation. J Physiol 545: 767–781, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo J, Schofield GG. Activation of muscarinic M5 receptors inhibits recombinant KCNQ2/KCNQ3 K+ channles expressed in HEK293T cells. Eur J Pharmacol 462: 25–32, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science 297: 609–611, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 51: 239–249, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci 29: 13684–13690, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell 37: 541–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose-responsive and express K-ATP channels. Endocrinology 144: 1331–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Jones S, Brown DA, Milligan G, Willer E, Buckley NJ, Caulfield MP. Bradykinin excites rat sympathetic neurons by inhibition of M-current through a mechanism involving B2 receptors and Gαq/11. Neuron 14: 399–405, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Lam DD, Przydzial MJ, Ridley SH, Yeo GSH, Rochford JJ, O′Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology 149: 1323–1328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci 9: 305–310, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Lopez HS, Adams PR. A G protein mediates the inhibition of the voltage-dependent potassium M-current by muscarine, LHRH, substance P and UTP in bullfrog sympathetic neurons. Eur J Neurosci 1: 529–542, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Maolood N, Meister B. Dynorphin in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus. Neuroscience 154: 1121–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 20. McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci USA 86: 8098–8102, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meister B, Gomuc B, Suarez E, Ishii Y, Durr K, Gillberg L. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur J Neurosci 24: 2731–2740, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic, 2001 [Google Scholar]
- 23. Pfaffinger PJ, Leibowitz MD, Subers EM, Nathanson NM, Almers W, Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron 1: 477–484, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23: 9529–9540, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30: 1560–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5-HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharmacol 72: 885–896, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Qui J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30: 1560–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 148: 4937–4951, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Roepke TA, Qui J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci 31: 11825–11835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 275: 24089–24095, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Schweitzer P. Cannabinoids decrease the K+ M-current in hippocampal CA1 neurons. J Neurosci 20: 51–58, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schweitzer P, Madamba S, Siggins GR. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature 346: 464–467, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Shapiro MS, Wollmuth LP, Hille B. Angiotensin II inhibits calcium and M-current channels in rat sympathetic neurons via G proteins. Neuron 12: 1319–1329, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci 25: 7449–7458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71: 488–497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang HS, Pan ZM, Shi WM, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282: 1890–1893, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30: 2472–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci 25: 3400–3413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ. GnRH activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 149: 2459–2466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60: 582–589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 6: 398–405, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]