Abstract
Associations between exponential childhood growth superimposed on low birth weight and adult onset cardiovascular disease with glucose intolerance/type 2 diabetes mellitus exist in epidemiological investigations. To determine the metabolic adaptations that guard against myocardial failure on subsequent exposure to hypoxia, we compared with controls (CON), the effect of intrauterine (IUGR), postnatal (PNGR), and intrauterine and postnatal (IPGR) calorie and growth restriction (n = 6/group) on myocardial macronutrient transporter (fatty acid and glucose) -mediated uptake in pregestational young female adult rat offspring. A higher myocardial FAT/CD36 protein expression in IUGR, PNGR, and IPGR, with higher FATP1 in IUGR, FATP6 in PNGR, FABP-c in PNGR and IPGR, and no change in GLUT4 of all groups was observed. These adaptive macronutrient transporter protein changes were associated with no change in myocardial [3H]bromopalmitate accumulation but a diminution in 2-deoxy-[14C]glucose uptake. Examination of the sarcolemmal subfraction revealed higher basal concentrations of FAT/CD36 in PNGR and FATP1 and GLUT4 in IUGR, PNGR, and IPGR vs. CON. Exogenous insulin uniformly further enhanced sarcolemmal association of these macronutrient transporter proteins above that of basal, with the exception of insulin resistance of FATP1 and GLUT4 in IUGR and FAT/CD36 in PNGR. The basal sarcolemmal macronutrient transporter adaptations proved protective against subsequent chronic hypoxic exposure (7 days) only in IUGR and PNGR, with notable deterioration in IPGR and CON of the echocardiographic ejection fraction. We conclude that the IUGR and PNGR pregestational adult female offspring displayed a resistance to insulin-induced translocation of FATP1, GLUT4, or FAT/CD36 to the myocardial sarcolemma due to preexistent higher basal concentrations. This basal adaptation of myocardial macronutrient transporters ensured adequate fatty acid uptake, thereby proving protective against chronic hypoxia-induced myocardial compromise.
Keywords: myocardium, metabolism, substrate, glucose transporter, fatty acid transport protein
epidemiological investigations have established a link between postnatal catch-up growth superimposed on low birth weight with adult cardiovascular disease in both men and women (4, 12, 21). Ischemic heart disease may occur due to the increased incidence of hypertension, type 2 diabetes mellitus, dyslipidemia, atherosclerosis, and obesity in the face of diminutive coronary arterial dimensions and perturbed vascular response in adult intrauterine growth-restricted (IUGR) offspring (3, 7). This myocardial phenotypic presentation may be triggered initially by adaptive responses to inadequacy of metabolic fuels necessary for producing the energy (ATP) required for myocardial contraction. This catch-up growth phenomenon has been studied extensively in cohorts of human population (2, 22) as well as many established animal models of growth restriction (10, 19, 26, 29, 30, 34, 46, 48, 49). Moreover, these changes could be sex and tissue specific with decrease in organ weight (11), reduction in number of nephrons leading to hypertension and glomerulosclerosis (6), and increased hepatic cholesterol and triglyceride content in male offspring (8).
The fetal/neonatal myocardium relies primarily on glucose, whereas the adult myocardium usually utilizes fatty acids to generate ATP (1).
Glucose is transported into the fetal/neonatal myocardium primarily via sarcolemma-associated facilitative glucose transporter isoform 1 (GLUT1, 50 kDa). In contrast, in the adult myocardium, the intracellular endosome-vesicular glucose transporter isoform 4 (GLUT4, 45 kDa), which is expressed sixfold higher than GLUT1 (1), translocates to the sarcolemma in response to insulin, hypoxia, and contraction and plays a major role in glucose uptake (1, 25, 35). However, the adult myocardium under basal conditions relies mainly on fatty acids to fuel 70% of the energy metabolism (9, 16, 23, 38).
Long-chain fatty acids are transported across bilipid sarcolemmal layers by both passive diffusion and a carrier mediated process. Based on our current understanding, a possible scenario for transsarcolemmal protein mediated uptake of albumin bound fatty acids from the circulation could entail the following multistep process. Initial aggregation of fatty acids on the sarcolemmal membrane can be mediated by plasma membrane fatty acid binding protein (FABP-pm, 43 kDa) and fatty acid translocase (FAT/CD36 protein, 88 kDa) (25, 47). This function by FAT/CD36, a class B scavenger receptor protein, could provide the necessary gradient for initiating fatty acid transport via fatty acid transporter proteins (FATP). Intracellular FAT/CD36 is present in an endosomal compartment distinctly different from that of GLUT4 and translocates to the sarcolemma in response to insulin, hypoxia, and contraction (36). In addition, this protein can also translocate to the outer mitochondrial membrane, thereby facilitating entry of fatty acids into the mitochondrial matrix toward enzymes that catalyze β-oxidation (37, 39). Both the cardiac-specific FATP6 (FATP6, 70 kDa; colocalizes with FAT/CD36) and the insulin-responsive FATP1 (71 kDa) are instrumental in transporting fatty acids across sarcolemmal bilipid membranes (1). Cytoplasmic fatty acid binding protein (FABP-c, 15 kDa) then assists in moving fatty acids from the inner sarcolemmal layer into the cytoplasm (25) for subsequent activation by acyl-CoA synthetases and mitochondrial β-oxidation or storage in the form of triglycerides.
We therefore hypothesized that IUGR and/or postnatal growth-restricted (PNGR) adult offspring would demonstrate adaptive perturbations in myocardial fatty acid and glucose transporters to overcome diminished substrate availability prompted by early nutritional restriction. These perturbations would, in turn, contribute toward the protection of myocardial function during subsequent hypoxic exposures. We tested this hypothesis in a well-characterized calorie-restrictive rat model of IUGR and PNGR (44) and focused primarily on the pregestational young female adult, since phenotypic inheritance to the next generation has been demonstrated (31, 42). It has been characterized in a well-established model of intrauterine growth restriction that offspring of embryos, reproduced by a female exposed to both in utero and postnatal growth restriction and transferred to a normal female, still exhibit metabolic derangements, including altered fasting glucose levels and hyperinsulinemia (42).
METHODS
Animals.
Sprague-Dawley rats (60 days old, 200–250 g) (Charles River Laboratories, Hollister, CA) were housed in individual cages and exposed to 12:12-h light-dark cycles at 21–23°C. Each animal had ad libitum access to standard rat chow. The National Institutes of Health guidelines were followed as approved by the Animal Research Committee of the University of California, Los Angeles.
Maternal calorie restriction model.
Pregnant rats (semi-nutrient restricted) received 50% of their daily food intake (11 g/day) beginning from day 11 (d11) through d21 of gestation, which constitutes mid- to late gestation, compared with their control counterparts, who received ad libitum access to rat chow. Both groups had ad libitum access to drinking water.
Postnatal animal maintenance.
At birth, the litter size was culled to six. Newborn rats were cross-fostered to be reared by either a mother who continued to be semi-nutrient restricted (20 g/day food intake) through lactation (44) or a control mother with ad libitum access to rat chow. This scheme resulted in four groups, with control mothers rearing control (CON) or prenatally semi-nutrient restricted pups (IUGR), and pre- and postnatally semi-nutrient restricted mothers rearing prenatally semi-nutrient restricted pups (IPGR) or control pups (PNGR) (Table 1). Pups were weaned to standard rat chow with ad libitum access to food at postnatal day 21 onward and maintained in individual cages (44).
Table 1.
Study design of early calorie restriction
| CON | IUGR | PNGR | IPGR | |
|---|---|---|---|---|
| Prenatal calorie restriction | − | + | − | + |
| Postnatal calorie restriction | − | − | + | + |
Study design demonstrating the 4 experimental groups generated by cross-fostering postnatal rat pups. Semi-calorie-restricted mothers received 50% of daily nutrient intake from mid- to late pregnancy (embryonic day 11 to 21) and through lactation (postnatal day 1 to day 21). Experimental groups namely control with no pre- or postnatal nutrient restriction (CON), IUGR (intra–uterine growth restriction) with prenatal nutrient restriction, PNGR (postnatal growth restriction) with postnatal nutrient restriction, and IPGR (intrauterine and postnatal nutrient restriction) with both pre- and postnatal nutrient restriction.
In vivo insulin administration.
At day 60, female animals from all four experimental groups received either vehicle or insulin (8 U/kg) intraperitoneally (27). Animals were deeply anesthetized with inhalational isoflurane after 20 min, a predetermined optimal time point (27, 44) for peak plasma insulin concentration, blood samples were collected, and hearts and skeletal muscle were harvested following euthanasia.
Assessment of plasma metabolites.
Plasma was separated in the collected blood samples and analyzed for glucose by the glucose oxidase method and free fatty acids by a colorimetric assay (50). Insulin and adiponectin were assessed by double-antibody radioimmunoassay using rat standards and anti-rat insulin and adiponectin antibodies (Linco Research, St. Charles, MO; sensitivity for insulin = 0.1 ng/ml and adiponectin = 0.16 ng/ml).
Myocardial and skeletal muscle homogenate preparation.
Myocardium and skeletal muscles were rapidly separated from surrounding tissues, washed, quickly snap-frozen in liquid nitrogen, and stored at −70°C. Homogenates were prepared as previously described (43). Briefly, tissue was powdered under liquid nitrogen and homogenized with 15 strokes in a tight-fitting Potter-Elvenjhem homogenizer in cell lysis buffer (1:8 wt/vol; Cell Signaling; Danvers, MA), containing 20 mM Tris·HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1μg/ml leupeptin, 1% IGAPAL, 0.5% sodium cholate, 1% protease inhibitor cocktail (Sigma P8340), 1 mM PMSF, and sodium fluoride. These homogenates were centrifuged at 3,000 rpm at 4°C for 15 min and the supernatant sonicated (15 s × 3), centrifuged at 10,000 rpm for 15 min, and collected for determination of protein concentration by Bio-Rad assay prior to storage at −70°C.
Myocardial subfractionation.
Plasma membrane (PM) subfractions were isolated as previously described and the relative purity confirmed (13). Briefly, 1 g of tissue was thawed in 250 mM sucrose, 1 mM EDTA, 100 μM PMSF (pH7.4) (buffer A), homogenized on ice with a Brinkman polytron at setting 3 for 30 s, then further homogenized with 15 strokes of a Teflon pestle, and centrifuged at 17,000 g for 30 min. The supernatant was removed and centrifuged at 48,000 g for 30 min, this supernatant was then recentrifuged at 250,000 g for 1 h, and the pellet was collected. This pellet was resuspended in 1–2 ml of sucrose buffer and layered on a 38% sucrose cushion (38 g/100 ml sucrose, 20 mM HEPES, 1 mM EDTA, pH 7.4) and then centrifuged at 100,000 g for 65 min (ultracentrifuge, Beckman SW28, 22,500 rpm). The opaque white interface was carefully removed and diluted in buffer A and centrifuged at 17,000 g for 10 min to obtain the PM subfraction. These PM subfractions were sonicated (60 sonic, Dismembrator; Fisher Scientific, Pittsburgh, PA) using two 50-s cycles of 5–7 W. The resulting suspension was centrifuged at 10,000 g at 4°C for 10 min and stored at −70°C until Western blot analysis. The purity of these subfractions was validated as previously described, employing spectrophotometric assays for marker enzymes consisting of the K+-sensitive p-nitrophenolphosphatase for the sarcolemma and the EGTA-sensitive Ca2+ATPase as a marker for assessing contamination by the sarcoplasmic reticulum (5, 18).
Western blot analysis.
Twenty-five to fifty micrograms of myocardial homogenates and 50 μg of skeletal muscle homogenates were separated by SDS-PAGE and electro blotted onto nitrocellulose membranes. Membranes were then blocked in 5% nonfat dry milk in phosphate-buffered saline containing 0.1% Tween 20 (PBST) for 1 h overnight, incubated with specific primary antibodies: 1:2,500 dilution for GLUT4 (myocardium) (Abcam, Cambridge, MA), 1:100 dilution for FABP-c (Abcam), 1:1,000 dilution for FAT/CD36 (generous gift from Dr. M. Febbraio), and 1:1,000 dilution for FATP6, FATP4. and FATP1 for 1 h at room temperature or overnight at 4°C with gentle agitation. In addition, the structural protein vinculin (1:4,000 dilution of the antibody) was also assessed as the internal control to correct interlane loading variability. The membranes were then washed three times for 15 min each with PBST and incubated with the appropriate secondary horseradish peroxidase-conjugated antibody for 1 h at room temperature. After the membranes were washed three times for 15 min each, protein bands were visualized with the enhanced chemiluminescence method (Amersham Biosciences, Piscataway, NJ). The optimal time of exposure was predetermined to ensure that the optical densities were in the linear range for the protein concentrations employed. Quantification of protein bands was performed using the Image Quant software. Optical densities were reported as protein of interest/vinculin for all four experimental groups.
Surgical catheter placement.
Eighty-five- to one hundred-day-old female rats were anesthetized with inhalational isoflurane. The surgical site was shaved, and, under sterile conditions, a skin incision was made to expose the jugular vein. Heparin-flushed catheters (Braintree scientific, Braintree, MA) were placed in the jugular vein, secured, and externalized by tunneling under the skin. Animals were allowed to recover postsurgically for a minimum of 48 h.
[3H]R-bromopalmitate uptake studies.
Tracer infusate was freshly prepared on the day of the experiment. Briefly, [3H]R-bromopalmitate (American Radiolabeled Chemicals, St. Louis, MO) was dried in a glass tube under a nitrogen stream, and reconstituted in 4% BSA (Sigma Laboratories, St. Louis, MO) with unlabeled sodium palmitate, resulting in final concentrations of ∼11 μCi of [3H]R-bromopalmitate and 300 nmol sodium palmitate/ml of infusate (28). Each animal (n = CON 9, IUGR 6, PNGR 3, IPGR 5) received ∼1 ml of the infusate containing ∼11 μCi of [3H]R-bromopalmitate via the jugular vein catheter over 4 min, as previously described (28). Blood samples were collected at predetermined time points of 0, 2, 4, 6, 8, and 10 min. Animals were euthanized with pentobarbital sodium following blood collection. Myocardium, skeletal muscle [extensor digitorum longus (EDL), gastrocnemius, soleus], visceral white adipose tissue, and liver were rapidly collected and snap-frozen in liquid nitrogen. The nonmyocardial tissues served as comparison controls. Tissue extraction was conducted as previously described (20). Briefly, 100–200 mg of tissue was homogenized in vol/vol (2:2) of chloroform and methanol and centrifuged at 4,000 rpm for 10 min. Supernatant was collected in glass vials, 2 ml of water was added, and sample was extensively shaken and then centrifuged at 4,000 rpm for 10 min. The resulting lower chloroform phase was collected and radioactivity assessed.
2-Deoxy-[14C]glucose uptake studies.
Myocardial glucose uptake was estimated by measuring 2-deoxy-d-[1-14C]glucose 6-phosphate as previously described (15). Briefly, under basal conditions, a subset of the female animals (n = 6/group) was administered 80 μCi of the nonmetabolizable 2-deoxy-d-[1-14C]glucose (PerkinElmer, Boston, MA) analog via tail vein. After 45 min, animals were subjected to deep sedation via inhalational isoflurane. Blood was collected via cardiac puncture, and plasma was separated and stored at −80°C. Following euthanasia, tissues were removed, washed extensively in ice cold buffer, snap-frozen in liquid nitrogen and stored at −80°C for 2-deoxy-d-[1-14C]glucose uptake assessment. Briefly, frozen tissues were powdered under liquid nitrogen, suspended in 2H2O (1:7.5, wt/vol), and homogenized using a hand-held homogenizer (Omni TH115, Marietta, GA) for 30 s × 2. The homogenates were boiled for 10 min and centrifuged at 4,000 rpm at room temperature for 10 min to separate the supernatant, which was recentrifuged at 14,000 rpm for 10 min prior to undertaking resin chromatography. Supernatants were subjected to resin chromatography (1.6 g/8 ml; Dowex, Sigma Chemical) and eluted with 0.2 M formic acid/0.5 M ammonium acetate. Radioactivity in aliquots of the eluates was assessed in a scintillation counter (Beckman LS3801).
Hypoxia studies.
Sixty- to eighty-day-old female rats from all four experimental groups (n = 6/group) were housed in a hypoxic chamber (Oxycycler; Biospherix, Redfield, NY) for 2 (acute) or 7 (chronic) days containing 0.10 fractional inspired oxygen, resulting in an arterial partial pressure of oxygen of ∼40 mmHg. Rats had ad libitum access to food and water and were maintained under standard 12:12-h light-dark cycles.
Echocardiographic assessment.
Echocardiograms were performed on animals from all four experimental groups prior to and after hypoxic exposure. Rat electrocardiograms were obtained via standard lead II arrangement with subcutaneous platinum electrodes (Grass Instruments, Warwick, RI). Briefly, animals were lightly anesthetized with inhalational isoflurane and ultrasonically imaged with the Siemens Acuson Sequoia C256 instrument (Siemens Medical Solutions, Mountain View, CA) with a 15-MHz probe (33). Digitized 2-dimensionally guided M-mode images were analyzed with Access Point (Freeland Systems, Santa Fe, NM) software for dimensions of the left ventricular cavity [end-diastolic dimension (EDD), end-systolic dimension (ESD)], wall thickness [posterior wall thickness (PWT) and ventricular septal thickness (VST)] during systole and diastole. Ejection times and heart rates were determined from Doppler images. Left ventricular mass was calculated from the EDD, PWT, and VST values as previously described (41). Left ventricular fractional shortening (%LVFS) and velocity of circumferential fiber shortening (Vcf) were calculated as indexes of contractility (41).
Statistical analysis.
Data are expressed as means ± SE. Analysis of variance (ANOVA) models were used to compare the different treatment groups. In the presence of significant differences, intergroup differences were validated by the post hoc Fisher's paired least significant difference test. Significance was assigned when the P value was <0.05.
RESULTS
The body weights at birth in the IUGR group were significantly less than those of CON. Whereas the IUGRs' caught up to those of the CON group, the PNGR and IPGR groups remained lower at both day 21 and day 60 (Table 2). Heart weights at day 2 in the IUGR group were significantly lower than those of CON, and those of the PNGR and IPGR groups were lower than those of CON at day 21 of age, whereas those of IUGR caught up to those of CON. By day 60, the same two groups, namely PNGR and IPGR, demonstrated a lower heart weight than that of CON and IUGR. Relative heart weights expressed as percentage of body weight were no different in all four groups at day 2 and day 21; however, at day 60, both IUGR and PNGR demonstrated a decrease compared with CON and IPGR (Table 2).
Table 2.
Body and heart weights
| Body Weight, g |
Heart Weight/Body Weight, % |
|||||
|---|---|---|---|---|---|---|
| day 2 | day 21 | day 60 | day 2 | day 21 | day 60 | |
| CON | 6.3 | 67 | 235 | 0.51 | 0.44 | 0.51 |
| ± 0.2 (24) | ± 1 (10) | ± 7 (12) | ± 0.04 | ± 0.02 | ± 0.01 | |
| IUGR | 5* | 67 | 237 | 0.45 | 0.45 | 0.45* |
| ± 0.2 (29) | ± 1 (12) | ± 7 (12) | ± 0.04 | ± 0.02 | ± 0.01 | |
| PNGR | 24*† | 204*† | 0.51 | 0.46 | 0.45* | |
| ± 0.5 (12) | ± 7 (12) | ± 0.04 | ± 0.01 | ± 0.01 | ||
| IPGR | 21*† | 178*†‡ | 0.45 | 0.44 | 0.50 | |
| ± 1 (12) | ± 7 (6) | ± 0.04 | ± 0.02 | ± 0.02 | ||
Values are means ± SE; n is shown in parentheses. Body and heart weights (expressed as %body weight) at days 2, 21, and 60 in all 4 experimental groups.Fisher's PLSD =
P < 0.05 vs. CON,
vs. IUGR,
vs. PNGR.
Although plasma free fatty acid concentrations trended to the lower side in all three groups vs. CON (Table 3), triglyceride (TG) concentrations were significantly diminished in the IPGR group vs. PNGR alone. Plasma total cholesterol (TC) and unesterified cholesterol (UC) concentrations were lower in IUGR and IPGR vs. CON, with the PNGR group demonstrating values higher than IUGR's. High-density lipoprotein (HDL) concentration was higher in PNGR vs. CON and IUGR but lower in IPGR. No intergroup difference was observed in plasma adiponectin concentrations (Table 3). Circulating glucose concentrations were observed to be lower in IUGR and IPGR groups vs. CON (Table 3), whereas plasma insulin concentrations were lower in the IUGR and PNGR vs. CON groups (Table 3).
Table 3.
Plasma metabolic and hormonal profile under basal nonfasting conditions
| CON | IUGR | PNGR | IPGR | |
|---|---|---|---|---|
| FFA, mg/dl | 27 ± 6 | 25 ± 6 | 15 ± 3 | 21 ± 2 |
| TG, mg/dl | 26 ± 5 | 16 ± 3 | 36 ± 15 | 13 ± 3‡ |
| TC, mg/dl | 78 ± 4 | 57 ± 5* | 92 ± 13† | 49 ± 1*‡ |
| HDL, mg/dl | 17 ± 4 | 12 ± 3 | 42 ± 17*† | 6 ± 1‡ |
| UC, mg/dl | 22 ± 1 | 17 ± 1* | 25 ± 4† | 16 ± 1*‡ |
| Adiponectin, μg/ml | 15.3 ± 1.7 | 17.2 ± 2.4 | 13.5 ± 2.2 | 13.6 ± 1 |
| Glucose, mg/dl | 143 ± 6 | 122 ± 5* | 127 ± 15 | 106 ± 5* |
| Insulin, ng/ml | 2.1 ± 0.5 | 1.2 ± 0.2* | 0.8 ± 0.2* | 1.2 ± 0.4 |
Values are means ± SE. Plasma metabolite and hormonal profile is shown in all 4 experimental groups under basal nonfasting conditions (day 85–100). FFA, free fatty acids; TG, triglycerides; HDL, high-density lipoproteins; TC, total cholesterol; UC, unesterified cholesterol. Fisher's PLSD =
P < 0.05 vs. CONTROL,
vs. IUGR,
vs. PNGR. (n = CON 9, IUGR 8, PNGR 3, IPGR 4).
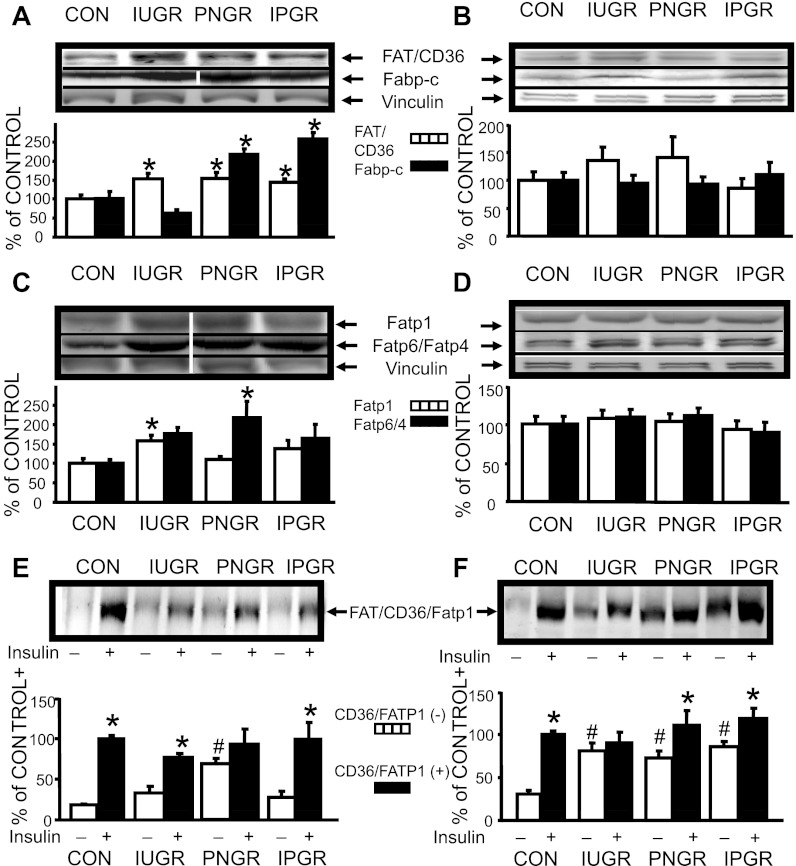
A 1.5-fold higher total myocardial FAT/CD36 protein concentration was seen in all three nutrient-restricted groups compared with CON (P < 0.05; Fig. 1A). In contrast, no intergroup difference in skeletal muscle FAT/CD36 protein concentration was observed (Fig. 1B). Again, total myocardial FABP-c concentration was higher only in PNGR (2.25-fold) and IPGR groups (2.5-fold) (P < 0.05; Fig. 1A), with no intergroup differences observed in skeletal muscle FABP-c concentration (Fig. 1B). A 1.5-fold higher total myocardial FATP1 concentration was observed in the IUGR group alone (P < 0.05) and a 2-fold higher total myocardial FATP6 was seen only in the PNGR group (P < 0.05; Fig. 1C) compared with the respective CON group. Again, no intergroup differences were noted in skeletal muscle FATP1 and FATP4 concentrations (Fig. 1D).
Fig. 1.
Myocardial and skeletal muscle fatty acid transporter proteins (FATP). A: myocardial FAT/CD36 and cytoplasmic fatty acid binding protein (FABP-c) proteins. Top: representative Western blots of FAT/CD36, FABP-c with vinculin (internal control) proteins. Samples from control (CON) and intrauterine growth restriced (IUGR) are from noncontiguous lanes from that of the postnatal growth-restricted (PNGR) and combined (IPGR) samples, as shown by white dividing line. Bottom: densitometric quantification of FAT/CD36-vinculin and FABP-c-vinculin protein concentrations expressed as %ON in all 4 experimental groups: CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs. CON. B: skeletal muscle FAT/CD36 and FABP-c proteins: Top: representative Western blots of FAT/CD36, FABP-c, and vinculin (internal control) proteins. Bottom: densitometric quantification of FAT/CD36-vinculin and FABP-c-vinculin protein concentrations expressed as %CON in all 4 experimental groups: CON (FAT/CD36 n = 8; FABP-c n = 6), IUGR (n = 9, 6), PNGR (n = 9, 6), and IPGR (n = 9, 6) at day 60. C: myocardial FATP1 and FATP6 proteins. Top: representative Western blots of FATP1, FATP6, and vinculin (internal control) proteins. Samples from CON and IUGR are from noncontiguous lanes from that of PNGR and IPGR samples, as shown by white dividing lines. Bottom: densitometric quantification of FATP1-vinculin and FATP6-vinculin protein concentrations expressed as %CON in all 4 experimental groups: CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs. CON. D: skeletal muscle FATP1 and FATP4 proteins: Top: representative Western blots of FATP1, FATP4, and vinculin (internal control) proteins. Bottom: densitometric quantification of FATP1-vinculin and FATP4-vinculin protein concentrations expressed as %CON in all 4 experimental groups: CON (n = 9), IUGR (n = 9), PNGR (n = 9), and IPGR (n = 9) at day 60. E: myocardium sarcolemmal FAT/CD36 protein. Top: representative Western blot of FAT/CD36 in the absence (−) and presence (+) of insulin stimulation in all 4 experimental groups. Bottom: densitometric quantification of FAT/CD36 protein concentrations in the absence (−) and presence (+) of insulin stimulation expressed as %CON (+) in CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs (−), #P < 0.05 vs. CON (−). F: myocardium sarcolemmal FATP1 protein. Top: representative Western blot of FATP1 in the absence (−) and presence (+) of insulin stimulation in all 4 experimental groups. Bottom: densitometric quantification of FATP1 protein concentrations in the absence (−) and presence (+) of insulin stimulation expressed as %CON (+), in CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs. (−), #P < 0.05 vs. CON (−).
Next, myocardial subfractions were examined in the basal and insulin-stimulated states for insulin-responsive proteins, namely FAT/CD36 and FATP1. The basal sarcolemmal FAT/CD36 protein concentration was higher only in PNGR vs. CON (P < 0.05). Insulin stimulation revealed a further higher concentration above that of basal in CON, IUGR, and IPGR groups (P < 0.05), with no such difference noted in PNGR (Fig. 1E). Basal FATP1 concentration was also higher in the sarcolemma of all three nutrient-restricted groups compared with CON (P < 0.05). Insulin stimulation enhanced to even higher FATP1 concentrations than that of basal in CON, PNGR, and IPGR groups (P < 0.05), with no such change in IUGR (Fig. 1F).
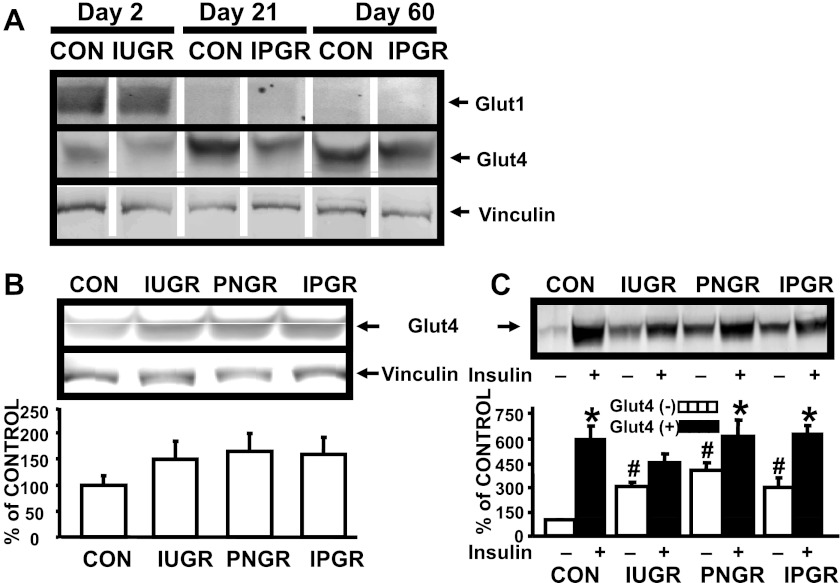
Detection of total myocardial GLUT1 at days 2, 21, and 60 revealed detectable bands at day 2 only and not at days 21 or 60 (100 μg of myocardial homogenate was loaded in each lane), regardless of the experimental group examined (Fig. 2A). In contrast, total myocardial GLUT4 protein bands were visible at all three developmental stages, with increasing intensity related to advancing age, namely at days 21 and 60 (25 μg of myocardial homogenate/lane; Fig. 2A). Myocardial total GLUT4 protein concentration was no different, although a trend toward an increase was evident in all three nutrient-restricted groups vs. CON at day 60 of age (Fig. 2B). In contrast, myocardial sarcolemmal GLUT4, similarly to FATP1, demonstrated higher basal concentrations in all three nutrient-restricted groups compared with CON (P < 0.05). Insulin stimulation resulted in an even higher concentration above that of the respective basal state in CON, PNGR, and IPGR (P < 0.05) but not in IUGR (Fig. 2C).
Fig. 2.
Myocardial and skeletal muscle glucose transporter protein. A: age-specific myocardial GLUT1 and GLUT4 proteins. Representative western blots of GLUT1, GLUT4, and vinculin (internal control) proteins at day 2 (CON and IUGR) and at days 21 and 60 (CON and IPGR each). B: myocardial GLUT4 proteins. Top: representative Western blot of GLUT4 and vinculin (internal control) proteins. Bottom: densitometric quantification of GLUT4-vinculin protein concentrations expressed as %CON in all 4 experimental groups: CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6). C: myocardium sarcolemmal GLUT4 protein. Top: representative Western blot of GLUT4 in the absence (−) and presence (+) of insulin stimulation in all 4 experimental groups. Bottom: densitometric quantification of GLUT4 protein concentrations in the absence (−) and presence (+) of insulin stimulation expressed as %CON in CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs. (−), #P < 0.05 vs. control (CON) (−).
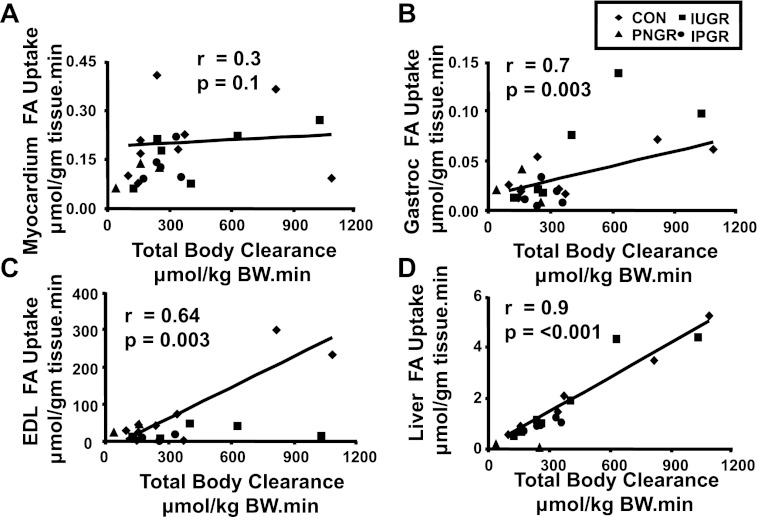
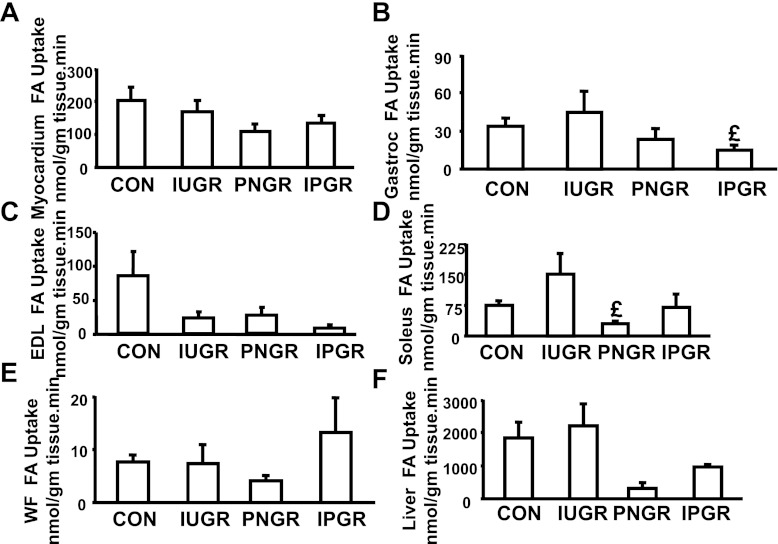
While myocardial (r = 0.3, P = 0.1), white adipose tissue (r = 0.4, P = 0.07; data not shown), and soleus skeletal muscle (oxidative fibers; r = 0.1, P = 0.07; data not shown) [3H]R-bromopalmitate uptake was not (Fig. 3A), gastrocnemius (Gastroc; mixed fibers; r = 0.7, P = 0.003), EDL (glycolytic fibers; r = 0.64, P = 0.003) skeletal muscle, and hepatic (r = 0.9, P < 0.001) fatty acid uptake were linearly associated with the total body fatty acid clearance rate (Fig. 3, B–D). Despite evident trends, no statistical differences were observed between the four experimental groups except for a lower soleus fatty acid uptake in the PNGR vs. IUGR and low gastrocnemius uptake in IPGR vs IUGR (Fig. 4).
Fig. 3.
Association between tissue fatty acid (FA) uptake and total body FA clearance. A–F: linear regression demonstrating correlations between total body FA clearance (x-axis, expressed as μmol·kg body wt−1·min−1) and myocardial (A), gastrocnemius (B), extensor digitorum longus (EDL; C), and liver (D) FA uptake (y-axes, expressed as μmol·g tissue−1·min−1) in 4 experimental groups: CON (n = 9), IUGR (n = 6), PNGR (n = 3), and IPGR (n = 5) at day 85–100. r and P values are depicted in individual panels.
Fig. 4.
Basal tissue [3H]R-bromopalmitate FA uptake. A–F: basal [3H]R-bromopalmitate FA uptake expressed in nmol·g tissue−1·min−1 is depicted in myocardium (A), gastrocnemius (B), EDL (C), soleus (D), white fat (WF; E), and liver (F) at day 85–100 of all 4 experimental groups: CON (n = 9), IUGR (n = 6), PNGR (n = 3), and IPGR (n = 5). Fisher's PLSD = £P < 0.05 vs. IUGR.
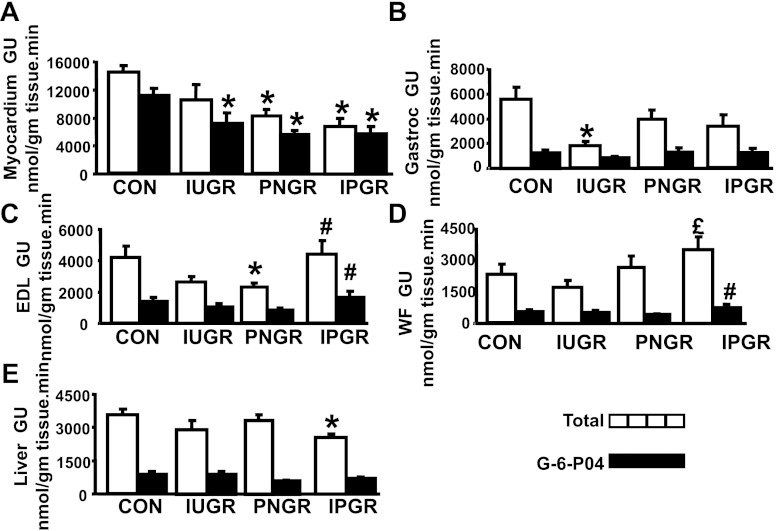
Myocardial 2-deoxy-[14C]glucose uptake measurements demonstrated a lower 2-deoxy-[14C]glucose and 2-deoxy-[14C]glucose 6-phosphate content in IUGR, PNGR, and IPGR groups compared with CON (Fig. 5A). While a lower Gastroc 2-deoxy-[14C]glucose uptake was observed in IUGR vs. CON (Fig. 5B), no intergroup difference in Gastroc 2-deoxy-[14C]glucose 6-phosphate was seen (Fig. 5B). EDL 2-deoxy-[14C]glucose uptake was lower in PNGR vs. CON and higher in IPGR vs. PNGR, with an associated increase in 2-deoxy-[14C]glucose 6-phosphate (Fig. 5C). White fat (WF) 2-deoxy-[14C]glucose uptake was higher in IPGR vs. IUGR with higher 2-deoxy-[14C]glucose 6-phosphate in IPGR vs. PNGR (Fig. 5D). Hepatic 2-deoxy-[14C]glucose uptake was lower in IPGR compared with CON (Fig. 5E). Overall, while a decrease in glucose uptake was seen in the myocardium, gastrocnemius, and liver, was particularly pronounced in the IPGR group, EDL and white adipose tissue instead demonstrated an increase.
Fig. 5.
Basal tissue 2-deoxy-[14C]glucose uptake. A–E: basal total deoxy-[14C]glucose uptake and [14C]glucose 6-phosphate content expressed in nmol·g tissue−1·min−1 is depicted in myocardium (A), gastrocnemius (B), EDL (C), WF (D), and liver (E) in all 4 experimental groups: CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Solid bars, total deoxy-[14C]glucose uptake (GU); stippled bars, phosphorylated deoxy-[14C]glucose content. Fisher's PLSD = *P < 0.05 vs. CON, £P < 0.05 vs. IUGR, #P < 0.05 vs. PNGR.
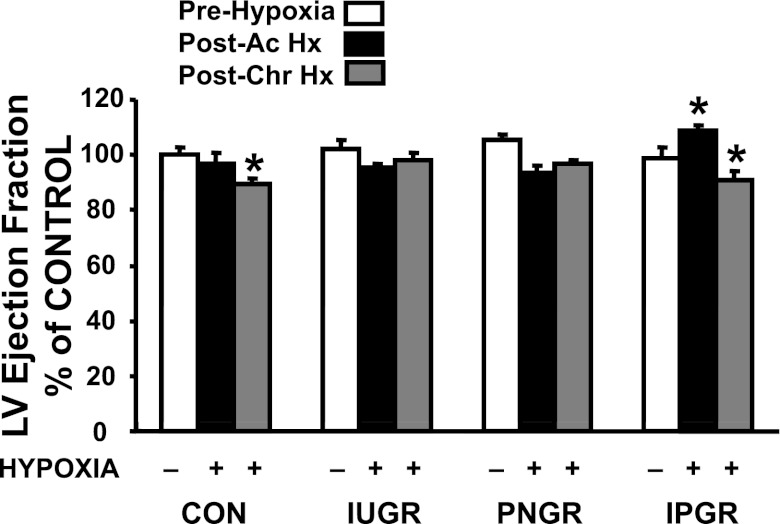
Cardiac parameters from echocardiography are shown in Table 4 and Fig. 6. No significant difference was noted in the heart rate between the four groups before and after subjecting the animals to hypoxia of either 2 days (acute) or 7 days (chronic) compared with the respective CON group, except for tachycardia of IPGR during post acute hypoxia (Table 4). Prehypoxic left ventricular mass was smaller in IPGR than in CON; however, a trend toward lower left ventricular mass was observed in all four groups post-chronic hypoxia compared with their respective prehypoxia CON (Table 4). Ejection fraction, an estimation of cardiac function, did not change post-acute hypoxia in CON, IUGR, and PNGR but was reduced in CON post-chronic hypoxia. In the IPGR group, post-acute hypoxia led to a greater ejection fraction; however, post-chronic hypoxia lowered the ejection fraction similarly to that seen in CON (Fig. 6). In contrast, post-acute and post-chronic hypoxia preserved the ejection fraction more so in IUGR than in PNGR (Fig. 6), with values similar to the prehypoxia condition within the same group and in the CON group. These ejection fraction data are consistent with the Vcf data in Table 4.
Table 4.
Echocardiographic assessments
| CON | IUGR | PNGR | IPGR | ||
|---|---|---|---|---|---|
| HR, (beats/min) | PreHx | 410 ± 32 | 393 ± 38 | 432 ± 49 | 417 ± 42 |
| PostAc. Hx | 402 ± 48 | 402 ± 38 | 455 ± 38 | 471 ± 28* | |
| PostChr. Hx | 408 ± 27 | 397 ± 44 | 419 ± 28 | 389 ± 15 | |
| VST, mm | PreHx | 1.4 ± 0.2 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.2 |
| PostAc. Hx | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 | |
| PostChr. Hx | 1.3 ± 0.05 | 1.6 ± 0.3 | 1.4 ± 0.2 | 1.3 ± 0.2 | |
| PWT, mm | PreHx | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.3 ± 0.1‡ | 1.4 ± 0.1 |
| PostAc. Hx | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 | |
| PostChr. Hx | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.1 | |
| ESD, mm | PreHx | 4.3 ± 0.6 | 4 ± 0.5 | 4 ± 0.4‡ | 4 ± 0.4 |
| PostAc. Hx | 4.4 ± 0.6 | 4.3 ± 0.4 | 4.2 ± 0.4 | 3.4 ± 0.4* | |
| PostChr. Hx | 4.7 ± 0.2 | 4 ± 0.6 | 4 ± 0.3 | 4.5 ± 0.5* | |
| EDD, mm | PreHx | 7.5 ± 0.6 | 7.2 ± 0.3 | 7.3 ± 0.5 | 6.7 ± 0.4 |
| PostAc. Hx | 7.3 ± 0.4 | 7.3 ± 0.7 | 7 ± 0.3 | 6.4 ± 0.4 | |
| PostChr. Hx | 7.3 ± 0.4 | 6.7 ± 0.7* | 6.8 ± 0.3 | 6.9 ± 0.3 | |
| VCF, cir/s | PreHx | 6.7 ± 0.7 | 6.7 ± 1.3 | 7.2 ± 0.8 | 6.7 ± 1 |
| PostAc. Hx | 6.2 ± 1.3 | 6.4 ± 0.5 | 6.8 ± 0.9 | 7.6 ± 0.7 | |
| PostChr. Hx | 5 ± 0.8 | 6.5 ± 1.3 | 6.8 ± 0.8 | 5.1 ± 0.8* | |
| LVM, mg | PreHx | 705 ± 131 | 714 ± 73 | 622 ± 93 | 573 ± 100‡ |
| PostAc. Hx | 678 ± 102 | 677 ± 115 | 608 ± 114 | 544 ± 88 | |
| PostChr. Hx | 662 ± 44 | 662 ± 145 | 594 ± 69 | 571 ± 103 | |
| LVM/BW | PreHx | 3.3 ± 0.5 | 3.1 ± 0.4 | 3.2 ± 0.6 | 3.5 ± 0.5 |
| PostAc. Hx | 3.5 ± 0.5 | 3.1 ± 0.5 | 3.4 ± 0.6 | 3.6 ± 0.5 | |
| PostChr. Hx | 2.8 ± 0.3* | 3 ± 0.6 | 3 ± 0.1 | 3 ± 0.5* |
Heart rate (HR), ventricular septal thickness (VST), posterior wall thickness (PWT), end systolic dimension (ESD), end diastolic dimension (EDD), velocity of circumferential fiber (VCF), left ventricular mass and left ventricular mass (LVM) to body weight (BW) ratio. Echocardiographic assessments at baseline (prehypoxia; preHx), after 2 days of hypoxia (postacute; postAc. Hx ) and after 7 days of hypoxia (postchronic; postChr. Hx) in 4 experimental groups. Values are means ± SE; n = 6 per treatment and per group. Fisher's PLSD
P < 0.05 vs. prehypoxia,
P < 0.05 vs. CON prehypoxia.
Fig. 6.
Echocardiographic assessment of myocardial performance under hypoxia. Left ventricular (LV) ejection fractions assessed by echocardiography are depicted at baseline under normoxia (prehypoxia), after 2 days of hypoxia (postacute; post-Ac Hx) and after 7 days of hypoxia (postchronic; post-Chr. Hx) and expressed as %prehypoxia baseline control in 4 experimental groups: CON (n = 6), IUGR (n = 6), PNGR (n = 6), and IPGR (n = 6) at day 60. Fisher's PLSD = *P < 0.05 vs. corresponding prehypoxia baseline value.
DISCUSSION
Early malnutrition during both intrauterine and postnatal life is associated with the subsequent development of dyslipidemia, insulin resistance, type 2 diabetes mellitus, and coronary artery disease, resulting in myocardial failure and mortality (3, 7). Myocardial disease does not occur in isolation but is a culmination of dyslipidemia, insulin resistance, type 2 diabetes, and hypertension that result in intramyocardiocyte fatty acid accumulation, which adversely affects myocardial function. Hence, the presence of insulin resistance and dyslipidemia are forerunners not only of metabolic dysregulation but also for the subsequent development of myocardial failure. Therefore, our present investigation focused on determining adaptations by the myocardial fatty acid transporter system against the backdrop of the glucose transporter system in the adult pregestational female rat offspring that were exposed to early-life caloric restriction during the intrauterine (IUGR) or postnatal (PNGR) periods or a combination of both (IPGR). We have previously demonstrated that during gestation the IUGR female rat offspring become hyperglycemic (14) and both the IUGR and PNGR female offspring transmit features of hyperglycemia and insulin resistance to their naturally born offspring (14). Given this possibility of transgenerational propagation of metabolic disturbances, it is essential to determine the metabolic adaptations present during the pregestational period prior to conception.
Our present observations of an increase in total protein expression of myocardial FAT/CD36, FABP-c, and FATP1 in the IUGR, PNGR, or IPGR group vs. CON supports the possibility of increased long-chain fatty acid transport intracellularly into the myocardiocyte (Table 5). Upon transport into the cell, fatty acids can be oxidized in the mitochondria toward generation of ATP or be esterified, thereby contributing toward intracellular lipid accumulation, which in turn adversely affects myocardial function (17, 24, 40). Similarly, an increase in myocardial GLUT4 concentrations has the propensity to enhance intracellular glucose transport, which fuels ATP generation or accumulates as glycogen, resulting in myocardiopathy. In a diabetic condition, hyperlipidemia and hyperglycemia contribute in this manner to myocardiopathy, particularly since both fatty acid and glucose oxidation cannot keep up with the increased intracellular transport of these macronutrients. Thus, intracellular accumulation of these two macronutrients contributes toward lipotoxicity and glucotoxicity, thereby disrupting myocardial performance (9, 32).
Table 5.
Summary of myocardial transporters and their respective macronutrient uptake compared with CON
| Transporters or Uptake | IUGR | PNGR | IPGR |
|---|---|---|---|
| FAT/CD36 | ↑ | ↑ | ↑ |
| FABPc | ND | ↑ | ↑ |
| FATP1 | ↑ | ND | ND |
| FATP6 | ND | ↑ | ND |
| Fatty acid uptake | ND | ND | ND |
| GLUT4 | ND | ND | ND |
| Glucose uptake | ↓ | ↓ | ↓ |
Summary of results related to myocardial transporters and their respective macronutrient uptake in IUGR, PNGR, and IPGR compared with CON is depicted. FABP, fatty acid binding protein; FATP, fatty acid transport protein; ND, No difference. Upward arrow represents an increase, downward arrow a decrease compared with CON.
In our present studies, rather than hyperglycemia and dyslipidemia, we encountered hypoglycemia and a trend toward lower circulating concentrations of fatty acids due to early nutritional restriction. In response to a diminished circulating fuel supply, expression of the key proteins FAT/CD36, FATP1, FATP6, and FABP-c was higher. This adaptation was targeted at overcoming a potential diminution of fatty acid availability to fuel the myocardial energy metabolism. The chronic effect of early nutrient restriction led to an increase in myocardial FAT/CD36 in the IUGR, PNGR, and IPGR groups, whereas FABP-c was higher only in the PNGR and IPGR groups. In contrast, FATP1 was higher only in the IUGR, whereas FATP6 was higher only in the PNGR group (Table 5). The reason for this differential regulation of the various protein components of the fatty acid transport system is unknown, since assessment of myocardial long-chain fatty acid uptake with 2-bromopalmitate showed no difference among the four experimental groups. The possible explanation for this disconnect between the myocardial long-chain fatty acid uptake and expression data may be that 2-bromopalmitate has different affinities for different fatty acid transporters or maybe the free fatty acid concentrations used during the infusion were too high, thereby masking any difference. One can speculate that, in the absence of these adaptive changes in the myocardial fatty acid transport proteins, fatty acid uptake by the myocardium would have suffered, thereby adversely affecting ATP production and myocardial performance. Due to the observed changes in the myocardial fatty acid transport system, no changes in myocardial performance assessed by the ejection fraction were seen between the four experimental groups under basal normoxic conditions.
When the changes in myocardial fatty acid transport system are compared with that of glucose transport, no changes in GLUT4 concentrations were apparent in either of three groups, i.e., IUGR, PNGR, and IPGR. This observation is different from that previously reported by us in skeletal muscle, where GLUT4 concentrations were diminished in all three groups (44). This absence of a significant change (perhaps a trend toward an increase) in myocardial GLUT4 expression is unable to preserve the glucose supply to fuel the myocardial energy metabolism in the face of low circulating glucose concentrations. In fact, myocardial glucose uptake was low in all three nutrient-restricted groups, resulting in low glucose 6-phosphate concentrations as well. Thus, unlike the fatty acid transport system that adapted to preserve the fatty acid supply, the glucose transport system failed to demonstrate a similar adaptability toward preserving the myocardial glucose supply. Our present results of myocardial GLUT4 are different from those of previous investigations surrounding either prolonged calorie restriction spanning the entire pregnancy, lactation, and beyond into adulthood until day 70 in the female rat (15) or acute uteroplacental insufficiency in days 21 and 120 male and female rats, resulting in diminished myocardial total GLUT4 expression and glucose uptake in the adult offspring (45). These two groups even demonstrated a concomitant decline in myocardial total GLUT1 expression (15, 45). These results contrast with our present findings of undetectable GLUT1 in CON and IPGR at days 21 and 60. Furthermore, our myocardial GLUT4 studies were confined to early nutritional restriction spanning late gestation and/or lactation only as opposed to prolonged caloric insufficiency into adulthood (15) or severe and acute fetal ischemic injury (45).
Of these myocardial transport proteins, FAT/CD36 and FATP1, similarly to GLUT4, are localized in an intracellular endosomal compartment, and in response to certain stimuli (namely insulin, contraction, hypoxia) can translocate to the sarcolemma. Hence, we further examined the sarcolemmal content of these three proteins in particular and observed an increase of FATP1 and GLUT4 in all three groups, IUGR, PNGR, and IPGR under basal conditions. In contrast, the FAT/CD36 receptor was increased only in the PNGR group. These changes in the basal state are in response to low circulating fatty acid and glucose concentrations, the associated hypoinsulinemia, and are geared toward preserving the fuel supply necessary for myocardial energy metabolism. Insulin stimulation further enhanced the sarcolemmal content of all three proteins above the basal concentration in the CON group. The same insulin responsiveness was evident and preserved in the PNGR and IPGR groups in the case of FATP1 and GLUT4; however, the IUGR group demonstrated metabolic inflexibility by expressing resistance to insulin-induced translocation to the sarcolemma. In contrast, insulin resistance in the case of FAT/CD36 was observed only in the PNGR group, whereas the IUGR group retained insulin sensitivity. Thus, collectively, the two groups that were exposed to intrauterine (IUGR) or to postnatal (PNGR) nutrient restriction displayed insulin resistance of translocation to the sarcolemma of either FATP1 or GLUT4 in the former or FAT/CD36 in the latter group. Again, this resistance may stem from adapting to the low availability of circulating macronutrients but may constitute an attempt at protecting the myocardium from excessive accumulation of fatty acids and glucose.
In addition to insulin responsiveness of the fatty acid transport system, similar to the glucose transport system (GLUT4), both contraction in skeletal muscle and hypoxia in the myocardium can also translocate FAT/CD36 and FATP1 to the sarcolemma via activation of AMPK. Thus, hypoxia-induced translocation can potentially overcome the insulin resistance of some of these key proteins, thereby protecting the energy resources necessary for myocardial performance. To this end, we assessed the effect of hypoxia (short term over 2 days and long term over 7 days) on myocardial function, which indirectly represents the adequate availability of fuels for ATP production. Although acute exposure to hypoxia did not affect myocardial function as determined by assessing the ejection fraction, chronic exposure led to compromising the ejection fraction in the two insulin-responsive groups CON and IPGR. In contrast, the IUGR and PNGR groups demonstrated no change in the ejection fraction under acute and chronic exposure to hypoxia. This lack of compromise supports the adequate availability of ATP. Thus, the insulin resistance of the fatty acid and glucose transport systems in the IUGR and PNGR groups perhaps maintains a balance between the enhanced macronutrient influx and oxidation against accumulation of macronutrients intracellularly, thereby protecting myocardial function. Thus, these key adaptations in myocardial macronutrient transport systems play a protective role in pregestational female adult offspring in countering myocardial failure by providing adequate fuel for ATP generation. Whether these key myocardial adaptations hold up during pregnancy, when a whole body state of insulin resistance ensues, requires future investigation.
We conclude that this is the first report of myocardial fatty acid transport system adaptations in adult pregestational female offspring exposed to early nutrient restriction. Given that the adult myocardial energy metabolism is predominantly reliant on fatty acids rather than glucose, key adaptations occur in fatty acid transporter protein expression and sarcolemma association targeted at preserving this macronutrient supply toward adequately fueling the ATP production necessary for myocardial performance. Perhaps myocardial priming to an early nutritional insult of a certain severity can offer protection and preservation of myocardial function upon reexposure to another environmental insult, namely hypoxia. The myocardial transporter adaptations incurred in response to the first insult may be responsible for preventing functional cardiac decompensation in the young pregestational female IUGR and PNGR adult. Furthermore, the metabolic inflexibility observed in response to exogenous insulin in the IUGR (FATP1 and GLUT4) and PNGR (FAT/CD36) young pregestational female may form a safeguard against the pathological intramyocardial accumulation of lipid or glycogen stores. These adaptations may provide the explanation for young females demonstrating resistance to myocardial dysfunction in response to early nutritional compromise and resultant growth failure.
GRANTS
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, HD-41230 and HD-25024 (to S. U. Devaskar). The echocardiographic studies were partially supported by the Laubisch Endowment at UCLA (to K. P. Roos). A. Abbasi was supported by NIH Grant T32 HD-07549.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.A., M.T., B.-C.S., and M.C.J. performed experiments; A.A., M.T., M.C.J., and K.P.R. analyzed data; A.A. prepared figures; A.A. drafted manuscript; A.A., M.T., B.-C.S., M.C.J., K.P.R., A.S., and S.U.D. approved final version of manuscript; M.T., M.C.J., K.P.R., and S.U.D. interpreted results of experiments; K.P.R. and S.U.D. edited and revised manuscript; S.U.D. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. M. Febbraio (Cleveland Clinic Foundation) for the generous gift of FAT/CD36 antibody and Dr. Luisa Iruela-Arispe (UCLA) for the Oxycycler employed in our studies. We acknowledge Shanthie Thamotharan and Cang Tran for experimental assistance.
REFERENCES
- 1. Abel ED. Glucose transport in the heart. Front Biosci 9: 201–215, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 41: 451–456, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ. Fetal origins of cardiovascular disease. Ann Med 31, Suppl 1: 3–6, 1999 [PubMed] [Google Scholar]
- 4. Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 353: 1802–1809, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bers DM. Isolation and characterization of cardiac sarcolemma. Biochim Biophys Acta 555: 131–146, 1979 [DOI] [PubMed] [Google Scholar]
- 6. Boubred F, Daniel L, Buffat C, Feuerstein JM, Tsimaratos M, Oliver C, gnat-George F, Lelievre-Pegorier M, Simeoni U. Early postnatal overfeeding induces early chronic renal dysfunction in adult male rats. Am J Physiol Renal Physiol 297: F943–F951, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578: 871–881, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi GY, Tosh DN, Garg A, Mansano R, Ross MG, Desai M. Gender-specific programmed hepatic lipid dysregulation in intrauterine growth-restricted offspring. Am J Obstet Gynecol 196: 477, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Coort SL, Bonen A, van d V, Glatz JF, Luiken JJ. Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: role of sarcolemmal substrate transporters. Mol Cell Biochem 299: 5–18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Grauw TJ, Myers RE, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate 49: 85–89, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Desai M, Gayle D, Babu J, Ross MG. Permanent reduction in heart and kidney organ growth in offspring of undernourished rat dams. Am J Obstet Gynecol 193: 1224–1232, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab 22: 155–171, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Fischer Y, Thomas J, Rosen P, Kammermeier H. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology 136: 412–420, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Garg M, Thamotharan M, Pan G, Lee PW, Devaskar SU. Early exposure of the pregestational intrauterine and postnatal growth-restricted female offspring to a peroxisome proliferator-activated receptor-γ agonist. Am J Physiol Endocrinol Metab 298: E489–E498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gavete ML, Agote M, Martin MA, Alvarez C, Escriva F. Effects of chronic undernutrition on glucose uptake and glucose transporter proteins in rat heart. Endocrinology 143: 4295–4303, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Glatz JF, Bonen A, Ouwens DM, Luiken JJ. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther 20: 471–476, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension 52: 181–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He J, Thamotharan M, Devaskar SU. Insulin-induced translocation of facilitative glucose transporters in fetal/neonatal rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284: R1138–R1146, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Holemans K, Aerts L, Van Assche FA. Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig 10: 392–399, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP, Pories WJ, MacDonald KG, Cline GW, Shulman GI, Dohm GL, Houmard JA. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284: E741–E747, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Jimenez-Chillaron JC, Patti ME. To catch up or not to catch up: is this the question? Lessons from animal models. Curr Opin Endocrinol Diabetes Obes 14: 23–29, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Karatekin G, Kutan AF, Nuhoglu A. Catch-up growth in fetal malnourished term infants. J Perinat Med 30: 411–415, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Lewin TM, Coleman RA. Regulation of myocardial triacylglycerol synthesis and metabolism. Biochim Biophys Acta 1634: 63–75, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Luiken JJ. Sarcolemmal fatty acid uptake vs. mitochondrial beta-oxidation as target to regress cardiac insulin resistance. Appl Physiol Nutr Metab 34: 473–480, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, Glatz JF. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflügers Arch 448: 1–15, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Mello MA, Cury L, Valle LB, Oliveira-Filho RM. Protein-calorie malnutrition in the young pregnant rat: factors involved in fetal growth impairment. Braz J Med Biol Res 20: 575–577, 1987 [PubMed] [Google Scholar]
- 27. Oak SA, Tran C, Pan G, Thamotharan M, Devaskar SU. Perturbed skeletal muscle insulin signaling in the adult female intrauterine growth-restricted rat. Am J Physiol Endocrinol Metab 290: E1321–E1330, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Oakes ND, Thalen P, Aasum E, Edgley A, Larsen T, Furler SM, Ljung B, Severson D. Cardiac metabolism in mice: tracer method developments and in vivo application revealing profound metabolic inflexibility in diabetes. Am J Physiol Endocrinol Metab 290: E870–E881, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Ogata ES, Swanson SL, Collins JW, Jr, Finley SL. Intrauterine growth retardation: altered hepatic energy and redox states in the fetal rat. Pediatr Res 27: 56–63, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Peterside IE, Selak MA, Simmons RA. Impaired oxidative phosphorylation in hepatic mitochondria in growth-retarded rats. Am J Physiol Endocrinol Metab 285: E1258–E1266, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond) 114: 381–392, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de RA, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52: 1793–1799, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Roos KP, Jordan MC, Fishbein MC, Ritter MR, Friedlander M, Chang HC, Rahgozar P, Han T, Garcia AJ, Maclellan WR, Ross RS, Philipson KD. Hypertrophy and heart failure in mice overexpressing the cardiac sodium-calcium exchanger. J Card Fail 13: 318–329, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadiq HF, deMello DE, Devaskar SU. The effect of intrauterine growth restriction upon fetal and postnatal hepatic glucose transporter and glucokinase proteins. Pediatr Res 43: 91–100, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Schroeder RE, Doria-Medina CL, Das UG, Sivitz WI, Devaskar SU. Effect of maternal diabetes upon fetal rat myocardial and skeletal muscle glucose transporters. Pediatr Res 41: 11–19, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2: 477–488, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell 4: 299–308, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Steinbusch LK, Schwenk RW, Ouwens DM, Diamant M, Glatz JF, Luiken JJ. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol Life Sci 68: 2525–2538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes. Part I: general concepts. Circulation 105: 1727–1733, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J., Jr Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94: 1109–1117, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Sangiorgi F, Lee PW, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 292: E1270–E1279, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Thamotharan M, McKnight RA, Thamotharan S, Kao DJ, Devaskar SU. Aberrant insulin-induced GLUT4 translocation predicts glucose intolerance in the offspring of a diabetic mother. Am J Physiol Endocrinol Metab 284: E901–E914, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 288: E935–E947, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Tsirka AE, Gruetzmacher EM, Kelley DE, Ritov VH, Devaskar SU, Lane RH. Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. J Endocrinol 169: 373–380, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Van Geijn HP, Kaylor WM, Jr, Nicola KR, Zuspan FP. Induction of severe intrauterine growth retardation in the Sprague-Dawley rat. Am J Obstet Gynecol 137: 43–47, 1980 [DOI] [PubMed] [Google Scholar]
- 47. van de V, Glatz JF, Stam HC, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72: 881–940, 1992 [DOI] [PubMed] [Google Scholar]
- 48. van M E. Alterations in the rate of fetal and placental development as a consequence of early maternal protein/calorie restriction. Biol Neonate 31: 324–332, 1977 [DOI] [PubMed] [Google Scholar]
- 49. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol 129: 101–123, 1986 [DOI] [PubMed] [Google Scholar]