Abstract
Previous studies have demonstrated that parathyroid hormone (PTH) binding to the PTH/PTH-related peptide receptor (PPR) stimulates G protein coupling, receptor phosphorylation, β-arrestin translocation, and internalization of the ligand/receptor complex. The extracellular signal-regulated mitogen-activated protein kinases 1/2 (ERK1/2 MAPK) are downstream effectors of PPR. In the current study, we investigated the role of PPR phosphorylation in the PTH regulation of the ERK1/2 MAPK pathway. Short treatment with PTH (0–40 min) of LLCP-K1 cells stably expressing a wild-type (WT) or a phosphorylation-deficient (PD) PPR (WT-PPR or PD-PPR cells, respectively) results in similar activation of ERK1/2. Interestingly, PTH stimulation of ERK1/2 in the WT-PPR cells then decreases as a result of longer PTH (60 min) treatment, and inhibition of ERK1/2 by PTH is observed at 90 min. Strikingly, the PD-PPR cells exhibit prolonged ERK1/2 activation up to 90 min of PTH treatment. An ERK1/2-dependent increase in c-fos expression is observed in the PD-PPR cells. Subsequently, c-fos expression in the WT-PPR and PD-PPR cells was markedly attenuated by a specific ERK1/2 pathway inhibitor. Further investigations revealed that PTH treatment causes a robust recruitment of a green fluorescent protein-tagged β-arrestin2 (β-arrestin2-GFP) in the WT-PPR cells. In contrast, β-arrestin2 recruitment was reduced in the PD-PPR cells. Importantly, expression of a receptor phosphorylation-independent β-arrestin2 (R169E) in the PD-PPR cells restored the biphasic effect of PTH on ERK1/2 as in the WT-PPR cells. The study reports a novel role for receptor phosphorylation and β-arrestin2 in the subsequent inhibition of the ERK1/2 pathway and in control of gene expression.
Keywords: G protein-coupled receptor, internalization, arrestin, gene expression
parathyroid hormone (PTH), an important regulator of calcium and phosphate homeostasis and bone remodeling, exerts these effects by binding to the PTH/PTH-related peptide receptor (PPR or PTHR1) in bone and kidney. The PPR is a member of the G protein-coupled receptor (GPCR) family that couples to multiple G proteins, including Gs, Gq/11, Gi, G12, and G13, and activates adenylate cyclase/cAMP/protein kinase A (PKA) and phospholipase C (PLC)/diacylglycerol, inositol trisphosphate (IP3), Ca/protein kinase C (PKC) pathways (1, 2, 21, 38, 39, 42).
The extracellular signal-regulated mitogen-activated protein kinases (ERK1/2 MAPK) are serine/threonine-directed kinases that are important for gene expression, cell cycle and cell proliferation differentiation, and apoptosis. Similar to other GPCRs that couple to Gs and/or Gq (20, 40, 54), several studies performed on PTH target cells, osteoblastic and kidney cells, suggested that PTH activation of ERK1/2 MAPK involves PKA and PKC and receptor and nonreceptor tyrosine kinases (3, 6, 10, 14–16, 23, 27, 32, 44, 45, 52).
Receptor phosphorylation modulates ERK1/2 activation by some GPCR (8, 22). PPR undergoes agonist-dependent phosphorylation (36). When the potential phosphorylation sites in the carboxyl terminal tail of the PPR (7 serine sites) were mutated to alanine residues, the mutant PPR was no longer phosphorylated after PTH stimulation (50). The phosphorylation-deficient (PD) mutant PPR, stably expressed in LLCP-K1 cells, was impaired in PTH-dependent internalization and had an exaggerated response to PTH stimulation of cAMP and IP3 accumulation (50).
In the current study, LLCP-K1 renal tubular cells stably expressing a wild-type (WT) or a PD PPR were used to elucidate the role of receptor phosphorylation in PTH regulation of ERK1/2 MAPK. Our results suggest that, although early PTH stimulation of ERK1/2 MAPK occurs via mechanisms that do not seem to require receptor phosphorylation, subsequent PTH-induced inhibition of ERK1/2 MAPK response and regulation of c-fos expression involve receptor phosphorylation and β-arrestin2.
MATERIALS AND METHODS
Reagents and supplies.
Bovine [Nle8,18,Tyr34]PTH(1–34)NH2 (PTH) was synthesized by a solid-phase method (Endocrine Unit, Massachusetts General Hospital, Boston, MA), purified by HPLC, and characterized by amino acid hydrolysis, NH2-terminal sequencing, and mass spectrography. Tissue culture media were purchased from Cellgro (Manassas, VA). FBS was from Sigma (St. Louis, MO), and streptomycin-penicillin was from GIBCO-BRL (Gaithersburg, MD). Tissue culture flasks, plates, and other supplies were from Corning (Oneonta, NY) and Fisher Scientific (Pittsburgh, PA). U-0126 was from Biomol Research Laboratories (Plymouth Meeting, PA). LipofectamineLTX was from Invitrogen (Carlsbad, CA). Peroxidase-labeled goat anti-rabbit and goat anti-mouse antisera were from Sigma. Immobilin PVDF membranes were from Millipore (Bedford, MA). [3H]myoinositol and the chemilumenescence kit were from Perkin-Elmer (Boston, MA). Anti-β-arrestin 1 and 2 and anti-c-fos antibodies were from EMD-Bioscience (San Diego, CA). Anti-phosphospecific (active) and anti-total (active and inactive) ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Cell culture.
LLCP-K1 porcine renal tubular cells were cultured in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% FBS. All media contained 1 μg/ml streptomycin and 100 U/ml penicillin. The cells were incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. Media were replaced every other day.
Development of stable cell lines expressing β-arrestin2-GFP, WT β-arrestin2, or β-arrestin2 (R169E).
Development and characterization of LLCP-K1 cell lines stably expressing similar numbers of WT or PD PPR that are green fluorescent protein (GFP) tagged or not on the extracellular domain (Exon E2) (WT-PPR and PD-PPR cells, respectively) were described previously (49, 50). Cells expressing the GFP-tagged and untagged versions of WT or PD PPR were interchangeably used in the study to ensure reproducibility of the findings in independent stable LLCP-K1 cell lines. For simplification purposes, we refer to either version as WT-PPR or PD-PPR. The findings presented herein were similar and reproducible in all cell lines.
The WT-PPR or PD-PPR cells were transfected with human wild β-arrestin2-GFP, human WT-β-arrestin2, or β-arrestin2 (R169E) (22) pcDNA3 plasmid vector, which also encodes a neomycin resistance gene. Transfection was performed as described previously (48) using LipofectamineLTX. Briefly, the plasmid DNA (1 μg) was mixed with a 100-μl serum-free DMEM and 7 μl of LipofectamineLTX and incubated at room temperature (RT) for 30 min. The complex was then added to cells grown in a 12-well plate, prewashed once with 1 ml serum-free DMEM, and left with 800 μl serum-free DMEM (final volume is 900 μl/well). After 24 h of incubation, the medium was replaced with full growth medium containing 1.5 mg/ml G418 selection antibiotic. The cells that survived the treatment of G418 (G418-resistant cells) were then reseeded at very low density to select individual colonies. Individual colonies were picked, expanded, and then tested for β-arrestin2 expression using a fluorescent microscope and/or Western blot analysis. G418 was omitted from the growth medium after one month. Three independent colonies for each cell line with similar expression levels were characterized by Western blot and were expanded for use in the study. Control cells were cells that underwent the whole process of transfection but did not show expression of β-arrestin2.
Western blot analysis.
Cells seeded in six-well plates were grown as a monolayer until they reached 100% confluency (48 h). Cells were rinsed (2×) with PBS, incubated for 1 h in serum-free DMEM containing 20 mM HEPES buffer and 0.1% BSA at 37° in a CO2 incubator, and then treated as indicated. The medium was removed, and the cells were placed on ice and rinsed (2×) with ice-cold PBS. The cells were lysed on ice using lysis buffer (62.5 mM Tris, pH 6.8, 1% SDS, 1 mM EDTA, pH 8, 1 mM EGTA, pH 7.4, 0.5% aprotinin, and 5 mM phenylmethylsulfonyl fluoride), and the lysates were cleared by centrifugation for 5 min at 4°C. The cell lysates were resolved on 8% SDS-PAGE. The proteins were then transferred from the gel onto an Immobilin membrane, and the membranes were blocked with 5% nonfat dry milk-0.2% Tween 20 in PBS. After incubation with the appropriate primary (1:2,000 in blocking buffer, 3 h at RT, or overnight at 4°C) and peroxidase-labeled secondary antibodies (1:5,000 in blocking buffer, 2 h at RT), the immunoreactive bands were detected with chemiluminescence and visualized using autoradiography.
Confocal microscopy studies.
Cells seeded on cover slips in six-well plates were grown as a monolayer until they reached 60–80% confluency. Cells were rinsed (2×) with PBS and then challenged with PTH as indicated in serum-free DMEM containing 20 mM HEPES buffer and 0.1% BSA at 37° in a CO2 incubator. The medium was removed, and the cells were rinsed (2×) with cold PBS and fixed with 4% paraformaldehyde (in PBS) for 15 min at RT. Fixed cells were then washed (3×) with PBS, each wash lasting 5 min, and then mounted using Vectashield, and the cover slips were sealed on the slides with nail polish.
The confocal microscopy images were obtained using the Radiance 2000 Laser Scanning system configured with a ×40 1,30 oil objective. Green and red images were captured sequentially at 1,024/1,024 resolution with 2 optical zoom, iris 2, Gain 20, and offset 0. X-Y horizontal planes (Z-series) were performed from the basolateral to apical level of the cells with 0.2 μm Z-step. The corresponding X-Z section was performed in the middle of the cell after determining the Z-start and Z-stop. The images were processed using LaserSharp 2000 software and assembled and labeled using Adobe Photoshop and Microsoft PowerPoint softwares. The micrographs presented are X-Y sections. To ensure consistency of the results, each experiment was repeated at least three times, and each time the areas were selected blindly and randomly.
Measurement of intracellular IP3 accumulation.
Cells were seeded in 12-well plates and incubated for 24 h at 37°C. Accumulated IPs were measured as described previously (48). Full growth medium was removed, and cells were radiolabeled at 37°C for 12 h with 2 μCi/ml [H3]myoinositol in 1 ml inositol- and serum-free DMEM supplemented with 0.1% BSA and 20 mM HEPES. Cells were then washed two times with PBS and preincubated with 20 mM LiCl for 60 min in serum-free DMEM containing 20 mM HEPES and 0.1% BSA. PTH or vehicle (10 mM acetic acid) was added, and the incubation was continued for the indicated time at 37°C. The incubation was terminated by placing the cells on ice, discarding the incubation medium, rinsing 2× with ice-cold PBS, and adding 0.5 ml of ice-cold 5% trichloroacetic acid (TCA) for 20 min at RT. TCA extract was collected, and the cells were rinsed with 1 ml double-distilled (dd) H20. TCA extract was made to a final volume of 2 ml by adding 0.5 ml ddH2O (final conc. of TCA is 1.25%). Separation of the IPs in 1 ml of the TCA extract was performed by AG 1X8 anion exchange column chromatography (formate form, 100–200 mesh; Bio-Rad). Free [H]inositol and glycerophosphate inositol fractions were washed and eluted with 10 ml of 10 mM inositol and 5 ml of 5 mM Borax/60 mM sodium formate, respectively. Inositol monophosphate and inositol biphosphate were eluted with 5 ml of 0.7 M ammonium formate/0.1 M formic acid (17, 21). The IP3 fraction was collected after elution with 5 ml of 1.05 ammonium formate/0.1 M formic acid.
Radioactivity in the 5-ml IP3 fraction mixed with 10 ml of scintillation liquid was counted using a liquid scintillation counter (model LS 6000IC; Beckman).
Statistical analysis.
Each experiment was repeated at least three times. t-Test was used for data analysis, and significance was considered when the P value was <0.05.
RESULTS
LLCP-K1 cells stably expressing a PD PPR mutant activate ERK1/2 normally in response to short PTH treatment.
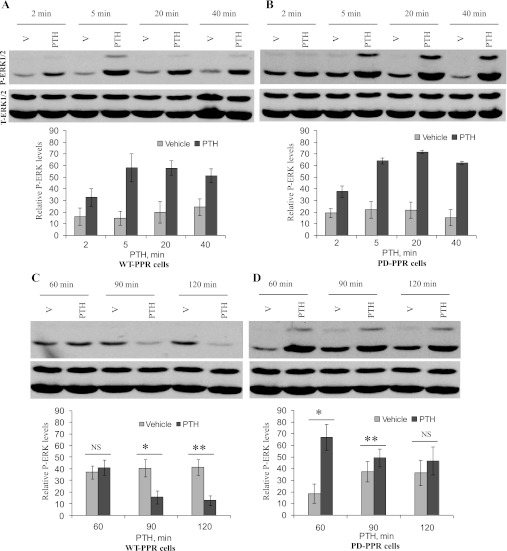
Receptor phosphorylation plays an important role in GPCR activation of MAPK (8, 22). Using Western blot analysis and anti-phosphospecific (active) ERK1/2 MAPK, we show that the PD-PPR cells activate ERK1/2 normally. PTH treatment of the WT-PPR and the PD-PPR cells (10 nM for 0–40 min at 37°C) similarly increased ERK1/2 activation (Fig. 1, A and B). The effects of PTH were time-dependent, with activation observed both in the WT-PPR and PD-PPR cells as early as 2 min, and maximal stimulation occurred between 5 and 20 min (Fig. 1, A and B).
Fig. 1.
Differential regulation of extracellular signal-regulated kinase (ERK) 1/2 in LLCP-K1 cells stably expressing a wild-type (WT-PPR) or a phosphorylation-deficient (PD-PPR) parathyroid hormone/parathyroid hormone-related peptide receptor. A and B: PD-PPR cells activate ERK1/2 normally in response to short parathyroid hormone (PTH) treatment. The WT-PPR and PD-PPR cells were serum starved for 1 h and then treated with 10 nM PTH for 0–40 min at 37°C. At the end of the incubation, the cells were placed on ice, washed 2× with ice-cold PBS, and lysed in SDS sample buffer. The cell lysates were analyzed on 8% SDS-PAGE. The samples were transferred to a nitrocellulose membrane and blotted with anti-phospho (active)-ERK1/2 antibody and a peroxidase-conjugated secondary antibody (blot on top). Detection was done with chemiluminescence, and the membranes were exposed to a film for 0.5 min. The blots were stripped and reprobed with anti-total (active and inactive) ERK1/2 antibody (blot on bottom). Representative images are displayed from one experiment. The respective graphs represent Western blot band densities of the 42-kDa phosphor-ERK2 (P-ERK) relative to its own total-ERK2 (T-ERK). The graphs are representative of results from 4 independent experiments; two experiments were performed in the green fluorescent protein (GFP)-tagged PPR cell lines and two in the untagged PPR cell lines. The data are expressed as means ± SD. C and D: PD-PPR cells show prolonged activation of ERK1/2 in response to long PTH treatment. The WT-PPR and PD-PPR cells were serum starved for 1 h and then treated with vehicle or 10 nM PTH (1–34) for 60, 90, and 120 min at 37°C. At the end of the incubation, the cells were processed for Western blot with anti-phospho ERK1/2 antibody (blot on top) and then with anti-total ERK1/2 antibody (blot on bottom) as described in A and B. Representative images are displayed from one experiment. The respective graphs represent Western blot band densities of the 42-kDa P-ERK relative to its own T-ERK. The graphs are representative of results from 4 independent experiments; two experiments were performed in the GFP-tagged PPR cell lines and two in the untagged PPR cell lines. V, vehicle. Data are expressed as means ± SD. Bars with * or ** indicate a P < 0.05 compared with its own vehicle control. NS indicates no significance with a P > 0.05.
LLCP-K1 cells stably expressing the PD mutant PPR exhibit prolonged activation of the ERK1/2 pathway in response to long PTH treatment.
In the WT-PPR cells, longer PTH treatment caused downregulation of ERK1/2 activation (Fig. 1C). PTH activation of ERK1/2 decreased after 60 min, and an inhibition of ERK1/2 is observed at 90 and 120 min of PTH treatment (Fig. 1C). In contrast, longer PTH treatment in the PD-PPR cells caused prolonged activation of ERK1/2 up to 90 min; PTH-treated cells had significantly (P < 0.05) higher ERK1/2 activation than vehicle-treated cells at 60 and 90 min, and no inhibition of ERK1/2 was observed up to 120 min (Fig. 1D). PTH had no effect on total (active and inactive) ERK1/2 during the time course tested (Fig. 1).
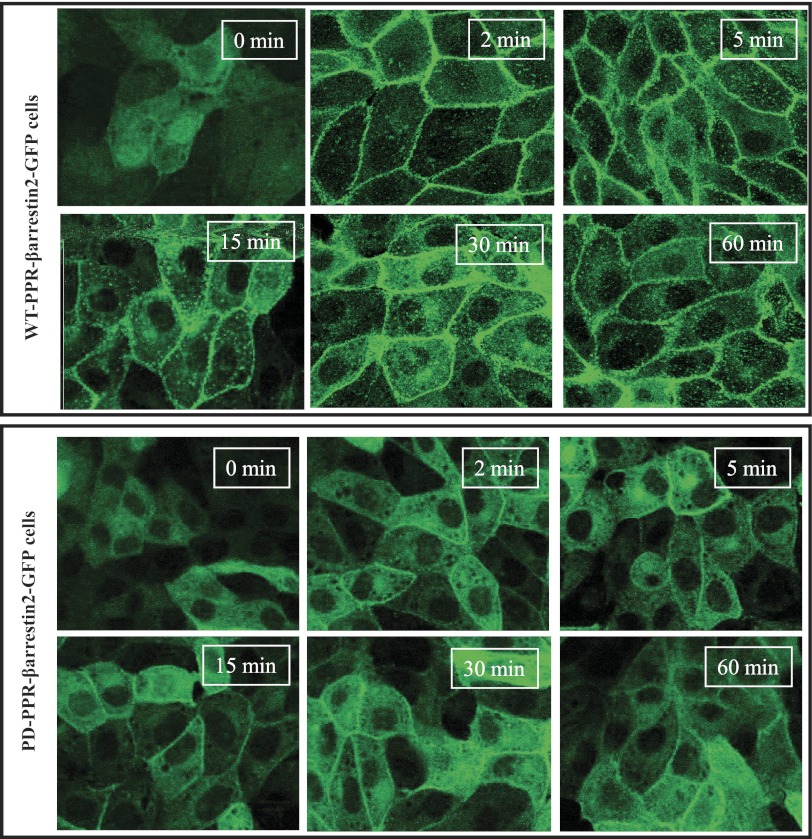
PTH-induced plasma membrane recruitment of β-arrestin2-GFP is attenuated in LLCP-K1 cells stably expressing the PD mutant PPR.
To examine the role of PPR phosphorylation in β-arrestin2 recruitment to the cell membrane, we developed WT-PPR and PD-PPR cell lines that stably express a GFP-tagged β-arrestin2 (WT-PPR-βarrestin2-GFP and PD-PPR-βarrestin2-GFP cells, respectively). The WT-PPR-arrestin2-GFP and PD-PPR-arrestin2-GFP stable cell lines were generated as described in materials and methods. The WT-PPR-βarrestin2-GFP and PD-PPR-βarrestin2-GFP cells were treated with PTH (100 nM for 2–60 min at 37°C). The cells were then fixed and examined using a confocal microscope. PTH treatment of the WT-PPR-βarrestin2-GFP cells caused a profound β-arrestin2-GFP translocation from the cytoplasm to the cell membrane. Translocation of β-arrestin2-GFP in the WT-PPR-βarrestin2-GFP cells was observed at 2 min (Fig. 2, top). A fraction of β-arrestin2-GFP remained at the cell membrane up to 60 min while in the meantime redistribution of a cytosolic fraction of β-arrestin2 was observed after 5 min of PTH treatment (Fig. 2, top). In contrast, the PD-PPR-βarrestin2-GFP cells showed only minor recruitment of β-arrestin2 to the cell membrane, which did not increase by prolonged PTH treatment (Fig. 2, bottom).
Fig. 2.
Decreased PTH-induced β-arrestin2-GFP membrane recruitment in the PD-PPR cells stably expressing β-arrestin2-GFP compared with the WT-PPR cells. The WT-PPR-βarrestin2-GFP and PD-PPR-βarrestin2-GFP cells were grown on cover slips in 6-well plates for 48 h. The cells were then treated with 100 nM PTH (0–60 min at 37°), washed with ice-cold PBS, and fixed with 4% paraformaldehyde for 20 min at RT, mounted, and examined under a confocal microscope. Images shown were taken at the X-Y horizontal planes (Z-series) in the middle of the cells. This experiment was repeated 3 times with similar results. These experiments were performed in the GFP-untagged WT-PPR and PD-PPR cells.
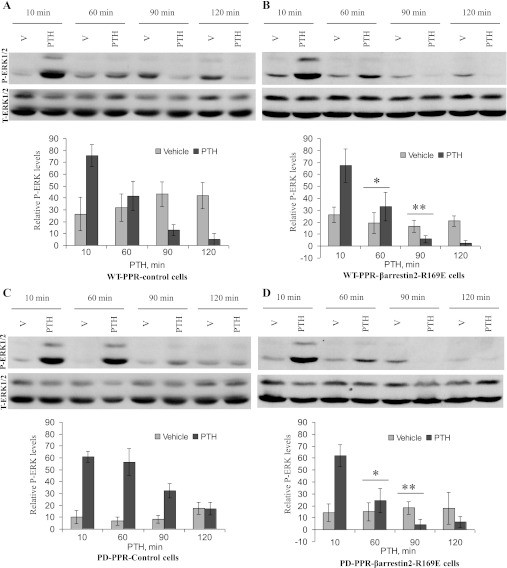
Stable expression of a phosphorylation-independent β-arrestin2 mutant in PD-PPR cells prevents the prolonged PTH activation of ERK1/2.
Arrestin binding to phosphorylated GPCRs involves disruption of the polar core within the arrestin molecule by the highly charged receptor-attached phosphates, converting arrestin to its high-affinity receptor-binding state (18, 19, 53). Subsequently, mutations destabilizing this polar core (R169E) increase the binding of arrestin to agonist-activated receptor without needing receptor phosphorylation (17–19, 24, 53). Therefore, to confirm the role of β-arrestin2 in subsequent PTH inhibition of ERK1/2, we generated WT-PPR or PD-PPR cells stably expressing the β-arrestin2-(R169E) mutant (WT-PPR-βarrestin-R169E and PD-PPR-βarrestin-R169E cells, respectively). Expression of the β-arrestin2 mutant was confirmed using Western blot analysis.
We next investigated the effects of PTH treatment (10 nM, 10–120 min) on ERK1/2 MAPK activation in the WT-PPR-βarrestin-R169E and PD-PPR-βarrestin-R169E cells. As shown above, activation of ERK1/2 in the PD-PPR control cells was monophasic and sustained in the PD-PPR cells compared with the WT-PPR control cells (Fig. 3, A and C). Overexpression of the β-arrestin2 mutant prevented the sustained ERK1/2 MAPK observed in the PD-PPR cells and restored the PTH-induced inhibition of ERK1/2 MAPKs at 90 and 120 min similar to that observed in the WT-PPR cells (Fig. 3D). PTH activation of ERK1/2 was similar in the WT-PPR control and WT-PPR-βarrestin-R169E cells (Fig. 3B).
Fig. 3.
Stable expression of a phosphorylation-independent mutant β-arrestin2 (R169E) prevented the prolonged PTH activation of ERK1/2 in the PD-PPR cells. The WT-PPR and PD-PPR cells stably expressing β-arrestin2 (R169E) were serum starved for 1 h and then treated with vehicle or 10 nM PTH for 10–120 min at 37°. The cells were then processed and analyzed for ERK1/2 activation as described in Fig. 1 (blots on top). The blots were stripped and reprobed with anti-total ERK1/2 antibody as described above (blots on bottom). Representative images are displayed from one experiment. The respective graphs represent Western blot band densities of the 42-kDa P-ERK relative to its own T-ERK. The graphs are representative of results from 4 independent experiments; all experiments were performed in the GFP-tagged PPR cell lines. The data are expressed as means ± SD. Bars with * or ** indicate a P < 0.05 compared with its own vehicle control.
Expression of similar levels of WT β-arrestin2 in the WT-PPR or PD-PPR cells did not alter PTH activation of ERK1/2 from mock-transfected control cells (data not shown).
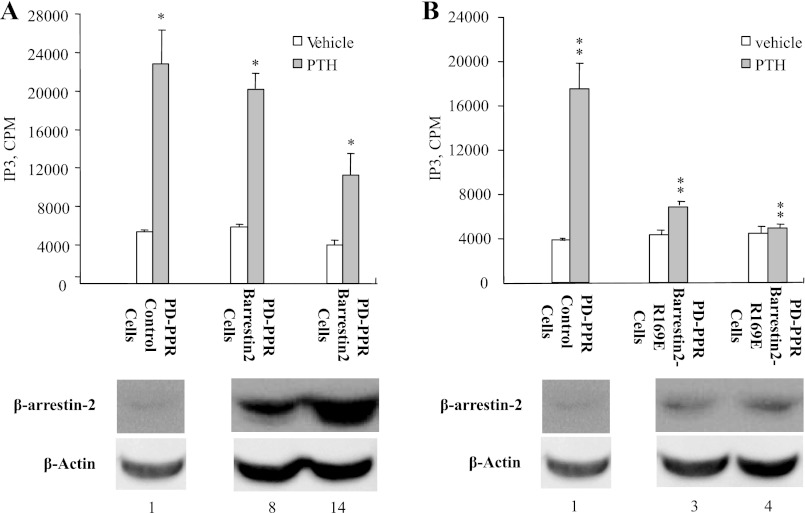
Stable expression of the phosphorylation-independent β-arrestin2 mutant prevents the excessive PTH stimulation of IP3 response in the PD-PPR cells.
To ensure that the mutant β-arrestin2 does interact with the activated PD-PPR, we compared the ability of the WT and mutant β-arrestin2 to uncouple the PD receptor from Gq/11 G proteins. The PD-PPR control, PD-PPR-βarrestin2, and PD-PPR-βarrestin-R169E cells were treated with PTH (100 nM for 40 min at 37°C) and assayed for IP3 accumulation. IP3 accumulation was remarkably high in the PD-PPR control cells as we reported previously (48) (Fig. 4A). Overexpression of low or high levels (Fig. 4A, bottom) of WT β-arrestin2 in the PD-PPR cells did not control the increase in PTH stimulation of IP3 formation (Fig. 4A, top).
Fig. 4.
Stable expression of β-arrestin2 (R169E) controls the exaggerated PTH activation of inositol trisphosphate (IP3) formation in the PD-PPR cells. The PD-PPR cells stably expressing control vector, WT-β-arrestin2, or β-arrestin2 (R169E) were serum starved for 1 h and then treated with vehicle or 100 nM PTH for 40 min at 37°. The cells were then placed on ice, washed 2× with ice-cold PBS, and assayed for IP3 as described in materials and methods. Each condition was performed in duplicate, and each experiment was repeated three times. The results are means ± SD of three experiments from two independently isolated stable cell lines, and all experiments were performed in the GFP-tagged PPR cell lines. Bars with * or ** indicate significance compared with the corresponding control (P value <0.05). For reference, corresponding Western blot for protein expression of endogenous β-arrestin (control), WT β-arrestin2, and β-arrestin2 (R169E) in the PD-PPR cells is shown on the bottom. Corresponding β-actin housekeeping protein is shown as a control. Band densities were quantified and normalized to β-actin. The number at the bottom of each blot indicates band density relative to its own β-actin band.
Importantly, expression of low levels (Fig. 4B, bottom) of the phosphorylation-independent mutant β-arrestin2 in the PD-PPR cells, however, prevented the increase in PTH stimulation of IP3 accumulation observed in the PD-PPR control cells (Fig. 4B, top).
As we have reported previously (48), the WT-PPR cells exhibit only a small increase (∼50% increase over basal) in IP3 accumulation and therefore were not included in the analysis.
LLCP-K1 cells stably expressing the PD mutant PPR show higher PTH stimulation of c-fos expression than those expressing the WT PPR.
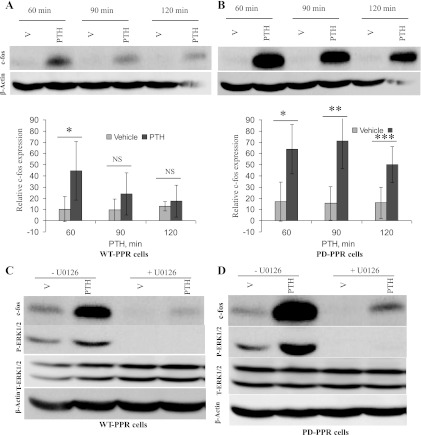
To examine the effects of PTH treatment on gene expression, the WT-PPR and PD-PPR cells were treated with PTH (10 nM for 60–120 min at 37°C). c-fos immunoreactivity in the cell lysates was then measured using Western blot. The PD-PPR cells exhibited increased c-fos expression compared with the WT-PPR cells. In the PD-PPR cells, PTH stimulation of c-fos expression was higher than in the WT-PPR cells at 60 min (Fig. 5, A and B). The levels of PTH-induced c-fos at 90 and 120 min then decrease both in the WT-PPR and PD-PPR cells. Despite the decrease in c-fos in the PD-PPR cells, the levels still remained significantly (P < 0.05) higher over basal at 90 and 120 min of PTH treatment compared with the WT-PPR cells (Fig. 5, A and B).
Fig. 5.
Enhanced c-fos expression in PD-PPR cells. A and B: PD-PPR cells show higher PTH stimulation of c-fos expression than the WT-PPR cells. The WT-PPR and PD-PPR cells were treated with PTH (10 nM for 60–120 min). The cells were then lysed and processed for Western blot analysis as described in Fig. 1 but using rabbit anti-c-fos antibody (blots on top). The blots were also stripped and reprobed with anti-β-actin antibody for loading control (blots on bottom). Representative images are displayed from one experiment. The respective graphs represent Western blot band densities of c-fos relative to β-actin. The graphs are representative of results from 3 independent experiments; two experiments were performed in the GFP-tagged PPR cell lines and one in the untagged PPR cell line. The data are expressed as means ± SD. Bars with *, **, or *** indicate a P < 0.05 compared with its own vehicle control. NS indicates no significance. C and D: c-fos expression is attenuated by inhibition of the ERK1/2 pathway in the WT-PPR and PD-PPR cells. The WT-PPR and PD-PPR cells were treated with PTH (10 nM for 60 min) in the presence or absence of the ERK1/2 pathway inhibitor U-0126 (4 μM). The cells were then lysed and analyzed for c-fos expression (first panels on top) using Western blot, as described above. The blots were also stripped and reprobed with anti-phospho-ERK1/2 antibody to ensure inhibition of the ERK1/2 pathway (second panels on top), anti-total-ERK1/2 antibody (third panels on top), and last with anti-β-actin antibody (panels on bottom). This experiment was repeated 3 times with similar results; two experiments were performed in the GFP-tagged PPR cell lines and one in the untagged PPR cell line.
The effects of PTH treatment on c-fos gene expression in the WT-PPR and PD-PPR cells were markedly but not completely blocked by pretreatment with the specific ERK1/2 pathway inhibitor U-0126 (4 μM for 60 min) (Fig. 5, C and D, top). Although U-0126 completely inhibited ERK/12 phosphorylation (Fig. 5, C and D, middle), U-0126 had no effect on PTH phosphorylation of cAMP-response element-binding protein transcription factor, ruling out any toxic effect of the drug (data not shown).
DISCUSSION
PPR phosphorylation is important for regulating PPR signaling and calcium ion homeostasis in vitro and in vivo (7, 33, 48, 50). Receptor phosphorylation has been shown to modulate GPCR activation of MAPK (8, 22). Also, internalization-dependent and -independent mechanisms for GPCR activation of ERK1/2 have been described (4, 11, 13, 26, 28, 29, 35, 47). Based on these reports and our previous finding that PTH-dependent PPR phosphorylation is required for PTH stimulation of PPR internalization, the current investigation was focused on unveiling the role of PTH-stimulated PPR phosphorylation in the regulation of ERK1/2 MAPK response. Our data show that PD-PPR cells, which express a PD internalization-impaired mutant PPR, stimulated ERK1/2 MAPKs in a similar time-dependent manner to the WT-PPR cells during the period of 2–40 min of incubation. Blocking receptor internalization using a dominant-negative dynamin GTPase mutant in HEK293 cells did, however, inhibit PPR signaling to ERK1/2, suggesting a role for PPR internalization in ERK1/2 activation (46). The discrepancy between our results and those of Syme et al. (46) is likely a reflection of the different molecular profile of HEK293 and LLCP-K1 cell systems and/or the PD mutant PPR and the mutant dynamin experimental models.
It has been shown that β2-adrenergic receptor, which couples to Gs, can couple to Gi upon phosphorylation by PKA (31). As a result, a mutant β2-adrenergic receptor that lacks the PKA phosphorylation sites was not able to couple to Gi and failed to activate ERK1/2 (31). Lack of inhibition of ERK1/2 in the PD-PPR cells is further in agreement with our previous finding that PPR is not a target for PKA phosphorylation (36), a prerequisite for β2-adrenergic receptor Gs/Gi switch.
Collectively these results suggest that receptor phosphorylation and internalization are not absolutely required for PPR coupling to the ERK1/2 pathway.
Although most of the studies have focused on elucidating the mechanisms involved in GPCR activation of ERK1/2, the molecular mechanisms involved in the regulation of the ERK1/2 pathway have been poorly investigated. The scope of the present study was therefore expanded to investigate the role of PPR phosphorylation in the regulation of the ERK1/2 MAPK pathway. To address this goal, we compared the effects of long PTH treatment (60–120 min) on ERK1/2 regulation in the WT-PPR and PD-PPR cells. It is interesting to observe in the WT-PPR cells that, contrary to short PTH treatment-induced ERK1/2 activation, prolonging PTH treatment (60–120 min) caused inhibition of ERK1/2. Furthermore, elimination of receptor phosphorylation as demonstrated in the PD-PPR cells caused sustained PTH activation of ERK1/2. Thus, our findings suggest that PTH downregulation of the ERK1/2 pathway is largely dependent on PPR phosphorylation.
β-Arrestins are multifunctional intracellular proteins originally identified on the basis of their ability to bind and uncouple GPCRs from G proteins and to mediate GPCR endocytosis, desensitization, and resensitization. Subsequent studies have further indicated that β-arrestins can act as scaffolds to create a functional module required for the interaction of the ERK1/2 pathway signaling components, such as the internalized receptor, Raf-1, MEK1, c-Src, and the activated ERK (5, 9, 12, 16, 30, 41, 43, 46).
In this study, we first characterized the intracellular trafficking of β-arrestin2 in response to PTH treatment of the WT-PPR cells stably coexpressing a GFP-tagged β-arrestin2. Whereas activated β2-adrenergic, dopamine D1A, and endothelin type A receptors traffic in endocytic vesicles without the plasma membrane-recruited β-arrestins (55), agonist-activated PPR-like ANG II type 1A and neurotensin receptors triggered a time-dependent redistribution of β-arrestin both to the cell membrane and to intracellular vesicular compartments with the internalized receptors. These results highlight the distinct pattern of the cellular distribution of β-arrestin proteins after GPCR stimulation (55), which may partly explain the functional diversity among individual GPCRs.
The diminished PTH-induced β-arrestin2-GFP translocation after elimination of PPR phosphorylation is in line with follitropin 1A and ANG II receptors whose association with β-arrestin2 required both receptor activation and phosphorylation (25, 37). However, PPR is in clear contrast to the human lutropin/choriogonadotropin receptor whose association with β-arrestin2 was independent of receptor phosphorylation but dependent on receptor activation (34).
More significantly, the findings of the prolonged ERK1/2 MAPK activation together with the weak β-arrestin2 recruitment in the PD-PPR cells prompted us to explore a possible role of β-arrestin2 recruitment in the PTH-induced inhibition of the ERK1/2 pathway exhibited by the WT-PPR cells. To assess this hypothesis, we took advantage of a phosphorylation-independent β-arrestin2 mutant (R169E) (18, 24) that we stably expressed in the PD-PPR cells. Attesting to a role of β-arrestin2 in regulating the ERK1/2 response, expression of β-arrestin2 (R169E) restored the PTH-induced inhibition of ERK1/2 MAPK in the PD-PPR cells similar to the WT-PPR cells. Because desensitization would only cause return to basal activation of ERK1/2, desensitization of the PLC response alone is unlikely to account for the later inhibition of the ERK1/2 pathway observed in the WT-PPR cells. Rather, PTH-induced downregulation of the ERK1/2 response in the WT-PPR cells following the initial activation (2–40 min) supports a role for a MAPK phosphatase. Moreover, the absence of PTH-induced inhibition of ERK1/2 in the PD-PPR cells and the reestablishment of this inhibition by expression of the phosphorylation-independent β-arrestin2 suggest that receptor phosphorylation and β-arrestin2 recruitment may be required for increasing expression of that phosphatase. This notion, however, requires further investigation. Partially phosphorylated mutant PPRs (S491/492/493A, S501A, and S504A), which maintain internalization but which exhibit significantly enhanced PLC signaling in response to PTH treatment, have been characterized (33). These signal-selective PPR mutants may be useful to address whether the sustained ERK1/2 response in the PD-PPR cells can be attributed to the impairment of internalization or the exaggeration of PLC signaling.
A role for a β-arrestin-dependent (5, 9, 12, 30, 43, 46) and for a β-arrestin-dependent G protein-independent (16, 41) mechanism in PPR and some GPCR activation of ERK1/2 has been indicated. Unexpectedly, using PD receptor models for the PPR and the follicle-stimulating hormone receptor (22), no reduction in ERK1/2 activation was detected despite the decreased β-arrestin recruitment. As discussed earlier in this section, besides the difference in the cell line systems used, this inconsistency may also arise from the different approach of disabling β-arrestin recruitment via PPR mutation vs. the complete knockdown of endogenous β-arrestin proteins using short-interfering RNA. It is conceivable that, as an early and upstream event, PPR phosphorylation modulates the ERK1/2 pathway via multiple mechanisms. Whereas phosphorylation may mediate ERK1/2 activation via stimulation of β-arrestin recruitment, phosphorylation may also decrease ERK1/2 activation by suppressing β-arrestin-independent mechanisms of ERK1/2 activation. Consequently, a decrease in ERK1/2 activation caused by impaired β-arrestin2 recruitment in the PD-PPR cells may be overshadowed by increased ERK1/2 activation as a result of lack of desensitization of the β-arrestin-independent pathways.
The ERK1/2 pathway is important for gene expression. We therefore further examined if prolonged PTH activation of ERK1/2 in PD-PPR cells results in enhanced gene expression. PTH treatment in the WT-PPR and PD-PPR cells induced c-fos expression at 60 min. PTH stimulation of c-fos expression, however, was enhanced in the PD-PPR cells. The ability to reduce c-fos expression by the U-0126 ERK1/2 pathway inhibitor indicates that the enhanced PTH-stimulated c-fos expression in PD-PPR cells is at least partly subsequent to sustained ERK1/2 MAPK activation.
Tohgo et al. (51) have reported that β-arrestin2 can sequester active ERK1/2 in the cytoplasm and that GPCRs that bind transiently to β-arrestin2 exhibit more efficient translocation of ERK1/2 to the nucleus and as a consequence have enhanced gene expression. Our results that the activated PD-PPR weakly recruits β-arrestin2 to the cell membrane compared with the WT-PPR and that the PD-PPR has enhanced gene expression are similar to that of Tohgo el al. Unlike our findings, those of Tohgo et al. were observed under short treatment and did not show an overall increase in ERK1/2 activation. Additionally, our unpublished results did not support a difference in nuclear translocation of ERK1/2 in WT and PD-PPR cells.
Taken together, our study demonstrates that PPR coupling to ERK1/2 MAPK does not require receptor phosphorylation or internalization. The study, however, presents for the first time a model for GPCR in which receptor phosphorylation, via a β-arrestin2-dependent mechanism, mediates a subsequent inhibition of the ERK1/2 MAPK pathway and controls c-fos expression. The study sheds light into the pathways involved in the regulation of ERK1/2 response. Although the occurrence of multiple natural GPCR mutations that completely abolish phosphorylation is unlikely, conditions that alter G protein receptor kinase(s) function are more likely. Altered G protein receptor kinase(s) can abnormally modulate GPCR phosphorylation and β-arrestin recruitment, causing dysregulation of the ERK1/2 MAPK pathway. Last, the findings of the current investigation may provide some explanation as to why intermittent (short) and continuous (long) PTH administrations and hyperparathyroidism have distinct effects on net bone mass.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1 K01 DK-64073 and RO1 DK-062285.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: H.A.T. conception and design of research; H.A.T. performed experiments; H.A.T. analyzed data; H.A.T. interpreted results of experiments; H.A.T. prepared figures; H.A.T. drafted manuscript; H.A.T. and A.B.A.-S. edited and revised manuscript; H.A.T. and A.B.A.-S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Vsevolod Gurevich at Vanderbilt University (Nashville, TN) for generously providing the wild-type and mutant β-arrestin2 (R169E) expression plasmids and Dr. Stefano Marullo at Institut Cochin Pavillon Gustave Roussy (Paris, France) for generously providing the β-arrestin2-GFP expression plasmid.
REFERENCES
- 1.Abou-Samra AB, Jueppner H, Potts JT, Jr, Segre GV. Inactivation of pertussis toxin-sensitive guanyl nucleotide-binding proteins increase parathyroid hormone receptors and reverse agonist-induced receptor down-regulation in ROS 17/28 cells. Endocrinology 125: 2594–2599, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89: 2732–2736, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed I, Gesty-Palmer D, Drezner MK, Luttrell LM. Transactivation of the epidermal growth factor receptor mediates parathyroid hormone and prostaglandin F2 alpha-stimulated mitogen-activated protein kinase activation in cultured transgenic murine osteoblasts. Mol Endocrinol 17: 1607–1621, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274: 1185–1188, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem 279: 35518–35525, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bacic D, Schulz N, Biber J, Kaissling B, Murer H, Wagner CA. Involvement of the MAPK-kinase pathway in the PTH-mediated regulation of the proximal tubule type IIa Na+/Pi cotransporter in mouse kidney. Pflugers Arch 446: 52–60, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bounoutas GS, Tawfeek H, Frohlich LF, Chung UI, Abou-Samra AB. Impact of impaired receptor internalization on calcium homeostasis in knock-in mice expressing a phosphorylation-deficient parathyroid hormone (PTH)/PTH-related peptide receptor. Endocrinology 147: 4674–4679, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Budd DC, Willars GB, McDonald JE, Tobin AB. Phosphorylation of the Gq/11-coupled m3-muscarinic receptor is involved in receptor activation of the ERK-1/2 mitogen-activated protein kinase pathway. J Biol Chem 276: 4581–4587, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Caunt CJ, Finch AR, Sedgley KR, Oakley L, Luttrell LM, McArdle CA. Arrestin-mediated ERK activation by gonadotropin-releasing hormone receptors: receptor-specific activation mechanisms and compartmentalization. J Biol Chem 281: 2701–2710, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D'Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem 279: 29121–29129, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cole JA. Parathyroid hormone activates mitogen-activated protein kinase in opossum kidney cells. Endocrinology 140: 5771–5779, 1999 [DOI] [PubMed] [Google Scholar]
- 12.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem 274: 4749–4753, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem 277: 22191–22200, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gentili C, Morelli S, Boland R, de Boland AR. Parathyroid hormone activation of map kinase in rat duodenal cells is mediated by 3′,5′-cyclic AMP and Ca(2+). Biochim Biophys Acta 1540: 201–212, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 281: 10856–10864, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gray-Keller MP, Detwiler PB, Benovic JL, Gurevich VV. Arrestin with a single amino acid substitution quenches light-activated rhodopsin in a phosphorylation-independent fashion. Biochemistry 36: 7058–7063, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Gurevich VV, Benovic JL. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol 51: 161–169, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Gurevich VV, Benovic JL. Visual arrestin binding to rhodopsin. Diverse functional roles of positively charged residues within the phosphorylation-recognition region of arrestin. J Biol Chem 270: 6010–6016, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Han XB, Conn PM. The role of protein kinases A and C pathways in the regulation of mitogen-activated protein kinase activation in response to gonadotropin-releasing hormone receptor activation. Endocrinology 140: 2241–2251, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254: 1024–1026, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Kara E, Crepieux P, Gauthier C, Martinat N, Piketty V, Guillou F, Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol Endocrinol 20: 3014–3026, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Khundmiri SJ, Ameen M, Delamere NA, Lederer ED. PTH-mediated regulation of Na+-K+-ATPase requires Src kinase-dependent ERK phosphorylation. Am J Physiol Renal Physiol 295: F426–F437, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem 274: 6831–6834, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy H, Galet C, Ascoli M. The association of arrestin-3 with the follitropin receptor depends on receptor activation and phosphorylation. Mol Cell Endocrinol 204: 127–140, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hebert TE. Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem 277: 35402–35410, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lederer ED, Sohi SS, McLeish KR. Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: role of ERK in sodium-phosphate cotransport. J Am Soc Nephrol 11: 222–231, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Luttrell LM, Daaka Y, Della Rocca GJ, Lefkowitz RJ. G protein-coupled receptors mediate two functionally distinct pathways of tyrosine phosphorylation in rat 1a fibroblasts. Shc phosphorylation and receptor endocytosis correlate with activation of Erk kinases. J Biol Chem 272: 31648–31656, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283: 655–661, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 98: 2449–2454, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. PKA-mediated phosphorylation of the beta1-adrenergic receptor promotes Gs/Gi switching. Cell Signal 16: 1397–1403, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Miao D, Tong XK, Chan GK, Panda D, McPherson PS, Goltzman D. Parathyroid hormone-related peptide stimulates osteogenic cell proliferation through protein kinase C activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem 276: 32204–32213, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Miedlich SU, Abou-Samra AB. Eliminating phosphorylation sites of the parathyroid hormone receptor type 1 differentially affects stimulation of phospholipase C and receptor internalization. Am J Physiol Endocrinol Metab 295: E665–E671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min L, Galet C, Ascoli M. The association of arrestin-3 with the human lutropin/choriogonadotropin receptor depends mostly on receptor activation rather than on receptor phosphorylation. J Biol Chem 277: 702–710, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Piketty V, Kara E, Guillou F, Reiter E, Crepieux P. Follicle-stimulating hormone (FSH) activates extracellular signal-regulated kinase phosphorylation independently of beta-arrestin- and dynamin-mediated FSH receptor internalization (Abstract). Reprod Biol Endocrinol 4: 33, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian F, Leung A, Abou-Samra A. Agonist-dependent phosphorylation of the parathyroid hormone/parathyroid hormone-related peptide receptor. Biochemistry 37: 6240–6246, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Qian H, Pipolo L, Thomas WG. Association of beta-Arrestin 1 with the type 1A angiotensin II receptor involves phosphorylation of the receptor carboxyl terminus and correlates with receptor internalization. Mol Endocrinol 15: 1706–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Rizzoli R, Bonjour JP. Effect of pertussis toxin on parathyroid hormone-stimulated cyclic AMP production in cultured kidney cells. J Bone Miner Res 3: 605–609, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Segre GV, Abou-Samra AB, Juppner H, Schipani E, Force T, Urena P, Freeman M, Kong XF, Kolakowski LF, Jr, Hock J. Characterization of cloned PTH/PTHrP receptors. J Endocrinol Invest 15: 11–17, 1992 [PubMed] [Google Scholar]
- 40.Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, Freissmuth M. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem 272: 5792–5799, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281: 1261–1273, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PH. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology 146: 2171–2175, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sneddon WB, Friedman PA. Beta-arrestin-dependent parathyroid hormone-stimulated extracellular signal-regulated kinase activation and parathyroid hormone type 1 receptor internalization. Endocrinology 148: 4073–4079, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Sneddon WB, Yang Y, Ba J, Harinstein LM, Friedman PA. Extracellular signal-regulated kinase activation by parathyroid hormone in distal tubule cells. Am J Physiol Renal Physiol 292: F1028–F1034, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Swarthout JT, Doggett TA, Lemker JL, Partridge NC. Stimulation of extracellular signal-regulated kinases and proliferation in rat osteoblastic cells by parathyroid hormone is protein kinase C-dependent. J Biol Chem 276: 7586–7592, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Syme CA, Friedman PA, Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J Biol Chem 280: 11281–11288, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Tang H, Nishishita T, Fitzgerald T, Landon EJ, Inagami T. Inhibition of AT1 receptor internalization by concanavalin A blocks angiotensin II-induced ERK activation in vascular smooth muscle cells. Involvement of epidermal growth factor receptor proteolysis but not AT1 receptor internalization. J Biol Chem 275: 13420–13426, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Tawfeek HA, Abou-Samra AB. Negative regulation of parathyroid hormone (PTH)-activated phospholipase C by PTH/PTH-related peptide receptor phosphorylation and protein kinase A. Endocrinology 149: 4016–4023, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tawfeek HA, Che J, Qian F, Abou-Samra AB. Parathyroid hormone receptor internalization is independent of protein kinase A and phospholipase C activation. Am J Physiol Endocrinol Metab 281: E545–E557, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Mol Endocrinol 16: 1–13, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278: 6258–6267, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Verheijen MH, Defize LH. Parathyroid hormone activates mitogen-activated protein kinase via a cAMP-mediated pathway independent of Ras. J Biol Chem 272: 3423–3429, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Vishnivetskiy SA, Paz CL, Schubert C, Hirsch JA, Sigler PB, Gurevich VV. How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem 274: 11451–11454, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Wan Y, Huang XY. Analysis of the Gs/mitogen-activated protein kinase pathway in mutant S49 cells. J Biol Chem 273: 14533–14537, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Barak LS, Anborgh PH, Laporte SA, Caron MG, Ferguson SS. Cellular trafficking of G protein-coupled receptor/beta-arrestin endocytic complexes. J Biol Chem 274: 10999–11006, 1999 [DOI] [PubMed] [Google Scholar]