Abstract
Sarcolipin (SLN) is a key regulator of sarco(endo)plasmic reticulum (SR) Ca2+-ATPase (SERCA), and its expression is altered in diseased atrial myocardium. To determine the precise role of SLN in atrial Ca2+ homeostasis, we developed a SLN knockout (sln−/−) mouse model and demonstrated that ablation of SLN enhances atrial SERCA pump activity. The present study is designed to determine the long-term effects of enhanced SERCA activity on atrial remodeling in the sln−/− mice. Calcium transient measurements show an increase in atrial SR Ca2+ load and twitch Ca2+ transients. Patch-clamping experiments demonstrate activation of the forward mode of sodium/calcium exchanger, increased L-type Ca2+ channel activity, and prolongation of action potential duration at 90% repolarization in the atrial myocytes of sln−/− mice. Spontaneous Ca2+ waves, delayed afterdepolarization, and triggered activities are frequent in the atrial myocytes of sln−/− mice. Furthermore, loss of SLN in atria is associated with increased interstitial fibrosis and altered expression of genes encoding collagen and other extracellular matrix proteins. Our results also show that the sln−/− mice are susceptible to atrial arrhythmias upon aging. Together, these findings indicate that ablation of SLN results in increased SERCA activity and SR Ca2+ load, which, in turn, could cause abnormal intracellular Ca2+ handling and atrial remodeling.
Keywords: fibrosis, action potential duration, calcium, sarcoplasmic reticulum Ca2+ adenosinetriphosphatase, arrhythmias
sarcolipin (sln), a 31-amino acid sarco(endo)plasmic reticulum (SR) membrane protein, is predominantly expressed in atria (2, 49). Studies on transgenic mouse models overexpressing SLN in the heart suggest that SLN is a key regulator of cardiac SR Ca2+-ATPase (SERCA) and mediator of β-adrenergic responses (1, 3, 20). Altered expression of SLN has been shown in atria of a diseased myocardium. The mRNA and protein levels of SLN are significantly decreased in the atrial myocardium of patients with chronic atrial fibrillation (AF) (39, 44). In atria of pacing-induced heart failure dogs, SLN protein expression is significantly increased, whereas, in atria of hearts prone to myocardial ischemia, SLN protein level is decreased (2). The expression levels of SERCA2a and phospholamban (PLN) are not altered in atria of human AF and in the above-mentioned animal models. Together, these studies suggest that SLN levels could affect the SERCA activity and intracellular Ca2+ (Ca2+i) handling in diseased atrial myocardium.
AF, the most common sustained arrhythmia, is associated with increased risk of stroke, symptoms of heart failure, and genetic disorders (29, 30, 36, 45). Several studies have suggested that abnormal Ca2+ handling is one of the major causes of atrial remodeling and AF (11, 14, 15, 52). The atrial tachycardia remodeling, which promotes AF, causes atrial contractile dysfunction, mainly via Ca2+-handling abnormalities (52). The reduced Ca2+ transients with slowed Ca2+ decay and reduced diastolic Ca2+ levels are suggested to cause atrial dilatation and AF (14, 42). Studies from a congestive heart failure dog model, which is susceptible to sustained AF, suggest that increased SR Ca2+ load may contribute to the generation of delayed afterdepolarizations (DAD) and triggered activities in the atrial myocytes (54). In mice expressing R176Q mutant ryanodine receptor 2 (RyR2), the Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated SR Ca2+ leak promotes AF (9). Thus alterations in the SR Ca2+ load and/or Ca2+ release mechanisms can contribute to atrial remodeling and contractile dysfunction.
Given that SLN is a key regulator of the cardiac SERCA pump (1, 3, 20), the selective downregulation of SLN in AF (39, 44) could affect the Ca2+i handling. In the present study, using a SLN knockout (sln−/−) mouse model (4), we determined if complete loss of SLN affects the Ca2+i handling and causes atrial remodeling.
MATERIALS AND METHODS
All animal procedures were performed with the approval of Institute Animal Care and Use Committee of the University of Medicine and Dentistry New Jersey-Newark campus, in accordance with the provision of the animal welfare act, the Public Health Service policy on Humane Care and Use of Laboratory Animals. Five to six- and twelve-mo-old sln−/− and C57/BL6 wild-type (WT) mice were used in this study. The Ca2+ measurements, electrophysiological, histopathological, and gene expression studies were carried out on 5- to 6-mo-old sln−/− and WT mice hearts.
Electrophysiological recording.
Generation of sln−/− mice was described previously (4). Atrial or ventricular myocytes were enzymatically isolated from WT and sln−/− mice hearts, as described earlier (38). All patch-clamp experiments were carried out at 34–36°C, using the ruptured (21) patch whole cell recording technique. For action potential (AP) recordings, patch pipettes (resistance 1–3 MΩ) were filled with internal solution containing (in mmol/l): 110 potassium-aspartate, 30 KCl, 5 NaCl, 10 HEPES, 0.1 EGTA, 5 MgATP, 5 Na2-phosphocreatine, 0.05 cAMP, pH 7.2 adjusted with KOH. The cells were superfused with Tyrode's solution containing (in mmol/l): 136 NaCl, 4.0 KCl, 0.33 Na2PO4, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4 adjusted with NaOH. APs were recorded under current clamp mode at pacing cycling length of 1 s. Whole cell currents were recorded under voltage-clamp configuration. For L-type Ca2+ current (ICa,L) recordings, patch pipettes were filled with internal solution containing (in mmol/l): 110 Cs-aspartate, 30 CsCl, 5 NaCl, 10 HEPES, 0.1 EGTA, 5 MgATP, 5 Na2-phosphocreatine, 0.05 cAMP, pH 7.2 adjusted with CsOH. The cells were perfused with a modified Tyrode's solution containing (in mmol/l): 120 NaCl, 20 tetraethylammonium-Cl, 0.33 Na2PO4, 1.0 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4 adjusted with NaOH. The myocytes were stimulated at a pacing cycling length of 6 s, with a double-pulse protocol. Following a 100-ms prepulse to −40 mV from the holding potential of −80 mV (to inactivate Na+ current and T-type Ca2+ current), ICa,L was elicited by a subsequent test depolarization step to 0 mV for 300 ms. For total outward K+ current (IK) recording, the pipette and superfusion solutions were the same as those for AP recording. Tetrodotoxin (10 μmol/l) and CdCl2 (0.5 mmol/l) were added into the Tyrode's solution to inhibit Na current and ICa,L. IK were elicited from a holding potential of −80 mV by a series of 400-ms test pulses from −40 to +40 mV in 10-mV increments. The inward Na+/Ca2+ exchange (NCX) current (INCX) was recorded by a rapid application of caffeine (10 mmol/l) for ∼2-s duration. The application of caffeine was always preceded by a set of ten 200-ms conditioning depolarizations from −40 to 0 mV to ensure a consistent degree of SR Ca2+ loading. A holding potential of −40 mV was maintained during caffeine application. Membrane current and voltage were measured with an Axopatch 200B patch-clamp amplifier controlled by a personal computer using a Digidata 1200 acquisition board, driven by pCLAMP 10 software (Axon Instruments).
SR Ca2+ load and Ca2+ transient measurements in isolated myocytes.
SR Ca2+ load and Ca2+ transient measurements using fluo 4-AM were carried out at 34–36°C, as described earlier (38). Myocytes were field stimulated at 0.5 Hz to maintain consistent SR Ca2+ load. The height of the 10 mmol/l caffeine-induced Ca2+ transient was used as a measure of total SR Ca2+ content. Fractional SR Ca2+ release was calculated by dividing the height of the last twitch transient by the height of the caffeine transient.
ECG recordings.
Standard lead II ECG was recorded from anesthetized mice with a differential amplifier (Warner DP301). The ECG signals were collected for a total duration of 30 min for each mouse, and all analyses were performed blinded to genotype.
Histopathology.
Myocardial tissues were sectioned at 6 μm and stained with hematoxylin and eosin, Masson's trichrome or Picro-Sirius Red. Fibrosis was identified as accumulation of collagen between the myofibrils. Quantitation of fibrosis in Sirius red stained images was carried out using Image-pro software.
Western blot analyses.
Total protein extracts from the WT and sln−/− mice atria were used for Western blot analyses using protein-specific antibodies, as described earlier (4). Signals detected by Super Signal WestDura substrate (Thermo Scientific) were quantitated by densitometry and then normalized to calsequestrin levels. To determine the basal phosphorylation of PLN and RyR, the blots were immunoprobed with phospho-serine (S) 16 PLN (Cyclacel, Dundee, U.), phospho S2808 RyR (Abcam, cat. no. ab59225) or S2814(26) antibody (a kind gift from Dr. Xander H. T. Wehrens, Texas). After the signals were detected, the membranes were stripped and reprobed with regular PLN or RyR antibody.
Microarray analysis.
Gene expression profiling was carried out using Affymetrix GeneChip Mouse Gene 1.0 ST Array. Microarray data were submitted to Gene Expression Omnibus with the accession number GSE23403. Microarray data normalized by the robust multichip analysis method were subjected to significant analysis of microarrays. Significant genes were selected using fold change >1.2 and false discovery rate < 5%. Gene expression changes were analyzed by the hierarchical clustering method using the MultiExperiment Viewer program (http://www.tm4.org/mev.html). Regulated genes were analyzed by their Gene Ontology (GO) annotations. A hypergeometric test was used to assess the significance of association of a particular GO term with a set of up- or downregulated genes. To avoid redundant GO terms, we removed those with >70% of genes overlapped with a more significant GO term.
Quantitative real-time PCR.
First-strand cDNA was synthesized using total RNA prepared from WT and sln−/− mice atria with the high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative real-time (qRT)-PCR was performed using Applied Biosystems 7500 RT-PCR system with Power SYBR Green Mastermix following the manufacturer's instructions. The data were analyzed by 7500 software version 2.0.2. Normalization of the signal was carried out based on the expression of cyclophilin D. The primers for qRT-PCR used were as follows: bone morphogenetic protein (BMP) 2 (forward 5′ GTTTGGCCTGAAGCAGAGAC 3′; reverse 5′ CGTCACTGGGGACAGAACTT 3′), BMP5 (forward 5′ TTCAAGGCAAGCGAGGTACT 3′; reverse 5′ GAAAAGAACATTCCCCGTCA 3′), cystatin 9 (forward 5′ CCGTGAACACATTCAACCAG 3′; reverse 5′ GGGTGACTCCCATCAGAGAA 3′), cathepsin K (forward 5′ AGGGAAGCAAGCACTGGATA 3′; reverse 5′ AGCACCAACGAGAGGAGAAA 3′), connexin 40 (forward 5′ GCCACCAAGTTCAGTGGAAT 3′; reverse 5′ TGCTATCACCACACCTCTCG 3′), matrix metalloproteinase 3 (MMP3) (forward 5′ CAGACTTGTCCCGTTTCCAT 3′; reverse 5′ GGAAGAGATGGCCAAAATGA 3′), S100A1 (forward 5′ GCCCTTCTGTCGAGAATCTG 3′; reverse 5′ TTGAAGTCCACTTCCCCATC 3′), and tissue inhibitor of MMP4 (forward 5′ ACCTCCGGAAGGAGTACGTT 3′; reverse 5′ CTGAGGGCAAGGCAGATAAG 3′).
Statistical analysis.
Data are presented as means ± SE. Statistical significance was assessed using paired, unpaired Student's t-tests, ANOVA analysis, or Fisher's exact test, with P < 0.05 considered significant.
RESULTS
Loss of SLN results in increased SR Ca2+ load in the atrial myocytes.
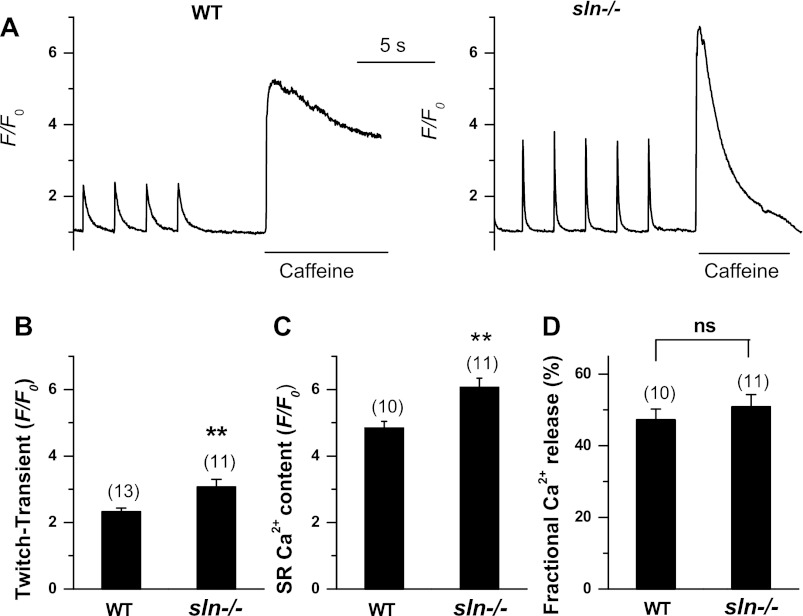
We first determined the effect of enhanced SERCA pump activity (4) on SR Ca2+ load and Ca2+ transients in the atrial myocytes of sln−/− mice. As shown in Fig. 1, A and B, the twitch Ca2+ transient was significantly increased in the sln−/− atrial myocytes (ratio of fluorescence over the basal diastolic fluorescence: WT = 2.2 ± 0.1, n = 13 vs. sln−/− = 3.1 ± 0.2, n = 11, P < 0.01). The fractional Ca2+ release was the same in WT and sln−/− atrial myocytes (Fig. 1D). The SR Ca2+ content as measured by caffeine-induced Ca2+ transient was significantly higher in the sln−/− atrial myocytes (Fig. 1, A and C; ratio of fluorescence over the basal diastolic fluorescence: WT = 4.7 ± 0.1, n = 10 vs. sln−/− = 6.1 ± 0.3, n = 11, P < 0.01). The Ca2+ transient (4) and SR Ca2+ load (data not shown) in the ventricular myocytes of sln−/− mice were not significantly different from that of WT mice.
Fig. 1.
Ca2+ transient amplitude and sarco(endo)plasmic reticulum (SR) Ca2+ content in sarcolipin knockout (sln−/−) atrial myocytes. A: twitch Ca2+ transients elicited by field stimulation at 0.5 Hz, and Ca2+ content measured as the height of the caffeine (Caff; 10 mmol/l)-induced Ca2+ transient in a representative atrial myocyte from wild-type (WT) and sln−/− mice. Ca2+ fluorescence intensity was recorded as the ratio F/F0 of the fluorescence (F) over the basal diastolic fluorescence (F0). B: summarized data for twitch Ca2+ transient in atrial myocytes. C: summarized data for SR Ca2+ contents in WT and sln−/− atrial myocytes. D: summarized data for fractional SR Ca2+ release, calculated by dividing the height of the last twitch transient by the height of the Caff transient. Values are means ± SE; no. of cells analyzed is indicated above each bar. **P < 0.05 compared with WT control myocytes. ns, Not significant.
Atrial myocytes from the sln−/− mice show prolonged AP durations.
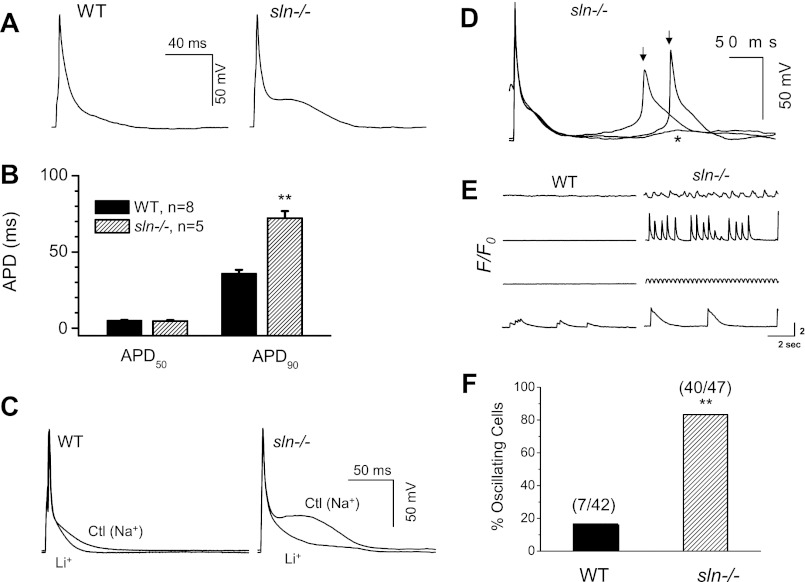
To determine whether the increased Ca2+ transients and SR Ca2+ load affect the cellular electrical properties, we measured the AP in isolated atrial myocytes. Results in Fig. 2A indicate that there is a substantial difference in the morphology of APs between WT and sln−/− atrial myocytes. An apparent late plateau was observed after the rapid repolarization phase in the sln−/− atrial myocytes, causing prolongation of the AP duration (APD) at 90% repolarization (APD90). There was no change in the APD at 50% repolarization (summarized in Fig. 2B).
Fig. 2.
Alternations of action potential (AP) morphology, occurrences of delayed afterdepolarizations (DADs), triggered activities, and spontaneous Ca2+ release in the atrial myocytes of sln−/− mice. A: representative AP recordings from the atrial myocytes of WT and sln−/− mice. Note the apparent late plateau phase in the AP recorded from sln−/− atrial myocyte. B: summary of AP duration (APD) values. The APD at 90% repolarization (APD90) is prolonged in atrial myocytes of sln−/− mice. C: comparison of the effects of Na+-free, Li+-containing Tyrode's solution on the AP properties, especially the late plateau in sln−/− and WT mice atrial myocytes. D: DAD (*) and triggered activities (↓) appear in atrial myocytes from sln−/− mice. E: representative traces of intracellular Ca2+ concentration fluorescence from WT and sln−/− atrial myocyte showing different incidences of spontaneous Ca2+ release (4 cells for each). F: percentage of oscillating atrial cells with spontaneous Ca2+ release in the sln−/− and WT mice. Values are means ± SE; no. of cells analyzed is indicated above each bar. **Significantly different from the WT atrial myocytes, P < 0.05 by Fisher's exact test.
To investigate if the INCX contributes to the late plateau and prolongation of late APD, we inhibited the forward mode of INCX by replacing Na+ with Li+ in the perfusate Tyrode's solution. The Li+ solution suppresses the late plateau and prevents the prolongation of APD90 (Fig. 2C). Together, these results suggest that the activation of forward mode of INCX can contribute to the late plateau and prolongation of APD90 in the atrial myocytes of sln−/− mice.
Spontaneous Ca2+ waves and triggered activities in the atrial myocytes of sln−/− mice.
Afterdepolarizations and triggered activities are the major causes of cardiac arrhythmias, such as atrial and ventricular fibrillation (9, 41, 51, 53). Our results show that DADs and triggered activities were more frequent in the atrial myocytes of sln−/− mice (Fig. 2D). The SR Ca2+ overload and spontaneous Ca2+ release can contribute to the transient inward currents, which cause DADs. Therefore, we calculated the percentage of spontaneously beating cells by monitoring spontaneous Ca2+ release (without stimulation). Results (Fig. 2, E and F) demonstrate that the number of atrial myocytes showing spontaneous Ca2+ waves/oscillations was abundant in the sln−/− mice (P < 0.05 by Fisher's exact test).
Increased ICa,L density in the atrial myocytes of sln−/− mice.
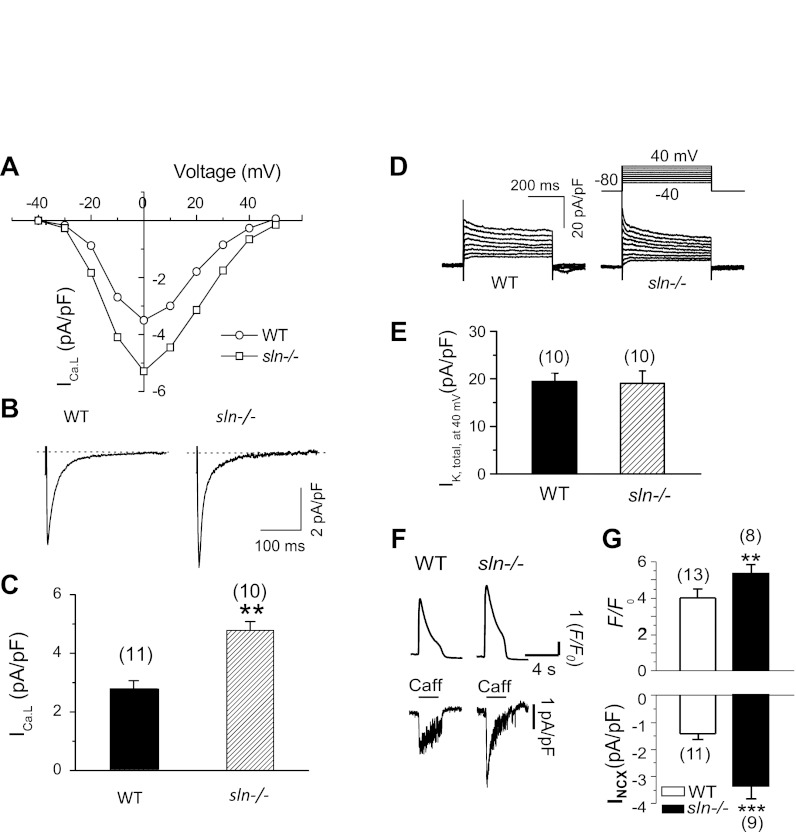
The ICa,L (8, 10, 12, 47) and IK (8, 12, 13, 16, 48) are the two major current components determining the APD. We therefore, evaluated the current density level of ICa,L and total IK (IK,total). The currents were recorded from 10–11 cells isolated from 4 mice in each group (WT vs. sln−/−). As shown in Fig. 3, A–C, the ICa,L density was increased in the atrial myocytes of sln−/− mice (pA/pF: WT = 2.8 ± 0.3 vs. sln−/− = 4.8 ± 0.3; P < 0.05). However, there was no alternation in current kinetics (e.g., current-voltage curve or inactivation). The outward IK,total densities were also not altered in the sln−/− atrial myocytes (Fig. 3, D and E). It should be noted that the IK,total showed less inactivation component, since the pipette solution had a low concentration of Ca2+ buffer. This phenomenon was consistent with a previous report on transient outward current modulation by CaMKII in human atrial cells (43).
Fig. 3.
L-type Ca2+ current (ICa,L), Na+/Ca2+ exchange current (INCX), and total outward K currents in the sln−/− atrial myocytes. A: representative current-voltage curve. B: ICa,L traces elicited at testing potential of 0 mV. C: summary of ICa,L densities. D: representative traces elicited with the voltage-clamp protocol shown. E: peak K currents at test potential of +40 mV in WT and sln−/− atrial myocytes. IK,total, total K current. F: representative tracings of Ca2+ transients (top) and INCX (bottom) after rapid application of Caff in atrial myocytes isolated from sln−/− or WT control hearts. G: average values of peak Ca2+ transient (F/F0, top) and peak INCX densities (bottom). Values are means ± SE; no. of cells analyzed is indicated above each bar. **P < 0.05; ***P < 0.01, sln−/− vs WT.
Increased INCX density in the atrial myocytes of sln−/− mice.
To obtain direct evidence for potential upregulation of INCX in the atrial myocytes of sln−/− mice, we measured INCX and SR Ca2+ release in response to caffeine (10 mmol/l) application. Results show that the amplitudes of both Ca2+ transients and peak INCX density were significantly increased in the atrial myocytes of sln−/− mice (Fig. 3, F and G). These results were consistent with AP morphology alteration, as shown in Fig. 2, A–C.
To determine cell size, we calculated the membrane capacitance, an index of cell surface area. The membrane capacitance of atrial myocytes isolated from the sln−/− mice atria were not different from that of atrial myocytes from WT mice (WT: 49.6 ± 2.1 pF vs. sln−/−: 54.5 ± 3.1 pF).
Increased RyR phosphorylation in the sln−/− atria.
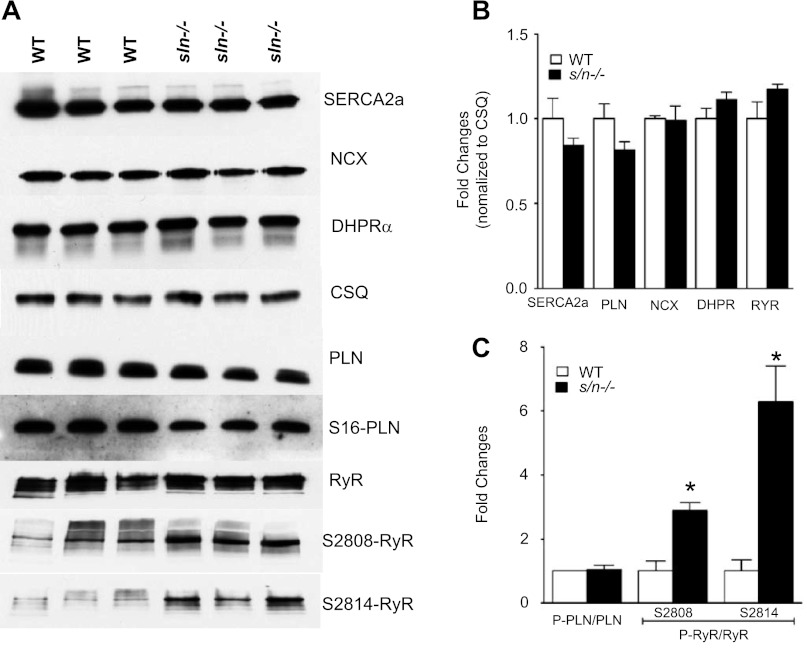
Next, we examined if altered expression of NCX or L-type calcium channel contributes to the changes in INCX or ICa,L activity. In addition, we quantitated the SERCA2a, PLN, calsequestrin (CSQ), and RyR protein levels. Our results show that the protein levels of SERCA2a, PLN, RyR, NCX CSQ, and the L-type calcium channel subunit, dihydropyridine receptor-α, were not altered in the sln−/− mice atria compared with that of age- and sex-matched WT control mice (Fig. 4, A and B).
Fig. 4.
Western blot analysis of Ca2+ handling proteins in the sln−/− mice atria. A: Western blots showing the protein levels of SR Ca2+-ATPase (SERCA) 2a, calsequestrin (CSQ), NCX, dihydropyridine receptor (DHPR-α), phospholamban (PLN), and ryanodine receptor (RyR). B: the fold change in expression levels of these proteins. The expression levels are normalized to CSQ levels. C: the ratios of serine 16 phosphorylated PLN (PS16-PLN) to total PLN and serine 2808 or 2814 phosphorylated RyR to total RyR; n = 3. Values are means ± SE. *Significantly different from the WT mice atria, P < 0.01.
To determine the phosphorylation status of PLN and RyR in the sln−/− atria, we measured the S16 phosphorylation of PLN and S2808 and S2814 phosphorylation of RyR by Western blot analysis using phosphor-specific antibodies. Results in Fig. 4, A and C, show that the basal phosphorylation of PLN was not altered, whereas the basal phosphorylation of RyR both at S2808 (2.87 ± 0.2-fold, P < 0.05) and S2814 (6.29 ± 1.14-fold, P < 0.05) was significantly increased in atria of sln−/− mice.
Atrial fibrosis in the sln−/− mice.
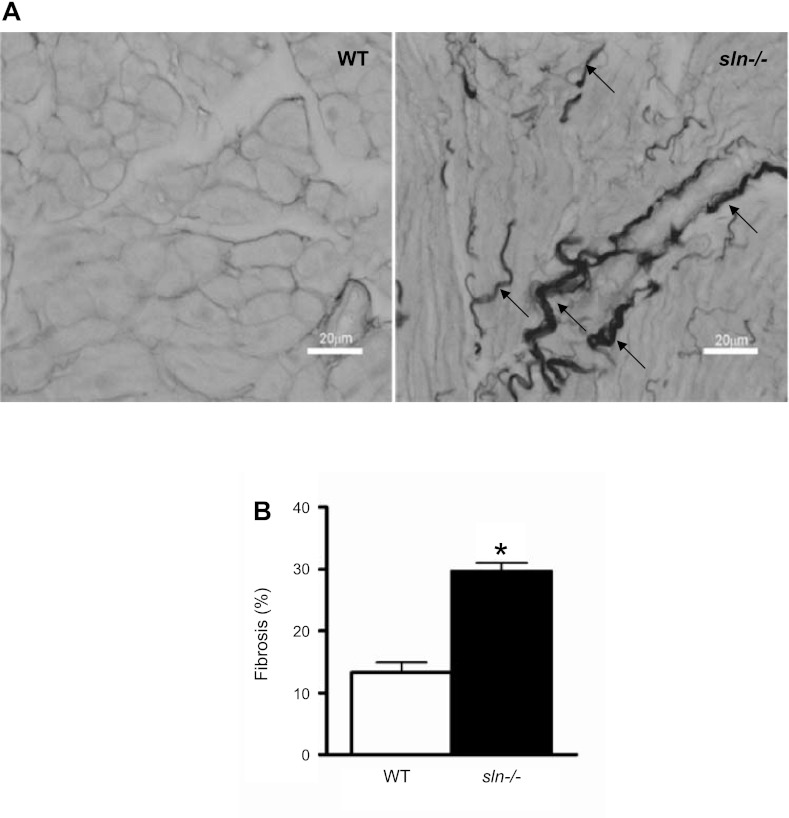
Ablation of SLN caused no apparent hypertrophic effect. The sizes of the sln−/− atria and atrial myocytes were not significantly different from that of age- and sex-matched WT control mice (data not shown). Hematoxylin and eosin staining showed no apparent pathological changes in the sln−/− atria. Masson's trichrome staining, on the other hand, showed increased fibrosis (data not shown). The Sirius Red staining that marks collagen revealed that the sln−/− atria has increased amount of interstitial fibrosis, as indicated by thick strands between the fibers (WT = 13.4 ± 1.5% vs. sln−/− = 29.7 ± 1.3%; P < 0.0001; n = 5; Fig. 5). There was no significant difference in Sirius Red staining between the ventricles of WT and sln−/− mice (data not shown).
Fig. 5.
Increased interstitial fibrosis in the sln−/− atria. A: representative images showing the Picro Sirius Red staining of atrial tissues from 5-mo-old WT and sln−/− mice. Original magnifications ×400. Bar represents 20 μm. Arrow indicates the collagen accumulation between the fibers. B: quantitation of Sirius Red stained connective tissue in atria of WT and sln−/− mice. Values are means ± SE; n = 5. *P < 0.0001.
Gene expression changes in the sln−/− atria mimic AF phenotype.
Gene expression profiling was carried out using Affymetrix GeneChip Mouse Gene 1.0 ST Array. Among 1,415 genes, 400 genes were upregulated, and 1,011 genes were downregulated (significant analysis of microarrays fold change >1.2; q value <0.05) in atria of sln−/− mice. Significantly regulated genes were classified using the GO database. These genes were in three major ontology categories, such as biological process, cellular component, and molecular function (Table 1). The GO analysis showed that downregulated genes tend to be associated with immune responses, fatty acid metabolism, and G protein-coupled receptor protein signaling pathway, whereas those upregulated tend to be associated with ion channel activity.
Table 1.
Significant Gene Ontology terms associated with differentially expressed genes in the sln−/− mice atria
| Association With Upregulated Genes | Association With Downregulated Genes | |
|---|---|---|
| Biological process | ||
| GO:0043933, macromolecular complex subunit organization | 9.05E-03 | 9.57E-01 |
| GO:0043085, positive regulation of catalytic activity | 8.43E-04 | 9.18E-01 |
| GO:0006457, protein folding | NA | 4.74E-03 |
| GO:0007186, G protein-coupled receptor protein signaling pathway | 1.45E-01 | 3.79E-03 |
| GO:0006629, lipid metabolic process | 7.32E-01 | 2.22E-03 |
| GO:0006633, fatty acid biosynthetic process | 7.30E-01 | 8.65E-04 |
| GO:0006952, defense response | 9.94E-01 | 3.52E-04 |
| GO:0006955, immune response | 9.97E-01 | 9.95E-07 |
| Cellular component | ||
| GO:0009986, cell surface | 5.02E-01 | 1.38E-03 |
| GO:0005783, endoplasmic reticulum | 9.07E-01 | 1.63E-04 |
| GO:0005615, extracellular space | 1.18E-01 | 1.38E-04 |
| Molecular function | ||
| GO:0005244, voltage-gated ion channel activity | 9.11E-03 | 8.89E-01 |
| GO:0005102, receptor binding | 2.55E-01 | 1.07E-03 |
| GO:0016860, intramolecular oxidoreductase activity | NA | 8.84E-05 |
| GO:0004930, G protein-coupled receptor activity | 4.74E-01 | 4.62E-05 |
Three Gene Ontology (GO) categories are shown, i.e., biological process, cellular component, and molecular function. P values indicating significance of association with up- and downregulated genes were derived from hypergeometirc test. Redundant GO terms were removed. See materials and methods for details. NA indicates no genes associated with a GO term.
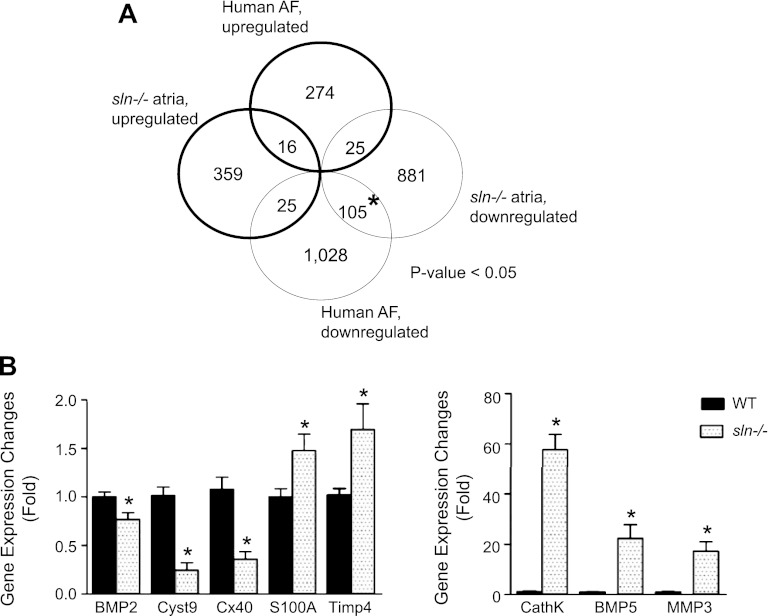
Next, we compared gene expression changes to the available human AF gene expression profiling (GEO accession no. GSE2240). Results show that ∼105 genes were significantly downregulated, and 25 genes were upregulated in common in the atria of human AF and sln−/− mice (Fig. 6A). We also found that the expression of procollagens type I, III, IV, V, VI, VIII, XIV, and XV; inflammatory genes like cathepsin K; MMPs such as MMP3, tissue inhibitor of MMP4; cytokines like BMP5; and S100A1 were significantly upregulated. On the other hand, the extracellular matrix protein connexin 40, and cystatin 9, an inhibitor of cathepsin, were selectively downregulated in the sln−/− mice atria (Table 2). To validate the microarray data, qRT-PCR analysis of selected genes was carried out. Results in Fig. 6B confirm and validate the microarray data. Together, these results indicate that gene expression changes in the sln−/− mice atria mimic gene expression profiling reported for human and various animal models of AF (27, 33, 37).
Fig. 6.
Genomewide analysis of gene expression regulation in sln−/− atria. A: comparative analysis of significantly regulated genes in atria of sln−/− mice (GEO accession no. GSE23403) vs. those in human patients with atrial fibrillation (AF) (GEO accession no. GSE2240). Significant genes were selected using cutoffs of fold change of 1.2 and 5% false discovery rate (significant analysis of microarrays). A P value (χ2 test) indicating significance of the numbers of overlapped genes is shown in the graph. *Statistically overrepresented. B: quantitative RT-PCR analyses of selected genes. BMP, bone morphogenetic protein; Cyst9, cystatin 9; Cx40, connexin 40; Timp4, tissue inhibitor of MMP4; CathK, cathepsin K; MMP3, matrix metalloproteinase 3. Values are means ± SE; n = 5. *Significant difference vs. WT, P < 0.05.
Table 2.
Expression profiles of collagen isoforms and extracellular matrix protein genes in the sln−/− mice atria
| Gene ID | GSB | Fold Change | P Value | Gene Description |
|---|---|---|---|---|
| 12156 | Bmp2 | −1.64 | 1.86E-02 | Bone morphogenetic protein 2 |
| 12160 | Bmp5 | 3.03 | 3.11E-02 | Bone morphogenetic protein 5 |
| 12818 | Col14a1 | 1.49 | 2.92E-01 | Collagen, type XIV, α1 |
| 12819 | Col15a1 | 1.84 | 1.75E-01 | Collagen, type XV, α1 |
| 12842 | Col1a1 | 1.71 | 3.50E-01 | Collagen, type I, α1 |
| 12843 | Col1a2 | 1.55 | 3.31E-01 | Collagen, type I, α2 |
| 12825 | Col3a1 | 2.07 | 3.22E-01 | Collagen, type III, α1 |
| 12826 | Col4a1 | 2.16 | 1.22E-01 | Collagen, type IV, α1 |
| 12827 | Col4a2 | 2.06 | 1.62E-01 | Collagen, type IV, α2 |
| 68018 | Col4a3bp | −1.16 | 1.86E-01 | Collagen, type IV, α3 |
| 12829 | Col4a4 | 1.19 | 3.58E-02 | Collagen, type IV, α4 |
| 12830 | Col4a5 | 1.31 | 1.73E-01 | Collagen, type IV, α5 |
| 94216 | Col4a6 | 1.07 | 3.01E-01 | Collagen, type IV, α6 |
| 12831 | Col5a1 | 1.35 | 2.96E-01 | Collagen, type V, α1 |
| 12833 | Col6a1 | 1.77 | 2.82E-01 | Collagen, type VI, α1 |
| 245026 | Col6a6 | −1.07 | 2.61E-01 | Collagen, type VI, α6 |
| 245026 | Col6a6 | −1.16 | 1.86E-01 | Collagen, type VI, α6 |
| 13013 | Cst9 | −3.43 | 7.06E-03 | Cystatin 9 |
| 13038 | Ctsk | 4.14 | 2.77E-03 | Cathepsin K |
| 14613 | Gja5 | −1.82 | 7.80E-03 | Gap junction protein, α5 |
| 17392 | Mmp3 | 2.27 | 4.55E-02 | Matrix metallopeptidase 3 |
| 20193 | S100a1 | 1.40 | 4.50E-03 | S100 calcium binding protein A1 |
| 110595 | Timp4 | 1.96 | 9.07E-03 | Tissue inhibitor of metalloproteinase 4 |
GSB, Gene Set Builder.
The sln−/− mice are susceptible to atrial arrhythmias upon aging.
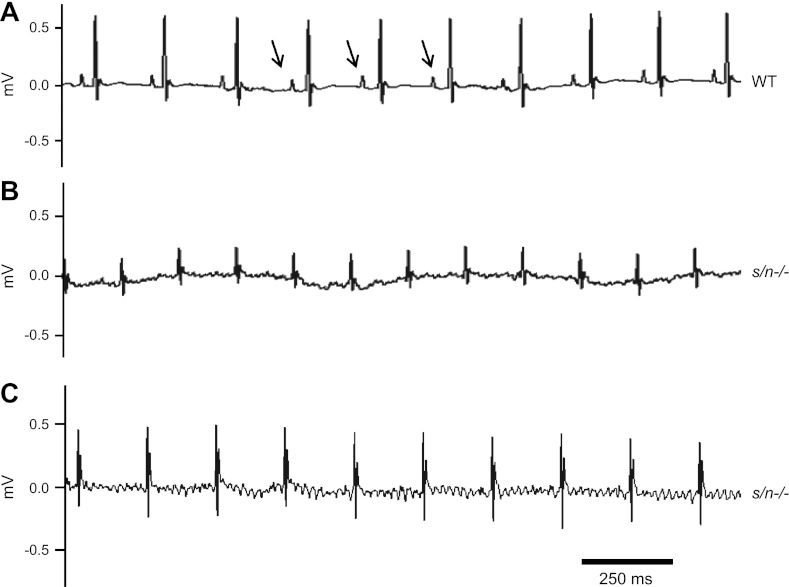
At 5 mo of age, the WT and sln−/− mice showed normal ECG pattern (data not shown). ECG recordings from the 12-mo-old WT mice showed normal P waves and QRS complexes (Fig. 7A). There were no atrial flutters or fibrillations observed in the WT mice (n = 7). However, in some (4 out of 6) of the 12-mo-old sln−/− mice, the P waves were completely replaced with small oscillatory waves (Fig. 7B), similar to that of AF/flutters. Some recordings (Fig. 7C) showed typical atrial flutter behavior associated with rather regular R-R intervals, but are likened to a sawtooth pattern at baseline (18). The means of the R-R intervals for the two groups (WT and sln−/−) of mice were not significantly different (data not shown) under the anesthetic conditions used for ECG recordings.
Fig. 7.
ECG recordings of 12-mo-old sln−/− mice. Representative surface ECG lead II recordings from 12-mo-old WT (A) and sln−/− (B and C) mice are shown. Arrows indicate the P waves.
DISCUSSION
In this study, using the sln−/− mouse model (4), we investigated the effect of enhanced atrial SERCA pump activity on cellular electrophysiology and structural remodeling. The key findings demonstrate that ablation of SLN in atria results in 1) increased SR Ca2+ load, Ca2+ transients, and spontaneous Ca2+ oscillation; 2) prolongation of APD due to the activation of forward mode of INCX; 3) increased ICa,L channel activity; and 4) structural remodeling and gene expression changes. Furthermore, our findings suggest that the sln−/− mice are susceptible to spontaneous atrial arrhythmias upon aging.
It is well documented that SERCA pump plays a significant role in maintaining the intracellular Ca2+ concentration ([Ca2+]i) in the heart (31, 32). SLN is a key regulator of the cardiac SERCA pump (1, 3, 5, 20), and its expression decreased in atria of patients with chronic AF (39, 44). To determine the consequences of SLN downregulation in SR Ca2+ handling, we generated a sln−/− mouse model (4). The data from the present study and an earlier publication (4) demonstrate that loss of SLN did not affect the Ca2+ transients and SR Ca2+ load in the ventricles, since it is a minor component. On the other hand, loss of SLN enhanced SERCA activity, caused SR Ca2+ overload, and increased both Ca2+ transients and INCX in atria. These observations, along with studies on transgenic mice overexpressing SLN (1, 3, 20), indicate that SLN levels can affect the SR Ca2+ cycling and SR Ca2+ load. Thus the downregulation of SLN in AF (39, 44) could contribute to the abnormal Ca2+i handling through enhanced SERCA pump activity and SR Ca2+ overload.
In addition to SR Ca2+ overload, spontaneous Ca2+ release, DADs, and triggered activities were more frequent in the atrial myocytes of sln−/− mice. The SR Ca2+ leak due to hyperphosphorylation of RyR2 by protein kinase A (PKA) is suggested to play an important role in atrial arrhythmogenesis (24, 50). A recent study demonstrates that an increase in CaMKII phosphorylation of RyR at S2814 can also enhances the SR Ca2+ leak and contributes to AF initiation in FKBP12.6 knockout mice (26). The increased basal phosphorylation of RyR, both at S2808 (a PKA target site) and S2814 (a CaMKII site) in atria of the sln−/− mice, suggests that hyperphosphorylated RyR may contribute to increased SR Ca2+ leak and spontaneous Ca2+ waves. It has been shown that the enhanced SR Ca2+ leak is sufficient to induce atrial arrhythmias without altering the SR Ca2+ load in human AF (23) and in a FKBP12.6-deficient mouse model (40). However, in congestive heart failure dogs, the generation of DADs and triggered activities were associated with higher SR Ca2+ load in atria (54). Similarly, atrial arrhythmias observed during acidosis were reported to be associated with an increase in SR Ca2+ load (35). Thus the enhanced SR Ca2+ load and spontaneous Ca2+ release could contribute to the generation of DADs and triggered activities via upregulated INCX in the sln−/− atrial myocytes.
The activation of the forward mode of INCX is attributable to the late plateau and prolongation of APD90 in the atrial myocytes of sln−/− mice. These observations, along with our laboratory's earlier studies showing enhanced SERCA pump activity and Ca2+ transients in the sln−/− mice atria (4), suggest that the high level of [Ca2+]i may promote inward INCX. The NCX levels have been shown to affect the APD and the late plateau phase (22, 34). However, the NCX protein level was not altered in the sln−/− atria (Fig. 4), suggesting that the increased INCX may be due to the functional modifications of NCX protein (6). Together, our studies demonstrate that alterations in Ca2+i cycling due to the enhanced SERCA pump activity and Ca2+ transients can cause abnormal APD dynamics via INCX.
The ICa,L activity was significantly increased in the sln−/− atrial myocytes. At present, we do not know the precise molecular mechanisms that enhance the ICa,L activity and its role in atrial remodeling in the sln−/− mice. PKA is shown to preassociate with ICa,L and RyRs, and thus the PKA-dependent phosphorylation can increases the ICa,L (55) and RyR (28) opening. However, a recent study demonstrated that PKA phosphorylation of S1928 is not functionally involved in β-adrenergic regulation of ICa,L (25). On the other hand, CaMKII-dependent phosphorylation of Cav1.2 at S1512 and S1570 has been shown to mediate the Ca2+ current facilitation and was suggested to contribute to arrhythmogenesis (7). Since the protein levels of dihydropyridine receptor-α were not altered (Fig. 4), we speculate that the upregulation of ICa,L activity could be through enhanced CaMKII-mediated phosphorylation. The increased ICa,L activity may be an adaptive mechanism to compensate the sustained activation of atrial SERCA pump in the absence of SLN and to maintain the cytosolic Ca2+ levels and contractile function of the heart. The increase in ICa,L activity could also trigger more RyR Ca2+ release and enhance the amplitude of Ca2+ transient, to overcome the shortening of Ca2+ transient duration.
In AF, electrical remodeling of atrial myocytes is characterized by shortening of APD, decreased SR Ca2+ content, ICa,L, and outward IK (46). On the other hand, the atrial myocytes of sln−/− mice exhibited increased SR Ca2+ load, Ca2+ transient, and ICa,L activity. Furthermore, these mice are susceptible to spontaneous atrial arrhythmias upon aging. Consistent with our findings, a recent study demonstrated that the enhanced Ca2+ cycling, including increased SR Ca2+ load, Ca2+ transient, and ICa,L, can promote AF (54). Although we do not have a ready explanation for these discrepancies, atrial arrhythmias may correlate to either enhanced or decreased ICa,L activity.
In addition to ion channel remodeling, the abnormal Ca2+i handling in the sln−/− atria induces gene expression changes. Some of the altered genes, in particular the key extracellular matrix protein genes, including collagens, fibrillin, and connexin 40, can contribute to the atrial structural remodeling in the sln−/− mice. The increased expression of transforming growth factor-β mRNA in the sln−/− atria suggests that the activation of transforming growth factor-β pathway could be involved in atrial fibrogenesis (17, 19) in the sln−/− mice.
Taken together, our studies demonstrate that structural and electrophysiological changes in the sln−/− mice atria mimic AF-associated atrial remodeling. It is also important to note that some (4 out of 6) of the 12-mo-old sln−/− mice developed spontaneous AFs/flutters, suggesting that the sln−/− mice may be susceptible to atrial arrhythmias. However, further studies using telemetry to detect AFs/flutters in both young and old sln−/− mice are warranted to validate these findings.
In conclusion, our studies indicate that SLN play a key role in maintaining atrial Ca2+ homeostasis. Loss of SLN function can enhance atrial SERCA pump activity and SR Ca2+ load, which, in turn, could cause abnormal Ca2+i handling and subsequent atrial remodeling, characterized by changes in ion channel function and altered gene expression.
GRANTS
This work was supported by funds from the Department of Cell Biology and Molecular Medicine, University of Medicine and Dentistry of New Jersey (UMDNJ)-New Jersey Medical School, Newark, NJ, and a UMDNJ Foundation grant (to G. J. Babu). M. Periasamy is supported by a National Heart, Lung, and Blood Institute (NHLBI) Grant (HL 080551). L.-H. Xie is supported by a NHLBI Grant (HL097878).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.-H.X. and G.J.B. conception and design of research; L.-H.X., M.S., J.Y.P., Z.Z., H.W., and G.J.B. performed experiments; L.-H.X., M.S., J.Y.P., Z.Z., B.T., and G.J.B. analyzed data; L.-H.X., M.P., and G.J.B. interpreted results of experiments; L.-H.X. and G.J.B. prepared figures; L.-H.X. and G.J.B. drafted manuscript; L.-H.X., B.T., M.P., and G.J.B. edited and revised manuscript; L.-H.X., M.S., J.Y.P., Z.Z., H.W., B.T., M.P., and G.J.B. approved final version of manuscript.
REFERENCES
- 1. Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini AO, Trivieri MG, Oudit GY, Morita T, Kusakari Y, Hirano S, Hongo K, Hirotani S, Yamaguchi O, Peterson A, Backx PH, Kurihara S, Hori M, MacLennan DH. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci U S A 101: 9199–9204, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol 43: 215–222, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 281: 3972–3979, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A 104: 17867–17872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babu GJ, Zheng Z, Natarajan P, Wheeler D, Janssen PM, Periasamy M. Overexpression of sarcolipin decreases myocyte contractility and calcium transient. Cardiovasc Res 65: 177–186, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bers DM, Despa S. Na+ transport in cardiac myocytes. Implications for excitation-contraction coupling. IUBMB Life 61: 215–221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaich A, Welling A, Fischer S, Wegener JW, Kostner K, Hofmann F, Moosmang S. Facilitation of murine cardiac L-type Ca(v)1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci U S A 107: 10285–10289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res 44: 121–131, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119: 1940–1951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation 110: 2651–2657, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Dobrev D. Atrial Ca(2+) signaling in atrial fibrillation as an antiarrhythmic drug target. Naunyn Schmiedebergs Arch Pharmacol 381: 195–206, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Dobrev D. Electrical remodeling in atrial fibrillation. Herz 31: 108–112; quiz 142–103, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation 112: 3697–3706, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J Cardiovasc Pharmacol 52: 293–299, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Dobrev D, Teos LY, Lederer WJ. Unique atrial myocyte Ca2+ signaling. J Mol Cell Cardiol 46: 448–451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol 557: 583–597, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Everett TH, 4th, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm 4: S24–S27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedrichs GS. Experimental models of atrial fibrillation/flutter. J Pharmacol Toxicol Methods 43: 117–123, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Gramley F, Lorenzen J, Koellensperger E, Kettering K, Weiss C, Munzel T. Atrial fibrosis and atrial fibrillation. The role of the TGF-beta(1) signaling pathway. Int J Cardiol 143: 405–413, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Gramolini AO, Trivieri MG, Oudit GY, Kislinger T, Li W, Patel MM, Emili A, Kranias EG, Backx PH, Maclennan DH. Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc Natl Acad Sci U S A 103: 2446–2451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110: 1358–1363, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kushnir A, Marks AR. The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol 59: 1–30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Cav 1.2 phosphorylation site S1928A mutant mice. J Biol Chem 283: 34738–34744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res 110: 465–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin CS, Pan CH. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol Life Sci 65: 1489–1508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1: 62–73, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Olgin JE, Verheule S. Transgenic and knockout mouse models of atrial arrhythmias. Cardiovasc Res 54: 280–286, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res 77: 265–273, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol 33: 1053–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med 12: 189–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pott C, Goldhaber JI, Philipson KD. Homozygous overexpression of the Na+-Ca2+ exchanger in mice: evidence for increased transsarcolemmal Ca2+ fluxes. Ann N Y Acad Sci 1099: 310–314, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol 295: H1669–H1683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91: 265–325, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 80: 9–19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shanmugam M, Gao S, Hong C, Fefelova N, Nowycky MC, Xie LH, Periasamy M, Babu GJ. Ablation of phospholamban and sarcolipin results in cardiac hypertrophy and decreased cardiac contractility. Cardiovasc Res 89: 353–361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shanmugam M, Molina CE, Gao S, Severac-Bastide R, Fischmeister R, Babu GJ. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca(2+) uptake in human atrial fibrillation. Biochem Biophys Res Commun 410: 97–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm 5: 1047–1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spencer CI, Sham JS. Effects of Na+/Ca2+ exchange induced by SR Ca2+ release on action potentials and afterdepolarizations in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 285: H2552–H2562, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Sun H, Gaspo R, Leblanc N, Nattel S. Cellular mechanisms of atrial contractile dysfunction caused by sustained atrial tachycardia. Circulation 98: 719–727, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res 85: 810–819, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Uemura N, Ohkusa T, Hamano K, Nakagome M, Hori H, Shimizu M, Matsuzaki M, Mochizuki S, Minamisawa S, Ishikawa Y. Down-regulation of sarcolipin mRNA expression in chronic atrial fibrillation. Eur J Clin Invest 34: 723–730, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Van Wagoner DR. Recent insights into the pathophysiology of atrial fibrillation. Semin Thorac Cardiovasc Surg 19: 9–15, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol 32: 1101–1117, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 85: 428–436, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 80: 772–781, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J 389: 151–159, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111: 2025–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92: 1954–1968, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Wit AL, Boyden PA. Triggered activity and atrial fibrillation. Heart Rhythm 4: S17–S23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol 1: 93–102, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A 87: 753–757, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]