Abstract
Shrinkage-induced inhibition of the Caenorhabditis elegans cell volume and cell cycle-dependent CLC anion channel CLH-3b occurs by concomitant phosphorylation of S742 and S747, which are located on a 175 amino acid linker domain between cystathionine-β-synthase 1 (CBS1) and CBS2. Phosphorylation is mediated by the SPAK kinase homolog GCK-3 and is mimicked by substituting serine residues with glutamate. Type 1 serine/threonine protein phosphatases mediate swelling-induced channel dephosphorylation. S742E/S747E double mutant channels are constitutively inactive and cannot be activated by cell swelling. S742E and S747E mutant channels were fully active in the absence of GCK-3 and were inactive when coexpressed with the kinase. Both channels responded to cell volume changes. However, the S747E mutant channel activated and inactivated in response to cell swelling and shrinkage, respectively, much more slowly than either wild-type or S742E mutant channels. Slower activation and inactivation of S747E was not due to altered rates of dephosphorylation or dephosphorylation-dependent conformational changes. GCK-3 binds to the 175 amino acid inter-CBS linker domain. Coexpression of wild-type CLH-3b and GCK-3 with either wild-type or S742E linkers gave rise to similar channel activity and regulation. In contrast, coexpression with the S747E linker greatly enhanced basal channel activity and increased the rate of shrinkage-induced channel inactivation. Our findings suggest the intriguing possibility that the phosphorylation state of S742 in S747E mutant channels modulates GCK-3/channel interaction and hence channel phosphorylation. These results provide a foundation for further detailed studies of the role of multisite phosphorylation in regulating CLH-3b and GCK-3 activity.
Keywords: GCK-3, cell volume, chloride channel, C. elegans
clc anion transport proteins function as Cl− channels and Cl−/H+ exchangers and are found in all major groups of life including archaebacteria (5, 22, 33). The structural bases of CLC gating and ion transport have been studied extensively. However, little is known about the cell signaling pathways and biophysical mechanisms that regulate CLC transport protein activity.1
Several CLC anion transport proteins have been shown to be regulated by phosphorylation events. However, there is little understanding of the signaling cascades that mediate phosphorylation or of the physiological context under which such regulation occurs. The picture is further complicated by apparent species differences and by the influence of experimental conditions on phosphorylation effects. For example, the type 1 serine/threonine protein phosphatase (PP1) inhibitor calyculin A inhibits swelling-induced activation of rat CLC-2, suggesting that dephosphorylation activates the channel (42). In contrast, a rabbit CLC-2 splice variant (28) is activated by protein kinase A (PKA)-mediated phosphorylation, but the activity of rat CLC-2 is unaffected by this kinase (36). Rabbit CLC-2 expressed in Xenopus oocytes is phosphorylated and inhibited by p34cdc2/cyclin B kinase (17).
CLC-3 is a member of CLC gene subfamily that encodes electrogenic Cl−/H+ exchangers (5). Several groups, however, have argued that CLC-3 functions as a phosphorylation-regulated outwardly rectifying anion channel. Like CLC-2 though, experimental conditions impact the effects of phosphorylation on CLC-3 activity. For example, several groups (e.g., 8, 20, 38) have shown that intracellular dialysis with activated calcium/calmodulin-dependent protein kinase II activates Cl− currents associated with CLC-3 expression. Other groups have argued that CLC-3 is responsible for swelling-activated outwardly rectifying anion currents (ICl,swell) that are inhibited by PKA or PKC-mediated phosphorylation (12, 35). A short isoform of CLC-3 may function in this volume-regulated anion channel activity (30, 39). However, these results are clouded by studies showing that CLC-3 knockout mice exhibit an ICl,swell similar to that of wild-type animals (44). On the basis of more detailed studies of current regulation, it has recently been suggested that the short CLC-3 isoform may be a component of the ICl,swell channel that alters its regulation by phosphorylation events (46).
We have exploited the molecular, genetic, and physiological tractability of the nematode Caenorhabditis elegans to characterize CLC anion channel function and regulation both in vivo and in heterologous expression systems. The C. elegans clh-3 gene encodes two CLC anion channel splice variants, CLH-3a and CLH-3b. CLH-3b is expressed in the worm oocyte and is activated by meiotic cell cycle progression and cell swelling (41). Activation requires protein dephosphorylation that is mediated by the PP1 phosphatases GSP-1 and GSP-2 (42). The Ste20 kinase GCK-3 binds to CLH-3b and inhibits its activity by mediating phosphorylation of two serine residues (11, 15). GCK-3 activity is regulated during cell volume and meiotic cell cycle changes by the MAP kinases MEK-2 and MPK-1 (14). GCK-3 is a homolog of the SPAK and OSR1 kinases, which regulate the activity of the volume sensitive K-Cl and Na-K-2Cl cotransporters and play central roles in systemic salt and water homeostasis (9, 24).
Eukaryotic CLC proteins are distinguished from most of their bacterial counterparts by extensive cytoplasmic COOH termini containing two cystathionine-β-synthase (CBS) domains termed CBS1 and CBS2 (e.g., Refs. 13, 29, 31, 32). CBS1 and CBS2 are connected by a linker with a highly variable sequence. The CBS linker in CLH-3b is 175 amino acids long, and the last 101 amino acids comprise a “regulatory domain.” GCK-3 binds to the regulatory domain via a SPAK interaction motif (11). Inhibition of CLH-3b by GCK-3 requires concomitant phosphorylation of S742 and S747, which are located downstream from the GCK-3 binding site (15).
Phosphorylation of S742 and S747 is mimicked by substituting the serine residues with glutamate. S742E/S747E double mutant channels are constitutively inhibited and as expected cannot be activated by swelling. S742E and S747E mutant channels are fully active in the absence of GCK-3 and are inhibited when coexpressed with the kinase (15).
S742E and S747E mutant channels that have been phosphorylated by coexpression with GCK-3 exhibit distinct responses to cell swelling. Wild-type and S742E mutant channels show identical degrees of activation with 1 min of cell swelling. In contrast, the activity of the S747E mutant channel is largely unaffected by the same stimulus (15).
Differential regulation of CLH-3b by dephosphorylation of S742 versus S747 has important implications for understanding channel regulation as well as the function and regulation of the kinases and phosphatases that control channel phosphorylation state (see Ref. 15 and discussion). The purpose of the current investigation was to characterize regulation of the S742E and S747E mutant channels in detail. Using a cell line lacking contaminating endogenous volume-sensitive Cl− currents, we were able to monitor channel activity over long time courses during cell swelling and shrinkage. Our studies demonstrate that S747E mutant channels activate and inactivate in response to cell volume changes. However, they do so much more slowly than either wild-type channels or the S742E mutant. Slower activation and inactivation of S747E is not due to altered rates of dephosphorylation- or dephosphorylation-dependent conformational changes. Instead, our findings suggest that the phosphorylation state of S742 in S747E mutant channels modulates GCK-3 binding and hence channel phosphorylation. Our results provide a foundation for further detailed studies of the role of multisite phosphorylation in regulating CLH-3b and GCK-3 activity, and for understanding multisite phosphorylation regulation of other ion transport proteins.
MATERIALS AND METHODS
Transfection and whole cell patch-clamp recording of human embryonic kidney 293 cells.
Human embryonic kidney (HEK293) cells were cultured in 35-mm diameter tissue culture plates in Eagle's minimal essential medium (MEM; GIBCO, Gaithersburg, MD) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), nonessential amino acids, sodium pyruvate, 50 U/ml penicillin, and 50 μg/ml streptomycin. After reaching 40–50% confluency, cells were transfected using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) with 1.0 μg green fluorescent protein (GFP), 1.5 μg CLH-3b, and 1.5 μg GCK-3 ligated into pcDNA3.1. Point mutations were generated using a QuikChange Lightning Multi Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA). All mutations were confirmed by DNA sequencing.
Following transfection, cells were incubated at 37°C for 24–30 h. Approximately 2 h prior to patch-clamp experiments, cells were detached from growth plates by exposure to 0.25% trypsin containing 1 mM EDTA (GIBCO) for 45 s. Detached cells were suspended in MEM, pelleted by centrifugation, resuspended in fresh MEM, and then plated onto poly-l-lysine-coated coverslips. Plated coverslips were placed in a bath chamber mounted onto the stage of an inverted microscope. Cells were visualized by fluorescence and differential interference contrast microscopy.
Transfected HEK293 cells were identified by GFP fluorescence and patch clamped using a bath solution containing 90 mM NMDG-Cl, 5 mM MgSO4, 1 mM CaCl2, 12 mM HEPES free acid titrated to pH 7.0 with CsOH, 8 mM Tris, 5 mM glucose, 95 mM sucrose, and 2 mM glutamine (pH 7.4, 310 mosM), and a pipette solution containing 116 mM NMDG-Cl, 2 mM MgSO4, 20 mM HEPES, 6 mM CsOH, 1 mM EGTA, 2 mM ATP, 0.5 mM GTP, and 10 mM sucrose (pH 7.2, 275 mosM). Cells were swollen by exposure to a hypotonic (250 mosM) bath solution that contained no added sucrose. Cells were shrunken by exposure to a bath solution made hypertonic (400 mosM) by sucrose addition. Swelling-induced currents were measured at −100 mV. Experimental protocols were performed on at least two independently transfected groups of cells. Whole cell currents are reported relative to whole cell capacitance to correct for differences in cell membrane area.
Patch electrodes were pulled from 1.5 mm outer diameter silanized borosilicate microhematocrit tubes; electrode resistance ranged from 4–8 MΩ. Currents were measured with an Axopatch 200B (Axon Instruments, Foster City, CA) patch-clamp amplifier. Electrical connections to the patch-clamp amplifier were made using Ag/AgCl wires and 3 M KCl/agar bridges. Data acquisition and analysis were performed using pClamp 8 software (Axon Instruments).
Western blotting.
Expression levels of CLH-3b linkers transfected into HEK cells were estimated using V5 epitope-tagged wild-type, S742E, S747E, or S742A/S747E linkers. Briefly, HEK cells were lysed 28 h after transfection using a modified RIPA buffer containing 0.05% SDS, 1.0% Triton X-100, 150 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, and 1.5 mM KH2PO4, pH 7.4. Lysates were centrifuged for 20 min at 10,000 g and 4°C to remove cellular debris. Protein concentrations in the collected supernatants were measured using BCA protein assay reagent (Thermo Fisher Scientific, Waltham, MA). Aliquots of the supernatants containing equal amounts of total protein (60 μg) were loaded onto a SDS-polyacrylamide gel, separated by electrophoresis, and then transferred from gel to nitrocellulose membranes. Linkers were detected by incubating blots with a polyclonal anti-V5 antibody (Invitrogen, Carlsbad, CA). To confirm equal gel lane protein loading, blots were also probed with a polyclonal anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Antibodies were detected using the SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific) and a G:Box Bio Imaging System (Syngene, Cambridge, UK).
Cell volume measurements.
Cell volume changes were measured in patch-clamp experiments to ensure that they were comparable for the different channel mutants tested. HEK cells were visualized at a single focal plane located at the point of maximum cell diameter by video-enhanced differential interference contrast (DIC) microscopy. Relative cell volume change was estimated by measuring cell diameter at this point before and during osmotic perturbations, and by assuming that the shape of the cell approximated a sphere. Observed volume changes were similar in all studies and are not reported.
Quantification of CLH-3b current properties.
Coexpression of CLH-3b with GCK-3 causes striking changes in channel voltage sensitivity and the kinetics of hyperpolarization-induced activation (11). Since CLH-3b is inwardly rectifying and active only at hyperpolarized voltages, we used current-to-voltage plots (e.g., Fig. 1A) to estimate channel activation voltage. A line was first drawn by linear regression analysis of currents measured between 0 mV and 60 mV where channel activity is low (see Fig. 1A). A second line was then drawn by linear regression analysis of currents measured between the first test voltage at which inward current was detected and a second voltage 20 mV more negative. The point at which these two lines intersect is defined as the activation voltage.
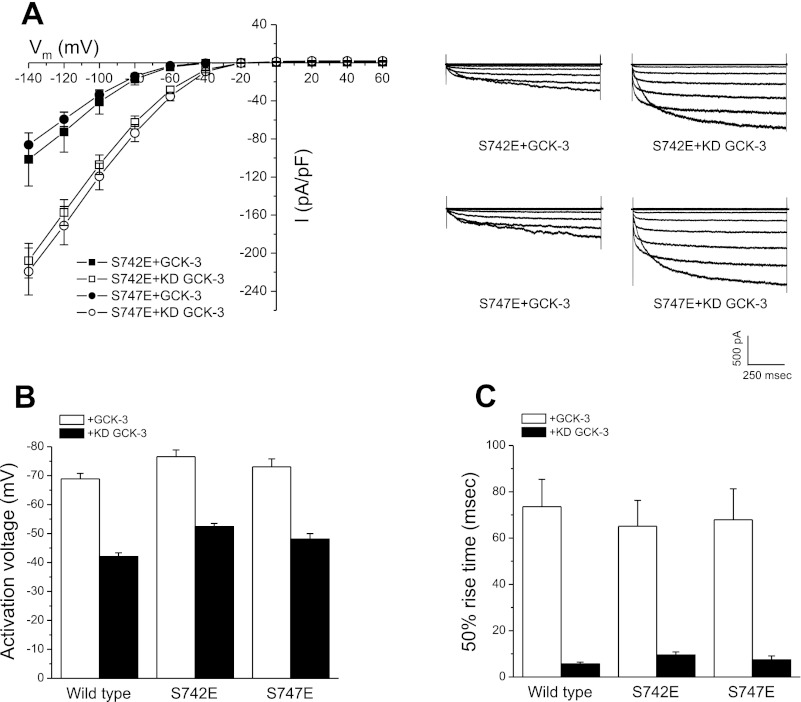
Fig. 1.
Steady-state properties of S742E and S747E mutant CLH-3b channels expressed with functional or kinase dead (KD) GCK-3. GCK-3-mediated phosphorylation of S742 and S747 hyperpolarizes CLH-3b activation voltage, slows hyperpolarization-induced current activation, and decreases whole cell current amplitude at hyperpolarized voltages (11). A: steady-state current amplitude and current-to-voltage relationships (left) and representative whole cell current traces (right). B: activation voltages. C: voltage-dependent activation kinetics measured as 50% rise time of S742E and S747E mutant channels expressed with or without functional GCK-3. Steady-state channel properties of the two mutants are similar. I, current; Vm, membrane potential. Values are means ± SE (n = 6–7).
CLH-3b channels are gated open by membrane hyperpolarization. Channel activation kinetics are described by either mono- or biexponential fits describing slow or fast and slow time constants, respectively. The nature of the fit is dictated by channel phosphorylation, which appears to inhibit a fast gating process (11). To simplify presentation and interpretation of activation kinetics under different experimental conditions, time constants are not used. Instead, the time required for whole cell current to reach 50% activation when membrane voltage is stepped from 0 mV to −100 mV for 1 s is quantified. This time is defined as the 50% rise time.
Rates of current change induced by cell swelling or shrinkage were quantified by stepping membrane voltage from 0 mV to −100 mV for 500 ms every 1 s. Initial rates of current activation by swelling and inactivation by shrinkage were determined by linear regression analysis performed during the initial 15–25 s of current change.
Statistical analyses.
Data are presented as means ± SE; n is the number of cells recorded from. Statistical significance was determined using Student's two-tailed t-test for paired or unpaired means. When comparing three or more groups, statistical significance was determined by one-way analysis of variance with a Tukey post hoc test. P ≤ 0.05 was taken to indicate statistical significance.
RESULTS
GCK-3-mediated phosphorylation of CLH-3b decreases current amplitude, hyperpolarizes channel activation voltage, and slows the kinetics of hyperpolarization-induced channel activation (11, 15). To begin characterizing the functional properties of the S742E and S747E mutants, the channels were coexpressed with either functional or kinase dead (KD) GCK-3 and steady-state channel properties were quantified. As shown in Fig. 1A, the current-to-voltage relationships for the mutants were indistinguishable (P > 0.3) at all voltages tested, both in the presence and absence of GCK-3 activity. Steady-state activation voltages (Fig. 1B) and activation kinetics (Fig. 1C) of S742E and S747E were similar to those of wild-type channels. These results demonstrate that the steady-state properties and regulation of CLH-3b are unaffected by substitution of either S742 or S747 with glutamate.
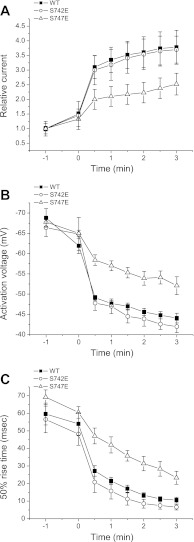
Our previous studies indicated that the S742E and S747E channels exhibit different sensitivity to cell swelling (15). However, detailed characterization of this difference was not possible due to the presence of a contaminating endogenous swelling-activated Cl− current in HEK cells. To circumvent this problem, we isolated a line of HEK cells lacking this current. As shown in Fig. 2, the wild-type and S742E mutant channels showed similar (P > 0.1) rates of swelling-induced activation as measured by changes in current amplitude (Fig. 2A), activation voltage (Fig. 2B), and voltage-dependent activation kinetics (Fig. 2C). However, the S747E mutant activated more slowly than either the wild-type or the S742E channels. Changes in current amplitude, activation voltage, and 50% rise time measured 3 min after induction of cell swelling were all significantly (P < 0.05) different compared with wild-type and S742E channels and reflect slower activation of the S747E mutant.
Fig. 2.
Swelling-induced changes in the activity of wild-type (WT) CLH-3b channels and S742E and S747E mutants coexpressed with functional GCK-3. Cells were swollen at time 0 by exposure to a 250 mosM bath solution. Rates of swelling-induced increase of current amplitude (A), depolarization of activation voltage (B), and decrease of 50% rise time (C) were significantly (P < 0.05) slower in S747E mutant channels compared with either wild-type channels or the S742E mutant. Values are means ± SE (n = 4–7).
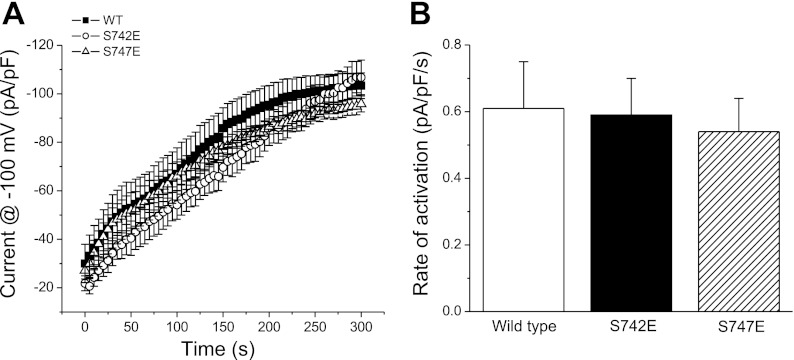
Activation of CLH-3b by cell swelling requires dephosphorylation of either S742 or S747 (15). Given that the steady-state properties of the wild-type and two mutant channels are identical (Fig. 1), the data in Fig. 2 suggest that the reduced rate of swelling-induced activation in the S747E mutant is due to 1) a reduced rate of S742 dephosphorylation and/or 2) a reduced rate of dephosphorylation-induced conformational change in the S747E mutant. We tested these two possibilities by inhibiting cellular kinase activity using an ATP- and Mg2+-free patch pipette solution. Channel activation under these conditions should depend only on rates of dephosphorylation and associated conformational changes. As shown in Fig. 3, A and B, rates of activation of wild-type and mutant channels were not significantly (P > 0.8) different when kinase activity was inhibited. These results demonstrate that in the absence of active GCK-3, the S747E mutation does not alter rates of channel dephosphorylation or associated conformational changes that activate the channel.
Fig. 3.
Effect of inhibition of kinase activity on wild-type CLH-3b channels and S742E and S747E mutants coexpressed with functional GCK-3. Kinase activity was inhibited by dialyzing cells with an ATP- and Mg2+-free patch pipette solution. Changes in current activity were measured immediately after whole cell access was obtained. The increase in current amplitude (A) and initial rates of current activation (B) were similar for the three channel types. Values are means ± SE (n = 5–7).
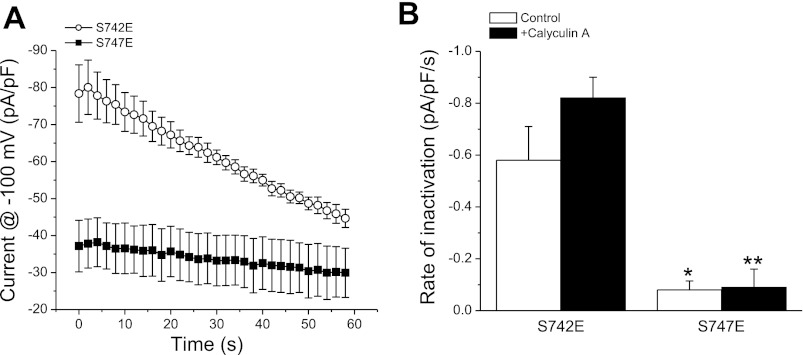
One interpretation of the data shown in Fig. 2 is that differences in swelling-induced channel activation reflect differences in the way active GCK-3 interacts with and/or phosphorylates the S742E and S747E mutants. Specifically, the slower rate of swelling-induced activation of S747E (Fig. 2) may be due to increased interaction of GCK-3 with the channel and/or an increased rate of phosphorylation of S742. If this is correct, then the two mutants should exhibit differences in shrinkage-induced channel inhibition. To test this possibility, mutant channels were activated by exposing cells to hypotonic medium for 3 min before shrinkage was induced by treatment with 400 mosM bath medium. Interestingly, the S747E mutant exhibited extremely slow apparent shrinkage-induced inactivation (Fig. 4A). The rates of current decline for S742E and S747E mutant channels were −0.58 pA·pF−1·s−1 and −0.08 pA·pF−1·s−1 (P < 0.01), respectively (Fig. 4B). Wild-type channels inactivated at a mean ± SE rate of −0.60 ± 0.13 pA·pF−1·s−1 (n = 6), which was not significantly (P > 0.9) different from that of the S742E mutant.
Fig. 4.
Shrinkage-induced inactivation of S742E and S747E mutant channels coexpressed with functional GCK-3. Cells were swollen for 3 min in hypotonic bath and then shrunken by exposure to a 400 mosM solution. A: time course of shrinkage-induced inactivation of S742E and S747E mutants following 3 min of cell swelling. B: initial rates of shrinkage-induced inactivation of S742E and S747E mutants in the presence or absence of 100 nM calyculin A. Cells were swollen for 1 min without calyculin A and then the drug was added to the bath media for the remainder of the experiment. Values are means ± SE (n = 5–6). *P < 0.01, **P < 0.0001 compared with the S742E mutant.
The rate of current decline of the S747E mutant was extremely slow, raising the concern that the decrease in current amplitude may not reflect channel inactivation per se, but instead could be due to shrinkage-induced changes in whole cell access resistance, intracellular Cl−, etc. To test this possibility, we examined the effect of shrinkage on S742A/S747E double mutant channels. Both S742 and S747 must be phosphorylated to inhibit CLH-3b activity (15). Because A742 cannot be phosphorylated, S742A/S747E mutant channels are constitutively fully active (15) and should not be affected by cell shrinkage. When cells expressing the S742A/S747E mutant were exposed to 400 mosM bath following 3 min of hypotonicity-induced swelling, current amplitude declined at a mean ± SE rate of −0.21 ± 0.09 pA·pF−1·s−1 (n = 5). This rate was not significantly (P > 0.2) different from that of the S747E mutant. Under steady-state conditions, S747E channels expressed with GCK-3 were fully inactivated similar to that of wild-type channels (Fig. 1, A–C). We conclude, therefore, that the rate of shrinkage-induced inactivation of S747E mutant channels is extremely slow and likely cannot be measured accurately under the experimental conditions used here.
To determine whether phosphatase activity influences channel inactivation, cells were treated with 100 nM of the PP1 inhibitor calyculin A. Initial studies demonstrated that a 2-min exposure to isotonic medium containing the drug was sufficient to completely prevent swelling-induced activation of wild-type channels coexpressed with GCK-3 (data not shown). We therefore activated the channels by exposing cells to drug-free hypotonic medium for 1 min followed by a 2-min exposure to hypotonic medium containing 100 nM calyculin A. Cells were then shrunken by exposure to 400 mosM medium containing calyculin A. As shown in Fig. 4B, calyculin A had no significant (P > 0.2) effect on the rates of shrinkage-induced current decline of the two mutants. Calyculin A also had no significant (P > 0.8) effect on S742A/S747E double mutant channels (mean ± SE rate of current decline = −0.19 ± 0.07 pA·pF−1·s−1; n = 4). Thus, the slower rate of inactivation of the S747E mutant must reflect differences in the way in which GCK-3 interacts with and/or phosphorylates the channel compared with S742E.
To test whether the S747E mutation alters interaction of GCK-3 with the channel, we coexpressed wild-type channels with GCK-3 and a 175 amino acid construct that comprises the linker domain located between the CBS1 and CBS2 motifs of the CLH-3b intracellular COOH terminus. The construct comprised G618 to P792 and contains the GCK-3 binding site (11) and serine residues 742 and 747 (15).
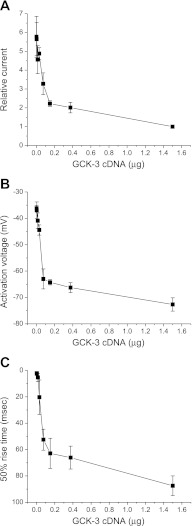
We first identified a level of GCK-3 expression that would induce partial inhibition of CLH-3b. Figure 5 shows the effects of transfecting HEK cells with increasing amounts of GCK-3 cDNA on CLH-3b whole cell current, channel activation voltage, and 50% rise time. Transfecting cells with 0.075 μg of GCK-3 cDNA resulted in approximately half-maximal inhibition of CLH-3b amplitude (Fig. 5A). Increasing GCK-3 cDNA level above 0.075 μg had relatively little additional effect on the hyperpolarization of channel activation voltage (Fig. 5B) and the slowing of voltage-dependent activation kinetics (i.e., 50% rise time; Fig. 5C) brought about by GCK-3-mediated channel phosphorylation.
Fig. 5.
Effect of GCK-3 expression level on steady-state functional properties of wild-type CLH-3b channels. Cells were transfected with 1.5 μg of CLH-3b cDNA and variable amounts of GCK-3 cDNA. Increasing GCK-3 expression levels inhibited whole cell current amplitude (A), hyperpolarized channel activation voltage (B), and slowed voltage-dependent activation kinetics measured as 50% rise time (C). Values are means ± SE (n = 4–6).
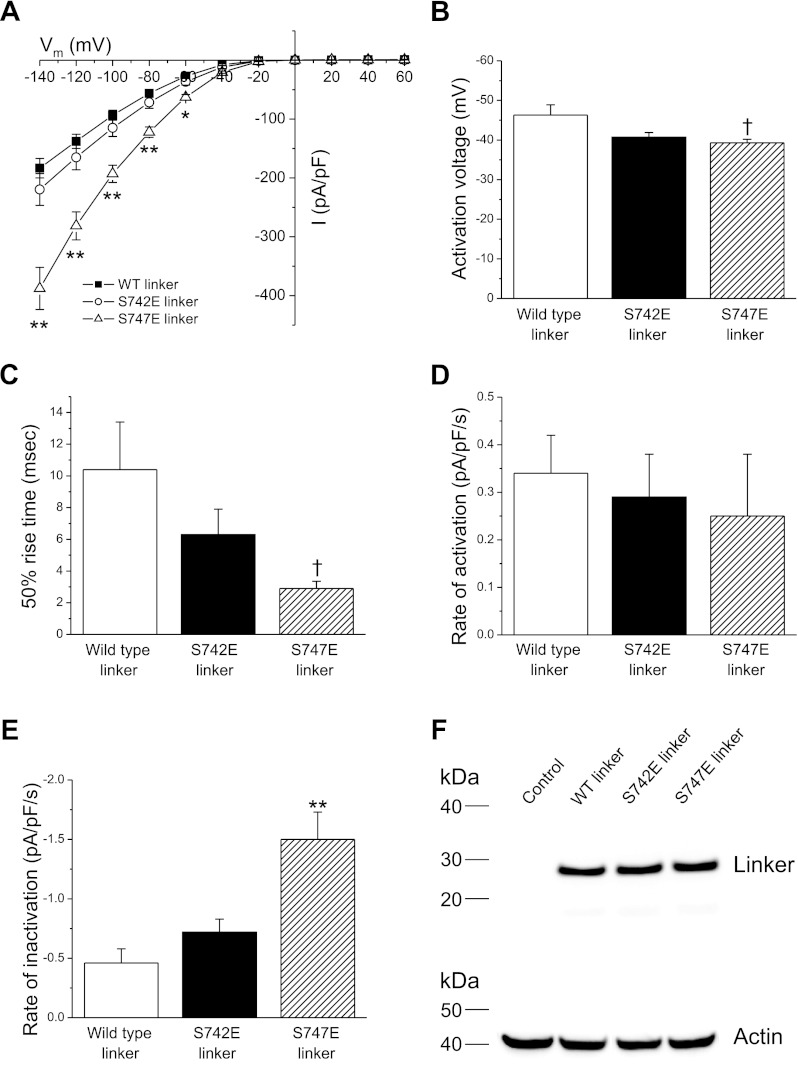
To assess the effect of wild-type and mutant linker expression on GCK-3 dependent regulation of CLH-3b, HEK cells were transfected with 1.5 μg, 0.075 μg, and 4 μg of wild-type CLH-3b, GCK-3, and linker cDNAs, respectively. The 4 μg concentration of linker cDNA was chosen to ensure that the linker would be expressed at a level greater than that of functional CLH-3b. Figure 6 shows the effect of expressing wild-type, S742E, or S747E linkers on steady-state CLH-3b activity. Current amplitude in cells expressing the S747E linker was significantly (P < 0.01) increased at all voltages more hyperpolarized than −40 mV compared with cells expressing either wild-type or S742E linker (Fig. 6A). In addition, CLH-3b activation voltage (Fig. 6B) was significantly (P < 0.05) more depolarized, and 50% rise time (Fig. 6C) was significantly (P < 0.05) faster in cells expressing the S747E linker compared with cells expressing wild-type linker.
Fig. 6.
Effect of expression of wild-type, S742E, or S747E linkers on functional properties of wild-type CLH-3b. Cells were cotransfected with 1.5 μg, 0.075 μg, and 4 μg of wild-type CLH-3b, GCK-3, and linker cDNAs, respectively. A: whole cell current amplitude and current-to-voltage relationships. B: activation voltages. C: 50% rise time. D and E: rates of swelling-induced activation (D) and shrinkage-induced inactivation (E). Values are means ± SE (n = 10–13). †P < 0.05, *P < 0.01, **P < 0.001 compared with cells expressing wild-type linker. F: Western blot showing expression levels of V5-tagged wild-type, S742E, and S747E linkers. Lanes were also probed with an anti-actin polyclonal antibody to assess protein loading. Control, nontransfected cells.
We next examined the effect of swelling and shrinkage on rates of CLH-3b activation and inactivation. Cells were swollen for 3 min by exposure to hypotonic bath solution and then shrunken by raising bath osmolality to 400 mosM. As shown in Fig. 6D, rates of swelling-induced channel activation were not significantly (P > 0.05) different for cells expressing the three different linkers. Surprisingly, however, in cells expressing the S747E linker the rate of shrinkage-induced inactivation was significantly (P < 0.001) increased compared with either wild-type or S742E linkers (Fig. 6E).
To ensure that equal amounts of the three linkers were expressed, we tagged their COOH termini with the V5 epitope and quantified expression level by Western analysis. As shown in Fig. 6F, wild-type, S742E, and S747E linkers exhibited similar degrees of expression. These data demonstrate that the effect of the S747E linker on channel activity reflects its functional properties rather than an altered expression level.
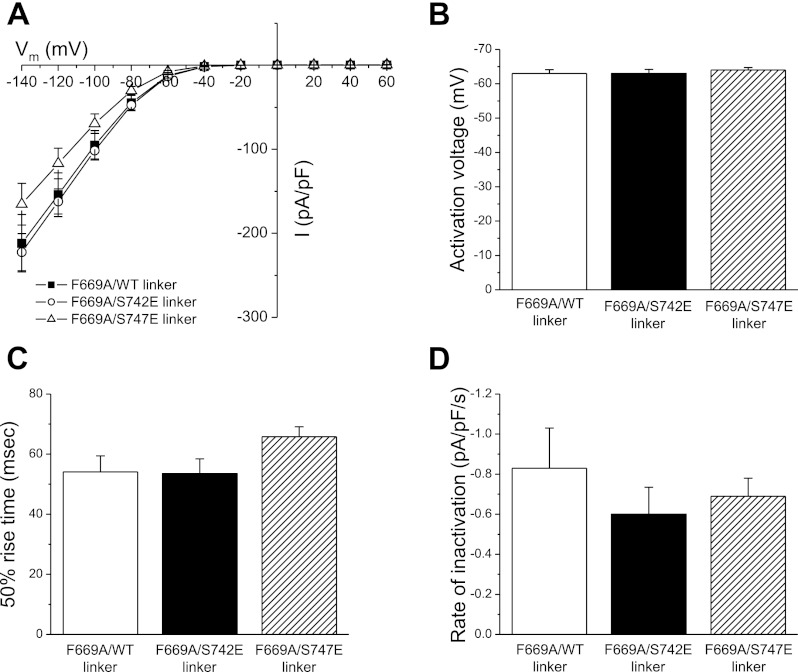
Data in Fig. 6 are consistent with the hypothesis that, under steady-state conditions, GCK-3 binds to the S747E linker more tightly than either wild-type linker or linker with the S742E mutation. This tighter interaction would in effect titrate GCK-3 away from wild-type CLH-3b, resulting in reduced phosphorylation and increased channel activity. If this hypothesis is correct, then disrupting the GCK-3/linker interaction should prevent the effects of S747E linker expression on wild-type CLH-3b activity (Fig. 6). As we have shown previously, GCK-3 binds to a four amino acid sequence, RFLI (residues 668–671), on the linker. Mutation of F669 to alanine prevents GCK-3 binding and phosphorylation-mediated channel inhibition (11). We coexpressed GCK-3 and wild-type CLH-3b together with wild-type, S742E, or S747E linkers containing the F669A mutation. As shown in Fig. 7, the F669A mutation completely reversed the effects of the S747E linker on channel activity. No significant (P > 0.05) differences were observed in basal current amplitude, activation voltage, and 50% rise time (Fig. 7, A–C), and in shrinkage-induced inactivation (Fig. 7D) and swelling-induced activation (data not shown) for cells expressing any of the three linkers.
Fig. 7.
Effect of expression of wild-type, S742E, or S747E linkers containing the F669A mutation on functional properties of wild-type CLH-3b. Cells were cotransfected with 1.5 μg, 0.075 μg, and 4 μg of wild-type CLH-3b, GCK-3, and linker cDNAs, respectively. A: whole cell current amplitude and current-to-voltage relationships. B: activation voltages. C: 50% rise time. D: rates of and shrinkage-induced inactivation. Values are means ± SE (n = 7–8).
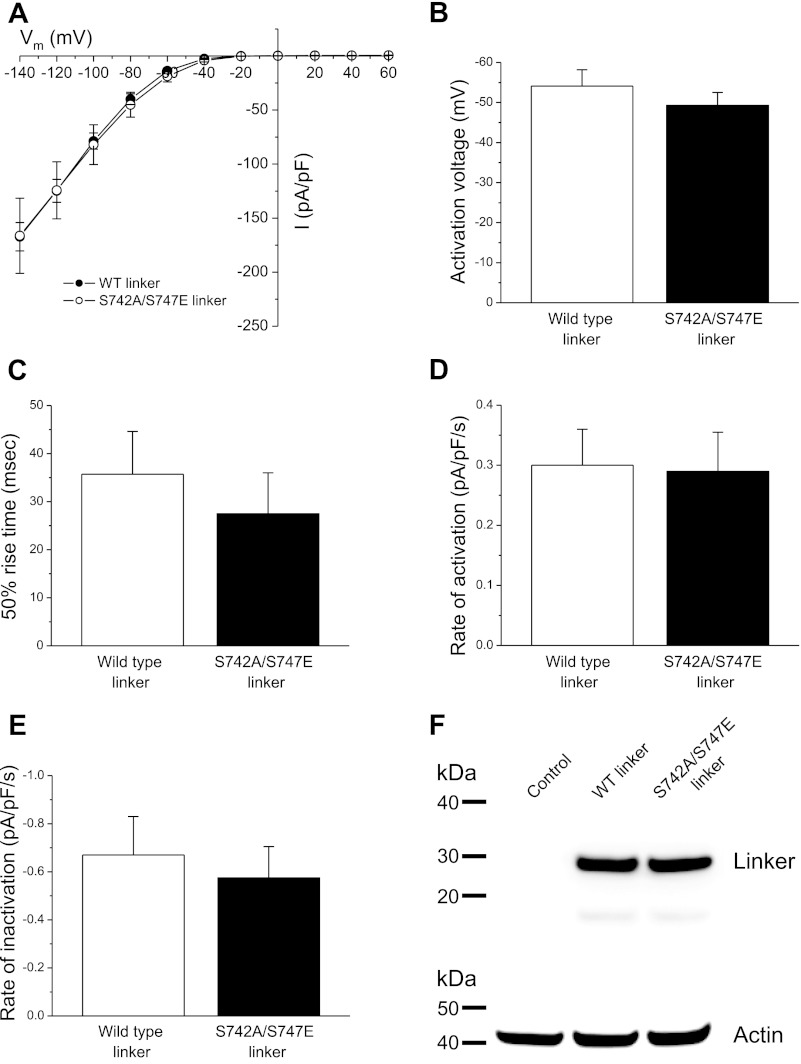
Data in Fig. 6 also suggest that the phosphorylation state of S742 in the S747E linker may modulate the interaction of GCK-3 with the channel. If this is correct, then preventing S742 phosphorylation should reverse the effects of the S747E linker on channel activity. To test this, we expressed wild-type CLH-3b and GCK-3 together with either wild-type linker or the S747E linker in which S742 was mutated to alanine (i.e., S742A/S747E) to prevent phosphorylation. As shown in Fig. 8, A–C, the steady-state current properties of CLH-3b in cells expressing either wild-type or S742A/S747E linkers were not significantly (P > 0.4) different. In addition, rates of swelling-induced activation (Fig. 8D) and shrinkage-induced inactivation (Fig. 8E) of CLH-3b were similar (P > 0.6) for cells expressing the wild-type or mutant linkers. Western analysis demonstrated that the two linkers were expressed at similar levels (Fig. 8F). A working model summarizing our findings is presented in the discussion section and shown in Fig. 9.
Fig. 8.
Effect of expression of wild-type or S742A/S747E linkers on functional properties of wild-type CLH-3b. Cells were cotransfected with 1.5 μg, 0.075 μg, and 4 μg of wild-type CLH-3b, GCK-3, and linker cDNAs, respectively. A: whole cell current amplitude and current-to-voltage relationships. B: activation voltages. C: 50% rise time. D and E: rates of swelling-induced activation (D) and shrinkage-induced inactivation (E). Values are means ± SE (n = 5–6). F: Western blot showing expression levels of V5-tagged wild-type and S742A/S747E linkers. Lanes were also probed with an anti-actin polyclonal antibody to assess protein loading. Control, nontransfected cells.
Fig. 9.
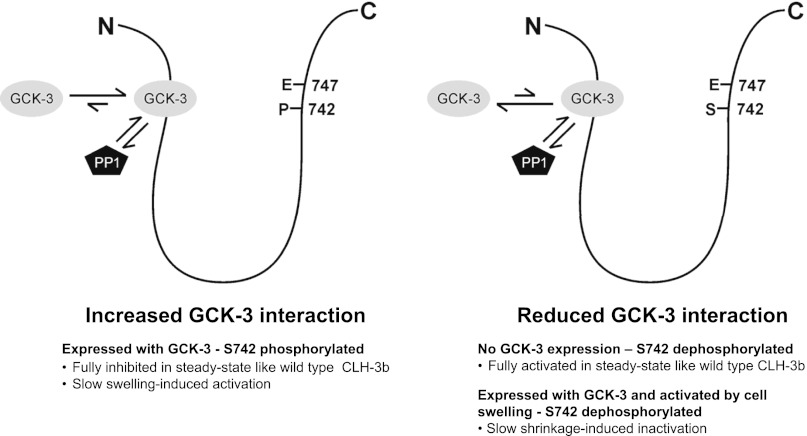
Working model of phosphorylation-dependent regulation of S747E mutant CLH-3b channels. Model assumes that GCK-3 and type 1 protein phosphatase (PP1) must compete for access to S742 and S747. Under steady-state conditions, S742 is fully phosphorylated and interaction of GCK-3 with the channel is high. This increased interaction slows swelling-induced channel dephosphorylation and associated channel activation (Fig. 2). When coexpressed with wild-type channels, the S747E linker functions as a sink for GCK-3, resulting in increased basal channel activity (Fig. 6, A–C). GCK-3 interaction is reduced by dephosphorylation of S742 in the S747E mutant. Reduced interaction slows shrinkage-induced inactivation of S747E mutant channels (Fig. 4). Dephosphorylation of the S747E mutant linker reduces its ability to interact with GCK-3, resulting in more rapid shrinkage-induced inactivation of coexpressed wild-type channels (Fig. 6E).
DISCUSSION
Multisite phosphorylation plays a central role in regulating the activity of numerous proteins. Multiple amino acid targets within a substrate protein can be phosphorylated/dephosphorylated in an ordered or random fashion via either processive or distributive mechanisms. For processive phosphorylation/dephosphorylation, the kinase/phosphatase binds once and modifies all target sites. In a distributive mechanism, the kinase/phosphatase binds, modifies a target amino acid, dissociates, and then must re-bind for a subsequent phosphorylation/dephosphorylation reaction to occur. Kinase and phosphatase binding can be competitive or independent, and the phosphorylation state of the substrate protein can modify binding kinetics and affinity. These different modes of phosphorylation and dephosphorylation dramatically impact the kinetics of phosphorylation/dephosphorylation reactions and the sensitivity of the substrate protein to concentrations of active kinase and phosphatase. Multisite phosphorylation thus provides an important mechanism for increasing protein regulatory modes and for fine-tuning protein function (reviewed in Refs. 7, 43).
Many transporters and ion channels have multiple phosphorylation sites. For example, the intracellular R-domain of CFTR contains multiple sites that are phosphorylated by PKA, PKC, and AMP-activated kinase and that function to inhibit or activate the channel (1, 21). Multisite phosphorylation of the voltage-gated K+ channel Kv2.1 provides a mechanism for graded changes in channel activation (34). Multisite phosphorylation of the brain voltage-gated Na+ channel Nav1.2a by PKC and PKA allows channel regulation through multiple signaling pathways (4). The swelling-activated K-Cl cotransporter KCC3 is phosphorylated at two threonine residues. Mutation of either residue to alanine activates the transporter 4- to 5-fold under isotonic conditions whereas combined mutation of both residues results in a ∼25-fold activation (37). Similarly, concomitant phosphorylation of three threonine residues is required for maximal activation of the shrinkage-activated Na-K-2Cl cotransporter NKCC2 (18).
The phosphorylation state of CLH-3b reflects a balance between kinase and phosphatase activity. It is generally accepted that the regulated step in cell volume-sensitive, phosphorylation-dependent transport processes is a change in kinase activity while phosphatase activity remains relatively constant (e.g., Refs. 23, 27). The SPAK and OSR1 kinases have been shown clearly to play important roles in systemic osmoregulation as well as in the detection of cell volume changes and activation of volume regulatory transport pathways in mammals (9, 24). Similarly, the SPAK/OSR1 homolog GCK-3 functions to regulate CLH-3b activity as well as whole animal salt and water balance in C. elegans (6, 11, 15). However, despite their well-defined roles in regulating volume-sensitive transport pathways, the precise mechanisms by which SPAK, OSR1, and GCK-3 signaling cascades detect and are controlled by cell volume changes remain unclear.
In response to swelling or meiotic maturation of the C. elegans oocyte, CLH-3b is activated via dephosphorylation mediated by the PP1 phosphatases GSP-1 and GSP-2 (42). As described in results, swelling-induced activation of heterologously expressed CLH-3b is inhibited by calyculin A, which at the low concentrations used is selective for PP1 phosphatases.
The molecular identity of the phosphatases that dephosphorylate CLH-3b in HEK cells is unknown. Three genes encode four PP1 isoforms in mammalian cells. The specificity of these four isoforms for the literally thousands of dephosphorylation reactions that occur in eukaryotic cells is mediated by their interaction with hundreds of regulatory or PP1-interacting proteins (3, 45). It is also unknown how GSP-1, GSP-2, or mammalian PP1s interact with CLH-3b. Predicted CLH-3b intracellular domains lack canonical PP1 docking motifs (40). PP1s could interact with CLH-3b via an undefined docking motif or via a PP1 interacting protein. Additional studies are clearly warranted to define the mechanisms of CLH-3b dephosphorylation.
Phosphorylation of CLH-3b is mimicked by substituting S742 and/or S747 with glutamate residues (15). Under steady-state conditions, S742E and S747E mutants had functional properties similar to those of wild-type CLH-3b expressed with either functional or kinase dead GCK-3 (Fig. 1). However, channels with the S747E mutation showed greatly reduced rates of swelling- and shrinkage-induced activation and inactivation, respectively (Figs. 2 and 4). These results demonstrate that serine-to-glutamate substitutions at either S742 or S747 did not alter basal channel activity. Instead, the S747E mutation altered only the rates of phosphorylation/dephosphorylation events and/or regulatory conformational changes.
Data shown in Fig. 3 suggest that, in the absence of active GCK-3, the S747E mutation did not alter rates of dephosphorylation and dephosphorylation-mediated conformational changes. Instead, the slower rate of swelling-induced activation of the S747E mutant reflected differences in the way in which GCK-3 interacts with the channel and/or phosphorylates S742. A simple interpretation for the slowed rate of swelling-induced activation of the S747E mutant (Fig. 2) is that glutamate substitution at S747 increased the rate of S742 phosphorylation. However, the greatly slowed rate of shrinkage-induced inactivation of the S747E mutant compared with S742E channels (Fig. 4) argues against this possibility.
We tested the possibility that mutation of S747 to glutamate alters the way in which GCK-3 interacts with the channel by coexpressing wild-type channels together with wild-type, S742E, or S747E inter-CBS linker domains. As shown in Fig. 6, A–C, the S747E linker increased steady-state wild-type channel activity compared with either wild-type or S742E linkers. In addition, shrinkage-induced inactivation was two- to threefold faster in cells expressing the S747E linker compared with wild-type or S742E linker expressing cells (Fig. 6E). These results are most consistent with a model in which the phosphorylation state of S742 in the S747E mutant modulates its interaction with GCK-3. We define GCK-3/CLH-3b interaction broadly to include binding of GCK-3 to the SPAK interaction motif (11), competition of GCK-3 with PP1-type phosphatases for interaction with phosphorylation sites, and the effects of mutations (i.e., serine to glutamate or serine to alanine) at S742 or S747 on the interaction of GCK-3 with these residues.
Figure 9 shows a working model that summarizes our results. Other interpretations of the data may be possible, but we feel that the model represents the simplest and most readily testable explanation of our findings. Specifically, we suggest that, under steady-state conditions, phosphorylation of S742 in the S747E mutant increases interaction of GCK-3 with the channel. We assume that GCK-3 and PP1 cannot interact simultaneously with CLH-3b phosphorylation sites. A tighter GCK-3/channel interaction would slow dephosphorylation and hence swelling-induced activation of the S747E channel (Fig. 2). Increased GCK-3 interaction would also explain the higher steady-state activity of wild-type CLH-3b channels coexpressed with S747E linker (Fig. 6, A–C). The mutant linker effectively functions as a kinase sink reducing phosphorylation and increasing the activity of wild-type channels.
Data shown in Fig. 8 support this hypothesis. When serine 742 was replaced by alanine, phosphorylation at this residue was prevented. Coexpression of CLH-3b and GCK-3 with the S742A/S747E linker reversed the effect of the S747E linker on channel activity (compare Fig. 6, A–E, and Fig. 8, A–E). In other words, the S742A/S747E linker, which cannot be phosphorylated, behaves the same as either the wild-type or S742E linker domains.
In response to swelling and dephosphorylation of S742, we postulate that the interaction of GCK-3 with the S747E channel is decreased. Reduced interaction would explain the slow shrinkage-induced inactivation of S747E channels. It would also explain the more rapid shrinkage-induced inactivation of wild-type channels coexpressed with S747E linker.
It is unclear at present whether our results have a physiological correlate or are simply a manifestation of substituting S747 with a glutamate residue. However, it is worth noting that multisite phosphorylation has been implicated in regulating the precise timing of cell cycle progression and associated events (e.g., Refs. 2, 16, 25). CLH-3b is expressed in vivo in the C. elegans oocyte (10, 41), and its activity is regulated by oocyte meiotic maturation (14, 41). Activation of CLH-3b plays an important role in coupling the timing of oocyte maturation to ovulation (41, 47). In addition, GCK-3 has been implicated in the regulation of multiple physiological processes in C. elegans including cell cycle control (26), development (19, 26), systemic osmotic homeostasis (6), and CLH-3b activity and oocyte ovulation (14, 41). Given the presence of dual regulatory phosphorylation sites on CLH-3b and the diverse physiological functions of GCK-3, it is likely that multisite phosphorylation plays important roles in regulation of CLH-3b channel activity. Complex multisite phosphorylation of CLH-3b may also play a role in regulating the localization and total cellular activity of GCK-3, which in turn could impact the regulation of other cellular processes. Our work provides an essential foundation for additional detailed studies into CLH-3b and GCK-3 interaction and regulation.
GRANTS
This work was supported by National Institutes of Health Grant R01 DK-51610 (to K. Strange).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.M. and K.S. conception and design of research; H.M. performed the experiments; H.M. and K.S. analyzed the data; H.M. and K.S. interpreted the results of the experiments; H.M. and K.S. prepared the figures; H.M. and K.S. drafted the manuscript; H.M. and K.S. edited and revised the manuscript; H.M. and K.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jerod Denton for critical reading of the manuscript and for many helpful discussions and suggestions.
Footnotes
This article is the topic of an Editorial Focus by Dayue Darrel Duan and Guangyu Wang (12a).
REFERENCES
- 1. Alzamora R, King JD, Jr., Hallows KR. CFTR regulation by phosphorylation. Methods Mol Biol 741: 471–488, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Barik D, Baumann WT, Paul MR, Novak B, Tyson JJ. A model of yeast cell-cycle regulation based on multisite phosphorylation. Mol Syst Biol 6: 405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 35: 450–458, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Molecular mechanism of convergent regulation of brain Na+ channels by protein kinase C and protein kinase A anchored to AKAP-15. Mol Cell Neurosci 21: 63–80, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chen TY, Hwang TC. CLC-0 and CFTR: chloride channels evolved from transporters. Physiol Rev 88: 351–387, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol 293: C915–C927, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cohen P. The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci 25: 596–601, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem 285: 11188–11196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409: 321–331, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Denton J, Nehrke K, Rutledge E, Morrison R, Strange K. Alternative splicing of N- and C-termini of a C. elegans ClC channel alters gating and sensitivity to external Cl− and H+. J Physiol 555: 97–114, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol 125: 113–125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol 113: 57–70, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a. Duan DD, Wang G. Double the keys, double the control: coupled phosphorylation sites provide novel molecular targets for precise control of ion channel function. Focus on “Differential regulation of a CLC anion channel by SPAK kinase ortholog-mediated multisite phosphorylation.” Am J Physiol Cell Physiol (April 11, 2012). doi:10.1152/ajpcell.00107.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estevez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol 557: 363–378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falin RA, Miyazaki H, Strange K. C. elegans STK39/SPAK ortholog-mediated inhibition of ClC anion channel activity is regulated by WNK independent ERK kinase signaling. Am J Physiol Cell Physiol 300: C624–C635, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falin RA, Morrison R, Ham A, Strange K. Identification of regulatory phosphorylation sites in a cell volume- and Ste20 kinase-dependent ClC anion channel. J Gen Physiol 133: 29–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frank CL, Ge X, Xie Z, Zhou Y, Tsai LH. Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J Biol Chem 285: 33324–33337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furukawa T, Ogura T, Zheng YJ, Tsuchiya H, Nakaya H, Katayama Y, Inagaki N. Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J Physiol 540: 883–893, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep 9: 70–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 276: 20093–20100, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hwang TC, Sheppard DN. Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol 587: 2151–2161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43: 3–36, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta 1802: 1150–1158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kim SY, Ferrell JE., Jr Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 128: 1133–1145, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kupinski AP, Muller-Reichert T, Eckmann CR. The Caenorhabditis elegans Ste20 kinase, GCK-3, is essential for postembryonic developmental timing and regulates meiotic chromosome segregation. Dev Biol 344: 758–771, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Lytle C. A volume-sensitive protein kinase regulates the Na-K-2Cl cotransporter in duck red blood cells. Am J Physiol Cell Physiol 274: C1002–C1010, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Malinowska DH, Kupert EY, Bahinski A, Sherry AM, Cuppoletti J. Cloning, functional expression, and characterization of a PKA-activated gastric Cl− channel. Am J Physiol Cell Physiol 268: C191–C200, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Markovic S, Dutzler R. The structure of the cytoplasmic domain of the chloride channel ClC-Ka reveals a conserved interaction interface. Structure 15: 715–725, 2007 [DOI] [PubMed] [Google Scholar]
- 30. McCloskey DT, Doherty L, Dai YP, Miller L, Hume JR, Yamboliev IA. Hypotonic activation of short ClC3 isoform is modulated by direct interaction between its cytosolic C-terminal tail and subcortical actin filaments. J Biol Chem 282: 16871–16877, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Meyer S, Dutzler R. Crystal structure of the cytoplasmic domain of the chloride channel ClC-0. Structure 14: 299–307, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Meyer S, Savaresi S, Forster IC, Dutzler R. Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5. Nat Struct Mol Biol 14: 60–67, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Miller C. ClC chloride channels viewed through a transporter lens. Nature 440: 484–489, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans 35: 1064–1068, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Nagasaki M, Ye L, Duan D, Horowitz B, Hume JR. Intracellular cyclic AMP inhibits native and recombinant volume-regulated chloride channels from mammalian heart. J Physiol 523: 705–717, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park K, Begenisich T, Melvin JE. Protein kinase A activation phosphorylates the rat ClC-2 Cl− channel but does not change activity. J Membr Biol 182: 31–37, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138: 525–536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol 556: 353–368, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rossow CF, Duan D, Hatton WJ, Britton F, Hume JR, Horowitz B. Functional role of amino terminus in ClC-3 chloride channel regulation by phosphorylation and cell volume. Acta Physiol (Oxf) 187: 5–19, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Roy J, Cyert MS. Cracking the phosphatase code: docking interactions determine substrate specificity. Sci Signal 2: re9, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Rutledge E, Bianchi L, Christensen M, Boehmer C, Morrison R, Broslat A, Beld AM, George A, Greenstein D, Strange K. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr Biol 11: 161–170, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Rutledge E, Denton J, Strange K. Cell cycle- and swelling-induced activation of a Caenorhabditis elegans ClC channel is mediated by CeGLC-7α/β phosphatases. J Cell Biol 158: 435–444, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salazar C, Hofer T. Multisite protein phosphorylation–from molecular mechanisms to kinetic models. FEBS J 276: 3177–3198, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29: 185–196, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33: 537–545, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto-Mizuma S, Wang GX, Liu LL, Schegg K, Hatton WJ, Duan D, Horowitz TL, Lamb FS, Hume JR. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J Physiol 557: 439–456, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell 15: 3938–3949, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]