ion channels are transmembrane pore-forming proteins that function as conductors of the cell to allow the flow of charged ions down their electrochemical gradient and, thus, control the voltage gradient across the plasma membrane (2). Ion channels are important for many cellular processes and functions including regulation of electrical activity, muscle excitation-contraction coupling, cell volume homeostasis, cell-cycle progression, cell migration, and apoptosis. Many of these cellular processes need to be precisely fine-tuned in a time-dependent manner. Numerous signaling transduction networks have been identified that control dynamic regulation of ion channel expression, localization, and function. Phosphorylation by kinases or dephosphorylation by phosphatases provides fundamental on/off switches to rapidly and reversibly convert ion channel functions in response to various cellular signals (8). For instance, osmotic perturbations such as hypertonic cell shrinkage and hypotonic cell swelling have been shown to regulate activity of several chloride (Cl−) channels (3, 6) and potassium (K+) channels (10) through phosphorylation and dephosphorylation of the target channel proteins, crucial to precise regulation of cell volume homeostasis. Understanding the detailed molecular mechanisms of covalent modification of channel proteins by phosphorylation/dephosphorylation has been a subject of intensive study.
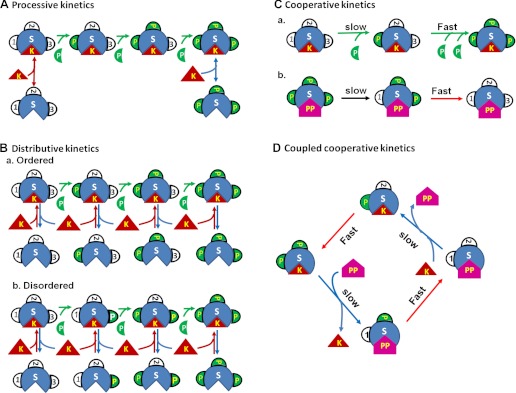
The regulation of ion channels by phosphorylation/dephosphorylation may involve multiple sites of phosphorylation. Multisite phosphorylation/dephosphorylation can follow either processive or distributive rules (7). Under the processive rule (Fig. 1A), the kinase phosphorylates more than one amino acid residue on its substrate during a single binding event, essentially the same as a single phosphorylation event. In contrast, during distributive phosphorylation, a single enzyme-substrate binding event results in phosphorylation of only one amino acid residue (Fig. 1B). After the substrate is phosphorylated, the kinase dissociates from its substrate and then must bind anew at a different site of phosphorylation for the next phosphorylation event. The target sites may be altered in a specific sequence (ordered distributive mechanism, Fig. 1B, a) or in a random fashion (disordered distributive mechanism, Fig. 1B, b). The initial binding of a kinase or a phosphatase to the substrate not only causes initial phosphorylation or dephosphorylation of one site but also promotes phosphorylation or dephosphorylation of the other phosphorylation sites of the substrate (cooperative kinetics, Fig. 1C). Multiple phosphorylation sites increase the number of phosphorylation states, and each distinct functional state can possess distinct biological properties (7). Therefore, multisite phosphorylation considerably increases the possibilities for regulating ion channel function and provides a more precise tool for dynamic regulation and fine-tuning of channel activity for specific cellular functions.
Fig. 1.
Distinct kinetic mechanisms of multisite protein phosphorylation (5, 7). A: processive kinetics: a single binding of the kinase (K) to the substrate (S) phosphorylates all sites of the substrate (full phosphorylation). B: distributive kinetics: multiple binding events are required for full phosphorylation of the substrate. The phosphorylation sites may be altered in a specific sequence (ordered, a) or in a random fashion (disordered, b). C: cooperative kinetics: the initial binding of kinase or phosphatase (PP) to the substrate not only causes initial phosphorylation (a) or dephosphorylation (b) of one site but also promotes phosphorylation or dephosphorylation of the other phosphorylation sites of the substrate. D: the coupled cooperative (or “double key”) kinetics. The phosphorylation of the two sites is closely coupled with the dephosphorylation process. When both sites are phosphorylated, it prevents phosphatase from replacing the kinase and binding to the substrates and slows down the dephosphorylation kinetics. The successful binding of the phosphatase to the substrate causes full dephosphorylation and also prevents the kinase from replacing the phosphatase and binding to the substrate in either a competitive (5) or noncompetitive manner, and thus, slows down the kinetics of phosphorylation of the first site.
In this issue of American Journal of Physiology-Cell Physiology, Miyazaki and Strange (5) investigate the unequal roles of two phosphorylation sites, S742 and S747, in the regulation of the Caenorhabditis elegans oocyte CLC channel (CLH-3b) activity in response to osmotic cell swelling and shrinkage. S742 and S747 are located on a 175 amino acid linker domain between two cystathionine-β-synthase domains. Three maneuvers were implemented to effect channel phosphorylation: treatment with the SPAK kinase ortholog GCK-3, treatment with the type 1 phosphatase (PP1) inhibitor calyculin A, or cell shrinkage. When substitution of serine residues with glutamate (S742E and S747E) was used to mimic channel phosphorylation, the S742E/S747E double mutant channels were constitutively inactive and could not be activated by cell swelling. In contrast, S742E and S747E mutant channels were fully active in the absence of GCK-3 and were inactive when coexpressed with the kinase. While both mutant channels responded to cell volume changes, the activation and inactivation of the S747E mutant channels in response to cell swelling and shrinkage, respectively, are much slower than that of either wild-type or S742E mutant channels. This slower response of S747E to cell swelling or shrinkage was not due to altered rates of dephosphorylation or to dephosphorylation-dependent conformational changes of the channels. Instead, the authors suggest that the fractional phosphorylation of S742 might regulate the binding of GCK-3 to the channel and, thus, the S747E channel activity (5). Although it is not clear whether GCK3 and PP1 share the same binding site on the channel, the results from this study support that coupled phosphorylation sites may provide novel molecular targets for precise control of ion channel function (Fig. 1D).
The finding that cell swelling or shrinkage may regulate the phosphorylation state of CLH-3b S742, which in turn modulates GCK-3 binding and subsequent channel phosphorylation and activity, provides novel mechanistic insight into the common mechanisms for regulation of the CLC channel activity in response to changes in cell volume as previously reported for CLC-2 (4) and CLC-3 channels (3). The findings may also apply to multisite phosphorylation regulation of other ion transport proteins (e.g., regulation of CFTR activity by inhibitory S737 and S768) (9). Recent proteomic and phosphoproteomic studies have identified even much larger numbers of phosphosites on a variety of ion channels, which sets the stage for further study of how cellular functions are dynamically regulated through multisite phosphorylation of ion channels (1).
GRANTS
G. Wang is supported by American Heart Association (AHA) Grant 10SDG4120011. D.
AUTHOR CONTRIBUTIONS
D.D.D. and G.W. prepared the figure; D.D.D. and G.W. drafted the manuscript; D.D.D. edited and revised the manuscript; D.D.D. and G.W. approved the final version of the manuscript.
REFERENCES
- 1.Baek JH, Cerda O, Trimmer JS. Mass spectrometry-based phosphoproteomics reveals multisite phosphorylation on mammalian brain voltage-gated sodium and potassium channels. Semin Cell Dev Biol 22: 153–159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 89: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol 113: 57–70, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa T, Ogura T, Zheng YJ, Tsuchiya H, Nakaya H, Katayama Y, Inagaki N. Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J Physiol 540: 883–893, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki H, Strange K. Differential regulation of a CLC anion channel by SPAK kinase ortholog-mediated multisite phosphorylation. Am J Physiol Cell Physiol (February 22, 2012). doi:10.1152/ajpcell.00419.2011. [DOI] [PMC free article] [PubMed]
- 6.Rutledge E, Denton J, Strange K. Cell cycle- and swelling-induced activation of a Caenorhabditis elegans ClC channel is mediated by CeGLC-7alpha/beta phosphatases. J Cell Biol 158: 435–444, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson M, Gunawardena J. Unlimited multistability in multisite phosphorylation systems. Nature 460: 274–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varedi KS, Ventura AC, Merajver SD, Lin XN. Multisite phosphorylation provides an effective and flexible mechanism for switch-like protein degradation. PLos One 5: e14029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G. State-dependent regulation of cystic fibrosis transmembrane conductance regulator (CFTR) gating by a high affinity Fe3+ bridge between the regulatory domain and cytoplasmic loop 3. J Biol Chem 285: 40438–40447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang GL, Wang GX, Yamamoto S, Ye L, Baxter H, Hume JR, Duan D. Molecular mechanisms of regulation of fast-inactivating voltage-dependent transient outward K+ current in mouse heart by cell volume changes. J Physiol 568: 423–443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]