Abstract
Purpose.
To determine whether Compound 49b, a novel PKA-activating drug, can prevent diabetic-like changes in the rat retina through increased insulin-like growth factor binding protein-3 (IGFBP-3) levels.
Methods.
For the cell culture studies, we used both human retinal endothelial cells (REC) and retinal Müller cells in either 5 mM (normal) or 25 mM (high) glucose. Cells were treated with 50 nM Compound 49b alone of following treatment with protein kinase A (PKA) siRNA or IGFBP-3 siRNA. Western blotting and ELISA analyses were done to verify PKA and IGFBP-3 knockdown, as well as to measure apoptotic markers. For animal studies, we used streptozotocin-treated rats after 2 and 8 months of diabetes. Some rats were treated topically with 1 mM Compound 49b. Analyses were done for retinal thickness, cell numbers in the ganglion cell layer, pericyte ghosts, and numbers of degenerate capillaries, as well as electroretinogram and heart morphology.
Results.
Compound 49b requires active PKA and IGFBP-3 to prevent apoptosis of REC. Compound 49b significantly reduced the numbers of degenerate capillaries and pericyte ghosts, while preventing the decreased retinal thickness and loss of cells in the ganglion cell layer. Compound 49b maintained a normal electroretinogram, with no changes in blood pressure, intraocular pressure, or heart morphological changes.
Conclusions.
Topical Compound 49b is able to prevent diabetic-like changes in the rat retina, without producing systemic changes. Compound 49b is able to prevent REC apoptosis through increasing IGFBP-3 levels, which are reduced in response to hyperglycemia.
We have developed a novel beta-adrenergic receptor agonist that prevents diabetic-like changes in the rat retina without systemic side effects. We have also determined the mechanism by which Compound 49b can prevent retinal endothelial cell apoptosis.
Introduction
The interplay of neuronal cells, vascular cells, and glial cells are thought to mediate the functional, histological, and biochemical changes observed in the diabetic retina in response to hyperglycemia.1–3 Interestingly, loss of sympathetic innervation via superior cervical ganglionectomy or loss of norepinephrine in dopamine beta hydroxylase knockout mice produces diabetic-like changes in retina, including formation of degenerate capillaries and pericyte ghosts, decreased retinal thickness, and cell loss in the ganglion cell layer, despite normal glucose levels.4 We hypothesize that interruption of normal sympathetic input through β-1- and β-3-adrenergic receptors, which are present on retinal endothelial cells,5 and β-1- and β-2-adrenergic receptors, which are present on retinal Müller cells,6 may mediate some of these effects. Indeed, treatment with β-adrenergic receptor agonists can reduce endogenous pro-apoptotic factors, TNFα and IL-1β, in both retinal endothelial cells (RECs)7 and retinal Müller cells.6 These observations suggest that enhancement of adrenergic input may help suppress apoptosis of retinal cells under various conditions of stress, notably diabetes-induced hyperglycemia.
Beta-adrenergic receptors in the retina initiate signal transduction as they do in most other tissues, leading to increased cAMP levels and increased PKA activity.8 By monitoring changes in these downstream targets of signal transduction, we can assess effectiveness of a variety of receptor agonists in both in vivo and in vitro models of diabetes. Previously, we have shown that topical delivery of isoproterenol to rodent eyes reaches the retina, resulting in increased protein kinase A (PKA) activity and CREB phosphorylation.8 The pivotal observation of these previous studies was that treatment with isoproterenol eye drops eliminates the functional, histological, and biochemical changes commonly seen in retinas of streptozotocin-induced diabetic rats, but with one significant side effect. Topical isoproterenol produced cardiovascular remodeling, suggesting that the topically applied drug reached the systemic circulation.
To circumvent adverse cardiovascular effects, we developed a novel drug, Compound 49b, which we now find to be a promising candidate for treatment of diabetic retinopathy. Compound 49b is similar in structure to isoproterenol with the addition of a N-substituent 3,4,5-trimethoxylphenethyl ring.
By screening the effects of multiple beta-adrenergic receptor agonists in two cell culture models, human REC and rat retinal Müller cells (rMC-1 cell line), Compound 49b was determined to be highly effective in preventing TNFα upregulation and cleavage of caspase 3 in both cell types at 50 nM versus10 μM required to elicit these effects with isoproterenol. Similar vascular protective effects were found in vivo in an 8-month study using topical application of Compound 49 in the well-characterized streptozotocin-induced type I diabetic rat model.9
Although multiple pathways are likely involved in the vascular protective response of Compound 49b, of particular interest is a recently described pathway involving insulin-like growth factor binding protein 3 (IGFBP-3). The standard IGF-dependent actions of the family of IGF binding proteins have been well described; however, since 1993, several IGF-independent actions have been discovered for IGFBPs, primarily related to cell fate and apoptosis.10,11 The role of IGFBP-3 in the control of cell growth remains an area under intense study, as IGFBP-3 may enhance or suppress cell growth, depending on specific conditions.12 In the retina, multiple forms of IGFBP (2–5) are secreted by REC.13 IGFBP-3 has been shown to enhance cell proliferation in REC and to decrease the formation of neovascular tufts in a murine model of oxygen-induced retinopathy.10,14,15 Together, these reports suggest that IGFBP-3 may provide vascular protection in the retina. IGFBP-3 is also neuroprotective in the retina and reduces injury-induced retinal inflammation.16
The findings we report here test the hypothesis that Compound 49b may be a promising treatment for diabetic retinopathy, preventing and/or slowing the progression of the neuronal and vascular changes through beta-adrenergic receptor-dependent maintenance of IGFBP-3 levels in the diabetic retina without causing deleterious cardiovascular remodeling.
Methods
Reagents
Reagents were purchased from commercial sources as follows: Bcl-xl antibodies, cytochrome C antibodies, and Bax antibodies from Cell Signaling Technology (Beverly, MA); FasL antibodies, Fas antibodies, and actin antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); IGFBP-3 antibody from GroPep (Novozymes, Adelaide, Australia); PKA Activity ELISA from Millipore (Bilerica, MA); human PRKACA siRNA, human PRKACB siRNA, and Non-Targeting siRNA number 1 from Dharmacon RNAi Technologies (Chicago, IL); human IGFBP-3 siRNAs and Lipofectamine RNAiMAX Transfection Reagent from Invitrogen (Carlsbad, CA); Cell Death Detection ELISA from Roche Applied Science (Nonnenwald, Penzberg, Germany); human IGFBP-3 immunoassay from R&D (Minneapolis, MN); secondary anti-mouse and anti-rabbit antibodies conjugated with horseradish peroxidase (HRP) from Promega (Madison, WI); and enhanced chemilluminescence (ECL) for immunoblot development and signal detection from Amersham Biosciences (Piscataway, NJ). Compound 49b was synthesized in Dr Duane Miller's laboratory (University of Tennessee Health Science Center).
Retinal Endothelial Cell Culture
Primary human retinal microvascular endothelial cells (REC) were acquired from Cell Systems Corporation (CSC, Kirkland, WA). Cells were grown in M131 medium containing microvascular growth supplements (Invitrogen), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B. Before the experiment, cells were transferred to high (25 mM) or normal (5 mM) glucose medium (Cell Systems), supplemented with 10% fetal bovine serum (FBS) and antibiotics for 3 days. Only primary cells within passage 6 were used. Cells were quiesced by incubating in high or normal glucose medium without FBS for 24 hours and used to perform the experiments unless otherwise indicated.
Müller Cells (rMC-1) Culture
Müller cells (rMC-1, kindly provided by Dr Vijay Sarthy at Northwestern University) were thawed and cultured in Dulbecco's modified Eagle's medium in high-glucose (25 mM) conditions. Medium was supplemented with 10% FBS and antibiotics. Once cells reached approximately 80% confluency, they were placed into high-glucose medium without FBS to induce serum starvation for 18 to 24 hours. Based on preliminary data from isoproterenol studies with TNFα,17 cells were treated with Compound 49b at the 1- and 24-hour time points following serum starvation. For consistency with the REC data, Müller cells were treated at 50 ηM, 100 ηM, and 10μM for all compounds. For all experiments, some dishes remained untreated as controls.
Transfections
RECs were transfected with specific siRNA molecules at a final concentration of 20 nM using RNAiMAX transfection reagent according to the manufacturer's instructions. Cells were used for experiments 48 hours after transfection.
ELISA Analysis
PKA activity was assessed by PKA kinase activity ELISA (Millipore), according to the manufacturer's instructions, following treatment with PKA siRNA and/or Compound 49b under normal or high glucose conditions. IGFBP-3 was detected by specific ELISA (R&D Systems), after siRNA and/or Compound 49b treatment in vitro. Cell Death ELISA analyses of whole cell pellets were used to quantify apoptosis. This is a strong measure for apoptotic cell death. For all ELISA analyses, equal protein amounts were loaded into each well, allowing for comparisons using optical density (OD).
Western Blot Analysis
After appropriate treatments and rinsing with cold PBS, RECs were lysed in the lysis buffer containing the protease and phosphatase inhibitors and scraped into the tubes. Retinal extracts were prepared by sonication. Equal amounts of protein from the cell or tissue extracts were separated on the precast tris-glycine gel (Invitrogen), blotted onto a nitrocellulose membrane. After blocking in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (wt/vol) BSA, the membrane was treated with appropriate primary antibodies followed by incubation with secondary antibodies labeled with HRP. Antigen-antibody complexes were detected by chemiluminescence reagent kit (Thermo Scientific, Pittsburgh, PA).
Acid Sphingomyelinase Activity Assay
Protein lysates from either RECs or retina were prepared in lysis buffer containing the protease and phosphatase inhibitors after appropriate treatments. Sphingomyelinase activity was measured (Amplex Red Sphingomyelinase Assay Kit; Molecular Probes, Eugene, OR) according to the manufacturer's protocol. Briefly, 100 μL of the Amplex Red reagent/HRP/choline oxidase/alkaline phosphatase/sphingomyelin working solution were added to each microplate well containing the equal amount of sample protein. The mixture was incubated for 30 minutes at 37°C and fluorescence was detected at 530 nm with emission at 590 nm.
Animal Preparation
Male Lewis rats purchased from Charles River (Wilmington, MA) were made diabetic by a single injection of 60 mg/kg streptozotocin (STZ; Fisher, Pittsburgh, PA).Blood glucose was measured bimonthly, and levels higher than 250 mg/dL were considered diabetic. Three groups of rats were used for the study: nondiabetic control, diabetic, and diabetic + 49b. The rats were put onto Compound 49b eye drop therapy within 1 week of STZ injection. Rats were killed at age 2 or 8 months to isolate retinae, and retinal cell lysates were used for detection of PKA activity, IGFBP-3, and apoptotic markers. All animal procedures met the Association for Research in Vision and Ophthalmology requirements and were approved by the Institutional Animal Care and Use Committee of the University of Tennessee and conform to National Institutes of Health guidelines.
Electroretinogram
Electroretinogram (ERG) analyses were carried out to evaluate the changes in the electrical activity of the retina as we have done previously.8 Briefly, rats were dark-adapted overnight. ERG responses were recorded from both eyes together using platinum wire corneal electrodes, forehead reference electrode, and ground electrode in the tail. Pupils were fully dilated using 1% tropicamide solution (Alcon, Fort Worth, TX). Methylcellulose (Celluvise; Allergan, Irvine, CA) drops were applied as well to maintain a good electrical connection and body temperature was maintained at 37°C by a water-based heating pad. ERG waveforms were recorded with a bandwidth of 0.3 to 500 Hz and samples at 2 kHz by a digital acquisition system and were analyzed a custom-built program (MatLab). Statistics was done on the mean ±SD amplitudes of the a- and b-wave of each treatment group.
Blood pressure (BP) was measured noninvasively monthly using a CODA noninvasive blood pressure machine (CODA Systems; Kent Scientific, Torrington, CT). Animals were placed on an electrical heated pad and an individual holder consisting of an occlusion cuff and volume pressure recording (VPR) cuff was placed at the base of the tail, allowing systolic BP, diastolic BP, mean BP, heart pulse rate, tail blood volume, and tail blood flow to be measured simultaneously.
IOP was measured monthly using a tonometer (TonoLab; Colonial Medical Supply, Franconia, NH). Briefly, the tip of the probe of the tonometer was placed at the cornea of the eye. During measurements, the tip of the probe hit the cornea six times and gave the IOP reading of that eye. This procedure was carried out for both eyes.
Assessment of Retinal Thinning and Loss of Cells of the Ganglion Cell Layer
Formalin-fixed paraffin sections were stained with toluidine blue for light microscopy and morphometry of retinal thickness as described.8 Photomicrographs were assessed for retinal thickness and the number of cells in the ganglion cell layer (GCL) using methods previously described.8 The thickness of the retina and the cell count were measured using OpenLab software (Improvision, Lexington, MA).
Vascular Analyses
Retinas from one eye of all control, diabetic, and diabetic+49b rats after 8 months were used to count degenerate capillaries. The eyes were enucleated, suspended in 10% buffered formalin for 5 days, and the retina was dissected in 3% crude trypsin solution (Difco Bacto Trypsin 250, Detroit, MI). The retinal vascular tree was dried onto a glass slide and stained with hematoxylin-periodic acid-Shiff. Degenerate capillaries were counted and identified as previously described.8 Pericyte ghosts were estimated from the prevalence of spaces in the capillary basement membranes, where pericytes have disappeared. Pericyte ghost numbers were determined in multiple mid-retina fields, and reported per 1000 capillary cells.9,18
Heart Analyses
Tissue sections from the heart of control, diabetic, and treated rats at the 8-month of diabetes time point were frozen with freezing medium and 20 μm sections were obtained via cryostat sectioning. Sections were fixed to glass slides and stained with Pico Red collagen stain. Pictures were taken at ×400 using a Retiga camera (QImaging, Burbay, BC, Canada) attached to a Nikon Biophot light microscope (Nikon, Tokyo, Japan) with Qcapture software (QImaging).
Vitreous Humor Analyses
These studies were completed by Huntingdon Life Sciences, Inc., following method development by Ryan Yates and Hui He (University of Tennessee Health Science Center). Briefly, five rats were treated with Compound 49b for 0.25, 0.5, 1, 2, 4, 8, 12, and 24 hours. Following a blood collection and after animals were killed, the eyes (in situ) were rinsed with ice-cold saline to remove residual drug, and vitreous humor from both eyes of each rat was collected and pooled. After pooling, samples were weighed and snap frozen. Pooled vitreous humor samples were assessed by liquid chromatography ass spectrometry. Control rat vitreous humor was obtained from Pel-freeze (Rogers, AR).
Statistics
All the experiments were repeated in triplicate. Data are presented as means ± SEM and were analyzed using the Kruskal-Wallis method, followed by Dunn's test using Prism 4.0 software (GraphPad Software Inc., La Jolla, CA). P less than 0.05 was considered significant. For Western blots, one representative blot is shown.
Results
50nM Compound 49b Significantly Reduces Cleaved Caspase 3 Levels and TNFα Levels
To determine the optimal dose and time course for Compound 49b, we cultured retinal endothelial cells and retina Müller cells in high-glucose medium (25 mM) and treated with various doses of Compound 49b for varying time points. Cell lysates were analyzed using a cleaved caspase 3 ELISA and a TNFα ELISA. We found that 50 nM was the most effective dose to reduce TNFα and cleaved caspase 3 levels in both REC and retinal Müller cells (Fig. 1). This dose was subsequently used for in vitro studies described below.
Figure 1.
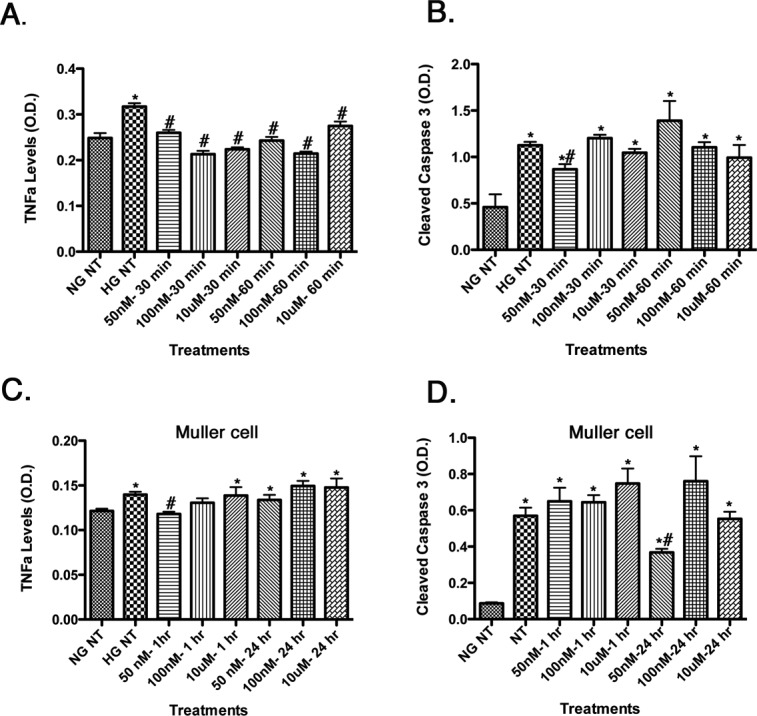
Dose and time testing for TNFα levels (left) and cleaved caspase 3 (right) for Compound 49b in REC (A, B). TNFα and cleaved caspase 3 levels in retinal Müller cells (C, D). Compound 49b reduced TNFα levels at all doses and time points tested in REC, while reducing TNFα at 1 hour at 50 nM in Müller cells. Compound 49b reduced cleaved caspase 3 at 50 nM at 30 minutes in REC and at 50 nM for 24 hours in Müller cells. This dose was used for all in vitro work. *P < 0.05 versus NG NT. #P < 0.05 versus HG NT. n = 3 at all doses and all groups.
High Glucose Produces a Significant Decrease in IGFBP-3, Which Is Blocked by Compound 49b
Retinal endothelial cells cultured in high-glucose medium (25 mM) had significantly reduced levels of IGFBP-3 (Fig. 2A). Similar results were obtained in retinal lysates from STZ-induced type 1 diabetic rats (Fig. 2B). When retinal endothelial cells or diabetic animals were treated with the novel β-adrenergic receptor agonist, Compound 49b, IGFBP-3 levels were restored toward control values.
Figure 2.
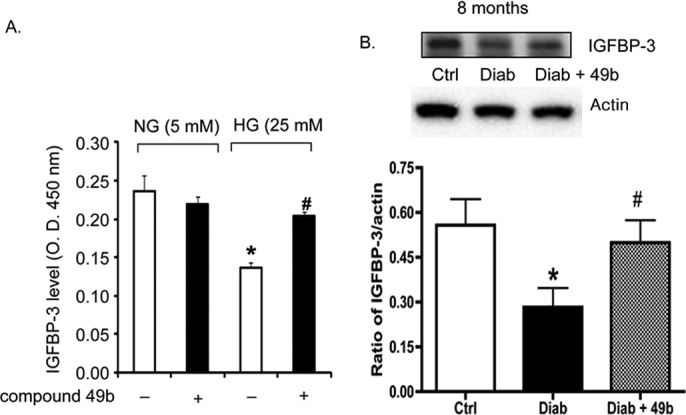
(A) Bar graph of IGFBP-3 levels in REC treated in medium containing normal glucose (NG-5mM) or high glucose (HG-25mM) and with or without treatment with 50 nM Compound 49b for 30 minutes. Equal protein was loaded for all samples; therefore, data are presented as optical density measurements. (B) Western blot analyses of the ratio of IGFBP-3 levels to actin in control (Ctrl), diabetic (diab-8months), and diabetic+Compound 49b (Diab+49b for 8 months). *P < 0.05 versus NG or Ctrl. #P < 0.05 versus HG alone or diabetes alone. n = 4 for cell culture and n = 5 for each animal group.
Compound 49b Increases IGFBP-3 to Reduce Retinal Endothelial Cell Apoptosis
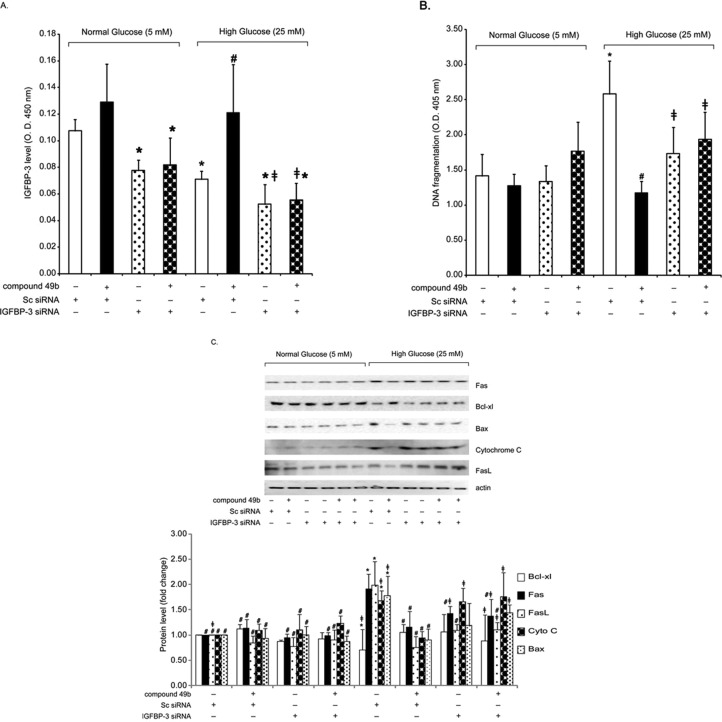
In the oxygen-induced retinopathy of prematurity model, IGFBP-3 shows antiapoptotic effects on retinal endothelial cells.10,14,19 We asked whether IGFBP-3 had the same effects in retinal endothelial cells cultured under hyperglycemic conditions. High-glucose treatment alone reduced IGFBP-3 levels (Fig. 3A) and significantly increased apoptosis (Fig. 3B). Treatment with Compound 49b reversed this effect and significantly reduced markers of apoptosis (Fig. 3C), including DNA fragmentation (Fig. 3B). When Compound 49b treatment was combined with IGFBP-3 siRNA, the ability of Compound 49b to inhibit apoptosis was eliminated. These results strongly suggest that under hyperglycemic conditions, IGFBP-3 is a required component in the antiapoptotic pathway(s) driven by β-adrenergic receptor agonists.
Figure 3.
For all panels, analyses were done on REC treated in medium containing normal glucose (NG-5mM) or high glucose (HG-25mM) and with or without treatment with 50 nM Compound 49b for 30 minutes. Cells in each group were also treated with IGFBP-3 siRNA for 24 hours before Compound 49b treatment. (A) Bar graph of IGFBP-3 levels in REC following treatments. (B) Bar graph of DNA fragmentation levels measured by the Roche Cell Death ELISA in REC following treatments. (C) Western blot analyses for apoptotic markers (Fas, Bax, Cytochrome C, and Fas ligand) and the antiapoptotic marker Bcl-xl in cells. *P < 0.05 versus NG scrambled siRNA. #P < 0.05 versus HG scrambled siRNA. ≠P < 0.05 versus HG Compound 49b-treated REC. n = 4 for all groups.
Compound 49b Increases IGFBP-3 Levels through PKA Activation
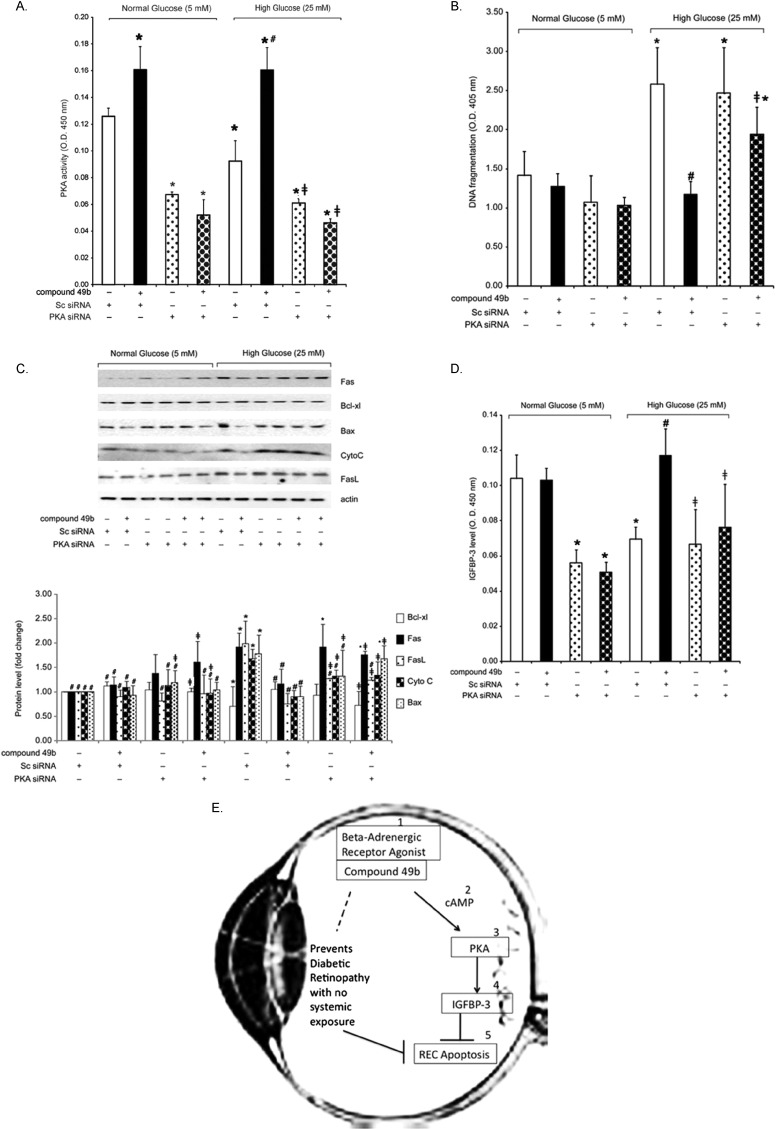
Because Compound 49b is based on the structure of isoproterenol, we verified that Compound 49b actions on IGFBP-3 are mediated through PKA-dependent pathways. Retinal endothelial cells cultured in high glucose showed significantly reduced PKA activity and increased apoptosis (Fig. 4A), whereas the addition of Compound 49b reversed these effects (Figs. 4B, 4C). When PKA siRNA was applied, Compound 49b did not reduce apoptotic markers (Fig. 4C). Likewise, Compound 49b could not increase IGFBP-3 levels when PKA activity was knocked down (Fig. 4D). Taken together, these results suggest that Compound 49b decreases hyperglycemia-induced apoptosis in retinal endothelial cell through β-adrenergic receptor mediated increases in PKA activity and IGFBP-3 (Fig. 4E).
Figure 4.
(A–D) Analyses were done on REC treated in medium containing normal glucose (NG-5mM) or high glucose (HG-25mM) and with or without treatment with 50 nM Compound 49b for 30 minutes. Cells in each group were also treated with PKA siRNA for 24 hours before Compound 49b treatment. (A) Control to verify that PKA activity was reduced following PKA siRNA application. (B) ELISA data from the Roche Cell Death ELISA measuring DNA fragmentation in the REC in all treatment groups. (C) Western blot analyses for apoptotic markers (Fas, Bax, Cytochrome C, and Fas ligand) and the antiapoptotic marker Bcl-xl in cells. (D) ELISA results of IGFBP-3 levels following PKA siRNA to demonstrate that Compound 49b does not increase IGFBP-3 in HG if PKA is knocked down. (E) Schematic of the hypothesis of the mechanism of action for the vascular protective effects of Compound 49b for REC. *P < 0.05 versus NG scrambled siRNA. #P < 0.05 versus HG scrambled siRNA. ≠P < 0.05 versus HG Compound 49b-treated REC. n = 4 for all groups.
Compound 49b Decreases Acid Sphingomyelinase in RECs Cultured in High Glucose and in the Diabetic Retina
In an attempt to offer a potential pathway between Compound 49b-PKA and the prevention of the diabetic retinal changes, we decided to investigate whether Compound 49b could reduce acid sphingomyelinase (ASM). Recent work has shown that ASM is increased rapidly in inflammatory cytokine signaling, and that reduction in ASM can prevent degenerate capillary formation in ASM–/– mice.20 Because Compound 49b can reduce TNFα and prevents the formation of degenerate capillaries, we evaluated whether Compound 49b decreased ASM. Data in both retinal endothelial cells cultured in normal and high glucose and in 8-month diabetic animals demonstrates that diabetes increases ASM levels, which are significantly reduced by Compound 49b treatment (Fig. 5). This may represent a novel pathway to explain the actions of Compound 49b on the diabetic retina.
Figure 5.
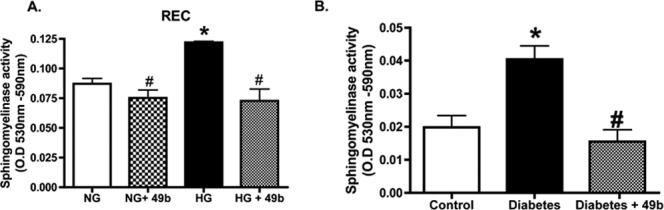
(A) Bar graph of ASM activity in REC treated in medium containing normal glucose (NG-5mM) or high glucose (HG-25mM) and with or without treatment with 50 nM Compound 49b for 30 minutes. Equal protein was loaded for all samples and data are presented as optical density measurements. (B) ASM activity in control (Ctrl), diabetic (diab+8months), and diabetic+Compound 49b (Diab+49b for 8 months). *P < 0.05 versus NG or Ctrl. #P < 0.05 versus HG alone or diabetes alone. n = 4 for cell culture and n = 6 for each animal group.
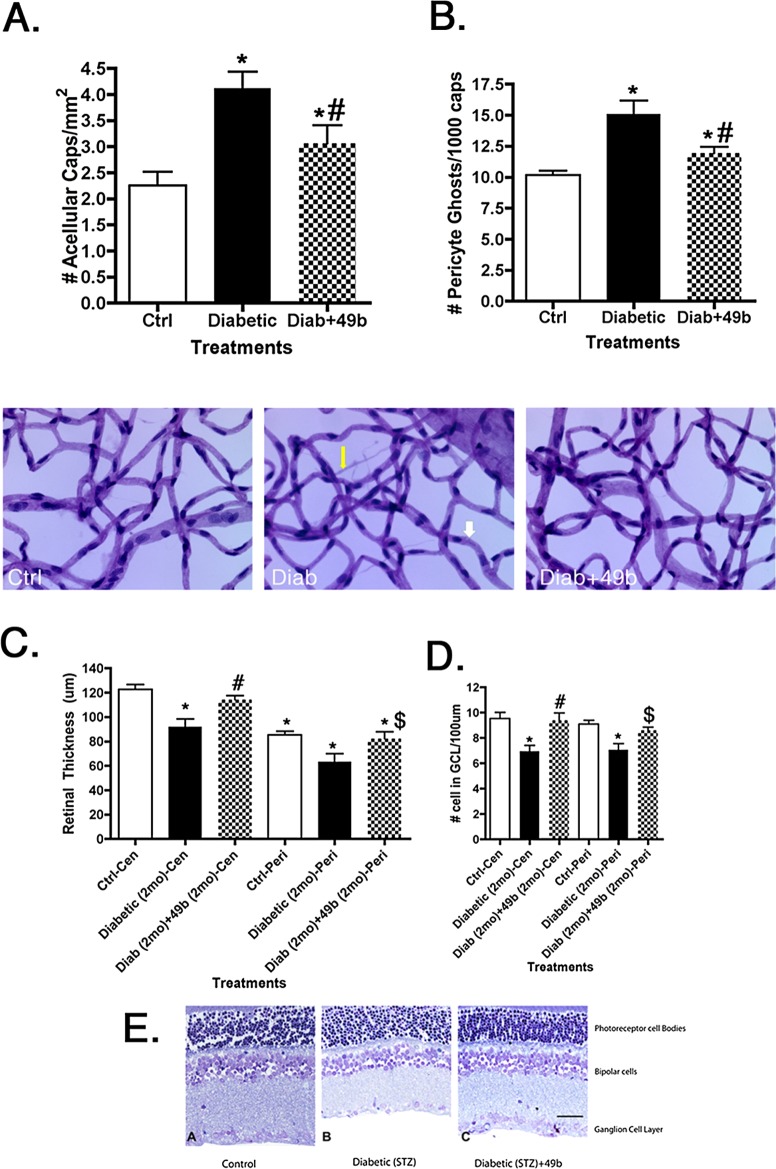
Compound 49b Is Effective In Vivo, Preventing Degeneration of Capillaries, Formation of Pericyte Ghosts, and Loss of Retinal Thickness in STZ-Induced Diabetes
To test the efficacy of Compound 49b in vivo, we analyzed the effects of 1 mM topical eye drops (4 drops of ∼10 μL volume given once per day) of Compound 49b applied to STZ-induced diabetic rats. In untreated diabetic retinas, two major morphological changes were noted within 8 months after induction of diabetes with STZ. These changes included (1) formation of degenerate capillaries (Fig. 6A), and (2) appearance of pericyte ghosts (Fig. 6B), both of which are established hallmarks of diabetic retinopathy. Each of these morphological changes was reduced after treatment with eye drops containing 1 mM Compound 49b. As predicted, the effective concentration of Compound 49b was significantly lower (by 50-fold) than we previously observed for isoproterenol.
Figure 6.
Data from diabetic animal studies. All work was done in control animals (Ctrl), diabetic only (Diabetic), and diabetic+Compound 49b (Diab+49b). (A) Bar graph of the numbers of degenerate capillaries in all groups after 8 months of diabetes and treatment. (B) Number of pericyte ghosts after 8 months of untreated diabetes (Diabetic) or 8 months of diabetes + daily Compound 49b treatment. Underneath (A, B) are representative images of control, diabetic only, and diabetic+49b. Yellow arrow shows a degenerate capillary and white arrow shows a pericyte ghost. (C) Retinal thickness from all groups after 2 months of diabetes or 2 months of diabetes+treatment in the central (Cen) and peripheral (Peri) retina. (D) Number of cells in the ganglion cell layer after 2 months of diabetes or diabetes+treatment in the central (Cen) and peripheral (Peri) retina. (E) Representative images of the retina from which counts in (C, D) were obtained. Scale Bar in Panel E is 5 μm. *P < 0.05 versus ctrl. #P < 0.05 versus Diabetic only. $P < 0.05 versus diabetic-peripheral regions. n = 5 for all animal groups.
Protection against apoptotic loss of vascular and neuronal cells was further confirmed by assessment of TNFα and cleaved caspase 3 levels with and without treatment with Compound 49b. We have previously shown that isoproterenol reduced TNFα and cleaved caspase 3 levels in the STZ model of diabetic retinopathy.8 Interestingly, Compound 49b not only decreased apoptosis of the vasculature, it also prevented loss of retinal thickness (Fig. 6C) and cell numbers in the ganglion cell layer (Fig. 6D) in the neural retina. These results suggest that Compound 49b prevents both the neuronal and vascular changes common to diabetic retinopathy.
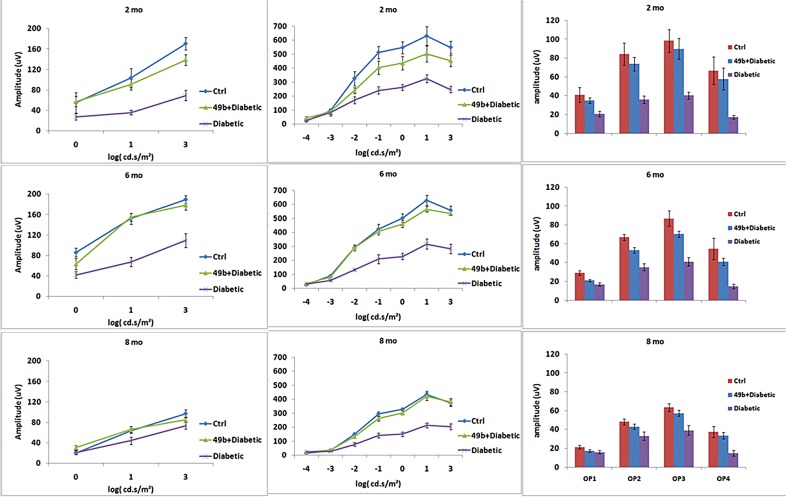
Compound 49b Prevents Diabetic Loss of Retinal Function as Measured by ERGs
It was important to determine if the morphological protection provided by Compound 49b was accompanied by functional protection against vision loss associated with diabetic retinopathy. B-wave and oscillatory potential amplitudes measured by ERGs represent standard measures of visual function in rats. Compound 49b prevented the loss of amplitudes of the B-wave and oscillatory potentials observed in diabetic animals at 2, 6, and 8 months of treatment (Fig. 7).
Figure 7.
ERG analyses from control (Ctrl), diabetic (diabetic), or diabetic + Compound 49b–treated animals (49b+diabetic) at 2 months, 6 months, and 8 months of treatment. The left panels represent the a-wave, middle panels are b-wave amplitudes, and the far right panels are the mean amplitudes of the oscillatory potentials. n = 5 for all groups at each time point.
No Heart Changes Are Noted after Compound 49b Treatment
Because systemic exposure to isoproterenol caused deleterious cardiac side effects, we wished to determine if delivery of the drug through topical eye drops would limit effects on the cardiovascular system. Figure 8 demonstrates that diabetes produces increased collagen content in the left ventricle of diabetic rats and that Compound 49b treatment does not alter this response. By this measure, it appears that systemic exposure to topical Compound 49b is limited.
Figure 8.
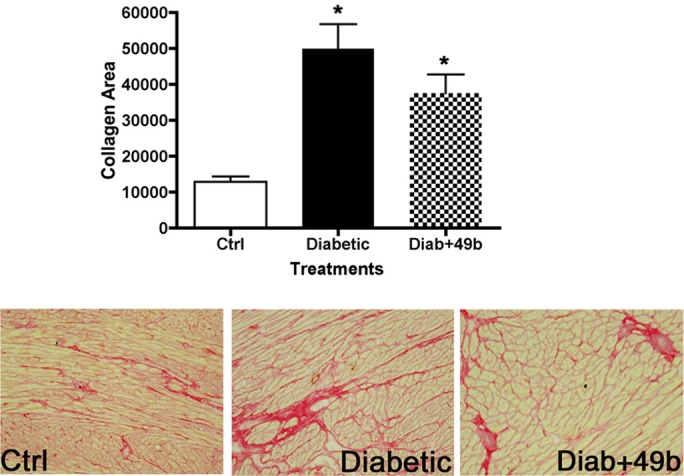
Analyses of the left ventricle for rats at 8 months of age. Data were collected from control (ctrl), diabetic only (diabetic), and diabetic+Compound 49b (Diab+49b) rats. The bar graph is the mean collagen area in the left ventricle of each group, with representative pictures below. All images were taken at ×20. Both diabetic and diabetic+49b had significantly more collagen than control samples of left ventricle. *P < 0.05 versus ctrl. n = 5 for all animal groups.
Importantly, we also found that Compound 49b does not alter systemic blood pressure or intraocular pressure (Table). The highly lipophilic nature of the drug may account for its selective accumulation and action at the site of application in the orbit.
Table.
Physiological Measurements in Control, Diabetic, and Diabetic+Compound 49b Rats after 8 Months of Diabetes and Treatment
|
n |
Body Weight (g) |
Blood Glucose (mg/dL) |
BP-Systolic |
BP-Diastolic |
IOP |
|
| Control | 6 | 507.6 ± 37.5 | 132.7 ± 7.3 | 100.0 ± 10.9 | 77.0 ± 9.4 | 8.92 ± 1.52 |
| Diabetic (8 mo) | 8 | 273.1 ± 40.5* | 724.6 ± 39.2* | 115.9 ± 14.1 | 87.8 ± 13.7 | 7.19 ± 1.05* |
| Diabetic (8 mo)+Compound 49b (8 mo) | 6 | 248.7 ± 8.3* | 695.8 ± 62.2* | 104.8 ± 13.7 | 81.2 ± 14.1 | 8.08 ± 1.17 |
Data are mean ± SD.
* P < 0.05 versus Control.
Figure 9 presents the levels of Compound 49b measured in the vitreous humor following topical delivery, suggesting that Compound 49b does reach the retinal region but is processed quickly.
Figure 9.
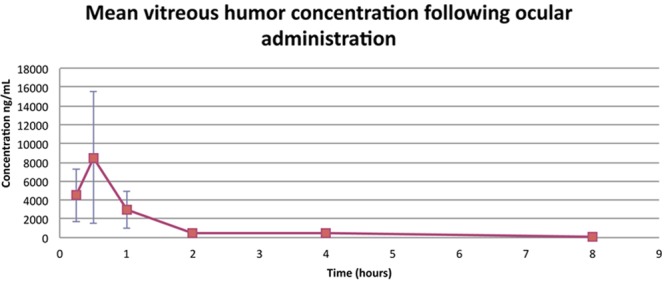
Mean concentration of Compound 49b in the vitreous humor of rats treated with 1 mM topical Compound 49b. n = 5.
Discussion
Endothelial damage is one of the earliest markers of microvascular damage from diabetes. Our goals were 2-fold: (1) determine whether 1 mM topical Compound 49b can prevent diabetes-induced changes to the retina; and (2) determine whether Compound 49b prevents apoptosis via the novel IGFBP-3 pathway. When Compound 49b was administered topically over 8 months to type 1 diabetic rats, the structural integrity of the diabetic retina was preserved. Monthly ERG analyses showed no loss of retinal electrical activity over the 8-month study in Compound 49b–treated rats compared with untreated diabetic rats. Supporting the local effect of Compound 49b, intraocular pressure, body weight, blood pressure, and glucose levels were not influenced by eye drop therapy. Ocular Compound 49b also did not appear to alter diabetes-induced changes to the left ventricle of the heart. Regarding Compound 49b's actions in the retina, Compound 49b was able to prevent pericyte loss and the formation of degenerate capillaries; both significant characteristics of diabetic retinopathy.21 Compound 49b improved neuronal measures in the retina, including prevention of loss of retinal thickness and cell number in the ganglion cell layer, both common changes in early diabetic retinopathy. Taken together, the results suggest that Compound 49b may work as a novel therapy for diabetic retinopathy.
To address the second goal of these studies, we used retinal endothelial cells in culture and investigated changes in IGFBP-3 when cells were exposed to hyperglycemia. To investigate whether β-adrenergic receptors can inhibit apoptosis of RECs within a diabetic milieu, we applied Compound 49b to RECs in vitro and to retina in vivo. These data suggest that Compound 49b can decrease the apoptotic markers, Fas, FasL, Bax and cytochrome C, and increase the antiapoptosis protein Bcl-xl compared with the diabetic group both in vitro and in vivo. This protection against apoptosis by Compound 49b suggests that restoring β-adrenergic input may counterbalance the apoptosis induced by high glucose. The mechanisms by which β-adrenergic receptors can be antiapoptotic for RECs are unknown.
In most tissues, β-adrenergic receptor stimulation will increase PKA levels to initiate cellular signaling.22 We found that Compound 49b increases PKA activity in diabetic rats and in REC cultured in high glucose. Inhibition of PKA eliminated the ability of Compound 49b to block REC apoptosis, suggesting that PKA is directly involved in the inhibition of apoptosis.
In the present study, we observed Compound 49b activates IGFBP-3 via a mechanism involving PKA to prevent REC apoptosis. The finding that downregulation of PKA completely suppressed Compound 49b-induced IGFBP-3 activation indicates that Compound 49b activates the IGFBP-3 release via PKA. The observation that downregulation of PKA and IGFBP-3 eliminates Compound 49b's antiapoptotic effects in REC supports a critical role for IGFBP-3 downstream of PKA in mediating Compound 49b's protective effects. The capacity of Compound 49b–PKA–IGFBP-3 signaling appears to be quite significant in inhibiting diabetic retinopathy in pathophysiologic models and could be used in clinical therapeutic treatment as well.
Our results support the idea that IGFBP-3 has IGF-1–independent effects that affect cell mobility and survival and may be a critical regulator of vascular development.10,14 Although we focused on the Compound 49b–PKA–IGFBP-3 apoptosis signaling pathway, IGFBP-3 can activate the antiapoptosis signaling by binding to IGFBP-3 receptor23 or other mechanisms,11 such as by activating the sphingosine kinase or phosphorylation of the protein.24 Recent work has shown that ASM is increased in the diabetic retina and may be involved in the deleterious effects in the retina.20,25 Our data with ASM suggest that Compound 49b significantly decreases ASM, suggesting that this may be a pathway by which Compound 49b is protective to the retina. Additional studies on the actions of Compound 49b on sphingosine kinase and regulation of IGFBP-3 through the sphingolipids are warranted.
The goal of this study was to establish the signaling pathway that mediated the protective effects of beta-adrenergic receptor agonist activation. We found that despite increased glucose levels, Compound 49b maintained IGFBP-3 levels. Additionally, we found that the antiapoptotic actions of a novel β-adrenergic receptor agonist, Compound 49b, can preserve the retinal neuronal and vascular health in a diabetic retinopathy rat model. Taken together, these studies suggest that maintenance of normal β-adrenergic receptor signaling in spite of hyperglycemia may be a novel antiapoptotic therapy for both the neural and vascular retina.
Footnotes
Supported by Juvenile Diabetes Research Foundation Grants 2-2008-1044 and 2-2011-597 (JJS), Oxnard Foundation (JJS), International Retinal Research Foundation (JJS), University of Tennessee Health Science Center Neuroscience Institute (JJS), National Institutes of Health National Research Service Award Minority Grant F31EY019240-02 (RJW), Hamilton Eye Institute Research to Prevent Blindness Award (PI: Barrett Haik), National Eye Institute Vision Core Grant PHS 3P30 EY013080 (PI: Dianna Johnson), Public Health Service Grant EY00300 (TSK), and Medical Research Service of the Department of Veterans Affairs (TSK).
These authors contributed equally to this manuscript.
Disclosure: Q. Zhang, None; K. Guy, None; J. Pagadala, None; Y. Jiang, None; R.J. Walker, None; L. Liu, None; C. Soderland, Cell Systems Corp (E); T.S. Kern, None; R. Ferry, Jr, None; H. He, None; C.R. Yates, None; D.D. Miller, P; J.J. Steinle, P
References
- 1. Gardner TW., Antonetti DA., Barber AJ., LaNoue KF., Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47 (suppl 2) ; S253–262 . [DOI] [PubMed] [Google Scholar]
- 2. Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27283–290 . [DOI] [PubMed] [Google Scholar]
- 3. Calcutt NA., Cooper ME., Kern TS., Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8417–429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steinle JJ., Kern TS., Thomas SA., McFadyen-Ketchum LS., Smith CP. Increased basement membrane thickness, pericyte ghosts, and loss of retinal thickness and cells in dopamine beta hydroxylase knockout mice. Exp Eye Res. 2009;881014–1019 . [DOI] [PubMed] [Google Scholar]
- 5. Steinle JJ., Booz GW., Meininger CJ., Day JN., Granger HJ. Beta 3-adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem. 2003;27820681–20686 . [DOI] [PubMed] [Google Scholar]
- 6. Walker R., Steinle J. Role of beta-adrenergic receptors in inflammatory marker expression in Müller cells. Invest Ophthalmol Vis Sci. 2007;485276–5281 . [DOI] [PubMed] [Google Scholar]
- 7. Steinle JJ. Sympathetic neurotransmission modulates expression of inflammatory markers in the rat retina. Exp Eye Res. 2007;84118–125 . [DOI] [PubMed] [Google Scholar]
- 8. Jiang Y., Walker RJ., Kern TS., Steinle JJ. Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp Eye Res. 2010;91171–179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engerman RL., Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995;11109–120 . [DOI] [PubMed] [Google Scholar]
- 10. Chang KH., Chan-Ling T., McFarland EL, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A. 2007;10410595–10600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Granata R., Trovato L., Garbarino G, et al. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. FASEB J. 2004;181456–1458 . [DOI] [PubMed] [Google Scholar]
- 12. Vasylyeva TL., Chen X., Ferry RJ ., Jr . Insulin-like growth factor binding protein-3 mediates cytokine-induced mesangial cell apoptosis. Growth Horm IGF Res. 2005;15207–214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu LQ., Sposato M., Liu HY, et al. Functional cloning of IGFBP-3 from human microvascular endothelial cells reveals its novel role in promoting proliferation of primitive CD34+CD38- hematopoietic cells in vitro. Oncol Res. 2003;13359–371 . [DOI] [PubMed] [Google Scholar]
- 14. Lofqvist C., Chen J., Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A. 2007;10410589–10594 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giannini S., Cresci B., Pala L, et al. IGFBPs modulate IGF-I- and high glucose-controlled growth of human retinal endothelial cells. J Endocrinol. 2001;171273–284 . [DOI] [PubMed] [Google Scholar]
- 16. Kielczewski JL., Hu P., Shaw LC, et al. Novel protective properties of IGFBP-3 result in enhanced pericyte ensheathment, reduced microglial activation, increased microglial apoptosis, and neuronal protection after ischemic retinal injury. Am J Pathol. 2011;1781517–1528 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walker RJ., Steinle JJ. Role of beta-adrenergic receptors in inflammatory marker expression in Müller cells. Invest Ophthalmol Vis Sci. 2007;485276–5281 . [DOI] [PubMed] [Google Scholar]
- 18. Kern TS., Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13863–867 . [DOI] [PubMed] [Google Scholar]
- 19. Kielczewski JL., Jarajapu Y., McFarland EL, et al. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ Res. 2009;105 897–905 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Opreanu M., Tikhonenko M., Bozack S, et al. The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes. 2011;602370–2378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kern TS., Engerman RL. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Arch Ophthalmol. 1996;114306–310 . [DOI] [PubMed] [Google Scholar]
- 22. Wichelhaus A., Russ M., Petersen S., Eckel J. G protein expression and adenylate cyclase regulation in ventricular cardiomyocytes from STZ-diabetic rats. Am J Physiol. 1994;267H548–H555 . [DOI] [PubMed] [Google Scholar]
- 23. Han J., Jogie-Brahim S., Harada A., Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-kappaB signaling. Cancer Lett. 2011;307200–210 . [DOI] [PubMed] [Google Scholar]
- 24. Conover CA. Potentiation of insulin-like growth factor (IGF) action by IGF-binding protein-3: studies of underlying mechanism. Endocrinology. 1992;1303191–3199 . [DOI] [PubMed] [Google Scholar]
- 25. Kielczewski JL., Li Calzi S., Shaw LC, et al. Free insulin-like growth factor binding protein-3 (IGFBP-3) reduces retinal vascular permeability in association with a reduction of acid sphingomyelinase (ASMase). Invest Ophthalmol Vis Sci. 2011;528278–8286 . [DOI] [PMC free article] [PubMed] [Google Scholar]