Abstract
Purpose.
The purpose of our study is to determine whether neuroretinal function, measured by the multifocal electroretinogram, differs between males and females with type 2 diabetes and no retinopathy.
Methods.
This study included 70 eyes from 70 adult subjects (14 control males, 22 control females, 16 males with type 2 diabetes, and 18 females with type 2 diabetes). A template-scaling technique was used to obtain first-order P1 implicit times and N1-P1 amplitudes from photopic multifocal electroretinograms within the central 45 degrees.
Results.
The males with type 2 diabetes were significantly more abnormal than their female counterparts in two separate analyses of local neuroretinal function. First, the total number of retinal locations with an abnormally delayed implicit time (z score ≥ 2) was higher (P < 0.001) in the diabetic males (482 locations = 29.2%) compared to the diabetic females (298 locations = 16.1%). Second, in the response topographies that consisted of 103 means of local implicit times for each group, the diabetic males were significantly delayed (P < 0.025) at 23 corresponding positions (22.3%) compared to the diabetic females. At the same time, no corresponding stimulus locations were significantly delayed in the diabetic females compared to the diabetic males.
Conclusions.
Neuroretinal function is more abnormal in males than in females for adults with type 2 diabetes and no retinopathy. These results suggest that, relative to males, females may have some protection from, or resistance to, neurodegenerative changes that precede the development of background retinopathy in type 2 diabetes.
In adults with type 2 diabetes and no retinopathy, local neuroretinal function is significantly more abnormal in males compared to females. Consequently, the neuroretinal function of females appears to be protected from neurodegenerative changes in the retina associated with type 2 diabetes.
Introduction
The relationship between diabetic retinopathy and sex (male and female) is unclear. Many studies do not report sex to be an independent risk factor for retinopathy in type 2 diabetes.1–5 However, a smaller body of evidence suggests that, if there is an association, the risk of retinopathy in type 2 diabetes is greater for males. In the Los Angeles Latino Eye Study (LALES), male sex was an independent risk factor for the presence of diabetic retinopathy.6 The United Kingdom Prospective Diabetes Study (UKPDS) determined that males presented with more retinopathy than females at the time they were diagnosed with type 2 diabetes, after adjusting for systolic blood pressure and fasting blood sugar.7 In the Chennai Urban Rural Epidemiology Study (CURES), male sex was an independent risk factor for severity of diabetic retinopathy.8
Although diabetic retinopathy is diagnosed and graded by vascular changes, neurodegenerative changes often precede the appearance or progression of retinopathy. For example, loss of oscillatory potentials in the electroretinogram predicts progression to proliferative diabetic retinopathy with high-risk characteristics (for vision loss) better than color fundus photographs.9 Furthermore, delays in oscillatory potentials consistently precede the development of retinopathy.10–12 More recent work with the multifocal electroretinogram (mfERG) demonstrates that localized delays in retinal responses (in combination with other factors) are highly predictive of sites where diabetic retinopathy will develop.13–16
If the male sex poses an increased risk for diabetic retinopathy, then delays of the mfERG response in diabetic patients may be greater in males than in females before retinopathy. Although conventional electroretinographic (ERG) amplitudes in healthy subjects are larger in females compared to males,17,18 to our knowledge, mfERGs of males and females have not been compared. Consequently, using subjects who were healthy or who had type 2 diabetes and no retinopathy, our study examined the mfERGs of the two sexes to determine whether neuroretinal function in subjects with type 2 diabetes is more affected in males than in females.
Methods
Subjects
We included 70 subjects in this study: 14 control males, 22 control females, 16 males with type 2 diabetes, and 18 females with type 2 diabetes. The data for the subjects were obtained from previous investigations published from this laboratory.14–16 For this study, the patients with retinopathy or type 1 diabetes were excluded, and only the patients with type 2 diabetes and no retinopathy were included. Before any analysis, one control female (57.4 years old) also was excluded because it was unclear whether she was not diabetic. Subsequently, the youngest control subjects were excluded to make the average ages of the control groups match the groups with type 2 diabetes. The average ages of the control males and females who were excluded were 31.2 and 31.3 years, respectively. For subjects involved in longitudinal studies, only measurements from their first visit were used. The University of California Committee for Protection of Human Subjects had approved the research, all procedures adhered to the tenets of the Declaration of Helsinki, and all subjects provided written consent.
Subjects had best-corrected visual acuities of 20/20 or better, spherical equivalent refractive errors between −6.00 and +4.00 diopters, no significant media opacities, and no histories of ocular pathology, including diabetic retinopathy. Patients with type 2 diabetes had been diagnosed by a medical doctor, and were under the care of a physician at the time of testing. Two of the 34 patients with diabetes reported taking insulin. A retina specialist confirmed the absence of diabetic retinopathy in both eyes of all subjects by examining digital fundus photos subtending ∼50°, taken after mfERG testing.
The mean ages of the four subject groups were not significantly different. Control males and females were 52.8 ± 7.3 (range 37–61) and 52.3 ± 9.7 (range 31–65) years old, respectively. Diabetic males and females were 55.9 ± 9.3 (range 35–67) and 53.5 ± 5.7 (range 42–63) years old, respectively.
At the time of the mfERG measures, duration of diabetes, glycated hemoglobin (HbA1c), blood pressure, and blood glucose measurements were similar between the male and female patients with diabetes (Table 1). The time since the diagnosis of type 2 diabetes ranged from one to 10.5 years for males and one to 21.2 years for females. HbA1c was measured using A1C At-Home testing kits (Flexsite Diagnostic, Palm City, FL). Due to the retrospective nature of this study, HbA1c measures were available only for 8 of the 16 diabetic males, and 11 of the 18 diabetic females. In addition, blood pressure measurements had been made for only 7 of the 16 diabetic males and 11 of the 18 diabetic females.
Table 1.
Mean of Characteristics in the Patient Groups
|
Male |
Female |
PValue* |
|
| Diabetes duration (y) | 6.9 (2.4) | 8.1 (4.8) | 0.37 |
| HbA1c (%) | 8.1 (2.3) | 8.5 (1.5) | 0.73 |
| Blood glucose (mg/dL) | 201 (108) | 161 (80) | 0.24 |
| Systolic BP (mm Hg) | 132 (20) | 117 (14) | 0.14 |
| Diastolic BP (mm Hg) | 76 (11) | 73 (7) | 0.49 |
The numbers in parentheses represent SD. BP, blood pressure.
Student's two-tailed t-test.
Multifocal Electroretinograms
The mfERG methods have been described previously.14 Briefly, mfERGs were recorded using a Visual Evoked Response Imaging System (VERIS Science 4.3, EDI, San Mateo, CA). The stimulus consisted of a scaled 103-element hexagonal array. Before the procedure, corneas were anesthetized with 0.5% proparacaine, and pupils were dilated to at least 7 mm with 2.5% phenylephrine and 1.0% tropicamide.
Retinal signals were acquired using a Burian-Allen bipolar contact lens electrode (Hansen Ophthalmic Development Laboratory, Coralville, IA) filled with Celluvisc (carboxymethylcellulose 1%), and a ground electrode clipped to the right ear lobe. During the procedure, the fellow eye was occluded with light pressure to prevent blinking. Total recording time was approximately eight minutes per eye. Only the data obtained from the eye tested first were used.
For each of the 103 mfERG waveforms, implicit time was measured from the onset of the local flash to the peak P1 voltage, and response amplitude was the difference between the peak P1 voltage and the preceding N1 trough (Fig. 1). To derive the first-order P1 implicit time and N1-P1 amplitude, the Hood and Li template scaling method was applied to all exported waveforms.19 The 103 local waveform templates were created from 50 healthy subjects (21 males and 29 females).
Figure 1.
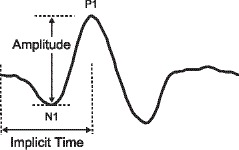
Waveform showing location of N1 trough and P1 peak.
Statistical Analysis
Statistical analyses were performed using Microsoft Excel for Mac 2008 and Stata (version 10.1). The exception was Fisher's exact tests, which were performed on the website (http://statpages.org/ctab2x2.html). Unless specified otherwise, P values ≤ 0.05 were considered significant.
The mfERG implicit times and amplitudes for the males and females were compared by four different methods: (1) number of abnormalities, (2) response topographies, consisting of 103 local means for each group, (3) frequency of abnormal eyes, and (4) whole eye averages. To tally the number of retinal locations with an abnormal implicit time or amplitude, raw measurements at each location for every subject were converted first to z scores. The z scores for all subjects were calculated from the mean and standard deviation of all control subjects combined (14 males and 22 females). Any implicit time that was delayed by a z score ≥2, and any amplitude that was attenuated by a z score ≤−2 were deemed abnormal (P < 0.023 for both). In this way, z scores were treated as a categorical variable in these analyses: either a retinal location was normal or abnormal.
The number of retinal locations with an abnormality was a cumulative sum. If, for example, all retinal locations in a subject group were abnormal, the sum would equal the number of subjects in the group multiplied by 103. When the number of abnormalities was converted to a frequency for a subject group, it approximated the percentage of total retinal area with significant neuroretinal dysfunction for that group.
A response topography for a subject group was constructed by calculating the average implicit time and amplitude for each of the 103 stimulus locations. In essence, it represented the average eye of a subject group. Response topographies allowed the average implicit time and amplitude at each retinal position of one subject group to be compared (as a continuous variable) to the local mean at the same location of another subject group. By comparing two subject groups in this way (location by location), one could determine if, and where on the retina, neural function differed. A difference was considered significant if the average measure of one group fell outside the 95% confidence interval of the other group. Confidence intervals were constructed using the appropriate t-statistic. To be conservative, the 95% confidence intervals from the group with the larger mean confidence interval (of the two groups being compared) were used.
Whole eye averages were calculated by averaging all 103 implicit times and amplitudes for each subject. In this way, a mean implicit time and a mean amplitude were each used as a global index to represent overall neuroretinal function for each subject. Whole eye averages from one subject group were compared to those of another using a two-tailed Student's t-test.
In a multivariate regression, performed to examine relationships between measured factors, 11 variables were considered: male/female sex, age, duration of diabetes, systolic blood pressure, diastolic blood pressure, blood glucose at time of testing, HbA1c, whole eye implicit time, whole eye amplitude, number of implicit time abnormalities, and number of amplitude abnormalities. The males and females were assigned arbitrarily a value, 1 and 0, respectively.
A full multivariate model was used to examine the association between sex and all other potential factors. Though the males and females with type 2 diabetes appeared to be similar in age, duration of diabetes, glycated hemoglobin, blood glucose at time of testing, and blood pressure (using t-tests), it was important to demonstrate (in other ways) that sex was not associated significantly with any of these variables. Otherwise, the possibility existed that a correlated factor, and not sex, was responsible for the results of this study. A full multivariate associative model also was performed for the number of implicit time abnormalities to determine whether or not associative models included sex as a significant variable or an important confounder.
Results
Implicit Time Analyses
Number of Local Implicit Time Abnormalities.
The number of retinal locations with an abnormal implicit time was significantly higher (P < 0.001) in the males with type 2 diabetes (482/1648 = 29.2%) compared to the females with type 2 diabetes (298/1854 = 16.1%, Table 2 and Fig. 2). In contrast, the number of abnormal implicit times in the control males (5/1442 = 0.3%) was similar (P = 0.052, 0.013 × 4 t-tests) to that for the control females (25/2266 = 1.1%).
Table 2.
Percentages of Retinal Locations with an Abnormal Implicit Time (z scores ≥2)
|
Control |
Type 2 Diabetic |
Fisher Exact P Value (Control vs. Diabetic) |
|
| Male | 0.3% | 29.2% | <0.001 |
| Female | 1.1% | 16.1% | <0.001 |
| Fisher Exact P value (male vs. female) | 0.013* | <0.001 |
Rows compare the control subjects to the patients with type 2 diabetes in a given sex. Columns compare the sexes.
Not significant when adjusted for four t-tests.
Figure 2.
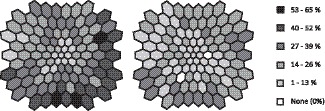
Retinal view in left-eye format showing the occurrence of implicit time abnormalities (z score ≥ 2) at each location in the stimulus array for the diabetic males (left) and the diabetic females (right).
Comparisons of Response Topographies Consisting of Local Implicit Time Means.
When the average implicit time at each of the 103 retinal locations for the males with type 2 diabetes was compared to the mean implicit time at the corresponding stimulus location for the females with type 2 diabetes (using 95% confidence intervals), 23 retinal locations (22%) were delayed significantly in the diabetic males. No corresponding stimulus locations were significantly faster in the diabetic males compared to the diabetic females. Conversely, the response topographies of the control males and the control females were similar, with only two stimulus locations that were significantly delayed (using 95% confidence intervals) in the control males. No corresponding stimulus locations were significantly faster in the control males compared to the control females.
When response topographies for the males with type 2 diabetes and the control males were compared, 96 of the 103 retinal locations (93.2%) were significantly delayed (using 95% confidence intervals) in the diabetic males. No location was significantly faster in the diabetic males relative to the control males. The response topography for the females with type 2 diabetes was significantly delayed (using 95% confidence intervals) at 54 retinal locations (52.4%) compared to the control females. No retinal location in the diabetic females was significantly faster relative to the control females.
Proportion of Abnormal Eyes (Based on Frequency of Implicit Time Abnormalities).
When an eye was defined as abnormal if it had ≥ 6 retinal locations that were abnormally delayed, the frequency of abnormal eyes was significantly higher (P = 0.001) in the males with type 2 diabetes (9/16 = 56%) compared to the control males (0/14 = 0%). In contrast, the females with type 2 diabetes did not have a significantly higher frequency (P = 0.11) of abnormal eyes compared to the control females: 6/18 = 33% versus 2/22 = 9%. The frequencies of abnormal eyes also were not statistically different between the control males and females, and between the diabetic males and females (Table 3).
Table 3.
Proportions of Eyes with Six or More Retinal Locations that Were Abnormally Delayed (z scores ≥2)
|
Control |
Type 2 Diabetic |
Fisher Exact P Value (Control vs. Diabetic) |
|
| Male | 0/14 | 9/16 | 0.001 |
| Female | 2/22 | 6/18 | 0.11 |
| Fisher Exact P value (male vs. female) | 0.51 | 0.30 |
Rows compare the control subjects to the diabetic patients in a given sex. Columns compare sexes.
Whole Eye Implicit Time Averages.
The three previous analyses examined local neuroretinal function to determine whether subject groups differed. The conventional method for comparing subject groups is a global approach averaging implicit times (across retinal locations) for each subject and then comparing subject groups (Fig. 3). When the 103 implicit times for each subject's eye were averaged to create an overall index of neuroretinal function (whole eye average), the males with type 2 diabetes had significantly longer implicit times (P = 0.011) than the control males: 29.7 ± 0.3 ms (average implicit time ± SE) versus 28.8 ± 0.2 ms. In contrast, the implicit times of the females with type 2 diabetes and the control females did not differ significantly (29.1 ± 0.2 versus 28.6 ± 0.2 ms). Whole eye implicit times were not significantly different between the control males and females, and between the diabetic males and females (though the diabetic males tended to have longer implicit times compared to the diabetic females).
Figure 3.
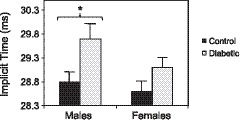
Average whole eye implicit times. Bars indicate 1 SE. The males with type 2 diabetes (29.7 ± 0.3 ms) had significantly longer latencies (*P = 0.011) than the control males (28.8 ± 0.2 ms). The implicit times of the females with type 2 diabetes (29.1 ± 0.2 ms) were similar to the control females (28.6 ± 0.2 ms).
Amplitude Analyses
Number of Local Amplitude Abnormalities.
The numbers of abnormal amplitudes were significantly higher (both P < 0.001) in the diabetic males (52/1648 = 3.2%) versus the control males (0/1442 = 0%), and in the diabetic females (46/1854 = 2.5%) versus the control females (1/2266 = 0%). However, the number of retinal locations with an abnormal amplitude was similar between the diabetic males and females, and between the control males and females (Table 4).
Table 4.
Percentages of Retinal Locations with Abnormal Amplitudes (z scores ≤−2)
|
Control |
Type 2 Diabetic |
Fisher Exact P Value (Control vs. Diabetic) |
|
| Male | 0% | 3.2% | <0.001 |
| Female | 0% | 2.5% | <0.001 |
| Fisher Exact P value (male vs. female) | 1.0 | 0.26 |
Rows compare the control subjects to the patients with type 2 diabetes in a given sex. Columns compare the sexes.
Comparisons of Response Topographies Consisting of Local Amplitude Means.
The response topographies for amplitude did not differ significantly between the control males versus the control females, control males versus diabetic males, control females versus diabetic females, and diabetic males versus diabetic females.
Proportion of Abnormal Eyes (Based on Frequency of Amplitude Abnormalities).
The frequency of abnormal eyes based on amplitude abnormalities was not significantly different between the females with type 2 diabetes (2/18 = 11%) and the control females (0/22), and between the males with type 2 diabetes (2/16 = 13%) and the control males (0/14). The frequency of abnormal eyes was not significantly different between the diabetic males and females, and between the control males and females.
Whole Eye Amplitude Averages.
No group differed significantly from the others when whole eye averages for amplitude were compared (Fig. 4).
Figure 4.
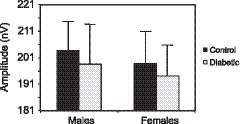
Amplitudes across whole eyes. Bars indicate 1 SE. Amplitudes were not significantly different among the groups. The values for the control males, males with type 2 diabetes, control females, and females with type 2 diabetes were 203.7 ± 11.1, 198.6 ± 15.1, 198.7 ± 12.6, and 194.2 ± 11.9 nV, respectively.
Multiple Regression Analysis
Sex.
Univariate analyses demonstrated that sex was not significantly correlated with any of the other 10 variables that were considered (age, duration of diabetes, HbA1c, systolic blood pressure, diastolic blood pressure, blood glucose at time of testing, whole eye implicit time, the number of implicit time abnormalities, whole eye amplitude, and the number of amplitude abnormalities). A full, stepwise, forward multivariate regression found that sex's association with the number of implicit time abnormalities approached significance (P = 0.053) when HbA1c was included as a confounder (P = 0.35).
Number of Implicit Time Abnormalities.
A full multivariate associative model also was performed for the number of implicit time abnormalities. The univariate analyses for the number of implicit time abnormalities demonstrated that it was correlated significantly with five variables: age, blood glucose at time of testing, whole eye implicit time, whole eye amplitude, and the number of amplitude abnormalities. No important confounders were identified.
In the multivariate analyses, whole eye implicit time was excluded, since it was correlated so strongly with the number of implicit time abnormalities that no other factor remained significant when it was included. Of the remaining four factors, two continued to be significant in multivariate regression analysis: blood glucose at time of testing (P < 0.001) and age (P = 0.001). Both variables had positive associations with the number of implicit time abnormalities.
Discussion
In adults with type 2 diabetes and no retinopathy, local neuroretinal function is significantly more abnormal in males compared to females. The male/female differences in this study manifested in two analyses: the number of abnormal implicit times and the comparison of response topographies consisting of local implicit time means. No differences in amplitude between the diabetic males and females were found.
Although local implicit times were significantly more abnormal for the males with type 2 diabetes compared to their female counterparts, local neuroretinal function did not differ significantly between the control males and females. If there was any difference, the control females might be more abnormal: the larger number of implicit time abnormalities in the control females compared to the control males approached significance (P = 0.052 after adjusting for four t-tests). Despite reports from conventional ERG studies of significantly larger amplitudes in normal females compared to normal males,17,18 amplitudes with the mfERG were similar between the control males and females in our study.
The univariate analysis for sex indicated that the differences in local neuroretinal function between the diabetic males and females in our study were not artifacts of an association with another variable. Multiple regression analysis also showed that a relationship between sex and the number of implicit time abnormalities in the diabetic subjects (with HbA1c as a confounder) approached significance (P = 0.053). Consequently, although t-tests indicated no difference in HbA1c between the two diabetic groups, multivariate analysis suggested that the females with diabetes tended to have higher HbA1c—perhaps reducing (and not exaggerating) the differences between the diabetic males and females in our study.
Multiple regression also revealed that implicit time abnormalities were associated significantly with two factors: increasing age (P = 0.001) and higher blood glucose at time of testing (P < 0.001). The first result seems logical; implicit time increases with age.20 In addition, random blood glucose levels tend to reflect long-term blood sugar control.21 In our study, blood glucose levels at time of testing were related significantly to HbA1c (P < 0.001). However, these results should be interpreted with caution, since HbA1c measures were lacking for many of the subjects.
Although local neuroretinal function differed significantly between the males and females with type 2 diabetes, the same data demonstrated that the two groups did not differ in overall neuroretinal function. This result confirms that neuroretinal dysfunction in type 2 diabetes is not uniform, as observed in previous studies.14,22–24 It should not be surprising that local retinal function, when considered in discrete patches, is different from overall retinal function, when all patches (normal and abnormal) are aggregated. As illustrated in our study, whole eye averages can be insensitive to significant (but localized) neuroretinal dysfunction when implicit times and amplitudes of 103 retinal locations are combined. Only when neuroretinal dysfunction becomes widespread (i.e., more uniform) will the analyses of local and global function necessarily agree.
Our results suggest that neuroretinal function is protected in females with type 2 diabetes, relative to their male counterparts. Though novel, our results are consistent with two studies. Animal experiments demonstrate that female neurons are less sensitive than male neurons to hypoxia induced by oxygen-glucose deprivation,25 and in sensory nerves of humans, longer latencies and lower amplitudes were found in males with type 2 diabetes compared to their female counterparts.26 A combination of facets makes our investigation unique: the data were obtained non-invasively, all subjects with type 2 diabetes were in a similar stage of disease (none had retinopathy), and our study used the mfERG, an instrument that previous investigations of this type have not included (or have not reported).
Delays of the mfERG implicit time indicate neuroretinal dysfunction, and they can be used (in combination with other factors) to predict retinal areas that are at risk for developing microaneurysms, dot hemorrhages, cotton wool spots, or exudates.13 Although our results cannot be used to predict the prevalence of retinopathy in the two sexes, the proportion of eyes with a significant number of implicit time abnormalities was similar in the males and females with type 2 diabetes. However, the greater frequency of implicit time abnormalities in the diabetic males, compared to the diabetic females, suggests that a greater percentage of retinal area in the males is dysfunctional compared to the females. To this end, we might expect that diabetic retinopathy would be more extensive initially in males versus females. The UKPDS finding, that males presented with more retinopathy than females at the time they were diagnosed with type 2 diabetes, is consistent with our findings.7 Moreover, in a recent study using the mfERG to examine diabetic macular edema, the best model predicted that males were six times more likely to have macular edema in a given retinal zone compared to females if systolic blood pressure, mfERG implicit time, and mfERG amplitude were held constant.27
A limitation of our study was the lack of blood pressure measurements and HbA1c levels for approximately half of the subjects. However, the literature reports that no relationship exists between the mfERG implicit time and either variable (Harrison et al. IOVS 2009;50:ARVO E-Abstract 1368).28 In addition, regression analysis for the number of implicit time abnormalities in this study found no significant relationship among the three. Instead of HbA1c, the number of implicit time abnormalities was related more to the blood glucose level at the time of the mfERG testing (a measurement taken for all subjects with diabetes in our study).
Another potential limitation of this study was the possibility of selection bias. Since males may seek medical care less often than females,29 the results of our study, like any study comparing the two sexes, might be confounded by behavioral, economical, and cultural differences between men and women. Selection bias could have caused an over- or under-estimation of the neuroretinal differences between the diabetic males and females observed in our study. For example, the recruitment of subjects from healthcare facilities may have led to an underestimation, since male and female subjects who don't receive regular healthcare (and presumably have poorer diabetic control) would have been less likely to receive an invitation to participate in this study. Conversely, early neglect of type 2 diabetes in the males, despite more comparable control to the females later, could have caused an overestimation or even a creation of differences that are, in fact, insignificant. In our study, however, the multiple regression analysis suggested that the males tended to have better diabetic control than the diabetic females. We also feel that males and females who pay for insurance and who volunteer for 2.5 hours of unnecessary medical testing (i.e., participation in this study) may be more likely to share similar perspectives regarding healthcare.
Delay of the mfERG implicit times is thought to be associated with areas of ischemia.30–33 One reason why females with type 2 diabetes may have had better vascular perfusion than men is estrogen. By increasing production of nitric oxide and nitric oxide synthase (NOS) genes, estrogen causes vasodilation.34 Vasodilation is one of several credible explanations for why estrogen attenuates retinal ischemia-reperfusion injury in rats.35
Although not reported in the preceding results, we also examined whether using sex-specific z scores would yield results that were similar to our main findings. These z scores were generated using the normative female data to calculate the female z scores, and the normative male data to calculate the male z scores. We observed that the differences between the diabetic males and females were larger than the ones reported in our study. Moreover, multivariate analysis for sex showed no significant association with any variable except the number of implicit time abnormalities (and HbA1c as a confounder). The association between sex and the number of implicit time abnormalities remained significant in the multivariate analysis for the number of implicit time abnormalities. To this end, the use of sex-specific z scores agreed with and strengthened the main findings of our study.
Studying the retinal differences in males and females with diabetes may provide insight into mechanisms of neuroprotection. More clues also may be discovered by using the mfERG to determine the effect of insulin,36 estrogen,37,38 or other substances thought to be neuroprotective. If the mfERG is used to assess the retina in these endeavors, local and global neuroretinal function must be examined. Once neuroprotective mechanisms are elucidated, perhaps therapies can be developed to prevent or delay the onset of diabetic retinopathy.
Acknowledgments
Jason Ng and Kavita Dhamdhere assisted in the collection of data.
Footnotes
Supported by NIH Grant EY02271 (AJA) and JDRF Grant 8-2008-823 (MAB).
Disclosure: G.Y. Ozawa, None; M.A. Bearse, Jr, None; K.W. Bronson-Castain, None; W.W. Harrison, None; M.E. Schneck, None; S. Barez, None; A.J. Adams, None
References
- 1. Hove MN., Kristensen JK., Lauritzen T., Bek T. The prevalence of retinopathy in an unselected population of type 2 diabetes patients from Arhus County, Denmark. Acta Ophthalmol Scand. 2004;82443–448 . [DOI] [PubMed] [Google Scholar]
- 2. Klein R., Klein BE., Moss SE., Davis MD., DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102527–532 . [DOI] [PubMed] [Google Scholar]
- 3. Wong J., Molyneaux L., Constantino M., Twigg SM., Yue DK. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care. 2008;311985–1990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tapp RJ., Zimmet PZ., Harper CA, et al. Six year incidence and progression of diabetic retinopathy: results from the Mauritius diabetes complication study. Diabetes Res Clin Pract. 2006;73298–303 . [DOI] [PubMed] [Google Scholar]
- 5. Tung TH., Liu JH., Lee FL., Chen SJ., Li AF., Chou P. Population-based study of nonproliferative diabetic retinopathy among type 2 diabetic patients in Kinmen, Taiwan. Jpn J Ophthalmol. 2006;5044–52 . [DOI] [PubMed] [Google Scholar]
- 6. Varma R., Macias GL., Torres M., Klein R., Peña FY., Azen SP, et al. Los Angeles Latino Eye Study Group. Biologic risk factors associated with diabetic retinopathy: the Los Angeles Latino Eye Study. Ophthalmology. 2007;1141332–1340 . [DOI] [PubMed] [Google Scholar]
- 7. Kohner EM., Aldington SJ., Stratton IM, et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116297–303 . [DOI] [PubMed] [Google Scholar]
- 8. Pradeepa R., Anitha B., Mohan V., Ganesan A., Rema R. Risk factors for diabetic retinopathy in a South Indian Type 2 diabetic population – the Chennai Urban Rural Epidemiology Study (CURES) Eye Study 4. Diabet Med. 2008;25536–542 . [DOI] [PubMed] [Google Scholar]
- 9. Bresnick GH., Palta M. Predicting progression to severe proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105810–814 . [DOI] [PubMed] [Google Scholar]
- 10. Bresnick GH., Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Arch Ophthalmol. 1987;105929–933 . [DOI] [PubMed] [Google Scholar]
- 11. Yonemura D., Kawasaki K. New Approaches to ophthalmic electrodiagnosis by retinal oscillatory potential, drug-induced responses from retinal pigment epithelium and cone potential. Doc Ophthalmol. 1979;48163–222 . [DOI] [PubMed] [Google Scholar]
- 12. Yoshida A., Kojima M., Ogasawara H., Ishiko S. Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetic patients without retinopathy. Ophthalmology. 1991;981266–1271 . [DOI] [PubMed] [Google Scholar]
- 13. Han Y., Bearse MA , Jr,, Schneck ME., Barez S., Jacobsen CH., Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45948–954 . [DOI] [PubMed] [Google Scholar]
- 14. Bearse MA , Jr,, Adams AJ., Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25425–448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng JS., Bearse MA , Jr,, Scheck ME., Barez S., Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;491622–1628 . [DOI] [PubMed] [Google Scholar]
- 16. Harrison WW., Bearse MA , Jr,, Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52772–777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birch DG., Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;1101571–1576 . [DOI] [PubMed] [Google Scholar]
- 18. Brülü J., Lavoie MP., Casanova C., Lachapelle P., Hébert M. Evidence of a possible impact of the menstrual cycle on the reproducibility of scotopic ERGs in women. Doc Ophthalmol. 2007;114125–134 . [DOI] [PubMed] [Google Scholar]
- 19. Hood DC., Li J. A technique for measuring individual multifocal ERG records. :Yager D. Non-Invasive Assessment of the Visual System. Washington, DC:Optical Society of America, Trends in Optics and Photonics; 199733–41 . [Google Scholar]
- 20. Seiple W., Vajaranant TS., Szlyk JP, et al. Multifocal electroretinography as a function of age: the importance of normative values for older adults. Invest Ophthalmol Vis Sci. 2003;441783–1792 . [DOI] [PubMed] [Google Scholar]
- 21. Gill GV., Hardy KJ., Patrick AW., Masterson A. Random blood glucose estimation in type 2 diabetes: does it reflect overall glycaemic control? Diabet Med. 1994;11705–708 . [DOI] [PubMed] [Google Scholar]
- 22. Han Y., Bearse MA , Jr,, Schneck ME., Barez S., Jacobsen C., Adams AJ. Towards optimal filtering of “standard” multifocal electroretinogram (mfERG) recordings: findings in normal and diabetic subjects. Br J Ophthalmol. 2004;88543–550 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han Y., Adams AJ., Bearse MA , Jr,, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;1221809–1815 . [DOI] [PubMed] [Google Scholar]
- 24. Bearse MA , Jr,, Han Y., Schneck ME., Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004;45296–304 . [DOI] [PubMed] [Google Scholar]
- 25. Lang JT., McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;633 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albers JW., Brown MB., Sima AA., Greene DA. Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Neurology. 1996;4685–91 . [DOI] [PubMed] [Google Scholar]
- 27. Harrison WW., Bearse MA , Jr,, Schneck ME, et al. Prediction, by retinal location, of the onset of diabetic edema in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;526825–6831 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klemp K., Larsen M., Sander B., Vaag A., Brockhoff PB., Lund-Andersen H. Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci. 2004;453812–3819 . [DOI] [PubMed] [Google Scholar]
- 29. Juel K., Christensen K. Are men seeking medical advice too late? Contacts to general practitioners and hospital admissions in Denmark 2005. J Public Health (Oxf). 2008;30111–113 . [DOI] [PubMed] [Google Scholar]
- 30. Ejstrup R., Scherfig E., la Cour M. Electrophysiological consequences of experimental branch retinal vein occlusion in pigs and the effect of dorzolamide. Invest Ophthalmol Vis Sci. 2011;52952–958 . [DOI] [PubMed] [Google Scholar]
- 31. Morén H., Gesslein B., Andreasson S., Malmsjö M. Multifocal electroretinogram for functional evaluation of retinal injury following ischemia-reperfusion in pigs. Graefes Arch Clin Exp Ophthalmol. 2010;248627–34 . [DOI] [PubMed] [Google Scholar]
- 32. Kofoed PK., Munch IC., Sander B, et al. Prolonged multifocal electroretinographic implicit times in the ocular ischemic syndrome. Invest Ophthalmol Vis Sci. 2010;511806–1810 . [DOI] [PubMed] [Google Scholar]
- 33. Tyrberg M., Ponjavic V., Lövestam-Adrian M. Multifocal electroretinogram (mfERG) in patients with diabetes mellitus and an enlarged foveal avascular zone (FAZ). Doc Ophthalmol. 2008;117185–189 . [DOI] [PubMed] [Google Scholar]
- 34. Mendelsohn ME., Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;3081583–1587 . [DOI] [PubMed] [Google Scholar]
- 35. Nonaka A., Kiryu J., Tsujikawa A, et al. Administration of 17beta-estradiol attenuates retinal ischemia-reperfusion injury in rats. Invest Ophthalmol Vis Sci. 2000;412689–2696 . [PubMed] [Google Scholar]
- 36. Reiter CE., Gardner TW. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Prog Retin Eye Res. 2003;22545–562 . [DOI] [PubMed] [Google Scholar]
- 37. Arnold S., Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;1101–11 . [DOI] [PubMed] [Google Scholar]
- 38. Russo R., Cavaliere F., Watanabe C, et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173583–590 . [DOI] [PubMed] [Google Scholar]


