Abstract
Hepcidin is a hepatocellular hormone that inhibits the release of iron from certain cell populations, including enterocytes and reticuloendothelial cells. The regulation of hepcidin (HAMP) gene expression by iron status is mediated in part by the signaling molecule bone morphogenetic protein 6 (BMP6). We took advantage of the low iron status of juvenile mice to characterize the regulation of Bmp6 and Hamp1 expression by iron administered in three forms: 1) ferri-transferrin (Fe-Tf), 2) ferric ammonium citrate (FAC), and 3) liver ferritin. Each of these forms of iron enters cells by distinct mechanisms and chemical forms. Iron was parenterally administered to 10-day-old mice, and hepatic expression of Bmp6 and Hamp1 mRNAs was measured 6 h later. We observed that hepatic Bmp6 expression increased in response to ferritin but was unchanged by Fe-Tf or FAC. Hepatic Hamp1 expression likewise increased in response to ferritin and Fe-Tf but was decreased by FAC. Exogenous ferritin increased Bmp6 and Hamp1 expression in older mice as well. Removing iron from ferritin markedly decreased its effect on Bmp6 expression. Exogenously administered ferritin and the derived iron localized in the liver primarily to sinusoidal lining cells. Moreover, expression of Bmp6 mRNA in isolated adult rodent liver cells was much higher in sinusoidal lining cells than hepatocytes (endothelial >> stellate > Kupffer). We conclude that exogenous iron-containing ferritin upregulates hepatic Bmp6 expression, and we speculate that liver ferritin contributes to regulation of Bmp6 and, thus, Hamp1 genes.
Keywords: iron, transferrin, sinusoidal, endothelial, Kupffer, stellate
the peptide hormone hepcidin regulates body iron homeostasis by determining the magnitude of dietary iron release from enterocytes and stored iron release from reticuloendothelial macrophages (22). The production of hepcidin is influenced by multiple signals, including those reflecting body iron status, erythropoietic demand, and inflammation (28). The regulation of the hepcidin (HAMP) gene by iron status involves the participation of the extracellular signaling molecule bone morphogenetic protein 6 (BMP6) (9, 54). Previous studies identify two distinct physiological pathways through which iron status regulates hepcidin: one is relatively acute (and associated with changes in circulating iron concentrations), and the other is chronic (and associated with changes in liver iron stores) (15, 52). Several observations suggest that ferri-transferrin (Fe-Tf) may serve as the “circulatory” iron signal (28). The molecular participants of the “iron stores” signal remain less well understood. Correlations between Bmp6 mRNA expression and liver iron concentrations (but not serum transferrin saturations) suggest that Bmp6 is a participant in the iron stores, but not Fe-Tf, pathway (15).
In addition to Fe-Tf, biological forms of iron taken up by the liver include non-transferrin-bound iron (NTBI), primarily in the form of iron citrate salts, and ferritin (5). Mechanisms of cellular uptake for each of these forms of iron are distinct (3, 5, 53). Fe-Tf is taken up via transferrin receptor-mediated endocytosis, and the iron is transported across the recycling endosome. NTBI enters cells via membrane ion transporters. Non-receptor-mediated uptake of transferrin-bound iron has also been demonstrated. The iron within ferritin cages enters cells via pinocytosis of ferritin (19, 48) and/or ferritin receptor-mediated endocytosis (51).
Ferritin is the major intracellular storage form of iron (4, 61), regardless of type of iron taken up by the cell. The ferritin shell is composed of heteromultimers of two subtypes, designated H and L, the relative contribution of which differs across tissues and cell types. Recent attention has been given to the potential roles for ferritin as not only a storage molecule, but also as an iron delivery system (44), a transcription factor (2), and a signaling molecule (13). Because ferritin is regulated by cellular oxidant state (25, 30) and has antioxidant properties (4), it may additionally serve as a modulator of the inflammatory response to oxidant injury (51). These expanded properties of ferritin, along with known associations between liver iron stores, liver ferritin concentrations, Bmp6 expression, and hepcidin expression, led us to hypothesize that liver ferritin may participate in the regulation of hepcidin expression. To test this hypothesis, we performed in vivo studies in which we administered non-heme iron as ferritin, Fe-Tf, or ferric ammonium citrate (FAC) exogenously to mice and examined liver Bmp6 and Hamp1 mRNA expression. Each of these forms of iron, ferritin (66), FAC (18), and Fe-Tf (52), has been administered intraperitoneally to rodents in studies of liver iron metabolism.
We took advantage of the inherently low baseline Bmp6 and Hamp1 mRNA expression and low liver iron concentrations in juvenile mice to maximize our ability to identify changes in response to iron administration. We found that exogenous iron-containing ferritin upregulates Bmp6 and Hamp1 mRNA expression. Moreover, we observed that exogenously administered ferritin and the iron delivered by this ferritin localize primarily to sinusoidal lining cells. Finally, we observed high Bmp6 expression in isolated sinusoidal lining cells, particularly endothelial cells, compared with hepatocytes. These studies suggest that iron in the form of ferritin and Fe-Tf can each regulate hepcidin, but by distinct mechanisms. Moreover, they implicate sinusoidal endothelial cells as a source of Bmp6 expression in the liver.
MATERIALS AND METHODS
Reagents.
All reagents were obtained from Sigma (St. Louis, MO) unless otherwise indicated. Human liver ferritin (Calbiochem) was demonstrated to be endotoxin-free (<0.2 endotoxin unit/mg) by commercial assay (PyroGene, Lonza) prior to usage. Rabbit anti-human ferritin immunoglobulin (catalog no. A-0133) was obtained from Dako (Carpinteria, CA). Anti-rat SE-1 antibody (catalog no. 10078) was obtained from IBL. Rat anti-mouse IgG2a/2b microbeads were obtained from Miltenyi Biotech.
Animals.
Wild-type FVB/n mice were bred and maintained under standard conditions. Juvenile mice were weaned at 21 days onto standard chow (Teklad Global 2018S) containing 225 ppm iron or onto chow (TestDiet 5755) containing 60 ppm iron ad libitum. Intraperitoneal injections of vehicle, human liver ferritin, BMP6, or FAC were administered in a total volume of 10 μl/g mouse. Mice were euthanized 6 h later by exposure to hypercapnia and exsanguination, and tissue samples were collected. All animal care was performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996), and studies were performed under an Institutional Animal Care and Use Committee-approved protocol.
Iron content of ferritin.
Ferritin iron content was measured as described elsewhere (14). To deplete iron, ferritin was treated with thioglycolic acid, purged with nitrogen, and dialyzed against 1% thioglycolic acid-0.05 mM HEPES for 3 days and then with 0.15 mM HEPES-0.1 mM NaCl for 1 day, as described elsewhere (43), yielding an iron concentration of <2 μg/g ferritin. Protein concentrations were determined by Bradford assay.
Iron histochemistry and ferritin immunohistochemistry.
Immunohistochemistry was performed on formalin-fixed paraffin sections (5 μm) using Vectastain ABC-Elite reagents (Vector Labs, Burlingame, CA). The primary antibody was rabbit anti-human liver ferritin (Dako; 1:100 dilution). Sections were counterstained in hematoxylin and visualized by light microscopy. Tissue sections were stained for iron using enhanced Perls' technique, as described elsewhere (64). Briefly, tissue sections were treated with 7% potassium ferrocyanide in 3% HCl at 37°C for 15 min, rinsed in water, and then reacted with diaminobenzidine (0.5 mg/ml in 0.1 M KCN and 0.03% H2O2) for 15 min to yield a brown product. Sections were counterstained with hematoxylin and visualized by light microscopy.
Cell isolation and culture.
Populations of sinusoidal lining cells were provided by the Non-Parenchymal Liver Cell Core of the Southern California Research Center for Alcoholic, Liver, and Pancreatic Diseases and Cirrhosis. Liver sinusoidal endothelial cells (LSECs) were isolated by magnetic cell sorting using SE-1 antibody, as described elsewhere (63), with modification. Briefly, livers were digested by collagenase perfusion, and the nonparenchymal cell population was enriched by centrifugation at 70 g for 1 min and suspension three times in Hanks' buffer. The resuspended cells were incubated with a monoclonal antibody to rat SE-1 and then with rat anti-mouse IgG2a/2b microbeads. Magnetic separation of the LSECs was performed using an autoMACS Pro separator (Miltenyi Biotec). Cell viability, ascertained by Trypan blue dye exclusion, was >95%. LSECs exhibited >95% purity, as determined by positive staining for acetylated low-density lipoprotein. The absence of contaminating Kupffer cells was ascertained by peroxidase staining. Kupffer cells were isolated by in situ sequential digestion of the liver with Pronase and collagenase followed by arabinogalactan gradient ultracentrifugation and adherence purification, as previously described (65, 69), except cells were collected at the arabinogalactan gradient interface of 1.043/1.058 and 1.058/1.085. This method achieves a final Kupffer cell purity >95% as assessed by peroxidase staining and latex bead phagocytosis. Stellate cells were purified by arabinogalactan gradient ultracentrifugation (70), with collection at the medium-1.034 interface. Kupffer cells, stellate cells, and endothelial cells were cultured in medium containing 2% serum for 6 h prior to analysis. Murine hepatocytes (Invitrogen, Carlsbad, CA) were assayed after 48 h in culture in serum-free Williams E medium supplemented with CM4000 (Invitrogen).
Real-time quantitative RT-PCR.
Liver tissue samples were homogenized in TRIzol (Invitrogen), and RNA was extracted according to the manufacturer's recommendations. RNA concentration was measured spectrophotometrically, and purity was verified by ratio of absorbance at 260 nm to absorbance at 280 nm. RNA integrity was confirmed by agarose gel electrophoresis and ethidium bromide staining. RNA samples were treated with DNase (Ambion). One-Step real-time RT-PCR was performed according to the manufacturer's directions (TaqMan, Applied Biosystems), as described elsewhere (16). Thermal cycling (40 cycles at 95°C for 15 s and 60°C for 1 min) was carried out using an PRISM 7700 (Applied Biosystems). Efficiency curves were determined by serial dilution. All results were within the linear range of the assay. Real-time RT-PCR results were analyzed by ΔΔCt and Pfaffl (efficiency curve) methods, with similar results obtained from each. The results from Pfaffl analyses are presented, with statistical analyses performed by commercial software (Rest 2009 version 2.0.13, Qiagen). All primers and probes are products of TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA), except murine Hamp1 and β-actin primers and probes (16), which were synthesized by Applied Biosystems.
RESULTS
Regulation of liver hepcidin expression by dietary iron and exogenous BMP6 administration in preweanling mice.
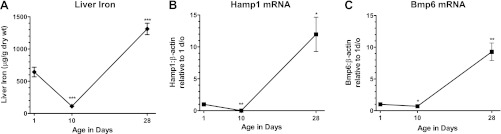
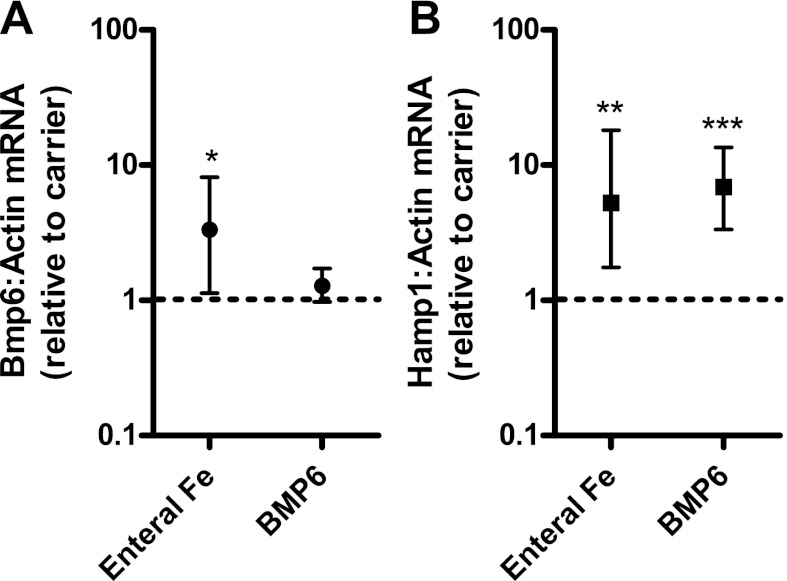
We first determined whether the inherently low iron status of preweanling mice (21) provided an opportune time frame to analyze the in vivo effects of iron administration on Bmp6 and Hamp1 mRNA expression. Because mice begin to access variable quantities of chow between 14 days of age and weaning at 21 days of age, we performed our preweanling analyses at 1 and 10 days, and postweaning analysis at 28 days (Fig. 1A). Liver iron concentrations were lowest at 10 days of age (mean 112 μg/g dry wt compared with 643 and 1,311 μg/g at 1 and 28 days of age). Liver Bmp6 mRNA expression followed a similar pattern (Fig. 1B). At 10 days, liver Bmp6 expression was 0.7-fold that measured in 1-day-old mice, and at 28 days, it was 9.3-fold higher than in 1-day-old mice. In agreement with previous reports (17, 21), we observed that liver Hamp1 expression was very low at 10 days relative to 1-day-old mice (0.007-fold, P < 0.001) and increased markedly by 28 days (12-fold over 1-day-old mice, P < 0.001; Fig. 1C). To ensure that mice at 10 days of age demonstrated a response to iron intake on hepatic Bmp6 and Hamp1 expression, iron (2 mg/kg) was administered as ferrous sulfate by oral-gastric tube. This treatment produced an increase in hepatic Bmp6 (3.3-fold, P < 0.05) and Hamp1 (5.3-fold, P < 0.05) mRNAs 6 h later (Fig. 2). To determine whether BMP6 itself would mediate an increase in Hamp1 expression at this age, we administered 5 μg of BMP6 intraperitoneally. We observed that liver Hamp1 expression was increased 6 h later (6.9-fold, P < 0.005). We concluded that mice at 10 days of age provide a suitable model system to investigate the regulation of Bmp6 and Hamp1 expression by iron.
Fig. 1.
Postnatal changes in liver iron concentrations and expression of liver bone morphogenetic protein 6 (Bmp6) and hepcidin (Hamp1) mRNAs. A: non-heme liver iron concentrations were measured in newborn mice at 1, 10, and 28 days of age (1 wk after weaning onto standard iron chow). B and C: liver Hamp1 and Bmp6 mRNAs were quantified by real-time RT-PCR and normalized to β-actin mRNA. Values are means ± SE (n = 3–5 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 2.
Effect of oral iron and exogenous BMP6 on liver Bmp6 and Hamp1 expression. Iron (2 mg/kg) was administered as ferrous sulfate (or carrier) via oral-gastric tube to 10-day-old mice (n = 4 per group). In other mice, 5 μg of human recombinant BMP6 (or carrier) was administered intraperitoneally. Liver Bmp6 and Hamp1 expression was measured 6 h later by real-time RT-PCR, and results were normalized to expression of β-actin. Values are means ± SE. Dashed line at “1” represents no difference relative to control (carrier) group. *P < 0.05, **P < 0.01, ***P < 0.001.
Exogenous administration of liver ferritin upregulates hepatic Bmp6 and Hamp1 mRNAs.
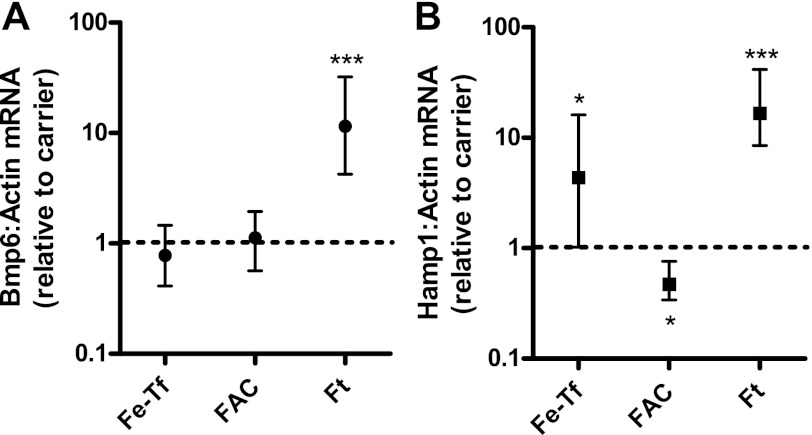
We examined the effect of three exogenously administered forms of iron, Fe-Tf, NTBI as FAC, and ferritin, on liver Bmp6 and Hamp1 expression. Each was administered intraperitoneally to 10-day-old mouse pups at a dose providing 2 mg/kg of iron, and the expression of liver Bmp6 and Hamp1 was measured 6 h later. Only ferritin was associated with an increase in Bmp6 expression (Fig. 3A). The mean increase in Bmp6 expression with ferritin was 11.5-fold (P < 0.001). Ferritin administration was also associated with an increase (mean 16-fold) in Hamp1 expression (Fig. 3B; P < 0.001). While Fe-Tf also increased Hamp1 expression (mean 4.4-fold, P < 0.05), there was no associated change in Bmp6 expression (mean 0.8-fold, P = 0.35) at this or at earlier (1 and 3 h) time points (not shown). FAC administration, on the other hand, was associated with a decrease in liver Hamp1 mRNA expression (mean 0.47-fold, P < 0.05) and no change in Bmp6 expression (mean 1.1-fold, P = 0.6). As expected, no significant change in total hepatic or splenic iron concentrations was observed with administration of these forms of iron at these doses (not shown).
Fig. 3.
Effect of parenteral administration of different forms of iron on Bmp6 and Hamp1 expression. Iron (2 mg/kg) was administered as ferri-transferrin (Fe-Tf) or carrier (n = 4 per group), ferric ammonium citrate (FAC) or carrier (n = 3 per group), or ferritin (Ft) or carrier (n = 7 per group) to 10-day-old mice. Liver Bmp6 and Hamp1 expression were measured 6 h later by real-time RT-PCR, and results were normalized to expression of β-actin. Values are means ± SE. Dashed line at “1” represents no difference relative to respective control (carrier) group. *P < 0.05, ***P < 0.001.
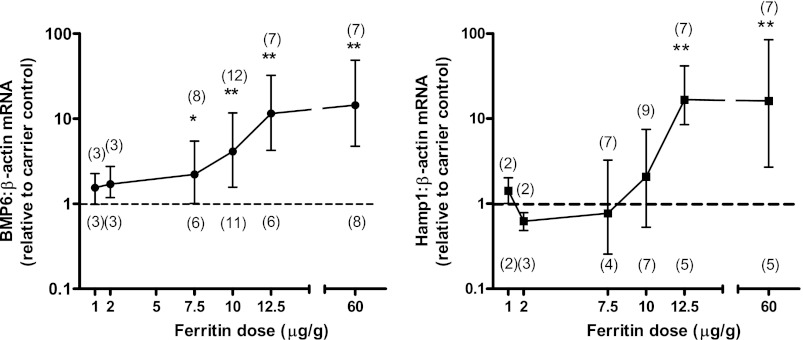
To analyze the dose response of ferritin on Bmp6 expression, we compared doses of ferritin from 1 to 60 μg/g (thereby administering iron at doses of ∼0.1–10 mg/kg). We found a dose-dependent increase in Bmp6 expression with increased ferritin administration up to 12.5 μg/g (Fig. 4). No additional response was observed at higher doses. No statistically significant effect of ferritin on Hamp1 expression was observed at doses <12.5 μg/g.
Fig. 4.
Dose-dependent effect of ferritin on liver expression of Bmp6 and Hamp1. Ferritin iron or carrier (doses indicative of ferritin iron administration) was administered to 10-day-old mice. Liver Bmp6 and Hamp1 expression was measured 6 h later by real-time RT-PCR, and results were normalized to expression of β-actin. Values are means ± SE; sample sizes are shown in parentheses above the curve for ferritin-injected mice and below the curve for carrier-injected mice. Dashed line at “1” represents no difference relative to control (carrier) group. *P < 0.05, **P < 0.01.
We subsequently determined whether the effects of ferritin administration on Bmp6 and Hamp1 expression were specific to preweanling mice. We administered ferritin at 9 μg/g to 28-day-old mice (1 wk after weaning onto 60 ppm iron-containing chow). As shown in Fig. 5, administration of ferritin was associated with an increase in Bmp6 (mean 5.4-fold, P < 0.005) and Hamp1 (mean 1.8-fold, P = 0.02) mRNAs.
Fig. 5.
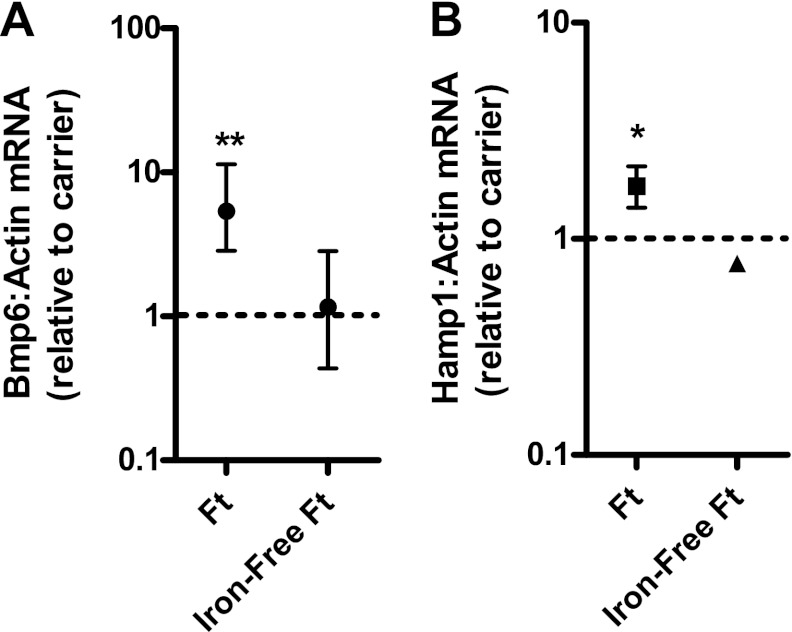
Effect of ferritin and iron-depleted ferritin administration on liver Bmp6 and Hamp1 expression in adult mice. Ferritin (9 μg/g, n = 4) or carrier (n = 3) was administered to adult female mice, and ferritin that had been depleted of iron (9 μg/g, n = 3) or carrier (n = 5) was administered to additional mice. Liver Bmp6 and Hamp1 expression were measured 6 h later by real-time RT-PCR, and results were normalized to expression of β-actin. Values are means ± SE. Dashed line at “1” represents no difference relative to control (carrier) group. *P < 0.05, **P < 0.01.
Removal of iron from ferritin attenuates its effect on Bmp6 expression.
To determine whether ferritin iron is required to effect changes in Bmp6 and Hamp1 expression, we tested the identical preparation of ferritin after iron removal. Administration of iron-depleted ferritin to adult mice was not associated with a change in Bmp6 or Hamp1 mRNA expression (Fig. 5). Similarly, in 10-day-old mice, while administration of iron-containing ferritin demonstrated the previously shown increase in Bmp6 mRNA expression (3.8-fold, P < 0.001), iron-depleted ferritin had no significant effect (1.4-fold increase, P = 0.1). While the increase in Hamp1 expression was 6.6-fold (P < 0.001) in the 10-day-old mice treated with iron-containing ferritin, a 4.7-fold increase, with marginal statistical significance (P = 0.04), was observed with iron-depleted ferritin.
Exogenously administered ferritin and iron delivered from ferritin localize primarily to sinusoidal lining cells.
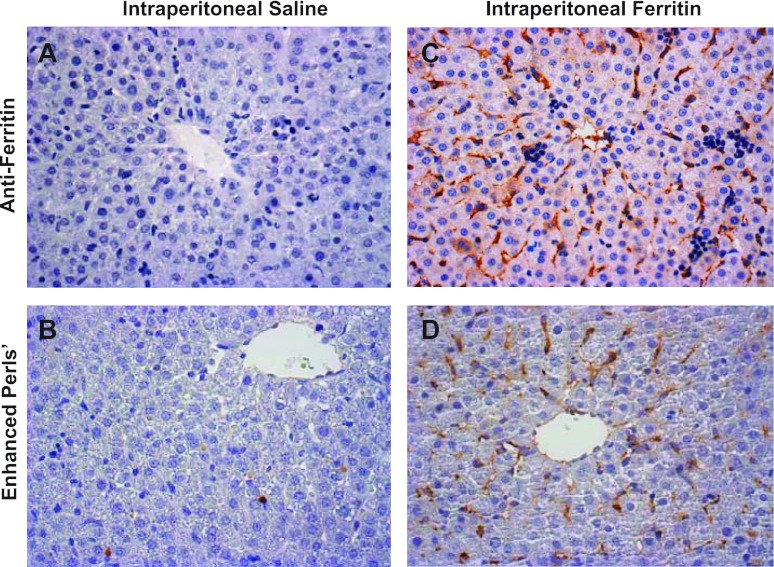
To identify the cell types accumulating exogenously administered ferritin and the iron derived from this ferritin, we performed immunohistochemical studies for ferritin and histochemical studies for iron on liver tissue from 10-day-old mice. Untreated mice at this age have very little immunoreactive ferritin (Fig. 6A) or stainable liver iron (Fig. 6B). Mice injected with ferritin demonstrated strong staining in sinusoidal lining cells (Fig. 6C) and much lighter staining in hepatocytes. Staining of greatest intensity was observed in cells with morphology and distribution consistent with endothelial cells. Cells with morphology and distribution consistent with Kupffer and stellate cells also demonstrated signal for ferritin (Fig. 6C). Mice injected with iron-depleted ferritin likewise demonstrated the strongest staining in these same cell populations (not shown). Histochemical staining for iron was performed on parallel liver sections. Mice injected with ferritin demonstrated iron primarily in sinusoidal lining cells (Fig. 6D). Tissue sections from mice injected with iron-depleted ferritin appeared similar to those from control mice (not shown). No ferritin immunoreactivity or histochemical iron staining was observed in the scattered erythroid cells normally observed in mouse liver at this age. These studies demonstrate that injected ferritin and the derived iron distribute primarily to nonparenchymal cell types. The greatest concentration of ferritin was observed in cells with an endothelial morphology.
Fig. 6.
Hepatic distribution of injected ferritin and iron. Saline carrier (A and B) or ferritin (60 μg/g; C and D) was administered intraperitoneally to 10-day-old mice, and animals were euthanized 6 h later. Liver sections were stained for ferritin with antibodies to human liver ferritin (A and C) or for iron with modified Perls' stain (B and D) and counterstained with hematoxylin. Images were photographed at ×400 magnification.
Basal liver Bmp6 expression is highest in nonparenchymal cells.
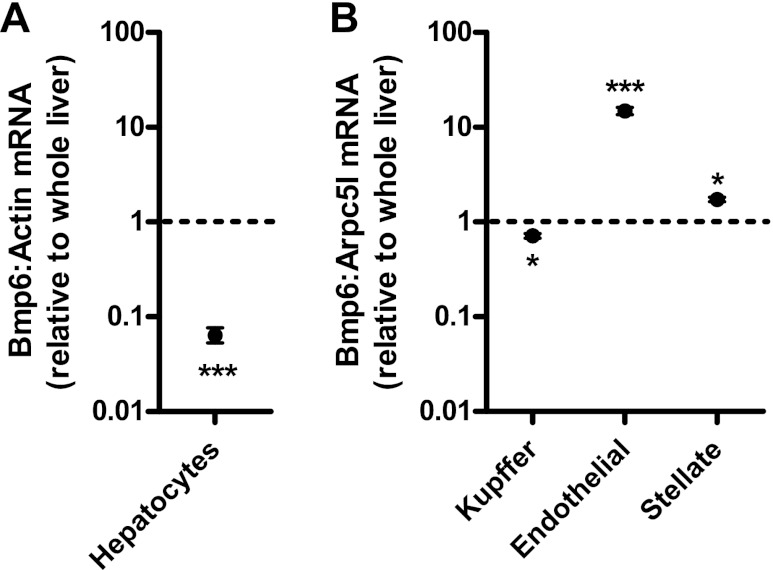
We next utilized isolated cultured liver cell populations to analyze cell types expressing Bmp6 in the liver. Expression of Bmp6 was much lower (0.06-fold, P < 0.001) in cultured mouse hepatocytes than whole liver (Fig. 7A). This observation suggests that the greatest contribution to Bmp6 expression in the liver was from nonparenchymal cells. To have sufficient quantity of nonparenchymal hepatic cell types for analysis, subsequent studies were performed in cells isolated from rats. We observed slightly less expression of Bmp6 in isolated cultured rat Kupffer cells than in whole liver (0.71-fold, P < 0.05; Fig. 7B). However, Bmp6 expression was much greater in cultured endothelial cells than in whole liver (14.8-fold, P < 0.001). Bmp6 expression was slightly greater in isolated stellate cells than in whole liver (1.7-fold, P < 0.05). These observations demonstrate that endothelial cells are a major contributor to Bmp6 mRNA expression in the liver.
Fig. 7.
Relative Bmp6 expression in isolated cultured primary liver cells. Bmp6 mRNA expression was quantified in primary cultures of mouse hepatocytes (A) or rat Kupffer, endothelial, and stellate cells (B) by real-time RT-PCR. Values are means ± SE. Dashed line at “1” represents no difference relative to control (whole liver RNA) group. *P < 0.05, *** P < 0.001.
DISCUSSION
Liver Hamp and Bmp6 expression has been shown to be regulated by changes in dietary iron intake and administration of parenteral iron; however, the biological forms of iron regulating these genes remain uncharacterized. Previous studies suggest that iron status regulates Hamp by more than one biological signal: one is acute (and possibly related to circulating iron concentrations), and the other is chronic (and associated with changes in liver iron concentrations). It has been suggested that the acute signal is mediated by Fe-Tf. We observed that exogenously administered Fe-Tf increased hepcidin expression without an associated change in Bmp6 expression. This observation is consistent with the proposed site of action of transferrin receptor 2, i.e., downstream of Bmp6 mRNA expression (16, 67). Biological forms of non-heme iron include not only Fe-Tf, but also NTBI (primarily in the form of citrate salts) and ferritin. We observed that exogenous FAC decreased hepcidin expression without a change in Bmp6 expression. This observation is consistent with the reported decrease in hepcidin expression in isolated hepatocytes and hepatoma cell lines in response to exposure to forms of NTBI (23). The observation that NTBI decreases hepcidin expression in vivo raises the possibility that circulating NTBI (as seen, e.g., in iron-overload conditions, such as hereditary hemochromatosis or β-thalassemia) (6) may contribute to hepcidin downregulation and exacerbate the pathogenesis of preexisting low-hepcidin states.
Most importantly, we found that administration of exogenous ferritin increased hepatic Bmp6 expression. Because liver iron concentration, liver ferritin concentration, and liver Bmp6 mRNA expression are associated with each other (15, 28), we next determined whether iron is required for the effect of ferritin on Bmp6 mRNA expression. We found that iron-depleted ferritin was not associated with an increase in Bmp6 mRNA expression. This observation suggests that exogenous ferritin is an effective means of delivering iron to a responsive cell type. It also, however, raises the possibility that iron-containing ferritin might be an endogenous regulator of Bmp6 expression. As such, the exogenously administered ferritin may have been delivered to the appropriate cell type to demonstrate such regulation. Intraperitoneally administered ferritin in animal model systems has been shown to distribute to multiple cell types, depending on dose administered and time assayed (7, 20, 35, 39, 66). Under our conditions, exogenously administered ferritin primarily distributed to sinusoidal lining cells, rather than hepatocytes, directing our attention to these cell types as potential sites of Bmp6 expression. While previous studies examining the cellular distribution of Bmp6 in the liver by immunohistochemistry found the strongest signal in hepatocytes (34), this observation does not necessarily identify the site of Bmp6 production. Expression of Bmp6 has been reported to be substantially higher in isolated Kupffer and stellate cells than hepatocytes (37). We likewise found substantially less Bmp6 mRNA expression in isolated hepatocytes than in whole liver. Additionally, we observed the greatest expression of Bmp6 mRNA not in Kupffer or stellate cells but, rather, in sinusoidal endothelial cells. Whether the pattern of BMP6 protein expression by these cell types parallels that of the mRNA remains to be determined.
Moreover, we identified endothelial cells morphologically as one of the sinusoidal cell types in which exogenously administered ferritin (and the derived iron) was most highly concentrated. Preferential accumulation in endothelial cells (and Kupffer cells) relative to hepatocytes was likewise reported upon exogenous administration of iron to piglets (10). Moreover, in various iron-overload conditions, including hereditary hemochromatosis, sinusoidal endothelial cells have been observed to accumulate iron (27, 33). The iron in these cells appears microscopically in the form of ferritin- and hemosiderin-containing lysosomes, rather than cytosolic ferritin. This observation suggests that the endothelial cell ferritin has been taken up, rather than synthesized de novo (45). In vivo studies demonstrate that intravascularly administered ferritin can enter endothelial cells by micropinocytosis (8). Studies on cultured endothelial cells suggest that ferritin can also be taken up by endocytosis (20). Endocytic uptake of ferritin has been observed in other liver cell types (51), including hepatocytes (1) and stellate cells (50). At least three molecules expressed in liver tissue have been shown to function as ferritin receptors: TIM-2 (12, 26) and transferrin receptor 1 (42) demonstrate activity as ferritin-H receptors, while SCARA5 functions as an ferritin-L receptor (31, 41). The contribution, if any, of these molecules to the uptake of iron from ferritin by the liver remains to be determined.
Independent of the identity of the responding cell type or the mechanism of ferritin uptake, our findings suggest that iron-containing ferritin might serve as a signaling molecule in the regulation of hepatic Bmp6 expression. The observation that serum ferritin concentrations are reflective of overall iron stores (68) and correlate with hepcidin levels raises the possibility that circulating ferritin might serve as the signaling molecule. Serum ferritin consists primarily of ferritin-L cages released by macrophages, but with very low iron content (14, 38). Nonetheless, some assays find that the iron content of human serum ferritin is substantial (29, 58) and that this iron content is increased in iron-overload states and decreased in iron deficiency (29). Also, the iron content and character of ferritin in the intrahepatic circulatory milieu may be distinct from that in the systemic circulation (47). Ultrastructural studies suggest that Kupffer cells in vivo release ferritin into the sinusoids and space of Disse (46). Cultured Kupffer cells secrete ferritin with a high iron content (57). Indeed, about half of the iron taken up by cultured Kupffer cells is subsequently released as ferritin (40). Cultured iron-loaded hepatocytes (40) and the hepatoma cell line HepG2 (24, 32, 40) likewise secrete ferritin into the media (the iron content of this ferritin was not reported). In vivo studies suggest that iron-containing ferritin is released from iron-loaded (11) or injured (45, 49) hepatocytes. Studies suggest that enterocytes can take up iron from dietary ferritin via endocytosis (56, 59); however, the absorbed iron appears to be released from the ferritin (56). As such, Kupffer cells or hepatocytes are more plausible sources than enterocytes for endogenous iron-containing ferritin in the regulation of liver Bmp6 expression.
Our studies demonstrate that exogenous iron-containing ferritin increases the hepatic expression of Hamp1 mRNA in association with the increase in Bmp6 mRNA. Liver Hamp1 mRNA expression in mice correlates with serum hepcidin levels and with downstream consequences on iron homeostasis (62). Interestingly, we observed that while administration of iron-depleted ferritin was associated with no change in liver Bmp6 mRNA expression, it was associated with a modest increase in Hamp1 expression. The basis for this observation is not clear. Possibly, an inflammatory signal (not dependent on STAT3 activation) contributed to hepcidin upregulation in response to the administered iron-depleted ferritin. The finding that iron-free ferritin induces inflammatory cytokines in cultured liver stellate cells supports this possibility (55).
Our observations suggest that iron-containing ferritin may serve as the iron “stores regulator,” mediating the upregulation of hepcidin expression in response to iron loading. If so, a “positive-feedback” loop might be anticipated, since cellular iron retention increases ferritin production (60). Such a feedback loop might be advantageous in facilitating the accumulation of stored iron; i.e., ferritin increases hepcidin, hepcidin mediates cellular iron retention, and cellular iron retention increases ferritin production. This feedback loop would be expected to be offset, however, by erythroid demand for iron; i.e., the hepcidin-mediated retention of cellular iron decreases serum iron concentrations and iron delivery (as Fe-Tf) to the erythron. Because erythroid signals suppressing hepcidin expression are of greater consequence than iron-stores signals (36), hepcidin levels would fall and iron would be released into the circulation. Once erythropoietic iron needs are met, dissipation of the erythroid signal would permit the iron-stores (ferritin) signal to upregulate hepcidin and replete reticuloendothelial iron stores. Cell-specific knockout of specific ferritin isoforms may be informative in testing these speculations and in determining the contribution of ferritin to the regulation of Bmp6 and Hamp1.
In conclusion, our data indicate that exogenously administered ferritin upregulates liver Bmp6 and Hamp1 expression in mice. The administered ferritin and iron contained therein accumulate primarily in liver sinusoidal lining cells. Sinusoidal lining cells, particularly endothelial cells, are a site of relatively high Bmp6 expression in the liver. Taken together, these observations implicate liver ferritin as a possible contributor to the iron “stores” signal in the BMP6-mediated regulation of hepcidin.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 DK-063016 (to R. E. Fleming), by the Saint Louis University Liver Center, and by the Non-Parenchymal Liver Cell Core (NIH Grant R24 AA-012885) of the Southern California Research Center for Alcoholic, Liver, and Pancreatic Diseases and Cirrhosis (NIH Grant P50 AA-011999).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.F. and R.E.F. are responsible for conception and design of the research; Q.F., M.C.M., A.W., and R.E.F. performed the experiments; Q.F., M.C.M., and R.E.F. analyzed the data; Q.F., A.W., R.S.B., and R.E.F. interpreted the results of the experiments; Q.F. and R.E.F. prepared the figures; Q.F. and R.E.F. drafted the manuscript; Q.F., R.S.B., and R.E.F. edited and revised the manuscript; Q.F., M.C.M., A.W., R.S.B., and R.E.F. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of Rosemary O'Neill.
REFERENCES
- 1. Adams PC, Powell LW, Halliday JW. Isolation of a human hepatic ferritin receptor. Hepatology 8: 719–721, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Alkhateeb AA, Connor JR. Nuclear ferritin: a new role for ferritin in cell biology. Biochim Biophys Acta 1800: 793–797, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci 66: 3241–3261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 1790: 589–599, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bonkovsky HL. Iron and the liver. Am J Med Sci 301: 32–43, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820: 403–410, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Bro-Rasmussen F, Egeberg J. The ultrastructure of eosinophilic granulocytes in the peritoneal cavity of rats following injection of ferritin. Scand J Haematol 3: 257–268, 1966 [DOI] [PubMed] [Google Scholar]
- 8. Bruns RR, Palade GE. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol 37: 277–299, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camaschella C. BMP6 orchestrates iron metabolism. Nat Genet 41: 386–388, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Caperna TJ, Failla ML, Steele NC, Richards MP. Accumulation and metabolism of iron-dextran by hepatocytes, Kupffer cells and endothelial cells in the neonatal pig liver. J Nutr 117: 312–320, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Cazzola M, Borgna-Pignatti C, de Stefano P, Bergamaschi G, Bongo IG, Dezza L, Avato F. Internal distribution of excess iron and sources of serum ferritin in patients with thalassemia. Scand J Haematol 30: 289–296, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Chen TT, Li L, Chung DH, Allen CD, Torti SV, Torti FM, Cyster JG, Chen CY, Brodsky FM, Niemi EC, Nakamura MC, Seaman WE, Daws MR. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med 202: 955–965, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coffman LG, Parsonage D, D'Agostino R, Jr, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci USA 106: 570–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116: 1574–1584, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Corradini E, Meynard D, Wu Q, Chen S, Ventura P, Pietrangelo A, Babitt JL. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology 54: 273–284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corradini E, Rozier M, Meynard D, Odhiambo A, Lin HY, Feng Q, Migas MC, Britton RS, Babitt JL, Fleming RE. Iron regulation of hepcidin despite attenuated Smad1,5,8 signaling in mice without transferrin receptor 2 or Hfe. Gastroenterology 141: 1907–1914, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P, Loreal O, Ilyin G. C/EBPα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem 277: 41163–41170, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Drysdale JW, Munro HN. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem 241: 3630–3637, 1966 [PubMed] [Google Scholar]
- 19. Easton JM, Goldberg B, Green H. Demonstration of surface antigens and pinocytosis in mammalian cells with ferritin-antibody conjugates. J Cell Biol 12: 437–443, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher J, Devraj K, Ingram J, Slagle-Webb B, Madhankumar AB, Liu X, Klinger M, Simpson IA, Connor JR. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol 293: C641–C649, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Frazer DM, Wilkins SJ, Anderson GJ. Elevated iron absorption in the neonatal rat reflects high expression of iron transport genes in the distal alimentary tract. Am J Physiol Gastrointest Liver Physiol 293: G525–G531, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Ganz T. Hepcidin and iron regulation, 10 years later. Blood 117: 4425–4433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gehrke SG, Kulaksiz H, Herrmann T, Riedel HD, Bents K, Veltkamp C, Stremmel W. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood 102: 371–376, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood 103: 2369–2376, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hailemariam K, Iwasaki K, Huang BW, Sakamoto K, Tsuji Y. Transcriptional regulation of ferritin and antioxidant genes by HIPK2 under genotoxic stress. J Cell Sci 123: 3863–3871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han J, Seaman WE, Di X, Wang W, Willingham M, Torti FM, Torti SV. Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLos One 6: e23800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hausmann K, Wulfhekel U, Dullmann J, Kuse R. Iron storage in macrophages and endothelial cells. Histochemistry, ultrastructure, and clinical significance. Blut 32: 289–295, 1976 [DOI] [PubMed] [Google Scholar]
- 28. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell 142: 24–38, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Herbert V, Jayatilleke E, Shaw S, Rosman AS, Giardina P, Grady RW, Bowman B, Gunter EW. Serum ferritin iron, a new test, measures human body iron stores unconfounded by inflammation. Stem Cells 15: 291–296, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci USA 102: 15048–15052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang J, Zheng DL, Qin FS, Cheng N, Chen H, Wan BB, Wang YP, Xiao HS, Han ZG. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest 120: 223–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hubert N, Lescoat G, Sciot R, Moirand R, Jego P, Leroyer P, Brissot P. Regulation of ferritin and transferrin receptor expression by iron in human hepatocyte cultures. J Hepatol 18: 301–312, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Iancu TC, Lichterman L, Neustein HB. Hepatic sinusoidal cells in iron overload. Ultrastructural observations. Isr J Med Sci 14: 1191–1201, 1978 [PubMed] [Google Scholar]
- 34. Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al Saati T, Coppin H, Roth MP. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood 114: 2515–2520, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Keane WF, Raij L. Impaired mesangial clearance of macromolecules in rats with chronic mesangial ferritin-antiferritin immune complex deposition. Lab Invest 43: 500–508, 1980 [PubMed] [Google Scholar]
- 36. Kemna EH, Kartikasari AE, van Tits LJ, Pickkers P, Tjalsma H, Swinkels DW. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis 40: 339–346, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Knittel T, Fellmer P, Muller L, Ramadori G. Bone morphogenetic protein-6 is expressed in nonparenchymal liver cells and upregulated by transforming growth factor-β1. Exp Cell Res 232: 263–269, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Koorts AM, Viljoen M. Ferritin and ferritin isoforms. I. Structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem 113: 30–54, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Leknes IL. The uptake of foreign ferritin by macrophages in the spleen, trunk kidney and liver of platy. J Fish Biol 59: 1412–1415, 2001 [Google Scholar]
- 40. Lescoat G, Hubert N, Moirand R, Jego P, Pasdeloup N, Brissot P. Iron load increases ferritin synthesis and secretion in adult human hepatocyte cultures. Liver 11: 24–29, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, Drexler IR, Chen X, Sanna-Cherchi S, Mohammed F, Williams D, Lin CS, Schmidt-Ott KM, Andrews NC, Barasch J. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell 16: 35–46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li L, Fang CJ, Ryan JC, Niemi EC, Lebron JA, Bjorkman PJ, Arase H, Torti FM, Torti SV, Nakamura MC, Seaman WE. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc Natl Acad Sci USA 107: 3505–3510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lonnerdal B, Bryant A, Liu X, Theil EC. Iron absorption from soybean ferritin in nonanemic women. Am J Clin Nutr 83: 103–107, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. J Neural Transm 118: 337–347, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Nielsen P, Gunther U, Durken M, Fischer R, Dullmann J. Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med 135: 413–418, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Ono T, Seno S. Transport of ferritin from Kupffer cells to liver parenchymal cells. Morphological and immunocytochemical observations. Int J Hematol 54: 93–102, 1991 [PubMed] [Google Scholar]
- 47. Osterloh K, Aisen P. Pathways in the binding and uptake of ferritin by hepatocytes. Biochim Biophys Acta 1011: 40–45, 1989 [DOI] [PubMed] [Google Scholar]
- 48. Petitpierre-Gabathuler MP, Ryser HJ. Cellular uptake of soluble and aggregated ferritin: distinction between pinocytosis and phagocytosis. J Cell Sci 19: 141–156, 1975 [DOI] [PubMed] [Google Scholar]
- 49. Prieto J, Barry M, Sherlock S. Serum ferritin in patients with iron overload and with acute and chronic liver diseases. Gastroenterology 68: 525–533, 1975 [PubMed] [Google Scholar]
- 50. Ramm GA, Britton RS, O'Neill R, Bacon BR. Identification and characterization of a receptor for tissue ferritin on activated rat lipocytes. J Clin Invest 94: 9–15, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramm GA, Ruddell RG, Subramaniam VN. Identification of ferritin receptors: their role in iron homeostasis, hepatic injury, and inflammation. Gastroenterology 137: 1849–1851, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, Ginzburg Y, Roth MP, Nemeth E, Ganz T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology 53: 1333–1341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta 1331: 1–40, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Roth MP, Coppin H. BMP6: a key player in iron metabolism. Med Sci (Paris) 25: 678–680, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, Santambrogio P, Arosio P, Ramm GA. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase Cξ/nuclear factor-κB-regulated signaling in rat hepatic stellate cells. Hepatology 49: 887–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. San Martin CD, Garri C, Pizarro F, Walter T, Theil EC, Nunez MT. Caco-2 intestinal epithelial cells absorb soybean ferritin by μ2 (AP2)-dependent endocytosis. J Nutr 138: 659–666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sibille JC, Kondo H, Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology 8: 296–301, 1988 [DOI] [PubMed] [Google Scholar]
- 58. ten Kate J, Wolthuis A, Westerhuis B, van Deursen C. The iron content of serum ferritin: physiological importance and diagnostic value. Eur J Clin Chem Clin Biochem 35: 53–56, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Theil EC, Chen H, Miranda C, Janser H, Elsenhans B, Nunez MT, Pizarro F, Schumann K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J Nutr 142: 478–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Theil EC, Goss DJ. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev 109: 4568–4579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Theil EC, Matzapetakis M, Liu X. Ferritins: iron/oxygen biominerals in protein nanocages. J Biol Inorg Chem 11: 803–810, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Tjalsma H, Laarakkers CM, van Swelm RP, Theurl M, Theurl I, Kemna EH, van der Burgt YE, Venselaar H, Dutilh BE, Russel FG, Weiss G, Masereeuw R, Fleming RE, Swinkels DW. Mass spectrometry analysis of hepcidin peptides in experimental mouse models. PLos One 6: e16762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tokairin T, Nishikawa Y, Doi Y, Watanabe H, Yoshioka T, Su M, Omori Y, Enomoto K. A highly specific isolation of rat sinusoidal endothelial cells by the immunomagnetic bead method using SE-1 monoclonal antibody. J Hepatol 36: 725–733, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Tomatsu S, Orii KO, Fleming RE, Holden CC, Waheed A, Britton RS, Gutierrez MA, Velez-Castrillon S, Bacon BR, Sly WS. Contribution of the H63D mutation in HFE to murine hereditary hemochromatosis. Proc Natl Acad Sci USA 100: 15788–15793, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsukamoto H, Lin M, Ohata M, Giulivi C, French SW, Brittenham G. Iron primes hepatic macrophages for NF-κB activation in alcoholic liver injury. Am J Physiol Gastrointest Liver Physiol 277: G1240–G1250, 1999 [DOI] [PubMed] [Google Scholar]
- 66. Unger A, Hershko C. Hepatocellular uptake of ferritin in the rat. Br J Haematol 28: 169–179, 1974 [DOI] [PubMed] [Google Scholar]
- 67. Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology 50: 1992–2000, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta 1800: 760–769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiong S, She H, Takeuchi H, Han B, Engelhardt JF, Barton CH, Zandi E, Giulivi C, Tsukamoto H. Signaling role of intracellular iron in NF-κB activation. J Biol Chem 278: 17646–17654, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Zhu NL, Asahina K, Wang J, Ueno A, Lazaro R, Miyaoka Y, Miyajima A, Tsukamoto H. Hepatic stellate cell-derived δ-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem 287: 10355–10367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]