Abstract
Endogenous CCK plays an important role in pancreatic regeneration after pancreatitis. We used primary culture of mouse pancreatic acinar cells to evaluate the effect of CCK on acinar cell morphology and gene expression and to determine signaling pathways required for proliferation of acinar cells in vitro. Over 4 days in culture, cells grew out from acini and formed patches of monolayer, which displayed a reduced expression of acinar cell markers including digestive enzymes and Mist1 and an increased expression of ductal and embryonic markers, including cytokeratin 7, β-catenin, E-cadherin, pdx-1, and nestin. There was no appearance of stellate cell markers. CCK enhanced cellular spreading, DNA synthesis, and cyclin D1 expression. When signaling pathways were evaluated, CCK stimulation increased c-Jun expression, JNK and ERK activity, and AP-1 activation. Chemical inhibitors of JNK and ERK pathways, dominant-negative JNK and c-Jun, and c-Jun shRNA significantly inhibited CCK-induced DNA synthesis, CCK-induced AP-1 activation, and cyclin D1 expression. Furthermore, dominant-negative c-Jun reduced the increased expression of β-catenin and the decreased expression of amylase during culture. These results show that MAPK/c-Jun/AP-1 pathway plays an important role in pancreatic acinar cell dedifferentiation and proliferation in culture. Monolayer culture can serve as a model to study acinar cell proliferation similar to regeneration after pancreatitis in vivo.
Keywords: cholecystokinin, AP-1, pancreatic cell culture
despite the low turnover rate of pancreatic cells in adult pancreas, the pancreas has the capacity to regenerate after injury, such as following pancreatitis (14, 26, 47), surgical resection (22, 36, 63), or endoplasmic reticulum stress (27). Following a moderate caerulein-induced pancreatitis in rats, which leads to extensive depletion of exocrine pancreas, full pancreatic function is restored in ∼1 wk (1, 14). In a study in mice, Jensen et al. (33) showed that after caerulein-induced pancreatitis, the surviving pancreatic acinar cells underwent dedifferentiation with a reduced expression of pancreatic acinar cell digestive enzymes and increased expression of genes associated with undifferentiated pancreatic progenitor cells, such as pdx-1, E-cadherin, β-catenin, and Notch components. The dedifferentiated acinar cells divided, grew, and then later acquired the characteristics of mature acinar cells.
CCK plays a significant role in the regeneration of exocrine pancreas following pancreatic injury. Endogenous CCK release or exogenous CCK administration accelerates pancreatic recovery from caerulein-, ethionine-, or arginine-induced acute pancreatitis in rats (24, 42, 50, 57). In contrast, pancreatic regeneration was delayed in CCKA receptor-deficient rat (45, 46). All these evidences suggest that endogenous CCK is required for the exocrine pancreatic regeneration and exogenous CCK administration can enhance this process. However, little is known of the signal transduction pathways mediating these effects in vivo.
Pancreatic acinar cells, when cultured as a monolayer on type I collagen, are known to lose their differentiated morphology with decreased amount of zymogen granules; subsequently the cells spread out, divide, and form a confluent monolayer (39). The dedifferentiated acinar cells express ductal cell specific antigen during culture and they are more ductlike (11), indicating changes in gene expression. The culture medium can also influence cell differentiation as a medium favoring acinar cell dedifferentiation to a ductlike phenotype increased the capacity of the cells to reenter the cell cycle (58). CCK is known to stimulate cultured acinar cells to divide and increase DNA synthesis, nuclear labeling and total content of DNA and protein (37). However, the mechanisms mediating CCK action on acinar cell growth are not well understood. CCK activates a variety of intracellular signaling pathways in dissociated pancreatic acinar cells in addition to the Ca2+ signaling that mediates secretion (64). These include mitogen-activated protein kinase pathways, which include c-Jun NH2-terminal kinase (JNKs) (10), extracellular signal-related kinase (12, 17), and p38 MAPK (66). Previous in vivo studies show that increased endogenous CCK activates JNK and ERK in mouse pancreas (17, 59). The expression of c-Jun, a downstream target of JNK and ERK, is also transiently increased in vivo (17), and it has been shown that CCK increases the mRNA expression of c-Jun in freshly isolated pancreatic acinar cells (40).
In the present study, we investigated the process of dedifferentiation and proliferation of cultured pancreatic acinar cells and the role of c-Jun in these processes. We found that pancreatic acinar cells cultured on collagen-coated plates gradually lost their differentiated appearance and expressed markers for undifferentiated pancreatic cells such as pdx-1 and nestin. They flattened out, divided, and formed islands of confluent monolayer. CCK stimulation increased JNK, ERK and AP-1 activity, whereas dominant-negative JNK, dominant-negative c-Jun, and short hairpin RNA (shRNA) targeting endogenous c-Jun reduced proliferation of acinar cells. Moreover, dominant-negative c-Jun partially inhibited dedifferentiation of acinar cells. Our data suggested that cultured acinar cells acquire embryonic characteristics similar to those shown during pancreatic regeneration following pancreatitis. c-Jun/AP-1 plays an important role in pancreatic acinar cell dedifferentiation and the subsequent CCK-induced acinar cell proliferation in culture.
MATERIALS AND METHODS
Materials.
Collagenase was from Crescent Chemical (Islandia, NY) and sulfated CCK octapeptide from Research Plus (Bayonne, NJ). Rabbit polyclonal antibodies to amylase, chymotrypsin, cyclin D2, cyclophilin A, JNK1, and mouse monoclonal antibody to PCNA were from Santa Cruz Biotechnology (Santa Cruz, CA); polyclonal antibody to α-smooth muscle actin was from Abcam (Cambridge, MA); and antibody to desmin was from Thermo Scientific (Fremont, CA). Other rabbit polyclonal antibodies, including anti-phospho-ERK1/2 (Thr 202/Tyr 204), anti-phospho-JNK1/2 (Thr 183/Tyr 185), and anti-ERK1/2, were from Cell Signaling (Beverly, MA), and anti-porcine elastase was from Rockland (Gilbertsville, PA). Rabbit monoclonal antibody to c-Jun was from Epitomics (Burlingame, CA). Mouse monoclonal antibodies to E-cadherin and cyclin D1 were from Zymed Laboratories (South San Francisco, CA), to β-catenin from BD Transduction Laboratories (San Jose, CA), to nestin from BD Pharmingen (San Diego, CA), and to β-actin from Sigma. Rat monoclonal antibody to bromodeoxyuridine (BrdU) was from Accurate Chemical and Scientific (Westbury, NY). Rat monoclonal TROMA III (anti-cytokeratin 19) and TROMA I (anticytokeratin 8) were from the Developmental Studies Hybridoma Bank, University of Iowa. [32P]ATP and [3H]thymidine were from Amersham Pharmacia Biotech (Piscataway, NJ). Type I collagen-coated plates were purchased from BD Biosciences Labware Discovery (Bedford, MA). The AP-1 oligonucleotide and gel shift binding buffer were from Promega (Madison, WI). TRIzol and all the primers used for quantitative real-time PCR were obtained from Invitrogen (Carlsbad, CA) and RNAlater was from Ambion (Austin, TX). SP600125, U0126, and PD98095 were from Calbiochem (San Diego, CA). All other chemical reagents were obtained from Sigma Chemical (St. Louis, MO).
Adenovirus expressing DN JNK2 and DN c-Jun (TAM 67) were provided by Dr. Juanita Merchant (University of Michigan) and Dr. David Brenner (UCSD), respectively. The modified pAdTrack plasmid, which was used for the construction of adenovirus expressing c-Jun shRNA, was provided by Dr. Liangyou Rui (University of Michigan).
Pancreatic acinar cell isolation, culture, and [3H]thymidine incorporation.
Pancreatic acini were isolated from male ICR mice as previously described (16, 38, 65). However, to obtain smaller acini, we carried out a brief period of exposure to a solution of sterile Ca2+-Mg2+-free Krebs-Henseleit-bicarbonate medium containing 50 mM EDTA before the second collagenase digestion. After isolation, cells were seeded into 6- or 24-well plates coated with type I collagen. Adenoviruses at 107 pfu/ml, unless otherwise noted were added at the same time as when the cells were plated. After 24 h culture, hormones or growth factors were added to the medium as indicated. For the studies involving the use of chemical inhibitors, the inhibitors were added 1 h before adding the agonists. The rate of DNA synthesis was measured by use of a previously described [3H]thymidine incorporation assay (39). Briefly, after the 24-h attachment period, the cells were treated with hormones and growth factors for 48 h, after which 0.1 μCi/ml [3H]thymidine was added for an additional 24 h [total time in culture 96 h (4 days)]. Subsequently, the medium was removed and the cells were washed with PBS and sonicated, and DNA was precipitated with 10% TCA. Incorporation of [3H]thymidine was expressed as percentage of total counts per minute observed in control cells.
Morphology, immunohistochemistry, and quantification of BrdU incorporation.
Acinar cells were cultured in 35-mm dishes for 24–96 h with BrdU (10 μM), a thymidine analog added to the culture medium during the last 12 h of incubation. Subsequently the cells were fixed on the culture dish with 4% paraformaldehyde in the phosphate-buffered saline (PBS) for 1 h. After being rinsed with PBS, DNA was denatured with 2 N HCl in 0.5% Triton-PBS for 30 min. The cultures were then exposed sequentially to a 1:10 dilution of rat anti-BrdU monoclonal antibody for 2 h and 1:200 dilution of donkey anti-rat secondary antibody conjugated with Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA). After being rinsed with PBS, the culture dishes were coverslipped with Prolong Gold antifade reagent containing 4,6-diamidino-2-phenylindole (DAPI; Invitrogen). Immunofluorescence staining of amylase and other proteins was carried out as described previously (49). Immunofluorescence was viewed with a Leica DMIRB inverted microscope with a ×20 objective lens. For overall morphology, cells were cultured in 24-well plates as described for [3H]thymidine incorporation and imaged as live cells with bright-field optics at ×20 and ×40 magnification through the plastic plate bottom by use of an inverted Nikon TE200 microscope. All images were recorded with a Olympus DP-71 digital camera.
RNA isolation, reverse transcription, and quantitative real-time PCR.
RNA isolation, cDNA synthesis, and quantitative real-time PCR followed the same procedures as previously described (17, 54). Briefly, total RNA was isolated from cultured mouse pancreatic acinar cells via RNeasy kit from Invitrogen. RNA quality was evaluated by agarose gel electrophoresis and spectrophotometry. RNA was reverse transcribed using TaqMan reverse transcription reagents with random hexamers as primers. Quantitative PCR was carried out using a Bio-Rad I-Cycler IQ real-time PCR detection system with 96-well plates.
The primers used for quantitative RT-PCR were designed with Primer Express software from ABI (Foster City, CA) based on gene sequences obtained from the GenBank NCBI Sequence Viewer (http://www.ncbi.nlm.nih.gov) and are given in Table 1.
Table 1.
Primer sequences used for quantitative real-time PCR
| Gene | Primer Sequence (5′ → 3′) | Accession Number |
|---|---|---|
| c-Jun | S: GAAGTGACGGACCGTTCTATGAC | NM_010591 |
| AS: GGAGGAACGAGGCGTTGAG | ||
| c-fos | S: TTCCTGGCAATAGCGTGTTC | NM_010234 |
| AS: TTCAGACCACCTCGACAATG | ||
| ATF-3 | S: CCACTCTGACCCCGAGTGA | AY329367 |
| AS: CTAAATCCTGGAATTGGTGAGACA | ||
| amylase2 | S: CCTTCTGACAGAGCCCTTGTG | NM_009669 |
| AS: GGATGATCCTCCAGCACCAT | ||
| chymotrypsin | S: AGTTCAACTCCTTCACCGTGCGTA | NM_025583 |
| AS: AGCAGGGAAGTCATCGTCAACAGT | ||
| lipase | S: AGAGGACCTTTGGAGCCATTGGAA | NM_026925 |
| AS: ATTGCGTCCACAAACTGAGCATCG | ||
| elastase 1 | S: CTGAAGCCCGGAGGAACTC | AK007392 |
| AS: TGGTGCCATGATCCTCCATA | ||
| β-catenin | S: TGCAGCTTCTGGGTTCCGATGATA | NM_007614 |
| AS: AGATGGCAGGCTCAGTGATGTCTT | ||
| E-cadherin | S: TCAAGCTCGCGGATAACCAGAACA | BC098501 |
| AS: ATTCCCGCCTTCATGCAGTTGTTG | ||
| nestin | S: AGCCATTGTGGTCTACGGAAGTGA | NM_016701 |
| AS: AGCACCTCTTGGTTCTCATCCACA | ||
| pdx-1 | S: ACTTAACCTAGGCGTCGCACAAGA | NM_008814 |
| AS: TTGGCATCAGAAGCAGCCTCAAAG | ||
| cytokeratin 7 | S: TGCAGTCGCAGATCTCAGACACAT | NM_033073 |
| AS: TGTGGTTGGCCATCTCCTCATACT | ||
| cytokeratin 19 | S: AGTTTGAGACAGAACACGCCTTGC | NM_008471 |
| AS: TCAGGCTCTCAATCTGCATCTCCA | ||
| Insulin | S: ACCTGGAGACCTTAATGGGCCAAA | NM_008386 |
| AS: ATGACCTGCTTGCTGATGGTCTCT | ||
| p48 | S: TGCGCTTGGCCATAGGCTACATTA | NM_018809 |
| AS: AGATGATAACCTTCTGGGCCTGGT | ||
| Mist1 | S: ACTATATCAAGTCGCTGACCGCCA | AF049660 |
| AS: TGGTATAATTTAGGGCCTGGCGCA | ||
| Hnf6 | S: AGAACATGGGAAGGACAGAGGCAA | U95945 |
| AS: AGCTGCTGGGAGATGGTGATTTGT | ||
| cyclin D1 | S: CAACGCACTTTCTTTCCAGA | NM_007631 |
| AS: TCTGTTCCTGGCAGGCA | ||
| cyclin D2 | S: CAGACGTGCGGGATGTTG | NM_009829 |
| AS: TGACGAACACGCCTCTCTT | ||
| cyclin D3 | S: TCTTCCTTCCCACTCAACCAGCTT | NM_007632 |
| AS: TTTGGCAACTGAGAAGGTTGGAGC | ||
| Hes1 | S: AACACGACACCGGACAAACCAAAG | NM_008235 |
| AS: TGGAATGCCGGGAGCTATCTTTCT |
S, sense; AS, antisense.
Preparation of cell lysate, nuclear proteins, and Western blots.
Pancreatic acinar cells were washed, scraped from the culture dish and sonicated with ice-cold buffer as previously described (19, 60). The supernatant was prepared for SDS-PAGE, after which protein was transferred to nitrocellulose membrane and Western blotting was conducted with specific antibodies (16). For all Western blot experiments duplicate culture wells were used for each experimental condition and run on adjacent lanes of SDS gels. After exposure of blots to X-ray film, the film was scanned and both lanes were used to quantitate the signal. For representative samples the images were electronically cut and only one lane of a representative gel was shown. For this reason, the lanes are separated by a white line.
For nuclear protein extraction the cells were washed with PBS and collected with PBS-EDTA, centrifuged at 3,000 rpm for 5 min. The pellet was resuspended in 250–500 μl (at least 6 vol) harvest buffer (10 mM HEPES pH 7.9, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100, 1 mM DTT, 10 mM tetrasodium pyrophosphate, 100 mM NaF, 17.5 mM β-glycerophosphate, 1 mM PMSF, 10 mg/l aprotinin, 10 mg/l leupeptin, 1 mM benzamidine), incubated on ice for 5 min and then centrifuged at 2,000 rpm for 10 min to pellet nuclei. The pellet was washed by resuspension in 500 μl of wash buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 10 mg/l aprotinin, 10 mg/l leupeptin) and centrifuged at 2,000 rpm for 5 min, after which the supernatant was removed and discarded. The pellet was resuspended in four volumes of buffer C (10 mM HEPES, pH 7.9, 500 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1% Nonidet P-40, 1 mM DTT, 1 mM PMSF, 10 mg/l aprotinin, 10 mg/l leupeptin) and incubated on ice for 15 min with intermittent vortex, after which the samples were centrifuged at 14,000 rpm for 10 min and the supernatant was saved.
EMSA.
EMSA was performed as previously described (17). Briefly, a double stranded oligonucleotide of AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) was labeled with γ-[32P]ATP. Pancreatic nuclear extract was incubated with the gel shift binding buffer for 20 min. Then the labeled AP-1 probe was added and incubation continued for another 30 min. Subsequently, the reaction mixture was subjected to a nondenaturing gel electrophoresis with TBE, dried, and exposed. Supershift assays were conducted by adding specific antibody to the binding reaction mixture 20 min before addition of the labeled oligonucleotide.
Construction of recombinant adenovirus.
The recombinant adenovirus expressing AP-1 luciferase reporter was constructed according to the method of He et al. (23). Briefly, the DNA segment encoding AP-1 luciferase reporter was excised from the original AP-1 luciferase reporter plasmid (Stratagene, La Jolla, CA) and cloned into pAdTrack-cytomegalovirus (CMV). The resultant plasmid was then transformed into AdEasier-1 Escherichia coli cells (BJ5183 derivatives, which already contain the AdEasy-1 plasmid). Recombinants were selected and confirmed by restriction endonuclease digestion assay and DNA sequencing. Finally, linearized recombinant plasmid was transfected into human embryonic kidney-293 cells for packaging. Recombinant adenoviruses were purified by use of a Vivapure AdenoPACK 100 kit (Vivascience; Hannover, Germany). The titers of the viral stocks were estimated by counting enhanced green fluorescent protein (EGFP)-expressing cells because pAdTrack-CMV also encodes EGFP driven by a separate CMV promoter. An adenovirus expressing β-galactosidase and EGFP was used as a control.
Luciferase assay.
After the initial 24-h culture, acinar cells were infected with 106 pfu/ml adenovirus expressing AP-1 luciferase reporter 1 h before the addition of CCK, and the cells were washed, harvested, and lysed 6 h later. Luciferase activity was detected with a luciferase assay kit (Promega, Madison, WI) as described previously (19).
shRNA construction and evaluation.
Three small interfering RNA (siRNA) sequences targeting against mouse c-Jun mRNA were designed by using the online siRNA target finder from Genescript and Invitrogen. For the adenovirus knockdown construct, shRNA sequences for jun1, jun2, and jun3 are (sense: 5′-3′) GATCCCCGAAACGACCTTCTACGACGATTTCAAGAGAATCGTCGTAGAAGGTCGTTTCTTTTTG; GATCCCCAAAGTCATGAACCACGTTATTCAAGAGATAACGTGGTTCATGACTTTTTTTTG; and GATCCCCCGCCAACCTCAGCAACTTCAATTCAAGAGATTGAAGTTGCTGAGGTTGGCGTTTTTC. The underlined are sense and antisense targeting sequences of c-Jun shRNA. The oligonucleotides were synthesized by IDT (Coralville, IA), and the complementary strands were first annealed and then cloned into the modified pAdTrack plasmid in which the shRNA expression was under the control of a human H1 promoter. Adenovirus expressing c-Jun shRNA and EGFP was constructed as described earlier. To evaluate the efficiency of shRNA to knock down c-Jun expression in cultured acinar cells, adenovirus expressing c-Jun shRNA or the nonspecific shRNA was added at the same time the cells were plated at a titer of 107 pfu/ml. After culture, the cells were harvested; protein or RNA was extracted followed by Western blot or quantitative real-time PCR.
Statistical analysis.
Results are expressed as means ± SE, which were obtained from three to five different experiments with duplicate or triplicate wells per condition in each experiment. One-way ANOVA with the Dunnett's test was carried out using GraphPad Prism software. P < 0.05 represents statistical significance.
RESULTS
Characterization of acinar cell morphology and dedifferentiation in monolayer culture.
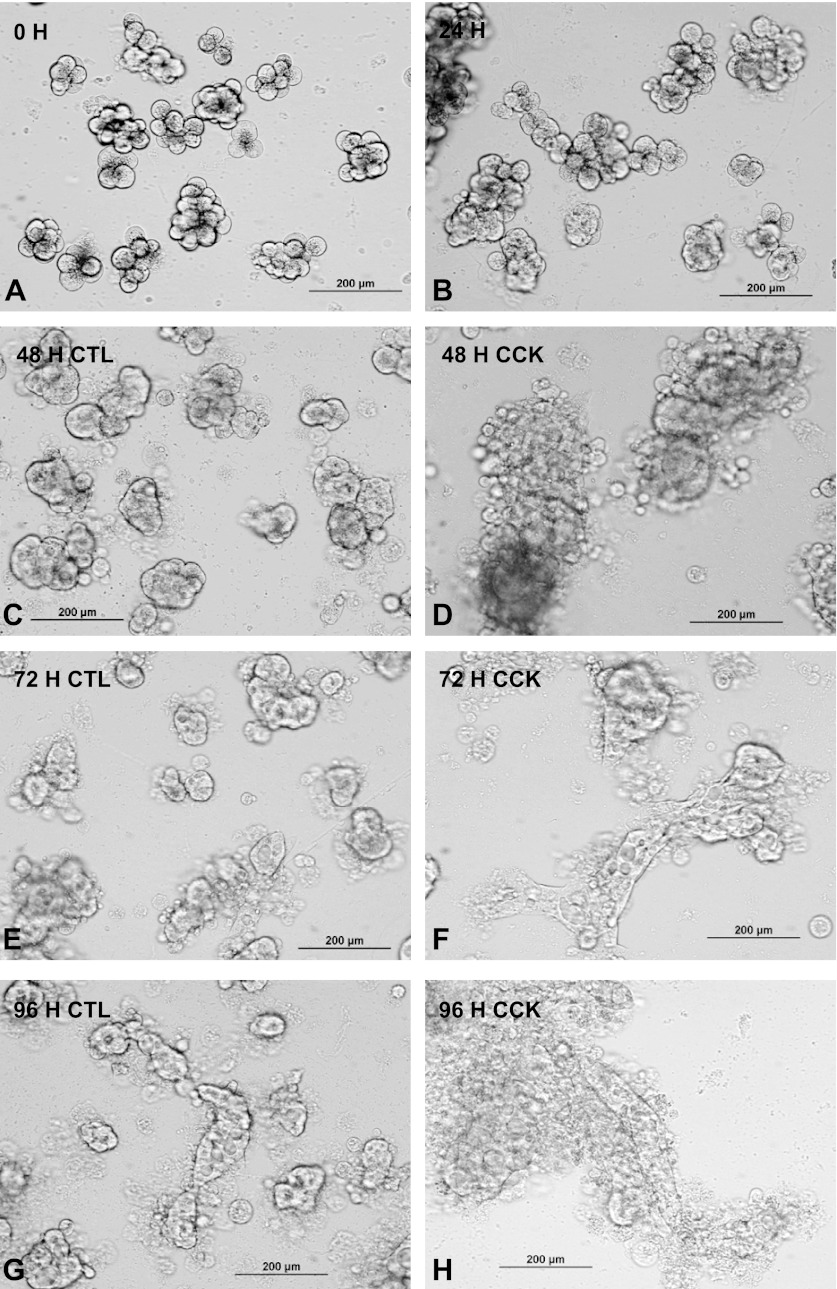
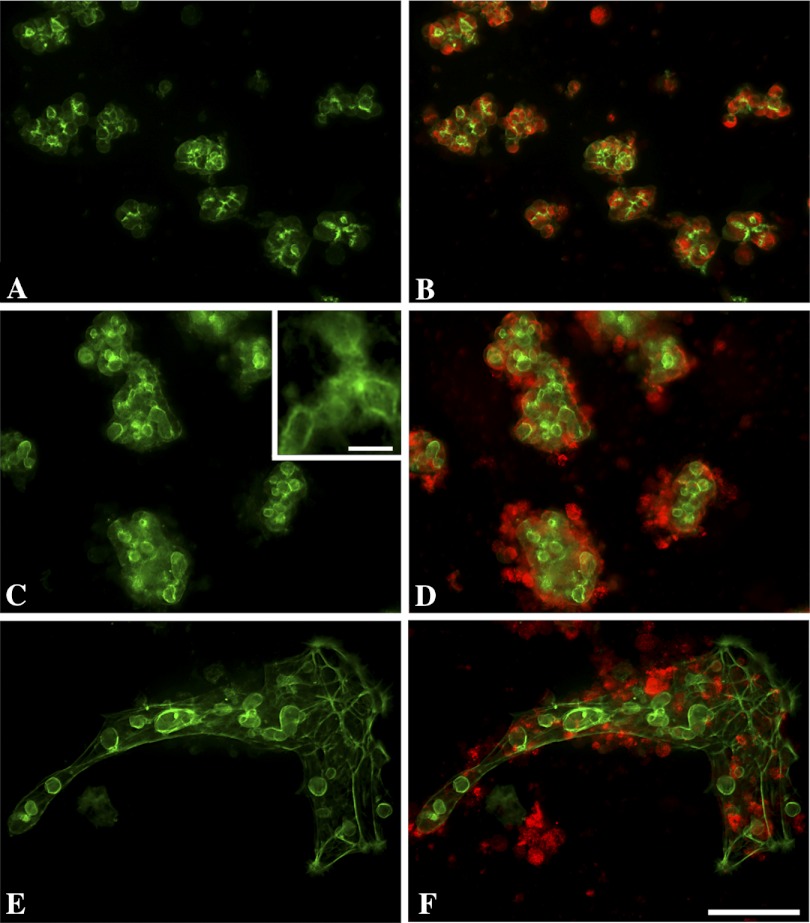
Mouse pancreatic acini were isolated following collagenase digestion and cultured for 0–96 h with or without CCK treatment (Fig. 1). Earlier studies have shown that the acinar preparation is highly enriched for acinar cells with only low levels of islet, duct, or vascular cells (2, 65). After 24 h culture, more than 90% of the cells had attached, usually clustered together as if derived from acini (Fig. 1B). They still preserved most of the characteristics of freshly isolated acini including the characteristic pattern of filamentous subluminal staining of actin with fluorescent phalloidin (Fig. 2A). Immunohistochemistry showed abundant amylase in 24 h cultured cells (Fig. 2B). After 48 h (Fig. 1, C and D) the cells began to flatten and spread out especially under the influence of CCK and this was even more pronounced after 72 h (Fig. 1, E and F). After 96 h (4 days) the filamentous actin staining had dramatically changed (Fig. 2, C and E) in that hollow spherical structures were seen somewhat similar to the appearance seen in suspension culture where individual acini become hollow spheres or cysts with the original acinar lumen becoming the cystic lumen (52, 53). A second pattern of actin staining after 96 h was along the margin of cells in an epithelial cell pattern. This second type of actin distribution was more prominent following CCK stimulation, and the flattening of the cell clusters was much more pronounced (Fig. 2F). In the 96-h cultures, amylase was still present often toward the periphery of the cell cultures (Fig. 2, D and F). Only a few cells in monolayer culture stained with Trypan blue (data not shown) in contrast to acini kept in suspension culture, where by 96 h at least half the cells were Trypan blue positive. Occasionally, isolated fibroblast-like cells were seen with abundant actin cables and no amylase staining.
Fig. 1.
Morphology of isolated pancreatic acinar cultures. Mouse isolated acini were plated in 24-well plates coated with collagen and imaged at 0 h (A), 24 h (B), 48 h (C and D), 72 h (E and F), or 96 h (G and H). CCK (1 nM) was added at 24 h to wells shown in D, F, and H. Live cells were imaged through the plastic culture well with an inverted microscope via a ×20 objective and recorded digitally. The acini are attached at 24 h and have flattened and begun to spread on the surface at 48 h. Spreading thereafter is enhanced by CCK. CTL, control.
Fig. 2.
Localization of filamentous actin and amylase in acinar cultures. A and B show, respectively, phalloidin staining of filamentous actin (green) alone or with amylase immunostaining (red) after 24-h culture. Note that the primary apical staining of F-actin is maintained and amylase is diffusely localized throughout the cell. C and D show control cultures after 96 h, and E and F show acinar cultures to which 1 nM CCK was added at 24 h. The actin staining is dominated by cystic structures (shown at higher magnification in inset) and bands of fibers along the cell borders. Amylase has been lost from the center of cell clusters but is distributed to peripheral regions of the cell clusters and in cells or debris outside of the actin bundles. CCK also promotes flattening and spreading out of the acinar cell clusters. Images taken with an inverted microscope and ×20 objective. The magnification bar for the 6 panels is 100 μm and the bar for the inset in C is 20 μm.
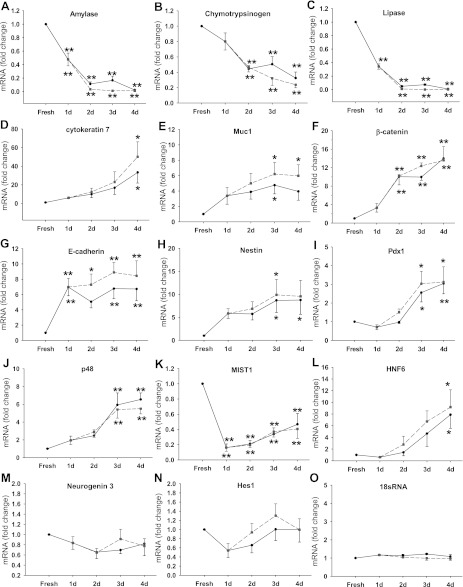
Quantitative real-time PCR was performed to measure the mRNA expression of markers for mature pancreatic cells including acinar, duct, and islet cells and for pancreatic embryonic cells. As shown in Fig. 3, during the culture, the mRNA expression of pancreatic digestive enzymes was significantly reduced, including amylase (Fig. 3A), chymotrypsinogen (Fig. 3B), lipase (Fig. 3C), and elastase (not shown); most of this decrease occurred over the first 2 days. Amylase and lipase were reduced to less than 2% of fresh acini, whereas chymotrypsin was somewhat higher, being reduced to 24% of fresh acini. The ductal markers, cytokeratin 7 (Fig. 3D) and cytokeratin 19 (data not shown) expression, increased over 20-fold. Mucin 1, a membrane-bound protein expressed by duct cells in pancreas (8), showed a sixfold increase during culture (Fig. 3E). By contrast, insulin, a marker of mature β cells remained very low at all time (data not shown). The expression of markers of less differentiated cells such as β-catenin (Fig. 3F) and E-cadherin (Fig. 3G) was increased. More importantly, markers of undifferentiated pancreatic cells including nestin (Fig. 3H) and pdx-1 (Fig. 3I) were also increased by ninefold and threefold, respectively. P48 is a transcription factor that plays an essential role in the specification of pancreatic fate in undifferentiated foregut endoderm, and its expression is restricted to pancreatic exocrine cells during the later stage of development. During culture its expression also showed a maximal 6.5-fold increase (Fig. 3J). Mist1 is also a transcription factor that is important for pancreatic development and its expression is restricted to acinar cells in adult pancreas. Mist1 expression decreased rapidly after 1 day in culture to 16% of fresh acini and then gradually increased to ∼40% of fresh acini after 4 days (Fig. 3K). HNF 6, a transcription regulator that is required for the development of both endocrine pancreas (32) and pancreatic duct epithelium (51), showed an increased expression of sixfold after 4 days of culture (Fig. 3L). However, neurogenin 3, another marker for pancreatic endocrine progenitor cells, showed no increase in culture (Fig. 3M). Hes1 is a downstream target gene of Notch signaling, which plays an active role in the differentiation of pancreatic progenitor cells (20). Hes1 expression did not change much during acinar cell culture (Fig. 3N). 18sRNA was used as control and its expression did not change (Fig. 3O). The changes in expression of all these genes were independent of CCK stimulation and may reflect in part the loss of 3D structure or extracellular matrix factors.
Fig. 3.
Characterization of acinar cell dedifferentiation in monolayer culture. Mouse pancreatic acinar cells were cultured for the time indicated. d, Days. RNA was extracted and samples were analyzed by quantitative real-time PCR as described in materials and methods. The expression of amylase (A), chymotrypsinogen (B), lipase (C), cytokeratin 7 (D), Muc1 (E), β-catenin (F), E-cadherin (G), nestin (H), pdx-1 (I), p48 (J), Mist1 (K), HNF6 (L), neurogenin 3 (M), Hes1 (N), and 18sRNA (O) was examined. Solid black and dashed gray lines represent the control and 1 nM CCK-treated groups, respectively. All results shown are expressed as fold increase compared with fresh acini and are means ± SE of 4–6 individual experiments. **P < 0.01; *P < 0.05 compared with the value of fresh acini.
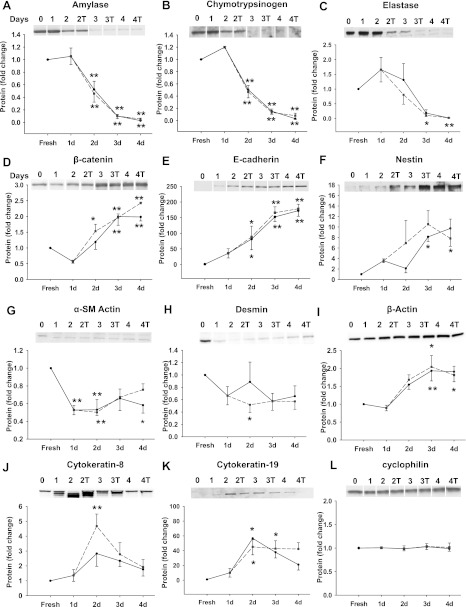
The protein expression of many of these genes was also examined by Western blot. Similar to the change of mRNA expression, amylase (Fig. 4A), chymotrypsin (Fig. 4B) and elastase (Fig. 4C) decreased to less than 5% of that in freshly isolated cells. The less differentiated cell markers β-catenin (Fig. 4D), E-cadherin (Fig. 4E), and nestin (Fig. 4F) showed a gradual increase in protein expression during culture. Pancreatic stellate cell markers desmin and α-smooth muscle actin both decreased during culture (Fig. 4, G and H). By contrast, the ductal marker, cytokeratin 19 increased 40-fold (Fig. 4K) and cytokeratin 8 and β-actin showed modest increases. Again, the protein expression of these genes was not significantly affected by CCK. Cyclophilin A was used as loading control and its expression did not change (Fig. 4L). Thus acinar cells in culture showed less expression of acinar cell markers and more markers of undifferentiated or ductal cells; markers of islets or stellate cells were not increased.
Fig. 4.
Protein expression during acinar cell dedifferentiation in monolayer culture. The protein expression of amylase (A), chymotrypsinogen (B), elastase (C), β-catenin (D), E-cadherin (E), and nestin (F), α-smooth muscle (SM) actin (G), desmin (H), β-actin (I), cytokeratin 8 (J), cytokeratin 19 (K), and cyclophilin (L) was measured by Western blot. Solid black and dashed gray lines represent the control and 1 nM CCK-treated groups, respectively. T, treatment with CCK. All results shown are expressed as fold increase compared with the fresh acini and are means ± SE of 4–6 individual experiments. For A, B, D, E, and L, duplicate culture wells were run on gels and imaged, and after quantification the image spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line was placed between the lanes. **P < 0.01; *P < 0.05 compared with the value of fresh acini.
Effect of secretagogues and growth factors on the proliferation of cultured acinar cells.
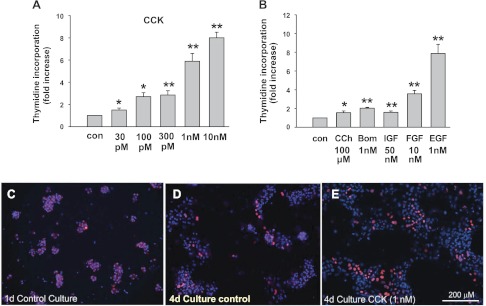
CCK is one of the main secretagogues that increase intracellular Ca2+ and the turnover of phosphatidylinositol in pancreatic acinar cells. Its effect on acinar cell proliferation was examined by the use of [3H]thymidine incorporation (Fig. 5). After the initial 24-h attachment period, the cells were treated with hormones and growth factors for 48 h after which 0.1 μCi/ml [3H]thymidine was added for an additional 24 h (total time in culture 4 days). We chose to add [3H]thymidine after 3 days of culture mainly because of a low initial [3H]thymidine incorporation and the smaller effect of CCK and other agonists during the first 2 days of culture. CCK increased the incorporation of [3H]thymidine into cultured acinar cells in a dose-dependent manner, with a minimal effect at 30 pM and a maximal effect of 8.0 ± 0.5-fold increase at 10 nM (Fig. 5A). Similar effects were also seen when laminin-coated culture wells were used (data not shown). In contrast, both bombesin and carbachol (CCh), also secretagogues, elicited a much smaller increase of DNA synthesis even at a concentration that is equivalent, in terms of the ability to stimulate amylase release, to the effective concentrations of CCK (Fig. 5B). Growth factors including IGF, FGF, and EGF also increased the incorporation of [3H]thymidine into DNA with EGF having the biggest effect (Fig. 5B). We also followed mitosis using BrdU incorporation by immunohistochemistry. After 1-day culture, there was little or almost no BrdU incorporation in acinar cells (Fig. 5C) similar to rates seen in vivo. After 4-day culture, overall BrdU incorporation increased, particularly in the CCK-treated group (Fig. 5, D and E).
Fig. 5.
Effect of secretagogues and growth factors on growth of cultured acinar cells. A and B: effect of secretagogues and growth factors on [3H]thymidine incorporation by cultured acinar cells. Cells were cultured for 24 h and then CCK (A), bombesin, carbachol (CCh), EGF, FGF, or IGF (B) were added at the concentration indicated. [3H]thymidine incorporation was performed as described in materials and methods. Results are expressed as fold increase of [3H]thymidine incorporation compared with control nontreated groups and are means ± SE of 4–6 individual experiments. **P < 0.01; *P < 0.05 compared with the control groups. C–E: bromodeoxyuridine (BrdU) incorporation in pancreatic acinar cells during culture. Acinar cells were cultured for 1 or 4 days with or without CCK stimulation. Blue, 4,6-diamidino-2-phenylindole (DAPI) staining; red, BrdU staining.
These results clearly indicate that CCK stimulates proliferation of acinar-derived cells.
Induction of cyclin D expression during acinar cell culture.
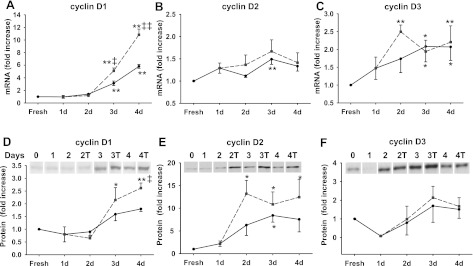
Cyclin D (including cyclin D1, D2, and D3) is the primary cyclin that regulates the G1-to-S phase transition of most cells. In response to mitogenic factors, cyclin D progressively accumulates during G1 phase and assembles with their catalytic partners Cdk4 and Cdk6 to phosphorylate members of the retinoblastoma (Rb) family of proteins. We examined cyclin D mRNA and protein expression during acinar cell culture (Fig. 6). Cyclin D1 expression significantly increased at both mRNA (Fig. 6A) and protein level (Fig. 6D) and CCK significantly enhanced both. Cyclin D2 protein expression also increased more with CCK treatment (Fig. 6E). We detected an increase of cyclin D3 mRNA expression (Fig. 6C), but its protein level did not change significantly compared with freshly isolated acini. Thus cyclin D1 appears to be the most likely cyclin D involved in CCK-induced acinar cell proliferation.
Fig. 6.
Induction of cyclin D expression during acinar cell culture. Acinar cells were cultured for the time indicated in control media (solid line) or with 1 nM CCK (dashed gray line). RNA or protein was extracted and samples were analyzed by quantitative real-time PCR or Western blot. The expression of cyclin D1, D2, and D3 mRNA (A–C) and protein (D–F) was measured. Results are expressed as fold increase compared with the fresh acini and means ± SE of 4–6 individual experiments. For D–F, duplicate culture wells were run on gels and imaged, and after quantification the image was spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line is placed between the lanes. **P < 0.01; *P < 0.05 compared with the value of fresh acini. ‡‡P < 0.01; ‡P < 0.05 compared with the non-CCK-treated group at the same time point (dashed vs. solid line).
Induction of c-jun expression by CCK in cultured acinar cells.
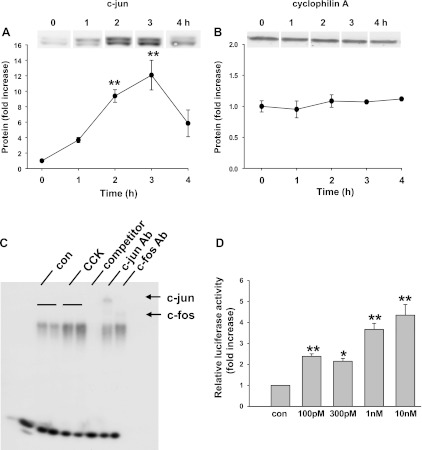
We examined the protein expression of c-Jun using Western blot (Fig. 7A). One nanomolar CCK stimulation of 24-h cultured acini rapidly upregulated the protein expression of c-Jun, which was detected at 1 h after CCK stimulation and peaked at 3 h with a 12.1 ± 2.0-fold increase. Cyclophilin A was used as loading control and its expression did not change with CCK treatment (Fig. 7B). c-Jun protein induction is also CCK dose dependent, and it showed a similar pattern as CCK-induced [3H]thymidine incorporation (data not shown).
Fig. 7.
Induction of c-Jun expression and AP-1 activation by CCK in cultured acinar cell. A and B: acinar cells were cultured for 24 h and then were stimulated with 1 nM CCK for the indicated time. c-Jun protein induction was measured (A), while cyclophilin A was used as control (B). Pooled data are means ± SE of 4–6 individual experiments and expressed as fold increase of non-CCK-treated control. **P < 0.01; *P < 0.05 compared with the values at time 0, non-CCK-treated control. C: acinar cells were cultured for 24 h followed by treatment with 1 nM CCK for 3 h. The DNA binding activity of AP-1 was examined by EMSA. Specificity of AP-1 DNA binding was determined by addition of excess of 50-fold unlabeled AP-1 consensus sequence (competitor). Supershift EMSA was performed using antibody to c-Jun. The results are representative from at least 3 independent experiments. D: AP-1 driven luciferase construct was delivered into acinar cells by adenoviral vector 1 h before CCK administration. The cells were stimulated with the designated concentration of CCK for 6 h. Relative luciferase activity was measured and the results were expressed as fold increase compared with the control non-CCK-treated group. Pooled data are means ± SE of 4 experiments. For A and B, duplicate culture wells were run on gels and imaged, and after quantification the image was spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line is placed between the lanes. **P < 0.01; *P < 0.05 compared with the non-CCK-treated control group.
JNK and ERK are upstream regulators of c-Jun induction. Their activation was measured by the use of phospho-specific antibodies (pJNK and pERK), which are activity state dependent. A 5.5-fold increase of pJNK and 4.5-fold increase of pERK activation was detected with pJNK peaking at 1 h and pERK at 5 min after 1 nM CCK treatment (data not shown).
AP-1 transcription activity was increased by CCK stimulation.
EMSA was performed to determine whether CCK stimulation can affect AP-1 DNA binding. Since CCK-induced c-Jun protein expression peaked at 3 h and c-Jun is one of the main components of AP-1, we evaluated the AP-1 DNA binding 3 h after CCK treatment. As shown in Fig. 7C, AP-1 DNA binding activity increased with CCK stimulation. The DNA protein complex was competed away by the cold probe and was supershifted by specific antibodies to c-Jun, indicating that c-Jun is involved in AP-1 DNA binding induced by CCK in cultured acinar cells. The degree of stimulation was lower than that in prior in vivo studies (17) primarily due to the presence of an appreciable basal level AP-1 DNA binding activity. AP-1 transcription activity was further evaluated by using a luciferase reporter assay that made use of an adenoviral vector to deliver AP-1 reporter construct. As shown in Fig. 7D, CCK dose dependently stimulated AP-1 driven luciferase expression, indicating an increase of AP-1 transactivation. Luciferase expression showed a significant increase with 30 pM CCK treatment and a greater increase with higher concentrations of CCK, reaching a 4.3 ± 0.5-fold increase with 10 nM CCK.
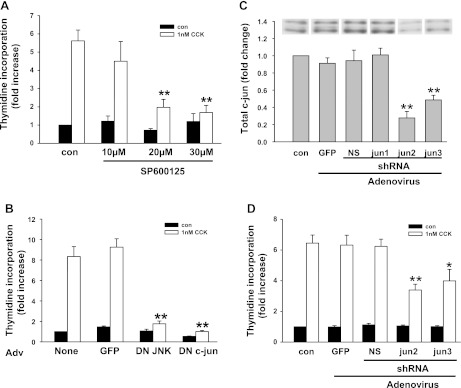
CCK-induced pancreatic acinar cell proliferation was inhibited by MAPK inhibitors, dominant-negative JNK, dominant-negative c-jun, and shRNA against c-jun.
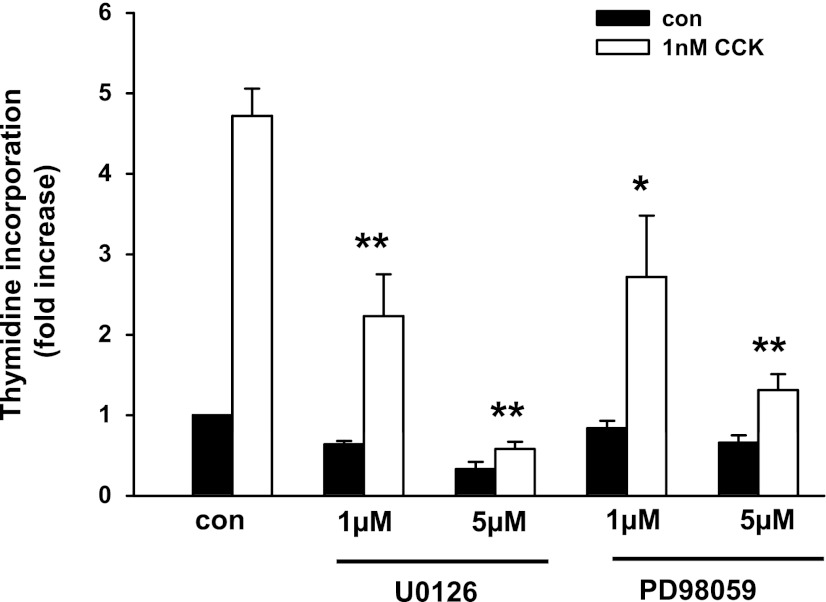
To determine whether MAPK pathways are required for CCK-induced acinar cell proliferation, we initially used specific chemical inhibitors to JNK and ERK pathways. The JNK inhibitor SP600125 reduced ∼50% of CCK-induced c-Jun expression (data not shown) and inhibited 80% of the [3H]thymidine incorporation at 30 μM (Fig. 8A). Two ERK pathway inhibitors U0126 and PD98059 also blocked acinar cell [3H]thymidine incorporation (Fig. 9), whereas the inactive analog U0124 had no effect.
Fig. 8.
Inhibition of CCK-induced pancreatic acinar cell proliferation by JNK inhibitor, dominant-negative JNK, dominant-negative c-Jun, and c-Jun short hairpin RNA (shRNA). A: effect of JNK inhibitor SP600125 on acinar cell [3H]thymidine incorporation. Acinar cells were preincubated with the inhibitor at the indicated concentration for 1 h followed by addition of 1 nM CCK. Values are means ± SE of 3–5 experiments. **P < 0.01 compared with control CCK-treated groups. B: effect of dominant-negative (DN) JNK and dominant-negative c-Jun on acinar cell [3H]thymidine incorporation. Adenovirus (Adv; 107 pfu/ml) was added when acinar cells were plated; 1 nM CCK was added 24 h later. Values are means ± SE of 3–5 experiments. C and D: reduction of CCK-induced acinar cell proliferation by c-Jun shRNA. Adenovirus was added at 107 pfu/ml when acinar cells were plated. C: cells were cultured for 24 h followed by protein extraction. The efficiency of shRNA to knockdown c-Jun expression was evaluated by Western blot. NS, nonspecific. **P < 0.01 compared with control group without any adenovirus infection. D: effect of c-Jun shRNA on CCK-induced acinar cell [3H]thymidine incorporation. For C, duplicate culture wells were run on gels and imaged, and after quantification the image was spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line is placed between the lanes. **P < 0.01 compared with control (no virus) CCK-treated groups. Values are means ± SE of 3–5 experiments. **P < 0.01; *P < 0.05 compared with CCK-treated group without any adenovirus infection.
Fig. 9.
Inhibition of CCK-induced pancreatic acinar cell proliferation by ERK pathway inhibitors. Acinar cells were preincubated with the inhibitors U0126 and PD 98059 at the indicated concentration for 1 h followed by addition of 1 nM CCK. [3H]thymidine incorporation was carried out as described in materials and methods. Values are means ± SE of 3–5 experiments. **P < 0.01; *P < 0.05 compared with control CCK-treated groups.
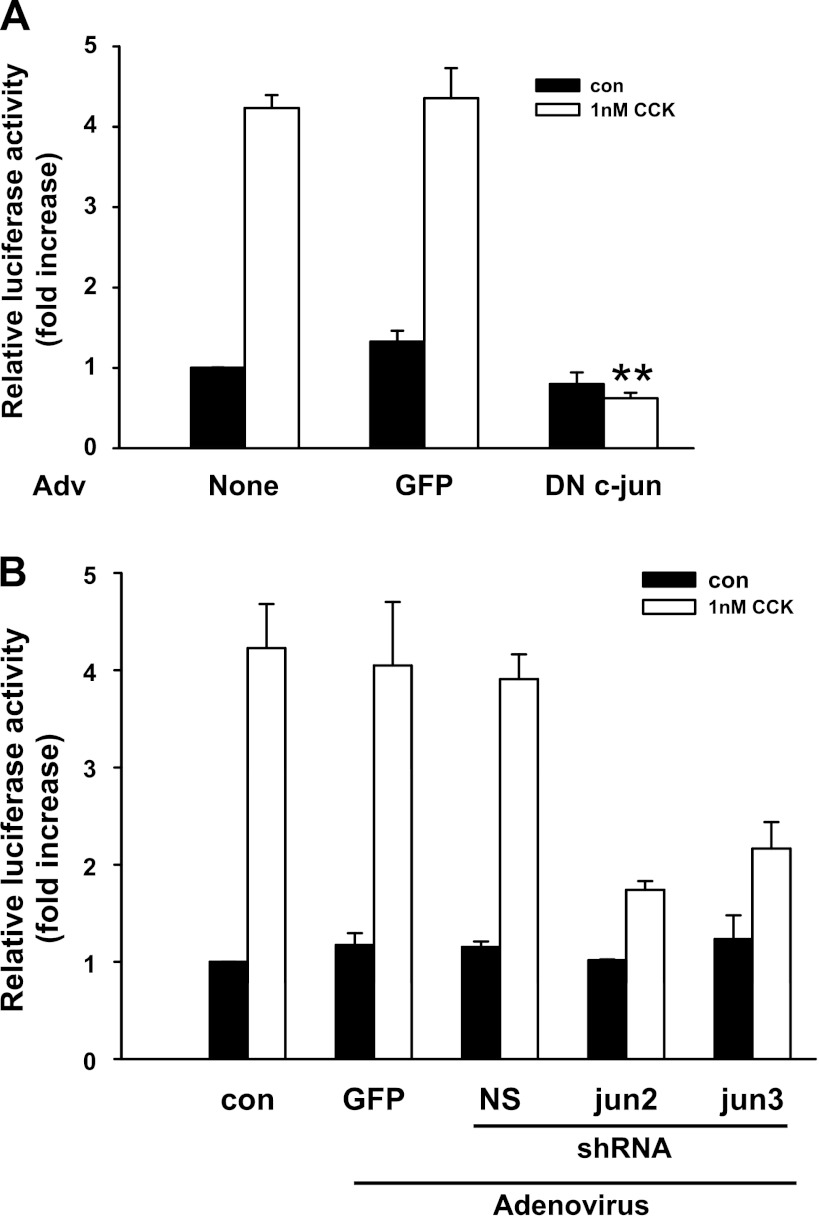
To further evaluate the role of JNK pathway components in acinar cell DNA synthesis, we examined the effect of dominant-negative JNK2 and dominant-negative c-Jun. Adenoviral vectors were used to express the dominant-negative mutants in acinar cells. The specificity of dominant-negative JNK2 was tested and shown to specifically inhibit CCK-induced c-Jun mRNA expression, whereas it did not affect the induction of c-fos and ATF3 (data not shown). Both of these dominant-negative mutants dramatically inhibited CCK-induced [3H]thymidine incorporation in acinar cells (Fig. 8B), whereas infection with a control adenovirus expressing GFP added at the same titer had no effect. This further confirmed the requirement of JNK and c-Jun for CCK-induced acinar cell growth.
Finally, we designed shRNA to knockdown endogenous c-Jun. We first tested the efficiency of three different c-Jun shRNA (jun1, jun2, and jun3) to knock down endogenous c-Jun expression by Western blot. The jun2 and jun3 shRNA reduced ∼60 and 40% of endogenous c-Jun protein expression, respectively (Fig. 8C), whereas jun1 and the nonspecific shRNA had no effect. Different titers of virus were tested and knockdown was maximal at 107 pfu/ml. In other studies (not shown), the mRNA for c-Jun was also reduced by jun2 and jun3 shRNA. As shown in Fig. 8D, adenovirus expressing jun2 or jun3 shRNA reduced 50 and 40% of CCK-induced [3H]thymidine incorporation, whereas infection with adenovirus expressing GFP or the nonspecific shRNA had no effect.
Effect of dominant-negative c-jun and c-jun shRNA on CCK-induced AP-1 transactivation.
To determine how c-Jun is involved in CCK-induced acinar cell growth, we first examined whether dominant-negative c-Jun has any effect on CCK-induced AP-1 activation. As shown in Fig. 10A, dominant-negative c-Jun completely blocked CCK-stimulated AP-1-driven luciferase expression, indicating that AP-1 activation was eliminated by dominant-negative c-Jun. Similarly, CCK-induced AP-1 activation was also significantly reduced by the two c-Jun shRNA (jun2 and jun3) molecules that reduced [3H]thymidine incorporation (Fig. 10B). This suggests that c-Jun acts through the activation of AP-1 to participate in CCK-induced acinar cell proliferation.
Fig. 10.
Inhibition of AP-1 luciferase activity by dominant-negative c-Jun and c-Jun shRNA. Adenovirus expressing GFP, dominant-negative c-Jun (A), or shRNA (B) was added at 107 pfu/ml when acinar cells were plated. Adenovirus expressing AP-1-driven luciferase was added 1 h before CCK administration at 106 pfu/ml. The cells were stimulated with 1 nM CCK for 6 h. Relative luciferase activity was measured and the results were expressed as fold increase compared with the control group. Pooled data are means ± SE of 4 experiments. **P < 0.01 compared with CCK-treated group without adenovirus infection.
Effect of dominant-negative c-jun on CCK-induced cyclin D1 induction.
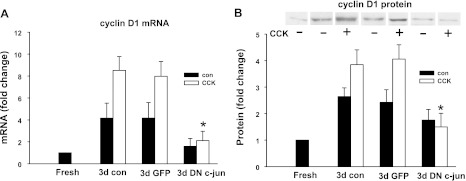
Cyclin D1 expression increased during culture and it was further upregulated by CCK treatment. To further evaluate how c-Jun regulates CCK-induced acinar cell proliferation, we tested the effect of dominant-negative c-Jun on cyclin D1 mRNA and protein expression. Acinar cells were cultured for 3 days in total with or without CCK treatment. As shown in Fig. 11A, cyclin D1 mRNA increased 4.2 ± 1.3-fold and 8.5 ± 1.3-fold in the control non-CCK-treated and CCK-treated group, respectively compared with acutely dissociated acini. Dominant-negative c-Jun inhibited 80% of cyclin D1 induction in both groups, whereas the control adenovirus expressing GFP had no effect. Similarly, dominant-negative c-Jun significantly blocked CCK-induced cyclin D1 protein expression (Fig. 11B). These data indicate a role of c-Jun in the regulation of cyclin D1 expression.
Fig. 11.
Inhibition of cyclin D1 induction by dominant-negative c-Jun. Adenovirus was added at 107 pfu/ml when acinar cells were plated. The cells were cultured for 72 h in control media or with 1 nM CCK and RNA or protein was extracted. Cyclin D1 mRNA (A) and protein (B) expression were examined. Pooled data are means ± SE of 3–4 experiments. For B, duplicate culture wells were run on gels and imaged, and after quantification the image was spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line is placed between the lanes. *P < 0.05 compared with CCK-treated group without adenovirus infection.
Effect of dominant-negative c-jun on amylase and β-catenin expression.
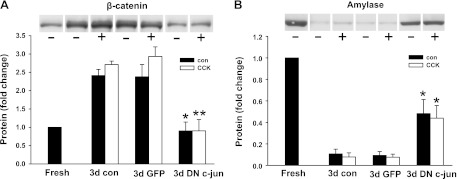
As shown earlier, acinar cells underwent dedifferentiation during culture. We then examined whether c-Jun is also required for acinar cell dedifferentiation. Decreased amylase expression and increased β-catenin expression were used as readout of acinar cell dedifferentiation. Acinar cells were cultured for 3 days with or without CCK treatment and then protein or RNA was extracted. As shown in Fig. 12, β-catenin expression increased and amylase expression decreased during culture. Dominant-negative c-Jun inhibited more than 70% of the increased expression of β-catenin at both mRNA and protein level. The reduced expression of amylase was also significantly blocked by dominant-negative c-Jun, and this occurred to a much greater degree at the protein level. These results indicate that basal activity of c-Jun is important for acinar cell dedifferentiation during culture.
Fig. 12.
Inhibition of the expression change of β-catenin and amylase during culture by dominant-negative c-Jun. Adenovirus was added at 107 pfu/ml when acinar cells were plated. The cells were cultured for 72 h in control medium or with 1 nM CCK followed by protein extraction. β-Catenin (A) and amylase (B) expression were examined. Pooled data are means ± SE of 3 experiments. For A and B, duplicate culture wells were run on gels and imaged, and after quantification the image was spliced to show only 1 lane per condition as described in materials and methods. For this reason a white line is placed between the lanes. **P < 0.01; *P < 0.05 compared with the corresponding groups without adenovirus infection.
DISCUSSION
We demonstrated the involvement of the JNK pathway through c-Jun and AP-1 in pancreatic acinar cell proliferation and dedifferentiation. We evaluated acinar cell morphology; the expression of the marker genes for mature differentiated pancreatic cells including acinar, ductal, stellate, islet, and progenitor cells; and the regulation of DNA synthesis during culture. Acinar cells showed a greatly reduced expression of pancreatic digestive enzymes, including amylase, chymotrypsinogen, and lipase; an increase in ductal markers such as cytokeratin 19; and an increased expression of genes associated with pancreatic embryonic development, including pdx-1, nestin, and p48. The expression of Mist1, an exocrine pancreatic-specific gene, decreased over time in culture, whereas the low level of insulin and the endocrine-specific factor neurogenin 3 did not change. There was a reduction of marker proteins for stellate cells. There was little expression of Hes1, which has been used as a readout of the activation of Notch signaling in acinar cells during recovery after pancreatitis (33). Mesenchymal factors may activate Notch signaling in acinar cells in vivo but are absent in acinar cell cultures. Thus in our study pancreatic acinar cells lost their characteristics of mature acinar cells and displayed embryonic features similar to exocrine precursor cells. However, they retained their sensitivity to CCK which when added stimulated cell division.
It has previously been shown that adult pancreatic acinar cells retain morphogenic plasticity and upon particular stimulation can change their differentiation commitment patterns toward other cell types with the results highly dependent on the culture conditions. Results of culture of pancreatic acini are highly dependent on the culture conditions. Most studies can be considered as suspension cultures, monolayer culture on plastic, monolayer cultures on a protein matrix (collagen, laminin, or Matrigel), or 3D cultures within an extracellular matrix gel. Suspension cultures with serum present dedifferentiate, do not proliferate, and undergo senescence (52, 53). By contrast, acini embedded in a matrix form cystlike structures with characteristics of acinar-to-ductal transformation (15, 43). Again there is little mitogenesis. Cells cultured as a monolayer lose their acinar characteristics and have been reported to assume a dedifferentiated or ductlike phenotype (3, 21, 29, 37, 39, 61). Most reports show that these cells proliferate and that this rate can be stimulated with CCK and growth factors (28, 37, 39, 62, 63). Other studies show that in the presence of appropriate factors pancreatic acinar cells can be transdifferentiated into insulin-secreting cells with secretory properties similar to those of native pancreatic β cells (44); this transdifferentiation is inhibited by active Notch signaling (4). In a recent in vivo study, acinar cells, infected with a combination of three transcription factors (Ngn3, pdx-1, and MafA) were reprogrammed into cells that closely resemble β cells (69). In our study, acinar cells cultured on the surface of collagen morphologically changed to epithelial-like cells with the parallel change on gene expression. In addition, we showed that CCK treatment did not affect the expression pattern of the various marker genes, indicating that acinar cell dedifferentiation was independent of CCK-activated signaling pathways.
Because the concentration of CCK used in this study (1 nM) can induce blebs in more sensitive freshly dissociated acini, there is a possibility that damage has been induced in our cultures and that this is an important step leading to CCK-induced proliferation. If significant acinar cell death had occurred, the flattened cells seen at 48–72 h could be the product of another precursor type such as centroacinar cells. We think this unlikely since there is no evidence for centroacinar cells responding to CCK, our cultures do not express centroacinar cell-specific markers, and some of the proliferating cells still contain amylase. However, future studies with lineage tracing will be necessary to resolve this possibility.
It has been shown that endogenous CCK is required for pancreatic regeneration after injury and that exogenous CCK accelerates the recovery. However, the signaling pathways mediating the trophic effect of CCK on pancreatic regeneration are not well established. In the present in vitro study, we used the dedifferentiated acinar cell model to study the mechanism by which CCK stimulates acinar cell proliferation. The cells divided to a limited extent even under basal conditions, indicating that there may be either tonic inhibitory factors in vivo, which are lost in the in vitro system, or there are autocrine or paracrine growth factors stimulating acinar cell growth in vitro. The dedifferentiation, which is independent of CCK, and the proliferation that is stimulated by CCK make these cultures an appropriate model to study acinar cell proliferation after damage such as occurs with pancreatitis. However, it may not be fully representative of in vivo events after injury and does not provide any information on redifferentiation that occurs as the final phase of the in vivo response to injury.
Besides CCK, other secretagogues such as CCh or bombesin were also shown to stimulate acinar cell [3H]thymidine incorporation, but with a much smaller effect than CCK. Thus CCK appears to initiate signals unique from other pancreatic secretagogues. EGF, FGF, HGF, and IGF-1 were able to stimulate acinar cell proliferation in monolayer culture as well, suggesting that these growth factors may also be involved in acinar cell growth in vivo (28, 37, 62, 63). In fact various growth factors have been reported to be highly expressed in pancreas after pancreatitis, including IGF-1 (22, 41), FGF 7 and 10 (31), and FGF21 (34).
Because of the uniform cell population and the ability to manipulate gene expression in cultured cells, it is feasible to use primary culture of dedifferentiated acinar cells to study the potential signaling pathways mediating the in vivo and in vitro trophic effect of CCK. The transcription factor c-Jun was our main interest in this study. c-Jun is an early response gene and belongs to the AP-1 transcription factor family. Its expression can be induced by endogenous CCK in vivo (17) or by exogenous CCK in freshly isolated acinar cells (40). It participates in the regulation of cell proliferation in a variety of cells. c-Jun−/− fibroblasts exhibit a severe growth defect (35, 55), and fetal hepatoblasts derived from c-Jun−/− fetuses also display reduced proliferation (13). More importantly, liver regeneration and hepatocyte proliferation are impaired in mice lacking c-Jun in the liver (6). Dominant-negative c-Jun also prevents vascular smooth muscle cell proliferation in vitro and in vivo (68). In the present study, CCK stimulation increased MAPK activation, c-Jun expression, and AP-1 activity in cultured acinar cells. Therefore it is likely that c-Jun/AP-1 is involved in CCK-induced acinar cell proliferation. To evaluate this hypothesis, we utilized chemical inhibitors to JNK and ERK pathways and overexpression of dominant-negative mutants. Finally, siRNA was used to knock down endogenous c-Jun expression. c-Jun shRNA inhibited CCK-induced acinar cell proliferation by 50%. The ability of three different approaches to block c-Jun action strongly supports our conclusion that c-Jun/AP-1 plays an essential role in dedifferentiated pancreatic acinar cell proliferation in vitro.
AP-1 protein complexes promote cell proliferation mainly through their ability to regulate the expression and function of cell cycle regulatory genes (56). Among these genes, cyclin D1 is one of the best studied. Its promoter region contains two AP-1 binding sites and the regulation of its expression by AP-1 has been well documented (5, 7, 25, 67). In our study, cyclin D1 showed an increased mRNA and protein expression during acinar cell culture, which was further enhanced by CCK. Furthermore, the elevated cyclin D1 expression was dramatically blocked by dominant-negative c-Jun, which also inhibited AP-1 activation. Thus CCK-induced acinar cell proliferation may be mediated by the activation of AP-1 and the subsequent induction of cyclin D1.
Besides the MAPK/c-Jun/AP-1 pathway, a variety of other signaling pathways can also be activated by CCK and may participate in acinar cell growth regulation, such as the PI3K/PKB/mTOR pathway and the Ca2+-calmodulin-NFAT pathway. Both pathways are required for CCK-induced adaptive pancreatic growth through division of mature acinar cells in vivo (9, 18, 60). Thus AP-1 may be one, but not the only, pathway that plays an essential role in CCK-induced acinar cell proliferation. Nicke et al. (48) showed that dominant-negative N17 Ras inhibited CCK-induced DNA synthesis in cultured rat acinar cells, indicating the involvement of Ras pathway in CCK-induced rat acinar cell proliferation.
In another study of pancreatic acinar cell growth in vitro (63), isolated mouse acinar cells were cultured on laminin-coated plates. They showed that PI3K played a critical role in IGF-1-induced acinar cell proliferation, whereas ERK activation was not required. This differs from our study using CCK in which ERK inhibition significantly blocked the trophic effect of CCK on acinar cells. This discrepancy could be due to the use of different agonists. IGF-1 and CCK may activate different set of signaling pathways in acinar cells, even if some pathways might be activated by both of them. Even the same pathway may have different cellular functions with different stimuli. This different result could also be due to different culture conditions, including extracellular matrix and culture medium.
Dominant-negative c-Jun dramatically inhibited the increased expression of β-catenin and the decreased expression of amylase, suggesting that the basal activity of c-Jun is necessary for the dedifferentiation of acinar cells. However, how c-Jun is involved in acinar cell dedifferentiation is not well understood. Hwang et al. (30) showed that c-Jun and AP-1 mediated interleukin-1β-induced dedifferentiation of chondrocytes by inducing the expression of the genes favoring dedifferentiation and suppressing the expression of the genes that are required for the maintenance of the differentiation characteristics. Thus it is possible that c-Jun/AP-1 works through similar mechanisms mediating acinar cells dedifferentiation.
In summary, pancreatic acinar cells in monolayer culture underwent dedifferentiation that was CCK independent and proliferation that was stimulated by CCK. The MAPK/c-Jun/AP-1 pathway plays an essential role in mediating both of these two processes. Monolayer culture of acinar cells appears similar to regeneration after pancreatitis and can be an appropriate model to formulate hypotheses that can be used to study acinar cell regeneration following injury in vitro. Furthermore, these cultured dedifferentiated acinar cells can be used to test factors required to redifferentiate acinar cells.
GRANTS
This research was supported by National Institutes of Health (NIH) grant DK059578 to J. A. Williams and by the combined Cell Biology and Imaging Core of the Michigan Gastrointestinal Peptide Center funded by NIH P30 DK034933 and the Michigan Diabetes Research and Training Center funded by NIH P60 DK020572.
DISCLOSURES
The authors have nothing to disclose beyond receiving support from the NIH.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brad Nelson for assistance on the morphological studies.
REFERENCES
- 1. Adler G, Hupp T, Kern HF. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch 382: 31–47, 1979 [DOI] [PubMed] [Google Scholar]
- 2. Amsterdam A, Jamieson JD. Studies on dispersed pancreatic exocrine cells. I Dissociation technique and morphologic characteristics of separated cells. J Cell Biol 63: 1037–1056, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arias AE, Bendayan M. Differentiation of pancreatic acinar cells into ductlike cells in vitro. Lab Invest 69: 518–530, 1993 [PubMed] [Google Scholar]
- 4. Baeyens L, Bonne S, Bos T, Rooman I, Peleman C, Lahoutte T, German M, Heimberg H, Bouwens L. Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 136: 1750–1760, e1713, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M. Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J 19: 2056–2068, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behrens A, Sibilia M, David JP, Mohle-Steinlein U, Tronche F, Schutz G, Wagner EF. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J 21: 1782–1790, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, Pestell RG, Greenberg ME. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol 18: 5609–5619, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131: 3457–3467, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Crozier SJ, Sans MD, Guo L, D'Alecy LG, Williams JA. Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice. J Physiol 573: 775–786, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem 271: 5686–5690, 1996 [DOI] [PubMed] [Google Scholar]
- 11. De Lisle RC, Logsdon CD. Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol 51: 64–75, 1990 [PubMed] [Google Scholar]
- 12. Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 267: G401–G408, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Eferl R, Sibilia M, Hilberg F, Fuchsbichler A, Kufferath I, Guertl B, Zenz R, Wagner EF, Zatloukal K. Functions of c-Jun in liver and heart development. J Cell Biol 145: 1049–1061, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elsasser HP, Adler G, Kern HF. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas 1: 421–429, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Githens S, Schexnayder JA, Moses RL, Denning GM, Smith JJ, Frazier ML. Mouse pancreatic acinar/ductular tissue gives rise to epithelial cultures that are morphologically, biochemically, and functionally indistinguishable from interlobular duct cell cultures. In Vitro Cell Dev Biol Anim 30A: 622–635, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Groblewski GE, Grady T, Mehta N, Lambert H, Logsdon CD, Landry J, Williams JA. Cholecystokinin stimulates heat shock protein 27 phosphorylation in rat pancreas both in vivo and in vitro. Gastroenterology 112: 1354–1361, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Guo L, Sans MD, Gurda GT, Lee SH, Ernst SA, Williams JA. Induction of early response genes in trypsin inhibitor-induced pancreatic growth. Am J Physiol Gastrointest Liver Physiol 292: G667–G677, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gurda GT, Crozier SJ, Ji B, Ernst SA, Logsdon CD, Rothermel BA, Williams JA. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology 139: 609–619.e9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gurda GT, Guo L, Lee SH, Molkentin JD, Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19: 198–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology 146: 1025–1034, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol 166: 97–103, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Hayakawa H, Kawarada Y, Mizumoto R, Hibasami H, Tanaka M, Nakashima K. Induction and involvement of endogenous IGF-I in pancreas regeneration after partial pancreatectomy in the dog. J Endocrinol 149: 259–267, 1996 [DOI] [PubMed] [Google Scholar]
- 23. He TC, Zhou S, Da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegyi P, Takacs T, Jarmay K, Nagy I, Czako L, Lonovics J. Spontaneous and cholecystokinin-octapeptide-promoted regeneration of the pancreas following l-arginine-induced pancreatitis in rat. Int J Pancreatol 22: 193–200, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9: 2105–2107, 1994 [PubMed] [Google Scholar]
- 26. Herman L, Fitzgerald PJ. Restitution of pancreatic acinar cells following ethionine. J Cell Biol 12: 297–312, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hess DA, Humphrey SE, Ishibashi J, Damsz B, Lee AH, Glimcher LH, Konieczny SF. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology 141: 1463–1472, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoshi H, Logsdon CD. Direct trophic effects of fibroblast growth factors on rat pancreatic acinar cells in vitro. Biochem Biophys Res Commun 196: 1202–1207, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Houbracken I, De Waele E, Lardon J, Ling Z, Heimberg H, Rooman I, Bouwens L. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology 141: 731–741.e4, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Hwang SG, Yu SS, Poo H, Chun JS. c-Jun/activator protein-1 mediates interleukin-1beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J Biol Chem 280: 29780–29787, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Ishiwata T, Naito Z, Lu YP, Kawahara K, Fujii T, Kawamoto Y, Teduka K, Sugisaki Y. Differential distribution of fibroblast growth factor (FGF)-7 and FGF-10 in l-arginine-induced acute pancreatitis. Exp Mol Pathol 73: 181–190, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F, Madsen OD, Carmeliet P, Dewerchin M, Collen D, Rousseau GG, Lemaigre FP. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol 20: 4445–4454, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128: 728–741, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Koester A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology 137: 1795–1804, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Johnson RS, Van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev 7: 1309–1317, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Lehv M, Fitzgerald PJ. Pancreatic acinar cell regeneration. IV. Regeneration after resection. Am J Pathol 53: 513–535, 1968 [PMC free article] [PubMed] [Google Scholar]
- 37. Logsdon CD. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol Gastrointest Liver Physiol 251: G487–G494, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Logsdon CD, Williams JA. Pancreatic acini in short-term culture: regulation by EGF, carbachol, insulin, and corticosterone. Am J Physiol Gastrointest Liver Physiol 244: G675–G682, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Logsdon CD, Williams JA. Pancreatic acinar cells in monolayer culture: direct trophic effects of caerulein in vitro. Am J Physiol Gastrointest Liver Physiol 250: G440–G447, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Lu L, Logsdon CD. CCK, bombesin, and carbachol stimulate c-fos, c-jun, and c-myc oncogene expression in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 263: G327–G332, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Ludwig CU, Menke A, Adler G, Lutz MP. Fibroblasts stimulate acinar cell proliferation through IGF-I during regeneration from acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 276: G193–G198, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Majumdar AP, Vesenka GD, Dubick MA. Acceleration of pancreatic regeneration by cholecystokinin in rats. Pancreas 2: 199–204, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132: 3767–3776, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA 102: 15116–15121, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miyasaka K, Ohta M, Masuda M, Funakoshi A. Retardation of pancreatic regeneration after partial pancreatectomy in a strain of rats without CCK-A receptor gene expression. Pancreas 14: 391–399, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Miyasaka K, Ohta M, Tateishi K, Jimi A, Funakoshi A. Role of cholecystokinin-A (CCK-A) receptor in pancreatic regeneration after pancreatic duct occlusion: a study in rats lacking CCK-A receptor gene expression. Pancreas 16: 114–123, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J Nutr 114: 467–471, 1984 [DOI] [PubMed] [Google Scholar]
- 48. Nicke B, Tseng MJ, Fenrich M, Logsdon CD. Adenovirus-mediated gene transfer of RasN17 inhibits specific CCK actions on pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 276: G499–G506, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Ohnishi H, Ernst SA, Wys N, McNiven M, Williams JA. Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am J Physiol Gastrointest Liver Physiol 271: G531–G538, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Parekh D, Ishizuka J, Townsend CM, Jr, Rajaraman S, Thompson JC. The effect of endogenous cholecystokinin released by bombesin and trypsin inhibitor on the regeneration of the pancreas. Ann Surg 218: 735–741, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pierreux CE, Poll AV, Kemp CR, Clotman F, Maestro MA, Cordi S, Ferrer J, Leyns L, Rousseau GG, Lemaigre FP. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology 130: 532–541, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Pinho AV, Rooman I, Reichert M, De Medts N, Bouwens L, Rustgi AK, Real FX. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut 60: 958–966, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Rooman I, Heremans Y, Heimberg H, Bouwens L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 43: 907–914, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Sans MD, Lee SH, D'Alecy LG, Williams JA. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol 287: G667–G675, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev 13: 607–619, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Song W, Yamaguchi H, Nakano I, Kimura T, Nawata H. Role of endogenous cholecystokinin in the regeneration of pancreatic tissue after acute hemorrhagic pancreatitis in rats. Fukuoka Igaku Zasshi 87: 14–22, 1996 [PubMed] [Google Scholar]
- 58. Sphyris N, Logsdon CD, Harrison DJ. Improved retention of zymogen granules in cultured murine pancreatic acinar cells and induction of acinar-ductal transdifferentiation in vitro. Pancreas 30: 148–157, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Tashiro M, Dabrowski A, Guo L, Sans MD, Williams JA. Calcineurin-dependent and calcineurin-independent signal transduction pathways activated as part of pancreatic growth. Pancreas 32: 314–320, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol 286: G784–G790, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Vila MR, Lloreta J, Real FX. Normal human pancreas cultures display functional ductal characteristics. Lab Invest 71: 423–431, 1994 [PubMed] [Google Scholar]
- 62. Vila MR, Nakamura T, Real FX. Hepatocyte growth factor is a potent mitogen for normal human pancreas cells in vitro. Lab Invest 73: 409–418, 1995 [PubMed] [Google Scholar]
- 63. Watanabe H, Saito H, Rychahou PG, Uchida T, Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology 128: 1391–1404, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol 63: 77–97, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol Endocrinol Metab Gastrointest Physiol 235: E517–E524, 1978 [DOI] [PubMed] [Google Scholar]
- 66. Williams JA, Sans MD, Tashiro M, Schafer C, Bragado MJ, Dabrowski A. Cholecystokinin activates a variety of intracellular signal transduction mechanisms in rodent pancreatic acinar cells. Pharmacol Toxicol 91: 297–303, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J 18: 188–197, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yasumoto H, Kim S, Zhan Y, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Iwao H. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Ther 8: 1682–1689, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455: 627–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.