Abstract
Many advances have been reported in the long-term culture of intestinal mucosal cells in recent years. A significant number of publications have described new culture media, cell formations, and growth patterns. Furthermore, it is now possible to study, e.g., the capabilities of isolated stem cells or the interactions between stem cells and mesenchyme. However, at the moment there is significant variation in the way these structures are described and named. A standardized nomenclature would benefit the ability to communicate and compare findings from different laboratories using the different culture systems. To address this issue, members of the NIH Intestinal Stem Cell Consortium herein propose a systematic nomenclature for in vitro cultures of the small and large intestine. We begin by describing the structures that are generated by preparative steps. We then define and describe structures produced in vitro, specifically: enterosphere, enteroid, reconstituted intestinal organoid, induced intestinal organoid, colonosphere, colonoid, and colonic organoid.
Keywords: cell culture, small intestine, colon, stem cells, crypt, intestinal organoid
in recent years, significant progress has been made in isolation and maintenance of intestinal cells derived from mammals in cell culture (14, 18–20). Although short-term organotypic cultures of native, nontransformed intestinal epithelial cells had been repeatedly reported in previous decades (7, 22), long-term maintenance and propagation of single cells or cell clusters was not feasible until the landmark publications by Sato et al. (20) and Ootani et al. (14) in 2009 that described for the first time specific cell culture conditions for the long-term culture of epithelial stem cells derived from small bowel mucosa. Since that time, multiple publications have reported on further progress in the field of in vitro cultures of intestinal cells (9, 10, 12, 17, 23–25). They can be viewed as the foundation for a whole new area of research. It will now be possible to characterize the growth patterns of individual cells isolated from the small intestinal or colonic epithelium, to investigate the effect of growth factors on single cells, to examine the interactions between pure epithelial cells and myofibroblasts in vitro (12), and to grow large quantities of different cells to serve as building blocks in bioengineered intestinal mucosa.
In many of these recent manuscripts, authors make great efforts to describe the isolated single cells, sheets of epithelial cells, or epithelial cell clusters as accurately as possible. The efforts to communicate what exactly the research team isolated and processed is hampered by the paucity of marker proteins for the different components of the intestinal stem cell niche. In addition, the exact developmental hierarchy of stem cells and progenitor cells in the small intestinal crypt-villus unit and the colon crypt-surface epithelium unit is only beginning to be elucidated. Similarly, many researchers are confronted with new cell formations they encounter under their microscopes and that do not resemble any structures that were previously known or described. Such formations are often the product of special cell culture conditions and the use of specific media. Since no universally accepted culture conditions exist for small intestinal and colonic progenitor and stem cells, different laboratories use different terminologies for particular cells or structures that may derive from them in cell culture. Accordingly, the creation of new words and abbreviations abounds in the literature as much as in the daily communications of fellow scientists. Among the members of the National Institutes of Health (NIH) Intestinal Stem Cell Consortium, we have found that the nomenclature for cells used in culture and structures derived from them is increasingly cumbersome owing to the lack of standardization. It is for this reason that we propose a systematic nomenclature for in vitro culture of intestinal stem cells and progenitors.
Structures That Are Isolated by Preparative Steps, e.g., Enzymatic Digestion, Chelation of Divalent Ions with EDTA, or Microdissection of Small Intestinal or Colonic Mucosa
Epithelial sheets.
Sheets of epithelial cells can be isolated from small intestinal or colonic mucosa. This isolation can be achieved, for example, by brief chelation (EDTA or EGTA) treatment that deprives the tissue of divalent ions. (5).
Isolated crypts.
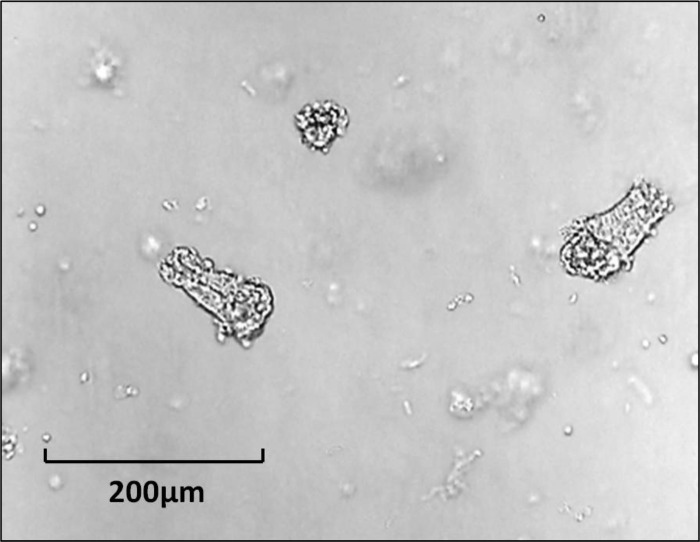
These structures contain the epithelial cells from the bottom of the crypt, the middle of the crypt, or the entire crypt. They may be harvested by exposure of intestinal mucosa to divalent ion chelators (Figs. 1 and 2) or dissection of intestinal mucosa under the dissecting microscope (13). As defined here, “isolated crypts” contain no mesenchymal cell elements (Fig. 3). Hence steps should be taken to confirm the absence of nonepithelial cells such as myofibroblasts, fibroblasts, or smooth muscle cells before using this term. This can be done, e.g., by Western blotting that confirms the presence of e-cadherin or epithelial cell adhesion molecule (EpCAM) but the absence of smooth muscle actin or vimentin (4).
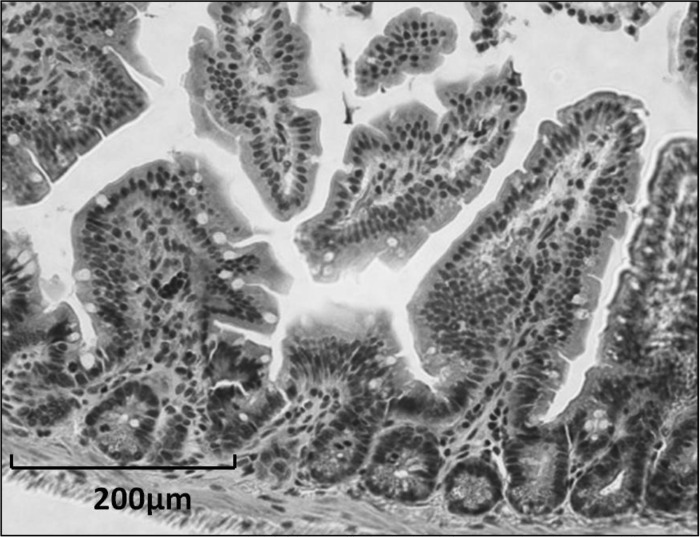
Fig. 1.
Murine small intestine; hematoxylin and eosin stain.
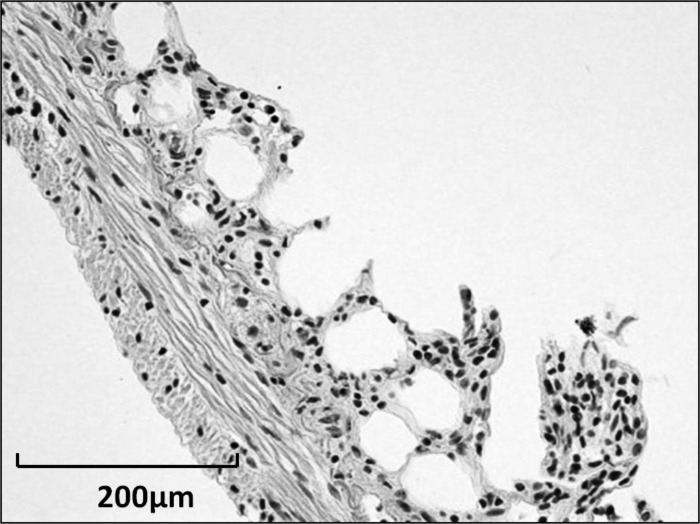
Fig. 2.
Murine small intestine after treatment with 3 mM EDTA solution to remove the epithelium.
Fig. 3.
Isolated crypts from murine jejunum.
Organoids (e.g., jejunal organoid, colon organoid).
These are cigar-shaped structures that consist of about 20 or more cells from the bottom of the crypt. These structures can be isolated from native tissue such that crypts and surrounding mesenchymal elements (e.g., myofibroblasts, fibroblasts, and smooth muscle cells) remain in contact during harvesting. A standard preparation uses incubation of mucosa with collagenase and dispase with subsequent gravity sedimentation (1, 3, 8). These structures are able to form neomucosa with a phenotype characteristic of their site of origin (2).
Mesenchymal cells/intestinal subepithelial myofibroblasts.
Mesenchymal cells, or intestinal subepithelial myofibroblasts (ISEMFs), can be isolated by first preparing organoids and then transferring cells into media containing FBS for several days to stimulate growth of mesenchyme while epithelial cells do not survive (15). Many of these cells have myofibroblast phenotype but the preparation can include fibroblasts or smooth muscle cells, which are typically distinguished based on expression of α-smooth muscle actin, vimentin, and desmin. They may even contain other stromal elements found within the lamina propria. A comprehensive review of this cell population has been published elsewhere (16). A significant variety of other mesenchymal cells are present in the mucosa. Although there is evidence for close interactions between epithelial cells and ISEMFs (12), the relationship between many other cell types and the mucosal epithelium remains to be elucidated.
Single intestinal epithelial cells.
These cells can be isolated by treatment of small intestinal or colonic mucosa with ion chelators and mechanical or enzyme-mediated separation into a single cell suspension and subsequent gravity sedimentation (20). The term “single” should only be used if careful efforts have been made to eliminate doublets, e.g., during FACS sorting. Similarly, the purity of the cell preparations should be confirmed by testing for the presence of epithelial cell markers and the absence of nonepithelial cells. This is readily achieved by FACS using EpCAM antibodies to positively select the epithelial cells and using CD45 antibodies to remove immune cells and CD31 antibodies to exclude endothelial cells (25).
Structures Produced During In Vitro Culture Using Defined Cell Culture Media Either by Culturing a Single Cell Species or by Coculture of Two or More Cell Types
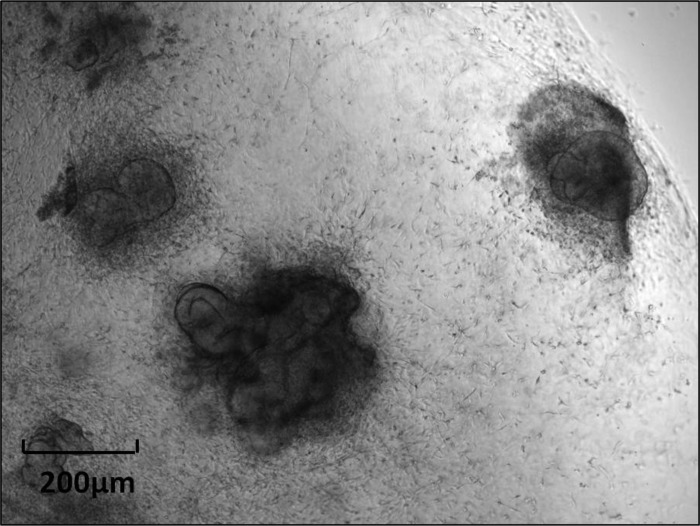
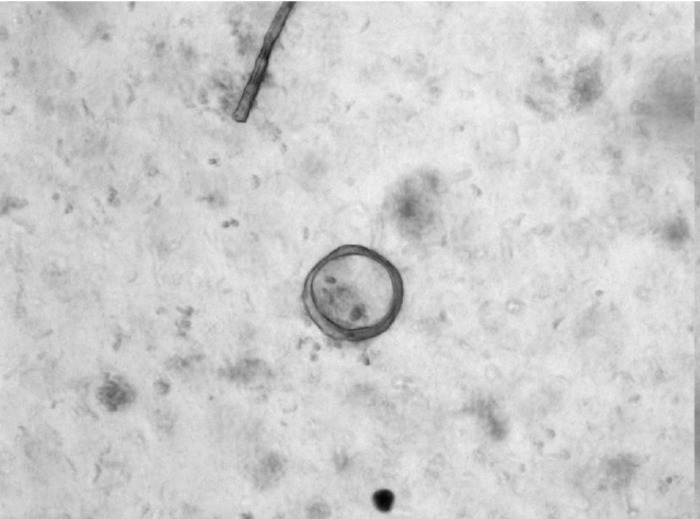
Enterosphere.
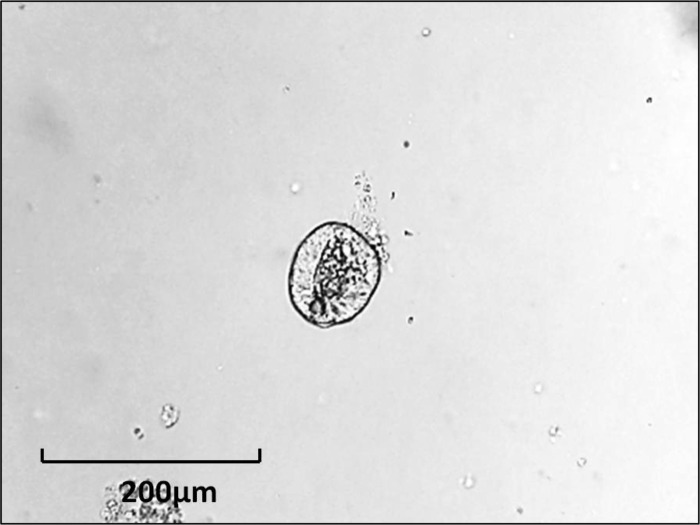
Enterosphere (EnS) is a spherical structure composed of several small intestinal epithelial cells that appears as a rounded-off epithelial cyst (Fig. 4). This term should used for all structures that have this morphology, irrespective of whether they were derived from single cells or from crypt fragments. Such structures form (e.g., from wild-type murine cells) within 3–24 h after the beginning of culture. In some cases, EnS may persist for weeks depending on the culture conditions (12). When transplanted into immunocompromised mice, enterospheres derived from human intestine will engraft and differentiate into different epithelial cell lineages (12).
Fig. 4.
Enterosphere from murine jejunum.
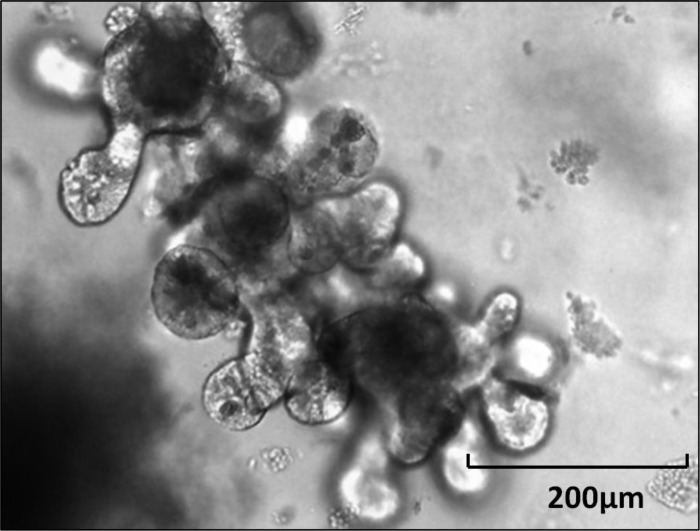
Enteroid.
Enteroid (EnO) is a multilobulated structure with a lumen that develops from an enterosphere by formation of budding crypts (Fig. 5). EnOs typically form between 3 and 7 days after the beginning of cell culture.
Fig. 5.
Enteroid from murine small intestine.
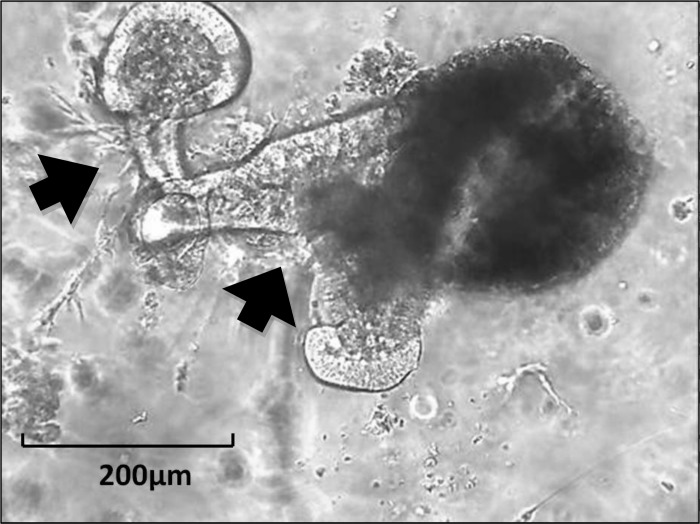
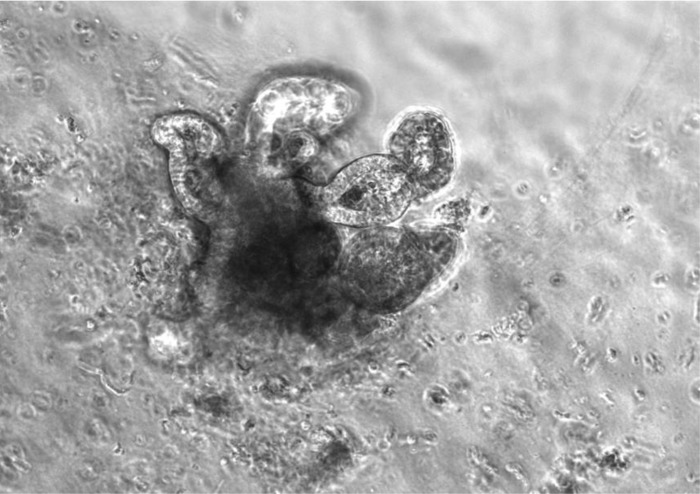
Reconstituted intestinal organoid.
Reconstituted intestinal organoid is a multicellular cluster structure that consists of small intestinal or colonic epithelial and mesenchymal cell elements (Fig. 6), e.g., a reconstituted cluster of isolated crypt cells and ISEMFs (12).
Fig. 6.
Reconstituted organoid from murine jejunum with budding of enterocytes and adjacent myofibroblasts (arrows).
Induced intestinal organoid.
This structure is also a multicellular cluster. It is isolated and cultured from induced embryonal or induced pluripotent stem cells by use of specific culture conditions that promote the differentiation into both intestinal epithelium and mesenchyme (21). Examples include induced human (iHIO) and murine (iMIO) intestinal organoids (Figs. 7 and 8).
Fig. 7.
Induced human intestinal organoid, from human foreskin fibroblasts.
Fig. 8.
Induced human intestinal organoid, as in Fig. 7, higher magnification.
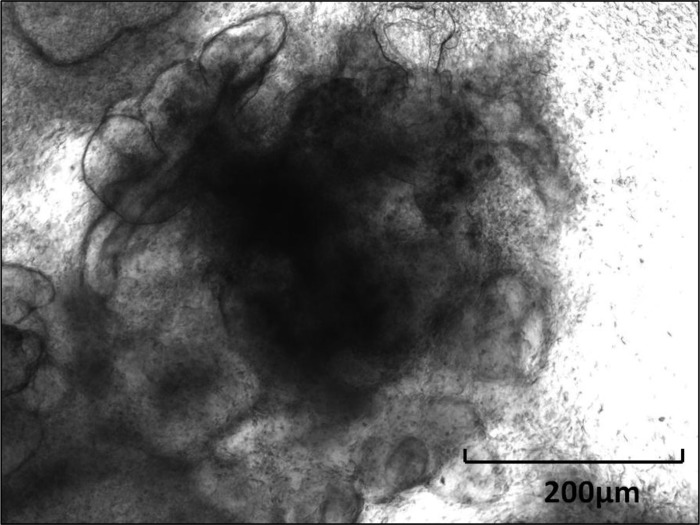
Colonosphere.
Colonosphere (CnS) is a spherical structure composed of several colonic mucosal epithelial cells that appears as rounded-off epithelial cyst (Fig. 9). It was been described that colonospheres form between 3 and 24 h after the beginning of culture (17).
Fig. 9.
Colonosphere.
Colonoid.
Colonoid (CnO) is a multilobulated structure with a lumen that develops from a colonosphere by formation of budding crypts (Fig. 10). Murine colonoids may form between 3 and 7 days after the beginning of cell culture (17).
Fig. 10.
Colonoid.
Colonic organoid.
This is a multicellular cluster structure that consists of colonic epithelial and mesenchymal cell elements, e.g., a reconstituted cluster of isolated crypt cells and subepithelial mesenchyme (M. Bjerknes and H. Cheng, unpublished data).
We propose that all cells and multicellular structures be named for the origin of their epithelial components. For example, iHIO clusters are designated “human” since they are derived from human pluripotent stem cells. Similarly, human intestinal epithelial cells that are cocultured with adjacent murine ISEMFs or form chimeric cell clusters with nonhuman ISEMFs should be termed “reconstituted human intestinal organoids.” We hope the fellow researchers with interest in intestinal stem cells will interpret the proposed nomenclature as a well-intentioned attempt at organization of terminology that represents a useful tool of communication at this point in time. We expect that the scientific community will see a rapid increase in publications in this field since a door has been opened that was closed for a long time. It is now conceivable to utilize our ability to isolate individual cells from the small intestinal and colonic epithelium and to culture and propagate them in many new ventures. It will be possible to study human cells, one at a time, to facilitate drug discoveries, to subject single cells of different species to genetic interventions, and to investigate any number of pharmacological manipulations at the single cell level over long periods of time. For this reason, a widely accepted agreement about the terminology in this area of research is important. We realize that this effort will need to be updated as the field of intestinal stem cell biology progresses. Nonetheless, we feel that semantic consensus will benefit our research area and facilitate the scientific discourse. In addition, it may form the basis of developing a common terminology for studies of other epithelial cells such as gastric or esophageal epithelial cultures.
GRANTS
This manuscript was generated as a project of the Intestinal Stem Cell Consortium, a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases (U01DK85547, U01DK85508, U01DK85527, U01DK85507, U01DK85551, U01DK85535, U01DK85532, U01DK85525, and U01DK85570).
ACKNOWLEDGMENTS
The authors are very grateful for critical reviews of the manuscript by the following individuals: Matthew Bjerknes (University of Toronto, Toronto, ON), David Breault (Harvard Stem Cell Institute, Boston, MA), Anthony Burgess (Ludwig Institute for Cancer Research, Melbourne, Australia), Hans Clevers (Hubrecht Institute, Utrecht, The Netherlands), P. Kay Lund (University of North Carolina, Chapel Hill, NC), Don Powell (University of Texas Medical Branch, Galveston, TX), Toshiro Sato (Keio University, Tokyo, Japan), Thaddeus Stappenbeck (Washington University, St. Louis, MO), James Wells (Cincinnati Children's Hospital, Cincinnati, OH), and Douglas Winton (Cambridge Research Institute, Cambridge, UK).
REFERENCES
- 1.Agopian VG, Chen DC, Avansino JR, Stelzner M. Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg 13: 971–982, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Avansino JR, Chen DC, Hoagland VD, Woolman JD, Haigh WG, Stelzner M. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg 201: 710–720, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Avansino JR, Chen DC, Hoagland VD, Woolman JD, Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery 140: 423–434, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu JF, Menard D. Isolation, characterization, and culture of normal human intestinal crypt and villus cells. Methods Mol Biol 806: 157–173, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bjerknes M, Cheng H. Intestinal epithelial stem cells and progenitors. Methods Enzymol 419: 337–383, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Booth C, O'Shea JA, Potten CS. Maintenance of functional stem cells in isolated and cultured adult intestinal epithelium. Exp Cell Res 249: 359–366, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci 101: 219–231, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol 298: G590–G600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Gallardo MM, Blasco MA, Sancho E, Clevers H, Batlle E. Isolation and in vitro expansion of human colonic stem cells. Nat Med 17: 1225–1227, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Kuo B, Urma D. Esophagus—anatomy and development. GI Motility online 2006. doi: :101038/gimo6 [Google Scholar]
- 12.Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, Lewis M, Stelzner M, Martin MG, Dunn JC. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PloS One 6: e26898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills SJ, Mathers JC, Chapman PD, Burn J, Gunn A. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut 48: 41–46, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plateroti M, Rubin DC, Duluc I, Singh R, Foltzer-Jourdainne C, Freund JN, Kedinger M. Subepithelial fibroblast cell lines from different levels of gut axis display regional characteristics. Am J Physiol Gastrointest Liver Physiol 274: G945–G954, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73: 213–237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramalingam S, Daughtridge GW, Johnston MJ, Gracz AD, Magness ST. Distinct levels of Sox9 expression mark colon epithelial stem cells that form colonoids in culture. Am J Physiol Gastrointest Liver Physiol 302: G10–G20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, Stange DE, Ferrante M, Vries RG, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141: 1762–1772, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stallmach A, Hahn U, Merker HJ, Hahn EG, Riecken EO. Differentiation of rat intestinal epithelial cells is induced by organotypic mesenchymal cells in vitro. Gut 30: 959–970, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Furstenberg RJ, Gulati AS, Baxi A, Doherty JM, Stappenbeck TS, Gracz AD, Magness ST, Henning SJ. Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 300: G409–G417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]