Abstract
Butyrate, an intestinal microbiota metabolite of dietary fiber, has been shown to exhibit protective effects toward inflammatory diseases such as ulcerative colitis (UC) and inflammation-mediated colorectal cancer. Recent studies have shown that chronic IFN-γ signaling plays an essential role in inflammation-mediated colorectal cancer development in vivo, whereas genome-wide association studies have linked human UC risk loci to IFNG, the gene that encodes IFN-γ. However, the molecular mechanisms underlying the butyrate-IFN-γ-colonic inflammation axis are not well defined. Here we showed that colonic mucosa from patients with UC exhibit increased signal transducer and activator of transcription 1 (STAT1) activation, and this STAT1 hyperactivation is correlated with increased T cell infiltration. Butyrate treatment-induced apoptosis of wild-type T cells but not Fas-deficient (Faslpr) or FasL-deficient (Fasgld) T cells, revealing a potential role of Fas-mediated apoptosis of T cells as a mechanism of butyrate function. Histone deacetylase 1 (HDAC1) was found to bind to the Fas promoter in T cells, and butyrate inhibits HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation in T cells. Knocking down gpr109a or slc5a8, the genes that encode for receptor and transporter of butyrate, respectively, resulted in altered expression of genes related to multiple inflammatory signaling pathways, including inducible nitric oxide synthase (iNOS), in mouse colonic epithelial cells in vivo. Butyrate effectively inhibited IFN-γ-induced STAT1 activation, resulting in inhibition of iNOS upregulation in human colon epithelial and carcinoma cells in vitro. Our data thus suggest that butyrate delivers a double-hit: induction of T cell apoptosis to eliminate the source of inflammation and suppression of IFN-γ-mediated inflammation in colonic epithelial cells, to suppress colonic inflammation.
Keywords: Fas promoter, histone deacetylase 1
compelling data from both human patients and animal model-based studies have shown that aberrant host immune response triggered by intestinal microbiota is a requisite for the onset of inflammatory diseases such as ulcerative colitis (UC) and colitis-associated colorectal cancer (15, 18, 47, 49). Although the etiology of UC is still not clear and the role of the proinflammatory cytokine IFN-γ in UC is controversial, recent Meta analysis and genome-wide association studies of large cohorts of human patients have surprisingly identified IFNG, the gene encoding for IFN-γ, as a UC susceptibility locus in humans (1, 30, 39).
IFN-γ is a proinflammatory cytokine that exerts its inflammatory function through signal transducer and activator of transcription 1 (STAT1) to regulate the expression of inflammatory mediators such as inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) in the colonic tissues (18). It has been reported that IFN-γ secretion is elevated in the peripheral blood (16) and IFN-γ expression level is increased in the inflamed colonic mucosa tissues in patients with UC (46). The expression and activation level of STAT1 is also significantly increased in colonic tissues of patients with UC (37). These observations thus suggest that IFN-γ might play a key role in human UC pathogenesis (16, 46) and inflammation-dependent spontaneous colorectal cancer development (2, 18). IFN-γ is secreted by activated T cells. Therefore, infiltration and persistence of activated T cells in the colonic tissues might be the source of IFN-γ and thereby a cause of colonic inflammation. Indeed, whereas IL-10 knockout (Il10−/−) mouse exhibits intolerance to intestinal microbiota and goes on to develop spontaneous colitis, Il10−/−Rag2−/− mouse fails to develop colitis, suggesting that UC is a T cell-mediated inflammatory disorder (27, 47).
A promising class of agents with both preventive and therapeutic potential to counteract inflammation-mediated UC and colorectal cancer is short-chain fatty acids, most notably butyrate (10, 22, 43). Butyrate is a major metabolite in colonic lumen arising from bacterial fermentation of dietary fiber and has been shown to be a critical mediator of the colonic inflammatory response (10, 21–22, 24, 40). One mechanism underlying butyrate function in suppression of colonic inflammation is inhibition of the IFN-γ/STAT1 signaling pathways (23, 40). Butyrate may exert its anti-inflammatory function through acting as a histone deacetylase (HDAC) inhibitor (10–11, 48); however, the specific molecular targets of butyrate as a HDAC inhibitor and molecular mechanisms of inhibition are not well-defined.
We conducted an in-depth analysis of butyrate function in both T cells and colonic epithelial cells and determined that butyrate delivers a double-hit to inhibit inflammation: first, butyrate inhibits IFN-γ-induced STAT1 activation and iNOS upregulation to suppress inflammation in colonic epithelial and carcinoma cells; second and more importantly, butyrate inhibits Fas promoter-bound HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation to enhance Fas-mediated apoptosis of T cells, resulting in termination of the uncontrolled T cell activation, thereby, eliminating the source of inflammation in the colonic tissue.
MATERIALS AND METHODS
Mice and cells.
BALB/c (H-2d) mice were obtained from the National Cancer Institute (NCI, Frederick, MD). C57BL/6J, Fas-deficient (Faslpr), and FasL-deficient (Fasgld) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). slc5a8−/− and gpr109a−/− mice were originally obtained from Dr. Thomas Boettger (Max-Planck-Instituts für Herz, Lundgenforschung, Germany) and Dr. Stefan Offermanns (University of Heidelberg, Heidelberg, Germany), respectively. All mice were housed, maintained, and studied in accordance with approved NIH and Georgia Health Sciences University guidelines for animal use and handling. CCD-841 and T84 cells were obtained from ATCC (Manassas, VA). CCD-841 and T84 cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA). ATCC characterizes these cells by morphology, immunology, DNA fingerprint, and cytogenetics.
Immunohistochemistry.
The human colorectal cancer progression tissue microarray (TMA) was obtained from the Cooperative Human Tissue Network. Immunohistochemical staining was carried out by the Georgia Pathology Service. CD3 antibody was obtained from Dako (Carpinteria, CA). STAT1 antibody was obtained from BD Biosciences (San Jose, CA).
Analysis of T cell activation.
Twenty-four-well plates were coated with anti-CD3 (clone 145-2C11; Biolegend, San Diego, CA) and anti-CD28 (clone: 28.2, Biolegend) mAbs. Spleen cells (1 × 106 cells/well) were then seeded in the mAb-coated plates. Cells were collected and stained with PE-conjugated CD4, APC-conjugated CD25, and FITC-conjugated CD69 mAbs (Biolegend) at different time points and analyzed by flow cytometry.
Cell proliferation assay.
To purify CD4+ or CD8+ T cells, spleen cells were incubated with anti-CD19, CD4 (for CD8 T cell purification), CD8 (for CD4 purification), CD11b, and NK mAbs (all from Biolegend) at 4°C for 20 min. Cells were washed twice with PBS and then incubated with anti-mouse IgG-conjugated and anti-Rat IgG-conjugated magnetic beads (Polysciences, Warrington, PA). The bound cells were removed by a magnetic stand. Proliferation assay was performed using the MTT proliferation kit (ATCC) according to manufacturer's instructions.
T cell apoptosis assay.
Spleen cells were collected, and live cells were isolated using the Lymphocyte Separation Medium (CellGrow). Cells were then seeded in anti-CD3/CD28-coated 24-well plates, along with butyrate, and incubated for 24 h. Cells were stained with PE-conjugated anti-CD4 or FITC-conjugated anti-CD8a and Alexa Fluor 647 Annexin V (Biolegend) and analyzed with flow cytometry.
Analysis of Fas protein.
For mouse Fas analysis, cells were stained with PE-conjugated anti-CD4 or PE-Cy5.5-conjugated anti-CD8 (Biolegend) and FITC-conjugated anti-Fas (Biolegend). For human Fas staining, cells were stained with anti-human Fas (clone DX2, Biolegend), followed by incubation with FITC-conjugated anti-mouse IgG. The stained cells were analyzed by flow cytometry.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assays were carried out essentially as previously described (50). Immunoprecipitation was carried out using anti-acetyl-H3K9 (Cell Signaling, Beverly, MA), HDAC1, and HDAC2 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies and agarose-protein A beads (Millipore, Billerica, MA). The Fas promoter DNA was detected by PCR using Fas promoter-specific primers (Table 1).
Table 1.
PCR primers
| Primer | Forward | Reverse |
|---|---|---|
| PTGS2 | 5′-CATCAAACAAAGGGGTCCAAGTTC-3′ | 5′-AAGACAGAGCAGCACGGGC-3′ |
| iNOS | 5′-CCAGAGGACCCAGAGACAAGC-3′ | 5′-GGCAGCACATCAAAGCGGC-3′ |
| STAT1 | 5′-CTTCTTCCTGAACCCCCCG-3′ | 5′-CCCATCATTCCAGAGGCACAG-3′ |
| STAT2 | 5′-TCTGGCACCTTCCTACTGCG-3′ | 5′-CAATGGCAACTCCTGGTCGTG-3′ |
| STAT3 | 5′-CGAGAGCAGCAAAGAAGGAGGG-3′ | 5′-GGGACATCGGCAGGTCAATG-3′ |
| STAT4 | 5′-GATGGTCACCTCACCTGGGC-3′ | 5′-TTGTGGCTCCGTGGAATCG-3′ |
| STAT5a | 5′-GCACCATAAGCCCCATTGG-3′ | 5′-CGTAGATAAGGTAGTTCAGGTCCCC-3′ |
| STAT5b | 5′-TCGGGGGCATCACCATTG-3′ | 5′-CCATCGGTATCAAGGACGGAG-3′ |
| STAT6 | 5′-CGTCTGGATGAAGTCCTGCG-3′ | 5′-TGGCGTTGTTGTCTTGGTTACC-3′ |
| IRF1 | 5′-TAACTCCAGCACTGTCACCGTG-3′ | 5′-TATGCCTATCCCAATGTCCCC-3′ |
| IRF2 | 5′-GCTGCCCTTATCCGAACGAC-3′ | 5′-GCGTAGGAAGACACAGGAGAAATC-3′ |
| IRF3 | 5′-CACGCTACACTCTGTGGTTCTGC-3′ | 5′-GCTGGCTGTTGGAGATGTGC-3′ |
| IRF4 | 5′-CCACGGACACACCTATGATGTTAG-3′ | 5′-AGAATGACGGAGGGAGCGG-3′ |
| IRF5 | 5′-CCATCCGTCTGTGCCAGTGTAAG-3′ | 5′-AACATCTCCAGCAGCAACCG-3′ |
| IRF6 | 5′-TGGCTACACAGAGATTCCAAACG-3′ | 5′-TGGGGGATGTCACACACTTGATAG-3′ |
| IRF7 | 5′-CCCCAGCAGTAAGAACTTCAGAGC-3′ | 5′-TAGTGTGGTGACCCTTGCCGCCTC-3′ |
| IRF8 | 5′-CGTGGAAGACGAGGTTACGCTG-3′ | 5′-GCTGAATGGTGTGTGTCATAGGC-3′ |
| IRF9 | 5′-CATCCCCATCTCCTGGAATGC-3′ | 5′-GGTGACTGCTCTGTGTGCTGTAAC-3′ |
| TGFβ1 | 5′-CCGCAACAACGCCATCTATG-3′ | 5′-TATCAGTGGGGGTCAGCAGCCG-3′ |
| Smad1 | 5′-AGCAGCACCTACCCTCACTCCC-3′ | 5′-GAACGCTTCACCCACACGG-3′ |
| Smad2 | 5′-AGCAAAAACAGCACTTGAGGTCTC-3′ | 5′-CAATACTGGGTCTGGACACATTACC-3′ |
| Smad3 | 5′-CAGGAGACAGACAGTGACCAGCAC-3′ | 5′-GTTGAGTGGAATCGGTGTTCTTTG-3′ |
| Smad4 | 5′-TGGGTCCGTGGGTGGAATAG-3′ | 5′-CCTGGAAATGGTTAGGGCGTC-3′ |
| Smad5 | 5′-CCTATGAGGAGCCCAAACACTG-3′ | 5′-TCTGAACAAAGATGCTGCTGTCAC-3′ |
| Smad6 | 5′-GCATTTTCTACGACCTACCTCAGG-3′ | 5′-GAACACCTTGATGGAGTAACCCG-3′ |
| Smad7 | 5′-GCTGTGTTGCTGTGAATCTTACGG-3′ | 5′-CACCACGCACCAGTGTGACC-3′ |
| β-actin | 5′-ATTGTTACCAACTGGGACGACATG-3′ | 5′-CTTCATGAGGTAGTCTGTCAGGTC-3′ |
| SOCS1 | 5′-TCCGCTCCCACTCCGATTAC-3′ | 5′-AACCACTCCCTCCGACCCAG-3′ |
| SOCS2 | 5′-CACGGAATGGGACTGTTCACC-3′ | 5′-CCTCTGGGTTCTCTTTCACATAGC-3′ |
| SOCS3 | 5′-AAGGAGCCAAACACAGCCAATAG-3′ | 5′-AAAAAAGCCACCCCAACACAC-3′ |
| SOCS4 | 5′-AACGACACACTGTTCCTATGAGTCC-3′ | 5′-CTGGGCTTATTCCTTTTTCTGGAG-3′ |
| SOCS5 | 5′-CCTTTTCCTGCTGGCTCGG-3′ | 5′-TGTGTATTTGTGCGTTGGGAGG-3′ |
| SOCS6 | 5′-CCCATCCGCTCCACATCAC-3′ | 5′-GCACATCCCGTCATCTAAAGGC-3′ |
| SOCS7 | 5′-TGTCCCTTCCCACTTCATCTACC-3′ | 5′-TGCCTTGTTTTCCCTATCTGTCTC-3′ |
| GPR109A | 5′-TGAGGCAGAGACAGATGGACAGAC-3′ | 5′-AGAAGTTGGGGAAAGATGGGC-3′ |
| SLC5A8 | 5′-CTTGTGAAGCATCTCCAGGGC-3′ | 5′-ACTCCAGGATAGCATCAATCAACC-3′ |
| SLC5a8-BS | 5′-ATTTGGATTGTGAATTGGAAAG-3′ | 5′-CCCCTAACACCCTAAAACCC-3′ |
| GPR109a-BS | 5′-TATGTTTATAATTGGGATTGGAGGT-3′ | 5′-AAACCCCACAAAAAACAAAAAATA-3′ |
| ChIP-Fas-P | 5′-GAACTTTCTCGCAACCCTTGG-3′ | 5′-GGCACGCACAAACCGCTTC-3′ |
| hiNOS | 5′-ACATCACCACACCCCCAACC-3′ | 5′-GAAAGCAGGAAGCCAGCAGAC-3′ |
BS: PCR primers for amplifying Bisulfite-modified genomic DNA for cloning and sequencing. ChIP-Fas-P: Primers for PCR amplification of the Fas promoter DNA immunoprecipitated in chromatin immunoprecipation (ChIP). iNOS, inducible nitric oxide synthase; STAT, signal transducer and activator of transcription; IRF, IFN regulatory factor; SOCS, suppressor of cytokine signaling.
HDAC activity assay.
HDAC activity assay was carried out essentially as previously described (44). Briefly, recombinant human HDAC1 and HDAC2 were assayed for their activity in vitro in the absence or presence of butyrate using the HDAC assay kit (BioVision, Mountain View, CA).
RT-PCR analysis.
RT-PCR was performed essentially as previously described (50). Briefly, total RNA was isolated from cells or tissues using Trizol (Invitrogen, San Diego, CA) according to the manufacturer's instructions and used for the first strand cDNA synthesis using the MMLV reverse transcriptase (Promega, Madison, WI). The cDNA was then used as template for PCR amplification. The sequences of primers are listed in Table 1.
Western blot analysis.
Western blotting analysis was performed essentially as previously described (50). The blot was probed with anti-pSTAT1 antibody (BD Biosciences), followed by reprobing with anti-β-actin antibody (Sigma, St. Louis, MO).
Sodium bisulfite treatment and genomic DNA sequencing.
CD4+ and CD8+ T cells were purified as described and used to isolate genomic DNA. The genomic DNA modification was performed as previously described (29). The bisulfite-modified genomic DNA was used as template for PCR amplification of the slc5a8 and gpr109a promoter regions. The primer sequences are listed in Table 1. The amplified DNA fragments were purified and cloned to PCR2.1 vector (Invitrogen). Individual clones were then sequenced.
Statistical analysis.
All statistical analysis was performed using SAS 9.2, and statistical significance was assessed using an α-level of 0.05.
RESULTS
STAT1 hyperactivation is linked to T cell accumulation in colonic tissues in UC.
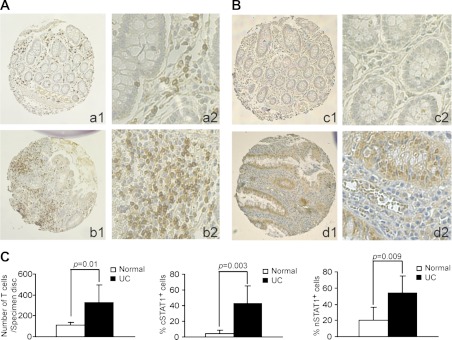
A TMA slide containing normal colon tissues from patients with colorectal cancer and colon tissues from patients with UC was stained for CD3 and STAT1 protein levels. As expected, CD3+ T cells were present in the mucosa (Fig. 1A, a1 and a2). However, five of the six UC colon tissue specimens showed greater T cell infiltration than the normal colon tissues (Fig. 1A, b1 and b2). Statistical analysis determined that the UC colon tissues have a significantly higher mean number of T cells than the normal colon tissue group (Fig. 1C). Less than 10% of normal colonic epithelial cells expressed detectable cytoplasmic STAT1 (cSTAT1), and a slightly higher percentage of them expressed nuclear STAT1 (nSTAT1) (Fig. 1B). Mucosal tissues from patients with UC exhibited higher percentages of both cSTAT1 and nSTAT1 than the normal mucosal tissues (Fig. 1B), and the differences were statistically significant (Fig. 1C). The intensity of nSTAT1 in mucosal epithelial cells from patients with UC is also significantly higher than that of normal mucosal epithelial cells (data not shown). Spearman rank correlation analysis revealed a statistically significant moderate and positive correlation between number of CD3+ T cells and the percentages of both cSTAT1- and nSTAT1-positive colonic epithelial cells (data not shown). These observations thus indicate that activation of the IFN-γ signaling pathway in the colonic mucosa is linked to accumulation of T cells in human patients with UC.
Fig. 1.
Signal transducer and activator of transcription 1 (STAT1) hyperactivation is linked to T cell accumulation in the colonic mucosa of human ulcerative colitis (UC) specimens. Immunohistochemical staining of CD3+ T cells (A), and cytoplasmic (cSTAT1) and nuclear (pSTAT1) protein (B) in normal colon tissues and colonic tissue specimens of human patients with UC. CD3 and STAT1 immunoreactivity is shown as the brown color, whereas cells that are unreactive are indicated by the blue counterstain. Shown are representative images. a and c: Normal colon tissues from patients with colon cancer b and d: colon tissues from human patients with UC. 1: Image of entire tissue microarray (TMA) tissue disc; 2: high magnification of 1. C: quantification of the number of CD3+ T cells and STAT1-positive cells in the colon tissues. The number of CD3+ T cells in each specimen printed on the TMA was counted, and cSTAT1 and nSTAT1 were scored. Left: difference in the number of T cells between normal (n = 7) and UC (n = 6) colon tissues. Middle: difference in the percentage of cSTAT1-positive colonic epithelial cells between normal and UC colon tissues. Right: difference in the percent of nSTAT1-positive colonic epithelial cells between normal and UC colon tissues.
Butyrate inhibits T cells activation.
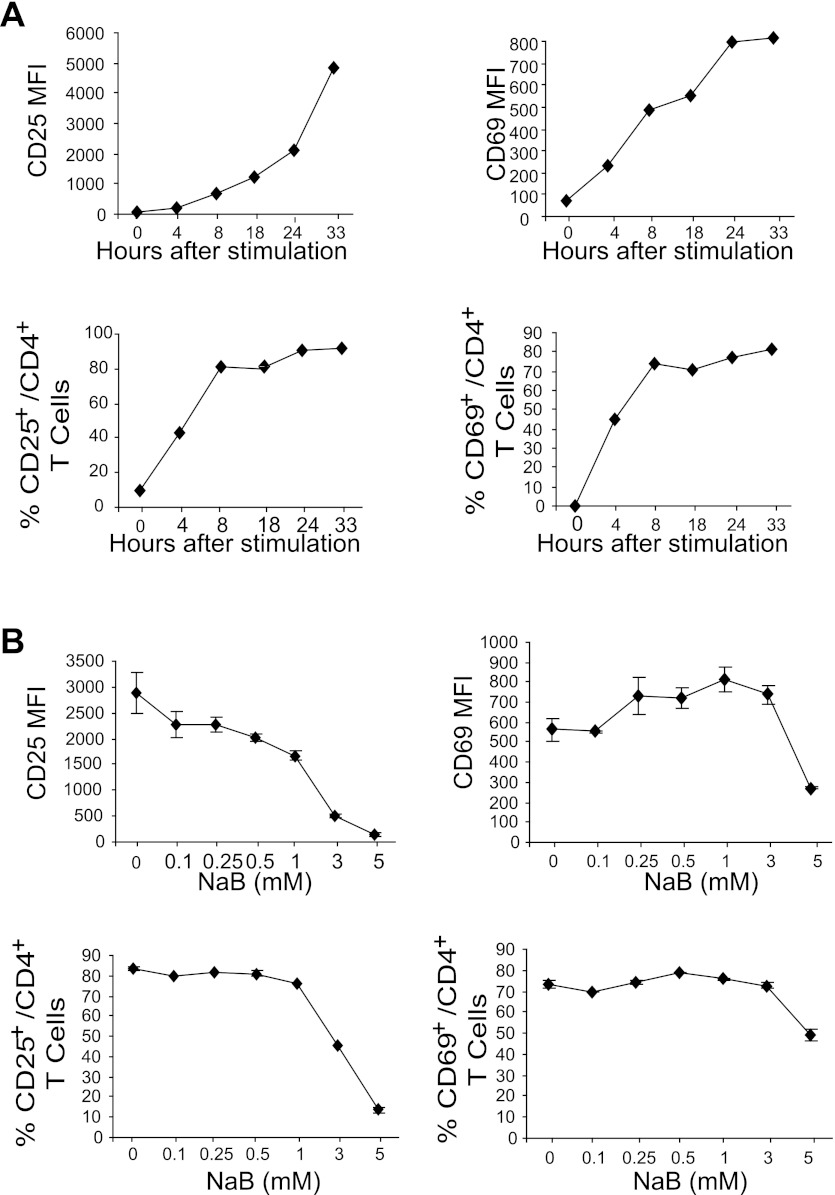
To determine the T cell activation kinetics, total mouse spleen cells were cultured in anti-CD3 and anti-CD28 mAb-coated plates. Cells were then collected overtime and stained for cell surface CD4, CD69, and CD25. CD4+ cells were then gated and analyzed for CD25 and CD69 mean fluorescence intensity and percentage of positive cells. Analysis of CD69+ and CD25+ cells among the CD4+ cell population indicated that T cell activation plateaus at ∼24 h, with more than 70% of T cells being activated (Fig. 2A). Next, spleen cells were stimulated with anti-CD3 and anti-CD28 mAbs in the presence of butyrate for 24 h and examined for CD4+ T cell activation. Butyrate at concentrations up to 3 mM did not inhibit the early activation marker CD69 expression intensity on CD4+ T cells nor the percentage of CD69+ CD4+ T cells (Fig. 2B). However, higher concentrations of butyrate (5 mM) did decrease CD69 level (Fig. 2B). The expression level of the late activation marker CD25 was inhibited by butyrate in a dose-dependent manner (Fig. 2B). Taken together, our data suggest that butyrate partially inhibits CD4+ T cell activation.
Fig. 2.
Butyrate inhibits CD4+ T cell activation. A: T cell activation kinetics. Spleen cells were cultured in anti-CD3/CD28 mAb-coated plates and analyzed by staining with CD4- and CD25-specific mAbs, or CD4- and CD69-specific mAbs, respectively, at the indicated time points. Percentages of CD25- and CD69-positive cells in the CD4+ T cell population and mean fluorescence intensity (MFI) of CD25 and CD69 were quantified. B: inhibition of T cell activation by butyrate. Spleen cells were cultured as described above in the presence of butyrate for 24 h and analyzed for CD25- and CD69-positive cells in the CD4+ T cell population, as well as the MFI of CD25 and CD69 of the CD4+ T cell population.
Butyrate inhibits T cell proliferation.
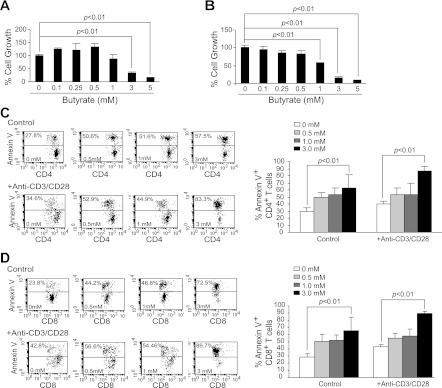
To determine whether the above-observed partial inhibition of T cell activation by butyrate is accompanied by inhibition of T cell proliferation, we purified both CD4+ and CD8+ T cells. The purified T cells were activated with anti-CD3/CD28 mAbs for 3 days in the presence of various concentrations of butyrate and analyzed for proliferation by MTT assay. Low concentrations of butyrate (<1 mM) did not exhibit a significant inhibitory effect on CD4+ T cells, but high concentrations of butyrate (>3 mM) significantly inhibited proliferation of both CD4+ and CD8+ T cells in vitro (Fig. 3, A and B).
Fig. 3.
Butyrate induces T cell apoptosis. Purified CD4+ (A) and CD8+ (B) T cells were cultured in anti-CD3/CD28 mAb-coated plates for 24 h. Butyrate was then added to the culture for another 24 h. Cellular proliferation/survival was analyzed using MTT assays. Cellular proliferation in the absence of butyrate was set as 100%. C: spleen cells were cultured in the absence (control) and presence (+Anti-CD3/CD28) of anti-CD3/CD28 mAbs for 24 h. Various concentrations of butyrate were then added to the cultures for another 24 h. Cells were collected and stained with Annexin V and CD4 mAb and analyzed by flow cytometry for apoptosis. Left: apoptosis profiles of CD4+ T cells are shown. Right: apoptotic cell death of CD4+ T cells was quantified by the formula: % Annexin V cells = % Annexin V-positive cells in the presence of butyrate − % Annexin V-positive cells in the absence of butyrate. Shown are representative results from 1 of 3 experiments. D: CD8+ T cells apoptosis in response to butyrate was analyzed as in C.
Butyrate enhances activation-induced cell death of T cells.
To determine whether the above-observed inhibition of proliferation is due to increased cell death, we activated T cells in the presence of butyrate and analyzed T cell apoptosis. Flow cytometry analysis indicated that butyrate increased apoptosis of resting CD4+ T cells (Fig. 3C) and CD8+ T cells (Fig. 3D) in a dose-dependent manner. Butyrate also significantly increased apoptosis of activated CD4+ T cells (Fig. 3C) and CD8+ T cells (Fig. 3D) in a dose-dependent manner. In summary, our data suggest that butyrate is a potent apoptosis inducer of CD4+ and CD8+ T cells.
Induction of apoptosis by butyrate in T cells is Fas-dependent.
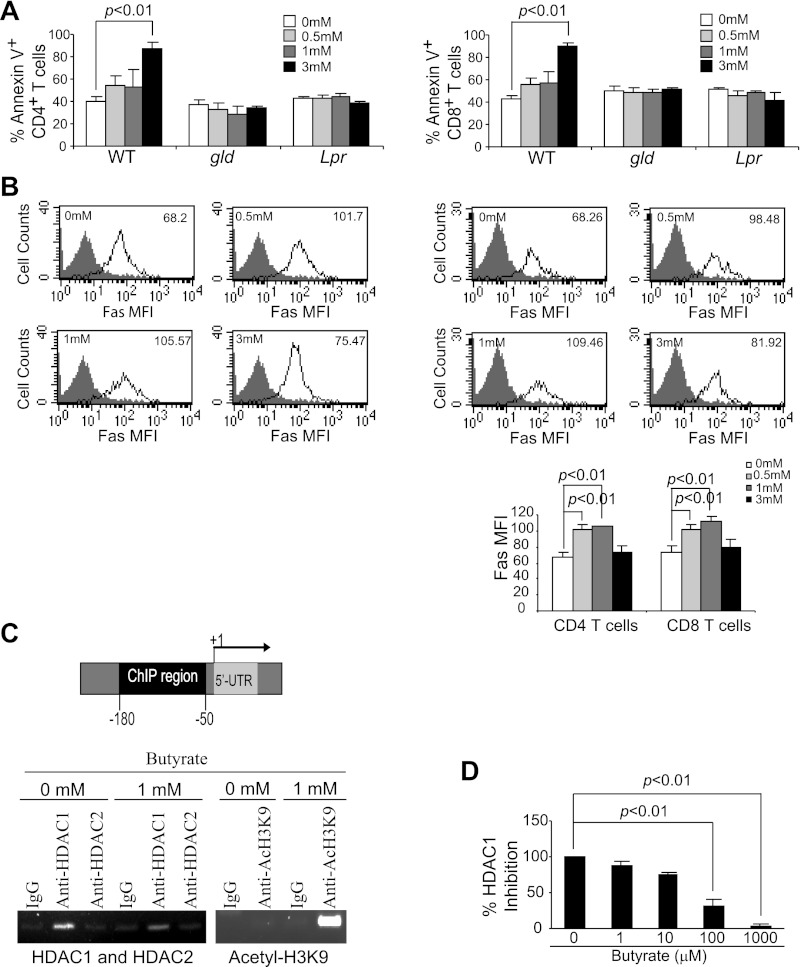
Termination of an immune response after clearance of pathogen-infected or diseased cells is primarily through elimination of effector T cells through Fas-FasL interaction to induce Fas-mediated apoptosis under physiological conditions (25). To determine whether butyrate-induced apoptosis is Fas-dependent, we examined the effects of butyrate on T cells isolated from FasL-deficient (Fasgld) and Fas-deficient (Faslpr) mice. As observed above, butyrate enhanced wild-type (WT) CD4+ and CD8+ T cell apoptosis (Fig. 4A). However, CD4+ and CD8+ T cells derived from Fasgld and Faslpr mice were resistant to butyrate-induced apoptosis (Fig. 4A). Our results thus indicate that butyrate induces apoptosis in T cells through the Fas-mediated apoptosis pathway.
Fig. 4.
Butyrate inhibits Fas promoter-bound histone deacetylase 1 (HDAC1) to induce Fas promoter hyperacetylation and Fas upregulation in T cells. A: induction of T cell apoptosis by butyrate is Fas dependent. Spleen cells from Fasgld and Faslpr were cultured in anti-CD3/CD28 mAb-coated plates with butyrate for 24 h. Cells were then collected and analyzed for apoptosis of CD4+ (A) and CD8+ (B) T cells as in Fig. 2. B: Fas expression level is upregulated by butyrate treatment. Spleen cells were cultured in anti-CD3/CD28-coated plates in the absence or presence of various concentrations of butyrate for 24 h. Cells were then stained with CD4- and Fas-specific mAbs (top, left) or CD8- and Fas-specific mAbs (top, right) and analyzed by flow cytometry. The Fas protein level is represented by the mean fluorescent intensity. The butyrate concentrations are indicated at the upper left corner of each plot, and the Fas MFI is indicated at the upper right corner of each plot. Shown is 1 representative result of 3 independent experiments. Bottom: Fas MFI in CD4+ and CD8+ T cells after butyrate treatment was quantified and presented. Data are means ± SD. C: HDAC1 association with the Fas promoter and butyrate-induced Fas promoter hyperacetylation. Left: mouse Fas promoter structure showing the chromatin immunoprecipitation (ChIP) PCR amplified region. Right: ChIP analysis of HDAC1, HDAC2, and acetyl-H3K9 association with the mouse Fas promoter. The HDAC1-, HDAC2-, and acetyl-H3K9-bound chromatin fragments were immunoprecipitated with the indicated antibodies and analyzed by PCR using Fas promoter sequence-specific primers. D: inhibition of HDAC1 activity by butyrate. Recombinant human HDAC1 was assayed for its activity using a commercially available kit in the absence or presence of butyrate. WT, wild-type.
Butyrate inhibits HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation.
To determine the effects of butyrate on Fas expression in T cells, spleen cells were stimulated with anti-CD3/CD28 mAbs in the presence of butyrate. Fas protein levels on CD4+ and CD8+ T cell surfaces were analyzed by flow cytometry. Low doses of butyrate significantly increased Fas protein levels in both CD4+ and CD8+ T cells. High-dose (3 mM) butyrate, however, exhibited minimal effects on Fas protein level (Fig. 4B), possibly attributable to protein degradation in the dying cells (Fig. 3).
To determine whether Fas expression is regulated by histone acetylation, we examined the association of HDACs with the Fas promoter in T cells. Mouse T cells were stimulated with anti-CD3/CD28 antibody-conjugated magnetic beads and subjected to ChIP using HDAC1- and HDAC2-specific antibodies. PCR amplification of the immunoprecipitated genomic DNA revealed that HDAC1 but not HDAC2 is physically associated with the Fas promoter region in the activated T cells in the absence and presence of butyrate (Fig. 4C). In vitro assays with purified HDAC1 indicated that butyrate inhibits HDAC1 activity (Fig. 4D). Next, we sought to determine whether butyrate-mediated inhibition of HDAC1 leads to an increase in Fas promoter histone acetylation. ChIP assay using an acetyl-H3-Lys9-specific antibody revealed that butyrate induces hyperacetylation of H3 in the Fas promoter region (Fig. 4C). Taken together, our data suggest that butyrate inhibits the Fas promoter-bound HDAC1 activity to hyperacetylate the Fas promoter, consequently resulting in the upregulation of Fas expression in T cells.
Butyrate transporter and receptor expression and function.
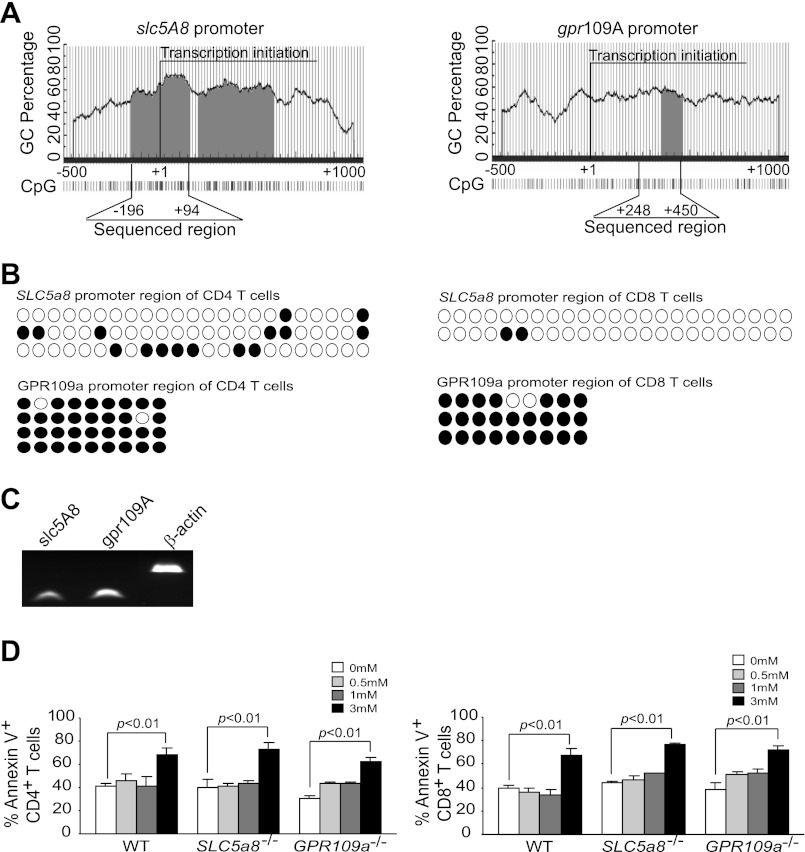
Butyrate can enter mammalian cells through the Na+-coupled transporter, slc5a8, via a Na+-dependent electrogenic process (31). However, the expression of slc5a8 is often silenced in colon cancer cells (26). Butyrate can also elicit its biological effects on colonic epithelial cells without transportation into cells, via the G protein-coupled butyrate receptor, gpr109a (44). To determine whether butyrate exerts its apoptosis-inducing function through the transporter, slc5a8, or the receptor, gpr109a, we examined the expression and functions of slc5a8 and gpr109a in T cells. The −3,000 to +1,000 region of the mouse slc5a8 promoter region contains two typical CpG islands (Fig. 5A), whereas, that same region of the gpr109a promoter contains a nontypical (>60% GC content) CpG island (Fig. 5A). DNA sequence analysis of the bisulfite-modified genomic DNA isolated from mouse CD4+ and CD8+ T cells revealed that the slc5a8 promoter region is only sporadically methylated in CD4+ and CD8+ T cells, whereas the gpr109a promoter region is heavily methylated in both CD4+ and CD8+ T cells (Fig. 5B). However, despite the fact that the slc5a8 promoter is partially methylated and the gpr109a promoter is heavily methylated, RT-PCR analysis indicated that both slc5a8 and gpr109a are expressed in T cells, albeit at low level (Fig. 5C).
Fig. 5.
Expression of the butyrate transporter and receptor in T cells and its relevance to butyrate-induced cell death. A: slc5a8 and gpr109a promoter structure. The 5′ regulatory region (−500 to +1,000 relative to the transcription initiation site) of the slc5a8 (left) and gpr109a (right) were analyzed with the program, Methprimer, for CpG island identification. The bisulfite sequencing PCR primer regions are indicated. B: methylation status of the slc5a8 (top) and gpr109a (bottom) promoter regions. ●: methylated CpGs; ○: unmethylated CpGs. C: slc5a8 and gpr109a expression in mouse T cells. CD4+ T cells were purified from WT mouse spleen and analyzed for slc5a8 and gpr109a expression levels by RT-PCR. D: sensitivity of T cells from slc5a8−/− and gpr109a−/− mice to butyrate. Spleen cells were cultured in anti-CD3/CD28-coated plates overnight, followed by incubation with butyrate at the indicated concentrations for 24 h. Cells were then stained with CD4-specific mAb and Annexin V (left), or CD8-specific mAb and Annexin V (right), respectively, and analyzed by flow cytometry as above. Data are means ± SD.
To determine whether slc5a8 or gpr109a mediates butyrate function in T cells, we stimulated T cells derived from WT, slc5a8-null (slc5a8−/−), and gpr109a-null (gpr109a−/−) mice with anti-CD3/CD28 mAbs in the absence and presence of butyrate and analyzed T cell apoptosis. We observed that CD4+ and CD8+ T cells isolated from slc5a8−/− and gpr109a−/− mice were still sensitive to butyrate-induced apoptosis (Fig. 5D), suggesting that GPR109A and SLC5A8 might compensate for each other's functions in T cells.
GPR109A and SLC5A8 mediate expression of inflammation-related genes in colonic tissue.
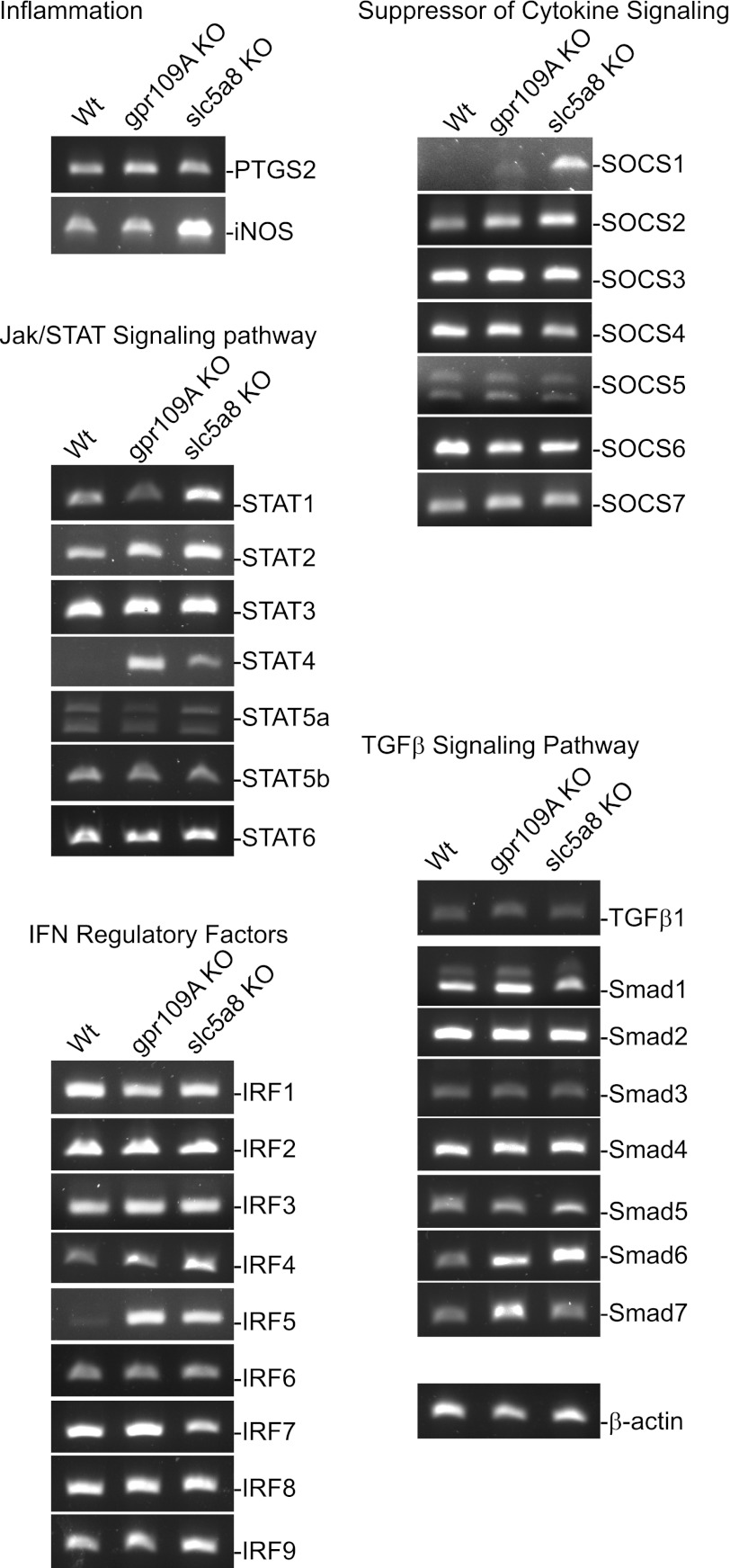
The above observations suggest that SLC5A8 and GPR109A might compensate for butyrate function in T cells. Because butyrate functions to inhibit colonic inflammation, we reasoned that deletion of slc5a8 or gpr109a alters the expression of inflammation-related genes in the colonic mucosa. Therefore, we collected colon mucosal tissues from conventional WT, slc5a8−/−, and gpr109a−/− mice and analyzed the expression levels of sets of genes with known functions in colonic inflammation. RT-PCR analysis revealed that iNOS, a marker of inflammation, is dramatically upregulated in colonic mucosa from slc5a8−/− mice. The expression levels of several genes of the IFN-γ and TGF-β signaling pathways, namely STAT1, STAT2, IFN regulatory factor (IRF)4, IRF5, suppressor of cytokine signaling (SOCS)1, and SMAD6, are upregulated in the colonic mucosa from slc5a8−/− mice (Fig. 6). STAT4, IRF4, IRF5, SMAD6, and SMAD7 are upregulated in the colonic mucosa from gpr109a−/− mice (Fig. 6). Taken together, these observations suggest that slc5a8 and gpr109a mediate expression of distinct genes that are involved in inflammatory response in colonic epithelial cells in vivo.
Fig. 6.
slc5a8- and gpr109a-mediated expression of genes in the cytokine and TGF-β signaling pathways in mouse colon tissues in vivo. Total RNA was isolated from fresh colons of WT, gpr109a−/−, and slc5a8−/− mice and used for RT-PCR analysis of the indicated genes. KO, knockout; iNOS, inducible nitric oxide synthase; SOCS, suppressor of cytokine signaling.
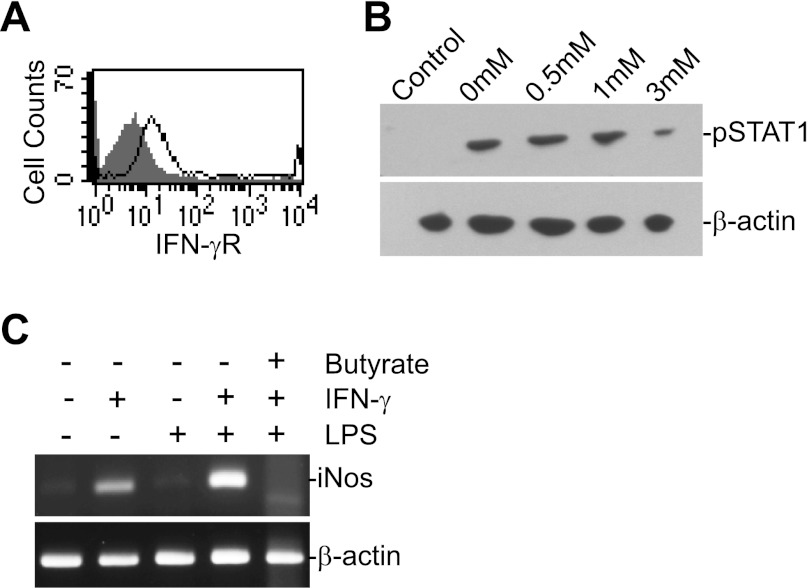
Butyrate inhibits IFN-γ signaling but not proliferation in human colonic epithelial cells.
To functionally determine whether butyrate mediates inflammatory response in colonic epithelial cells, we examined the effects of butyrate on proliferation, Fas expression, and IFN-γ signaling in the normal human colonic epithelial cell line CCD-841 in vitro. Flow cytometry analysis of cell surface Fas protein level indicated that exposure to butyrate resulted in no significant alteration in Fas receptor level. MTT assay revealed that, in contrast to what was observed in T cells, butyrate did not inhibit CCD-841 cell proliferation at a concentration as high as 10 mM (data not shown). IFN-γR is expressed on CCD841 cells (Fig. 7A). IFN-γ treatment in vitro induced STAT1 phosphorylation (pSTAT1), and butyrate inhibited this IFN-γ-induced STAT1 activation (Fig. 7B). Because iNOS is not expressed in CCD-841 cells and IFN-γ plus LPS did not induce iNOS expression in CCD-841 cells, we used colon carcinoma cell line T84 to determine the effects of butyrate on iNOS induction. IFN-γ and LPS induced a high level of iNOS expression, and butyrate effectively inhibited iNOS induction (Fig. 7C). Taken together, our data suggest that butyrate suppresses colonic inflammation at least in part through inhibiting iNOS upregulation in human colonic epithelial cells.
Fig. 7.
Butyrate inhibits IFN-γ-induced STAT1 activation and iNOS induction in human colonic epithelial cells in vitro. A: IFN-γR level on CCD-841 cells. CCD-841 cells were stained with IFN-γR-specific mAb and analyzed by flow cytometry. Shaded area: IgG isotype control, solid line: IFN-γR-specific staining. B: butyrate inhibits IFN-γ signaling. CCD-841 cells were cultured in the presence of butyrate at the indicated concentrations for 2 h. IFN-γ (100 U/ml) was then added and cultured for 24 h. The cells were analyzed by Western blotting with pSTAT1- and β-actin-specific mAbs, sequentially. C: colon carcinoma cell line T84 cells were cultured in the absence or presence of IFN-γ (100 U/ml) and LPS (10 μg/ml) or IFN-γ, LPS and butyrate (3 mM) for ∼20 h, and analyzed for iNOS transcript level by RT-PCR.
DISCUSSION
Multiple recent Meta analysis and genome-wide association studies of large cohorts of human patients have linked IFNG, the gene encoding for the proinflammatory cytokine IFN-γ, to human UC susceptibility (1, 30, 39). These findings firmly established a role of IFN-γ in the promotion of UC in humans. These data are consistent with several previous observations that increased mucosal expression of the IFN-γ gene, elevated secretion of IFN-γ protein, and increased levels of STAT1 expression and activation are associated with UC pathogenesis in human patients with UC (16, 37, 46). It has also been shown that treatment of mice with TNBS-induced colitis with IFN-γ neutralizing antibody can at least partially achieve reversal of colitis-associated weight loss (14). However, other studies indicated that IFN-γ signaling is not activated in colonic tissues of patients with UC (32). Furthermore, IFN-γ signaling might play a suppressive role against colonic inflammation and UC in an experimental colitis mouse model (32, 38). These contrasting observations might be due to the different experimental systems used in these studies. It is well-known that the host gut microbiota and immune cells are critical players in UC pathogenesis. The different composition of host gut microbiota may lead to different immune responses in different experimental system or human patients, thereby resulting in different immune cell and cytokine profiles in the gut. In addition, it is known that acute IFN-γ signaling facilitates the clearance of infection and pathogens (13), whereas chronic IFN-γ signaling mediates chronic inflammation and inflammation-associated diseases, including UC and colorectal cancer (18, 37). Therefore, it is very likely that acute IFN-γ production may suppress UC (38), whereas it is the sustained/chronic T cell activation that causes sustained IFN-γ signaling, leading to IFN-γ-dependent and inflammation-mediated diseases.
IFN-γ is produced primarily by activated T cells and NK cells. Therefore, the increase in IFN-γ/pSTAT1 is most likely due to the increase in infiltrating T cells in the colonic mucosa (20). Our results indicate that colonic mucosa from patients with UC harbor significantly more T cells than the normal colon tissues. This observation is consistent with human clinical data and animal-based studies that show a critical role of T cells in UC pathogenesis (6–7, 35, 49). Therefore, it is very likely that one of the mechanisms underlying UC pathogenesis is persistence and accumulation of activated T cells in the colonic mucosa that leads to overproduction of IFN-γ. Overproduction of IFN-γ leads to sustained IFN-γ signaling and STAT1 hyperactivation. IFN-γ-induced STAT1 activation is usually transient under normal physiological conditions. In contrast, overexpressed IFN-γ-induced STAT1 hyperactivation may result in altered expression patterns of IFN-γ-regulated genes such as iNOS that promotes chronic inflammation in the colonic mucosa to promote UC and colorectal cancer (18).
Butyrate is primarily derived from dietary fiber by anaerobic bacterial fermentation in the colon and exhibits potent anti-inflammatory activity (11, 48). In this study, we carried out a proof-of-concept study with mouse T cells and observed that butyrate enhances T cell apoptosis. T cells are typically activated in response to normal stimuli followed by elimination after clearance of the antigen. This is crucial for avoiding autoimmunity and uncontrolled immune responses. Because of the persistent exposure to a diverse number of antigens encountered in the gastrointestinal tract, gut lamina propria T lymphocyte (T-LPL) numbers are controlled by induction of Fas-mediated apoptosis (12). T-LPL often express high levels of Fas (12, 19); however, in cases of inflammatory bowel disease, Fas expression levels in T-LPL as well as T-LPL sensitivity to Fas-mediated apoptosis are often decreased (3, 5, 41, 42). In the functional level, it was reported that mice with Fas deficiency in the colon tissues are hypersensitive to DSS-induced colitis (34), whereas mice lacking FasL exhibited more severe and persistent colitis than control WT mice (36). Furthermore, treatment with anti-IL-12 mAb resulted in increased Fas-mediated apoptosis of T cells and reversal of colitis (14). These studies thus indicate that the Fas-mediated apoptosis pathway in both colonic epithelial cells and T cells plays a critical role in prevention of colitis in mouse models in vivo. Therefore, one mechanism underlying colitis pathogenesis might be increased resistance to Fas-mediated apoptosis in both colonic epithelial cells and T cells. Thus butyrate may function to inhibit colonic inflammation through increasing T cell sensitivity to apoptosis to eliminate the source of inflammation. Butyrate functioning as an apoptosis promoter has been reported in tumor cells. Several studies have shown that butyrate either directly induces solid tumor cell apoptosis through its transporter slc5a8 and receptor gpr109a (44–45) or through sensitizing the tumor cells to Fas-mediated apoptosis (8, 33). Our results thus extend the function of butyrate in apoptosis induction from tumor cells to T cells.
Butyrate elicits its apoptosis-inducing activity through its transporter SLC5A8 and receptor GPR109A in colon carcinoma cells (9, 31, 44–45). However, whereas knocking down either slc5a8 or gpr109a leads to altered expression of several inflammation-related genes in the colonic tissues, neither SLC5A8 nor GPR109A is essential for butyrate-mediated apoptosis in T cells, suggesting that the underlying molecular mechanisms of butyrate function in T cells might be different from that in colonic epithelial cells, and that SLC5A8 and GPR109A might compensate for each other in T cells. However, it should be noted that, in addition to GPR109A and SLC5A8, butyrate also has other transporters and receptors, including monocarboxylate transporter 1, GPR41, and GPR43 (4, 17, 28). Nevertheless, SLC5A8 and GPR109A mediate expression of distinct genes, including iNOS, in the colonic epithelial cells in vivo. These genes are known to play key roles in inflammation in vivo (Fig. 6).
We observed that butyrate directly inhibits HDAC1 enzymatic activity. We also demonstrated that HDAC1 is bound to the Fas promoter in T cells. Therefore, it is reasonable to assume that hyperacetylation of the Fas promoter is a consequence of butyrate-mediated inhibition of HDAC1 activity at the Fas promoter region. Hyperacetylation leads to Fas receptor upregulation and consequently increased sensitivity of T cells to activation-induced cell death. Our results thus identify HDAC1 as a molecular target of butyrate and demonstrate that butyrate regulates the death receptor Fas expression to regulate T cell apoptosis.
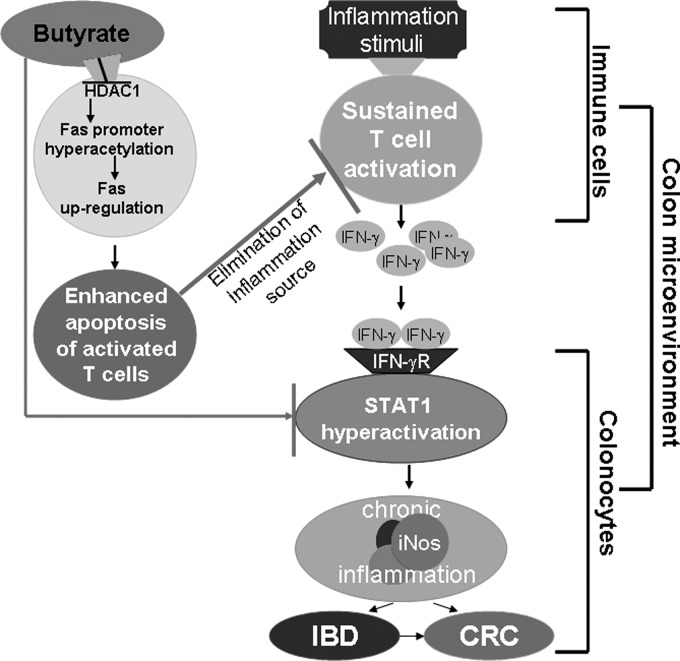
Although the end effects of butyrate are readily observable, questions still remain, however, on how exactly butyrate is utilized by the cell, the role of the SLC5a8 transporter and GPR109a receptor, and the manner in which butyrate interacts with HDAC1. Our data suggest that butyrate has beneficial effects on two fronts: eliminating the source of inflammation by increasing Fas-mediated apoptosis of T cells and reducing the expression of proinflammatory transcription factor, STAT1, in colonic epithelial cells. Based on these findings, we propose a model to illustrate the preventive and therapeutic roles of butyrate in UC. We propose that butyrate delivers a double-hit to suppress colonic inflammation: first, butyrate inhibits STAT1 hyperactivation in colonic epithelial cells to inhibit IFN-γ-mediated chronic inflammation; second, butyrate inhibits Fas promoter-bound HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation, resulting in enhanced apoptosis of T cells and thus decreased accumulation of T cells in the inflamed colonic mucosa (Fig. 8). Therefore, targeting the butyrate-HDAC1-Fas axis may offer an effective novel approach to eliminate the overreactive T cells in the treatment of inflammatory disease and inflammation-mediated colorectal cancer (35).
Fig. 8.
Model of butyrate function. Butyrate delivers a double-hit to suppress colonic inflammation: first, butyrate inhibits STAT1 hyperactivation in colonic epithelial cells to inhibit IFN-γ-mediated chronic inflammation; second, butyrate inhibits Fas promoter-bound HDAC1 activity to induce Fas promoter hyperacetylation and Fas upregulation, resulting in enhanced apoptosis of T cells, which leads to decreased accumulation of T cells in the inflamed colonic mucosa and consequently elimination of the source of inflammation. IBD, inflammatory bowel disease; CRC, colorectal cancer.
GRANTS
This work was supported by the National Institutes of Health (CA133085 to K. Liu), the American Cancer Society (RSG-09–209-01-TBG to K. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.A.Z. and M.T. performed experiments; M.A.Z., N.S., M.T., J.L.W., and K.L. analyzed data; M.A.Z., N.S., P.M.M., M.T., V.G., and K.L. interpreted results of experiments; M.A.Z. and K.L. prepared figures; M.A.Z. and K.L. drafted manuscript; M.A.Z., N.S., P.M.M., M.T., V.G., J.L.W., H.S., K.R., D.M., and K.L. approved final version of manuscript; N.S., P.M.M., V.G., H.S., K.R., D.M., and K.L. conception and design of research.
ACKNOWLEDGMENTS
We thank Ms. Kimberly Smith in the Georgia Pathology Services for the excellent technical assistance in the immunohistochemical staining of tissues. We also thank Dr. Jeanene Pihkala for assistance in flow cytometry analysis.
REFERENCES
- 1. Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis 17: 396–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boirivant M, Pica R, DeMaria R, Testi R, Pallone F, Strober W. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J Clin Invest 98: 2616–2622, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, Qiao L. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol 166: 6399–6403, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Chinen T, Kobayashi T, Ogata H, Takaesu G, Takaki H, Hashimoto M, Yagita H, Nawata H, Yoshimura A. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology 130: 373–388, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol 8: 458–466, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Chopin V, Toillon RA, Jouy N, Le Bourhis X. Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol 135: 79–86, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 557: 719–731, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daroqui MC, Augenlicht LH. Transcriptional attenuation in colon carcinoma cells in response to butyrate. Cancer Prev Res (Phila) 3: 1292–1302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 133: 2485S–2493S, 2003 [DOI] [PubMed] [Google Scholar]
- 12. De Maria R, Boirivant M, Cifone MG, Roncaioli P, Hahne M, Tschopp J, Pallone F, Santoni A, Testi R. Functional expression of Fas and Fas ligand on human gut lamina propria T lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J Clin Invest 97: 316–322, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fuss IJ, Marth T, Neurath MF, Pearlstein GR, Jain A, Strober W. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology 117: 1078–1088, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell 140: 859–870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonsky R, Deem RL, Landers CJ, Derkowski CA, Berel D, McGovern DP, Targan SR. Distinct IFNG methylation in a subset of ulcerative colitis patients based on reactivity to microbial antigens. Inflamm Bowel Dis 17: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, Kato S, Yoshimura A. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med 203: 1391–1397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hongo T, Morimoto Y, Iwagaki H, Kobashi K, Yoshii M, Urushihara N, Hizuta A, Tanaka N. Functional expression of Fas and Fas ligand on human colonic intraepithelial T lymphocytes. J Int Med Res 28: 132–142, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kappeler A, Mueller C. The role of activated cytotoxic T cells in inflammatory bowel disease. Histol Histopathol 15: 167–172, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr 137: 2576S–2579S, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res (Phila) 2: 200–208, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res 1: 855–862, 2003 [PubMed] [Google Scholar]
- 24. Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, Fan YY, Chapkin RS. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2+) loading and apoptosis in colonocytes. Cancer 117: 5294–5303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krammer PH. CD95's deadly mission in the immune system. Nature 407: 789–795, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA 100: 8412–8417, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology 141: 653–662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGough JM, Yang D, Huang S, Georgi D, Hewitt SM, Rocken C, Tanzer M, Ebert MP, Liu K. DNA methylation represses IFN-gamma-induced and signal transducer and activator of transcription 1-mediated IFN regulatory factor 8 activation in colon carcinoma cells. Mol Cancer Res 6: 1841–1851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagace C, Li C, Green T, Stevens CR, Beauchamp C, Fleshner PR, Carlson M, D'Amato M, Halfvarson J, Hibberd ML, Lordal M, Padyukov L, Andriulli A, Colombo E, Latiano A, Palmieri O, Bernard EJ, Deslandres C, Hommes DW, de Jong DJ, Stokkers PC, Weersma RK, Sharma Y, Silverberg MS, Cho JH, Wu J, Roeder K, Brant SR, Schumm LP, Duerr RH, Dubinsky MC, Glazer NL, Haritunians T, Ippoliti A, Melmed GY, Siscovick DS, Vasiliauskas EA, Targan SR, Annese V, Wijmenga C, Pettersson S, Rotter JI, Xavier RJ, Daly MJ, Rioux JD, Seielstad M. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 42: 332–337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor downregulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J Biol Chem 279: 13293–13296, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol 100: 64–72, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ogawa K, Yasumura S, Atarashi Y, Minemura M, Miyazaki T, Iwamoto M, Higuchi K, Watanabe A. Sodium butyrate enhances Fas-mediated apoptosis of human hepatoma cells. J Hepatol 40: 278–284, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Park SM, Chen L, Zhang M, Ashton-Rickardt P, Turner JR, Peter ME. CD95 is cytoprotective for intestinal epithelial cells in colitis. Inflamm Bowel Dis 16: 1063–1070, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology 140: 1838–1846, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sayani FA, Keenan CM, Van Sickle MD, Amundson KR, Parr EJ, Mathison RD, MacNaughton WK, Braun JE, Sharkey KA. The expression and role of Fas ligand in intestinal inflammation. Neurogastroenterol Motil 16: 61–74, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kuhbacher T, Hamling J, Folsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 51: 379–385, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheikh SZ, Matsuoka K, Kobayashi T, Li F, Rubinas T, Plevy SE. Cutting edge: IFN-gamma is a negative regulator of IL-23 in murine macrophages and experimental colitis. J Immunol 184: 4069–4073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W, Barmada MM, Klei L, Daly MJ, Abraham C, Bayless TM, Bossa F, Griffiths AM, Ippoliti AF, Lahaie RG, Latiano A, Pare P, Proctor DD, Regueiro MD, Steinhart AH, Targan SR, Schumm LP, Kistner EO, Lee AT, Gregersen PK, Rotter JI, Brant SR, Taylor KD, Roeder K, Duerr RH. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet 41: 216–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stempelj M, Kedinger M, Augenlicht L, Klampfer L. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J Biol Chem 282: 9797–9804, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Moller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 113: 160–167, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki A, Sugimura K, Ohtsuka K, Hasegawa K, Suzuki K, Ishizuka K, Mochizuki T, Honma T, Narisawa R, Asakura H. Fas/Fas ligand expression and characteristics of primed CD45RO+ T cells in the inflamed mucosa of ulcerative colitis. Scand J Gastroenterol 35: 1278–1283, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Tammariello AE, Milner JA. Mouse models for unraveling the importance of diet in colon cancer prevention. J Nutr Biochem 21: 77–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69: 2826–2832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, Prasad PD, Sterneck E, Ganapathy V. SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res 66: 11560–11564, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol 97: 2820–2828, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One 4: e6026, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40: 235–243, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Zimmerman M, Yang D, Hu X, Liu F, Singh N, Browning D, Ganapathy V, Chandler V, Choubey D, Abrams SI, Liu K. IFN-γ Upregulates Survivin and Ifi202 Expression to Induce Survival and Proliferation of Tumor-Specific T Cells. PLoS One 5: e14076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]