Abstract
Cystometric studies of bladder function in anesthetized neonatal rats have suggested specific changes in urodynamic parameters that coincide with the development of a mature bladder-to-bladder micturition reflex. Here, we used a conscious cystometry model that avoids the potentially confounding effects of anesthesia to characterize voiding patterns and urodynamic parameters during early postnatal development in healthy rat pups. Cystometry was performed on postnatal day (P)0, 3, 7, 14, and 21 rats with continuous intravesical instillation of NaCl via a bladder catheter. Micturition cycles were analyzed with respect to voiding pattern, nonvoiding contractions, infused volume, and basal, filling, threshold, and micturition pressures. Reproducible micturition patterns were obtained from all age groups. The time from stimulation to contraction was significantly longer (P ≤ 0.001) in ≤1-wk-old rats (∼10 s) than that in older rats (∼3 s). An interrupted voiding pattern was observed in ≤10-day-old subgroups. Micturition pressure progressively increased with age (from 21.77 ± 1.92 cmH2O at P0 to 35.47 ± 1.28 cmH2O at P21, P ≤ 0.001), as did bladder capacity. Nonvoiding contractions were prominent in the P3 age group (amplitude: 4.6 ± 1.3 cmH2O, frequency: ∼4.0 events/100 s). At P7, the pattern of spontaneous contractions became altered, acquiring a volume-related character that persisted in a less prominent manner through P21. Bladder compliance increased with age, i.e., maturation. In conclusion, conscious cystometry in rat pups resulted in reproducible micturition cycles that yielded consistent data. Our results revealed immature voiding and prolonged micturition contractions during the first 10 neonatal days and provide evidence for age-related changes in urodynamic parameters.
Keywords: postnatal development, cystometry
micturition is under the control of a highly complex system that integrates neural and smooth muscle components to produce coordinated and effective bladder performance (13). During development, an immature reflex precedes voluntary bladder control. The “rationale” for the switch in regulation is not exactly known, but continuing development of the central and peripheral neural pathways involved in the regulation of micturition is believed to underlie reorganization of the reflex (9, 19, 23, 28). Maturation occurs in an ordered fashion such that children first achieve awareness of bladder fullness, then daytime urine control, and finally nighttime urine control. The time period of postnatal “tuning” varies among species, requiring 2–5 yr in humans and generally 2–3 wk in rats. By the end of this period, the sacral/perigenital bladder reflex that prevails during ontogeny is downregulated, and a mature supraspinal micturition reflex is established/activated, allowing voluntary/spontaneous voiding (10, 21, 29). The exact determinants of the continued development are not known; however, increasing contribution of various neurotransmitters, changes in the sensitivity of urinary bladder smooth muscle contractility to stimulation, and functional maturation of synaptic transmission and higher brain centers have been shown to play a role (1, 8, 22, 27, 37). In addition to changes in bladder function, modulation of myogenic activity and progressive changes in urodynamic parameters have been described (23, 24, 33, 34). To date, the maturation of neural regulation of voiding in general and the concept of neonatal bladder function in particular have only been investigated in vitro (using whole bladder preparations or urinary bladder smooth muscle strips) or in anesthetized animals. To protect the integrity of the engaged neural circuits while avoiding the compromising effects of general anesthetics on smooth muscle contractility and/or neural transmission, we developed a model for studying bladder function in conscious, healthy rat pups using experimental cystometry. The aim of this study was to record and characterize voiding pattern and urodynamics in vivo at various stages of postnatal development.
MATERIALS AND METHODS
Animals.
All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Vermont. Animal care complied with Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health guidelines and was under the supervision of the University of Vermont's Office of Animal Care. All efforts were made to minimize animal discomfort and the number of animals used. Timed pregnant Wistar rats (Charles River Laboratories) were obtained 1 wk before parturition. After parturition, pups remained with their mother until the day of the experiment. Experiments were performed during the day portion of the animals' circadian cycle. Postnatal day (P)0, 3, 7, 14, and 21 male and female animals (n = 38) were used in this study.
Surgical procedures and cystometry.
After rat pups had been anesthetized with 3% isoflurane (Webster Veterinary Supply,), an upper midline abdominal incision (anteriorly from the umbilicus) was made to expose the urinary bladder and keep the stimulation area injury free. A flared-end polyethylene catheter (PE-10 BD, inner diameter: 0.28 mm and outer diameter: 0.61 mm, Intramedic, Clay Adams) was inserted and sutured (Prolene 7-0, Ethicon) into the tip of the bladder dome. The catheter was then routed subcutaneously and exteriorized at the back of the animal's neck. Both incisions (abdomen and neck area) were closed. After a 20-min recovery period, the unrestrained, unanesthetized rat was placed on a heated pad (∼33°C) in a Small Animal Cystometry Lab Station (Catamount), and the free end of the bladder catheter was connected to a remotely controlled syringe pump. Saline (22–25°C) was infused into the bladder continuously at a rate of 0.75 ml/h. In pups with no spontaneous voiding (≤2 wk old), each micturition was induced mechanically by gently stimulating the perigenital skin area with a cotton swab (35). Cystometrograms were recorded, and parameters were measured and analyzed simultaneously. Information about voided volumes in younger groups was not collected; “bladder capacity” refers to the infused volume reached at the time of a pup's unrest and initiation of stimulation. Bladder compliance was calculated from the slope between baseline pressure (the maximum compliance pressure at the start of the micturition cycle and infusion volume) and threshold pressure (bladder pressure and infused volume at which the voiding contractions commenced) in three micturition cycles (30).
Statistics and analysis.
Urodynamic parameters and voiding patterns were analyzed. Within-group comparisons were analyzed using paired Student's t-tests, and comparisons between different rat age groups were analyzed using unpaired t-tests (OriginLab software). Bladder capacity, basal, filling, threshold and micturition pressure, and frequency and amplitude of nonvoiding spontaneous changes in intravesical pressure from multiple consecutive micturition cycles (≥3 cycles) were averaged to provide a representative measure of bladder function. Nonvoiding contractions, defined as increases in bladder pressure >1 cmH2O (4) during the filling phase, were analyzed using Mini Analysis software (Synaptosoft). The frequency of nonvoiding contractions was measured during 100-s intervals, starting at the midpoint of the filling phase of two consecutive micturition cycles. Values are means ± SE.
RESULTS
Conscious cystometry was successful in all age groups. Voiding patterns were reproducible, with steady micturition cycles exhibiting evident filling and voiding phases. Perineal cutaneous stimulation initiated after the bladder had reached its estimated capacity, resulted in potent bladder contraction followed by micturition; although not quantified, the stimulus duration required to trigger micturition appeared to be longer in younger groups. Some young pups with full bladders exhibited behaviors (e.g., atypical movement or surveying surroundings) suggesting a degree of unrest or agitation. Developmental status was characterized according to 1) the presence/absence of intermediate voiding pattern, 2) changed bladder capacity, and 3) progressively increased micturition pressure.
Urodynamic parameters.
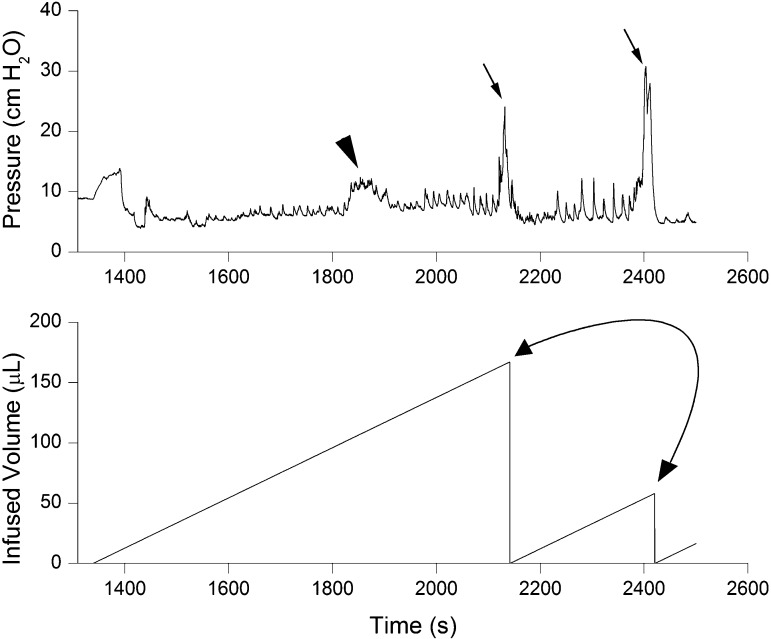
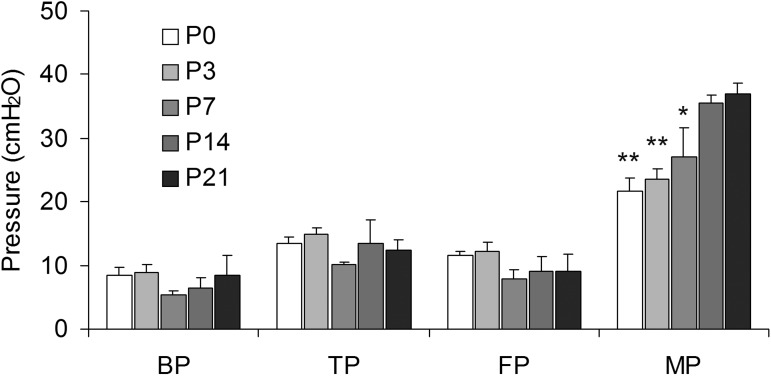
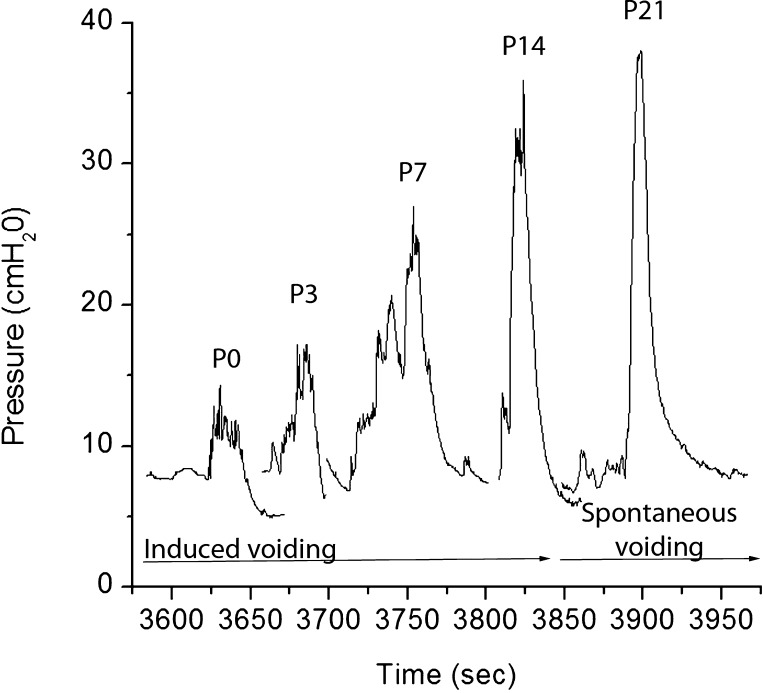
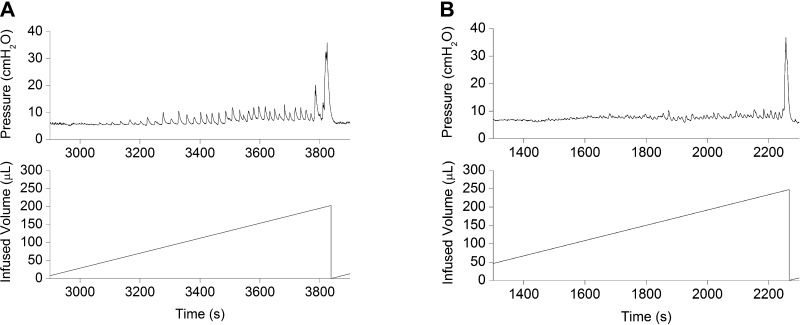
The bladder volume at which voiding was successfully triggered was reproducible, with no significant within-group variations. Younger animals voided frequently due to their small bladder capacities. An innocuous mechanical trigger at volumes ≤50% of the cystometric bladder capacity was unable to elicit effective detrusor contraction (i.e., micturition). During such attempts, intravesical pressure transiently increased by ∼7 ± 4 cmH2O (n = 3) and then returned to filling pressure values (Fig. 1). An interrupted voiding pattern characterized by two consecutive voidings within a short period of time was observed in some ≤10-day-old animals (Fig. 1); the amplitude of the second contraction was ∼20% greater than that of the first contraction. A comparison of urodynamic parameters showed a significant age-related increase in the amplitude of micturition contractions: P0, 21.77 ± 1.92 cmH2O (n = 6); P3, 23.51 ± 1.8 cmH2O (n = 8); P7, 27.04 ± 4.51 cmH2O (n = 8); P14, 35.47 ± 1.28 cmH2O (n = 8); and P21, 36.88 ± 1.82 cmH2O (n = 8). There was also a trend toward higher peak micturition pressure in male pups at P0, P3, P7, and P14 compared with female pups, although these differences did not reach statistical significance (P0, P = 0.052; P3, P = 0.06; P7, P = 0.059; and P14, P = 0.056). No differences in basal, filling, or threshold pressures between pups and adult rats were observed (Fig. 2). Micturition-contraction profiles changed during development. The total contraction duration at the micturition peak was longer in younger groups than in more mature groups: ≤1 wk, 10 ± 2.1 s (P ≤ 0.001); 1–2 wk, 6 ± 1.8 s (P ≤ 0.001); and 2–3 wk, 2 ± 0.91 s (n = 8 for each group; Fig. 3).
Fig. 1.
Representative cystometrogram illustrating unsuccessful stimulation at low bladder volume (arrowhead) and the pattern of interrupted voiding (arrows) characteristic of young rat pups. Note the continuing increases in the amplitude of myogenic contractions throughout the filling phase (volume-related pattern) uninterrupted by incomplete bladder emptying. The tracing represents one micturition cycle measured in a rat pup at postnatal day (P)8. Top: intravesical bladder pressure; bottom: infused volume.
Fig. 2.
Urodynamic parameters. Changes in basal (BP), filling (FP), threshold (TP), and micturition pressure (MP) during early postnatal development in rat pups are shown. The magnitude of the micturition contraction in younger groups was significantly lower compared with older groups. **P ≤ 0.001, P0 and P3 vs. P21; *P ≤ 0.05, P7 vs. P21.
Fig. 3.
Age-related changes in the micturition-contraction profile and peak micturition pressure. Each recording was obtained from a different animal.
Voiding patterns and nonvoiding bladder contractility.
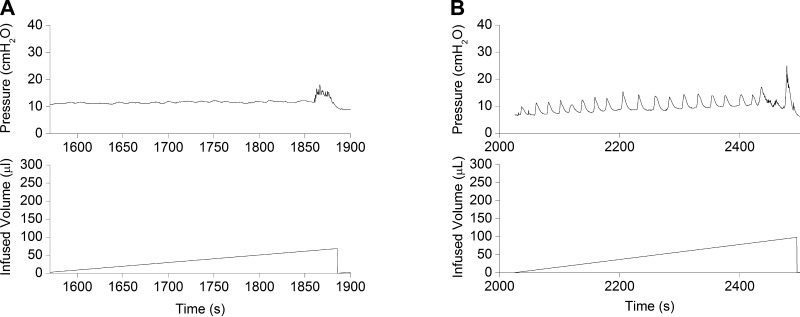
Detrusor contractile activity during filling, manifesting as fluctuations in bladder pressure, exhibited age-specific patterns, changing average amplitude, and frequency of individual events. At birth (P0), detrusor activity was absent (Fig. 4A). This initial inactivity was temporary and was replaced shortly thereafter with prominent high-amplitude (P3: 4.6 ± 1.3 cmH2O), low-frequency (∼4.0 events/100 s) rhythmic activity (Fig. 4B). After the first week, the amplitude of pressure spikes acquired a volume-dependent character (Fig. 5). Increases in bladder volume were associated with continuous increases in the magnitude of nonvoiding bladder contractions. No volume-induced changes in frequency were recorded. Micturition was consistently followed by a short period of markedly reduced contractile activity (resting activity). Interrupted voiding did not disrupt the progressive increase in the amplitude of volume-dependent rhythmic contractions. Only after the bladder was completely empty did spontaneous bladder activity reset to a new cycle (Fig. 1). Volume-dependent, nonvoiding contractions became less prominent, more frequent, and somewhat irregular (low amplitude, high frequency) before the mature pattern was achieved [amplitude/frequency (per 100 s): P1-P7, 3.63 ± 0.97 cmH2O/∼6 events; ≤P14, 2.28 ± 0.72 cmH2O/∼7 events; and ≤P21 1.71 ± 0.49 cmH2O/∼12 events (n = 6 for all groups)]. Mean bladder compliance increased with age from 18.77 ± 6.41 μl/cmH2O in newborns to 80.25 ± 23.01 μl/cmH2O at P21.
Fig. 4.
Representative tracings from one micturition cycle at P0 (A) and P3 (B) illustrating cystometric findings obtained in response to a continuous infusion of saline. Top: intravesical bladder pressure; bottom: infused volume.
Fig. 5.
Representative tracings from one micturition cycle at P14 (A) and P21 (B) illustrating cystometric findings obtained in response to a continuous infusion of saline. Top: intravesical bladder pressure; bottom: infused volume.
Bladder capacity.
Bladder capacity exhibited a linear increase with age during the first 3 wk after birth: ≤1 wk, 82.49 ± 10.13 μl (n = 6); 2–3 wk, 182.41 ± 13.62 μl (n = 8); and ≥3 wk, 310.00 ± 7.69 μl (n = 8, P ≤ 0.001).
DISCUSSION
Here, we used a conscious cystometry experimental model to study age-related changes in rat bladder function and micturition patterns before maturity. Our results demonstrate that conscious cystometry in young animals is a feasible method for monitoring functional maturation of the urinary bladder, providing reliable and reproducible data. It allows for an assessment of the intermediate micturition patterns, revealing critical time periods and switch points in postnatal development of the reflex circuitry.
Neonatal bladder function has generally been considered to be an automated process, one that is mediated by a segmental spinal micturition reflex without the involvement of higher regulatory centers (5, 11). In vivo cystometry, however, revealed incidents of behavioral unrest that coincided with a full bladder, suggesting that rat pups were aware of the need to empty their bladder. This phenomenon has been previously reported and may correspond to behavior observed in human newborns, indicating that the brain is already processing bladder fullness in the neonatal period. This interpretation is in agreement with recent findings suggesting that the voiding reflex pathway is anatomically developed at birth, although functional integration is immature (20, 29, 32).
In our study, adequate stimulation resulted in efficient voiding at all ages. Voiding was elicited by potent detrusor contractions that exhibited progressive increases in magnitude, most likely reflecting structural and/or physiological increases in bladder mass and/or an increase in Ca2+ sensitivity or the number of cellular Ca2+-binding sites during maturation, as previously reported (38, 40). Contraction properties (upstroke amplitude and relaxation) in young pups were comparable with those in adults; however, the durations of micturition contractions at peak were longer in younger groups. The prolonged detrusor contraction in young animals reported here could be related to differences in signal processing, transduction and response to stimulation induced by altered chemical ratio, and/or prolonged stimulation time, exaggerated responses to cutaneous stimulation, differences in bladder smooth muscle cell structure, or sensitivity to/utilization of extracellular Ca2+ between neonates and adult animals (8, 12, 21, 24, 26, 38–40). In neonates, bladder capacity was small, necessitating more frequent triggering of voiding; premature stimulation at low bladder capacity was unable to trigger micturition. With increasing age, the frequency of micturition decreased in proportion to the increase in bladder capacity. The pattern of interrupted voiding we occasionally observed in 1- to 2-wk-old rats was similar to that described in clinical studies. This pattern of voiding is considered to be an immature behavior reflecting dyscoordination between the sphincter and detrusor smooth muscle (29). However, in rats mechanically induced to micturate, the occurrence of interrupted voiding could be a transitional form between immature and mature voiding, although it presents similarly (two detrusor contractions in response to a single stimulation). Our study showed no changes in basal, filling, or threshold pressure among age groups. Bladder compliance increased with normal development. Previous reports (6, 23, 31) of a progressive decrease in threshold pressure can probably be attributed to differences in filling rates or effects of anesthesia used, both of which have been shown to affect urodynamic parameters.
After birth, many regions of the somatosensory nervous system undergo changes in connectivity, leading to transient functional stages before the adult pattern is achieved (3). In adults, the micturition trigger is achieved by reaching the threshold of tension-sensing mechanoreceptors in the bladder wall, which drives the afferent stimulus of the bladder-to-bladder micturition reflex (18). Distention of the bladder wall in young animals, however, fails to evoke a micturition contraction; instead, perineal cutaneous afferents act as forerunners of bladder-specific sensors within the perineal-to-bladder reflex (11). Considerable research attention has been paid to the autonomous, myogenic contractile activity of the bladder wall for its possible involvement in sensory processing and/or perception of bladder fullness (7, 14, 41). This activity has been suggested to generate afferent “noise” that contributes to the sensory stimulus modulation that affects the urge to urinate. Our study as well as previous reports based on in vitro bladder smooth muscle contractility experiments and/or anesthetized animals point to the fact that myogenic (phasic) activity changes with age. It is minimal perinatally, becomes prominent during approximately the first 2 postnatal weeks, and then diminishes, reaching the adult pattern of micturition. However, our in vivo results indicate that the time course for critical switch points in patterns is accelerated compared with that observed in in vitro or ex vivo studies. During conscious cystometry, there was only a transient absence of activity at P0, and a high-amplitude, low-frequency rhythmic myogenic activity developed shortly thereafter. This latter pattern lasted for <1 wk. At the beginning of week 2, this activity was replaced by distention-driven activity, suggesting that a fully functional, tension-sensing local bladder wall reflex mechanism is in place at this age. Interestingly, incomplete bladder emptying (i.e., interrupted voiding) did not disrupt the progressive increase in the amplitude of volume-related rhythmic contractions. Only after the bladder was completely empty was contractile activity during filling reduced to resting levels and a new micturition cycle initiated. Nonvoiding contractions are thought to be myogenic in origin but have been shown to receive excitatory (cholinergic, purinergic, peptidergic, and nitregic) and inhibitory (adrenergic) inputs (14–16, 25). The postnatal sympathoparasympathetic imbalance, reflecting a fully developed cholinergic (excitatory) and still-developing, subcompetent sympathetic (inhibitory) nervous system, may contribute to the elevated contractile activity of the detrusor during the first neonatal week. As part of the bladder sensory motor system, this elevated activity phenomenon may serve as an instructive role physiologically, facilitating bladder-specific sensory signal transduction, growth factor release, and the formation and/or strengthening of spinal synapses at a time when neural pathways are searching for appropriate connections (1, 8, 14). Further studies are required to elucidate the specific determinants of pattern switching and better understand the basic features and variations associated with the development of volume-driven bladder sensation. Although maturation of brain activity has been shown to be more important in unmasking the bladder-to-bladder reflex than maturation of bladder afferent activity, a role for bladder afferent maturation in shaping the distention-to-bladder sensation relation cannot be ruled out (35). Overall, our demonstration of postnatal changes in the urodynamic parameters in conscious rat pups is consistent with findings in anesthetized rats and rabbits (5, 24). Although these findings contrast with clinical studies reporting low or no increased detrusor activity in healthy infants, studying the physiologically elevated detrusor activity early after the birth and progressive changes induced by later development may be useful in the context of understanding regulation of bladder function and dysfunction (2, 14, 22, 36). Detrusor overactivity during filling remains the main focus in elucidating the pathophysiology of neurogenic bladder dysfunction, dysfunctional voiding, and idiopatic detrusor overactivity disorder in children (17). Therefore, the animal model presented here may be considered as an effective tool for systematically investigating the mechanisms underlying detrusor overactivity and the role it plays in pathophysiology of bladder dysfunction. Taken together, our results suggest that detrusor contractility undergoes changes during early postnatal development in rat pups and highlight the potential of further investigations to facilitate identification of the key modulators and/or new therapeutic targets.
Conclusions.
Here, we provide the first description of conscious cystometry in rat pups and discuss the development of the micturition reflex in terms of physiological patterns and urodynamic parameters. Our data demonstrating the progressive changes in bladder function that occur after birth significantly extend previous findings from in vitro and ex vivo studies and studies in anesthetized animals. The results show that conscious cystometry is a feasible method for monitoring functional maturation of the urinary bladder in young animals. The reliability of the data and the reproducibility of the measurements allow for further experimental manipulations, such as pharmacological interventions to test pathways that drive the maturation and/or physiology of the micturition reflex.
GRANTS
This work was funded National Institute of Diabetes and Digestive and Kidney Diseases Grant KO1-DK-079150.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.Z. and P.Z. conception and design of research; K.Z. and P.Z. performed experiments; K.Z. and P.Z. analyzed data; K.Z. and P.Z. interpreted results of experiments; K.Z. and P.Z. prepared figures; K.Z. and P.Z. drafted manuscript; K.Z. and P.Z. edited and revised manuscript; K.Z. and P.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Hill-Eubanks for helpful comments and editorial assistance.
REFERENCES
- 1. Araki I, de Groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. J Neurosci 17: 8402–8407, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachelard M, Sillen U, Hansson S, Hermansson G, Jodal U, Jacobsson B. Urodynamic pattern in asymptomatic infants: siblings of children with vesicoureteral reflux. J Urol 162: 1733–1738, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol 10: 138–145, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Biallosterski BT, van Koeveringe GA, van Kerrebroeck PE, Gillespie JI, de Wachter SG. Nonvoiding activity of the guinea pig bladder. J Urol 186: 721–727, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Bradley WE, Wright FS. Visceral reflex activity: development in postnatal rabbit. Science 152: 216–217, 1966 [DOI] [PubMed] [Google Scholar]
- 6. Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 69: 1193–1202, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Coolsaet BL, Van Duyl WA, Van Os-Bossagh P, De Bakker HV. New concepts in relation to urge and detrusor activity. Neurourol Urodyn 12: 463–471, 1993 [DOI] [PubMed] [Google Scholar]
- 8. de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol Behav 77: 689–692, 2002 [DOI] [PubMed] [Google Scholar]
- 9. de Groat WC, Araki I. Maturation of bladder reflex pathways during postnatal development. Adv Exp Med Biol 462: 253–263, discussion 311–320, 1999 [DOI] [PubMed] [Google Scholar]
- 10. de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res 92: 127–140, 1998 [DOI] [PubMed] [Google Scholar]
- 11. DeGroat WC, Douglas JW, Glass J, Simonds W, Weimer B, Werner P. Changes in somato-vesical reflexes during postnatal development in the kitten. Brain Res 94: 150–154, 1975 [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proc Natl Acad Sci USA 96: 7719–7722, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93: 478–483, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gillespie JI. Modulation of autonomous contractile activity in the isolated whole bladder of the guinea pig. BJU Int 93: 393–400, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gillespie JI. Noradrenaline inhibits autonomous activity in the isolated guinea pig bladder. BJU Int 93: 401–409, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Glassberg KI, Combs AJ, Horowitz M. Nonneurogenic voiding disorders in children and adolescents: clinical and videourodynamic findings in 4 specific conditions. J Urol 184: 2123–2127, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol 128: 593–607, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keating MA, Duckett JW, Snyder HM, Wein AJ, Potter L, Levin RM. Ontogeny of bladder function in the rabbit. J Urol 144: 766–769, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Kruse MN, De Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol Regul Integr Comp Physiol 258: R1508–R1511, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Longhurst P. Developmental aspects of bladder function. Scand J Urol Nephrol : 11–19, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Maggi CA, Santicioli P, Geppetti P, Frilli S, Spillantini MG, Nediani C, Hunt SP, Meli A. Biochemical, anatomical and functional correlates of postnatal development of the capsaicin-sensitive innervation of the rat urinary bladder. Brain Res 471: 183–190, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Maggi CA, Santicioli P, Meli A. Postnatal development of micturition reflex in rats. Am J Physiol Regul Integr Comp Physiol 250: R926–R931, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Maggi CA, Santicioli P, Meli A. Postnatal development of myogenic contractile activity and excitatory innervation of rat urinary bladder. Am J Physiol Regul Integr Comp Physiol 247: R972–R978, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol 291: R1049–R1059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pirker ME, Rolle U, Shinkai T, Shinkai M, Puri P. Prenatal and postnatal neuromuscular development of the ureterovesical junction. J Urol 177: 1546–1551, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Sann H, Walb G, Pierau FK. Postnatal development of the autonomic and sensory innervation of the musculature in the rat urinary bladder. Neurosci Lett 236: 29–32, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Shin T, Koyanagi T, Hara K, Kubota S, Nakano H. Development of urine production and urination in the human fetus assessed by real-time ultrasound. Asia Oceania J Obstet Gynaecol 13: 473–479, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Sillen U. Bladder function in healthy neonates and its development during infancy. J Urol 166: 2376–2381, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Smith PP, Deangelis AM, Kuchel GA. Evidence of central modulation of bladder compliance during filling phase. Neurourol Urodyn 31: 30–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 97: 1094–1101, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett 223: 197–200, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol 285: R809–R816, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Szigeti GP, Somogyi GT, Csernoch L, Szell EA. Age-dependence of the spontaneous activity of the rat urinary bladder. J Muscle Res Cell Motil 26: 23–29, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Wu HY, de Groat WC. Maternal separation uncouples reflex from spontaneous voiding in rat pups. J Urol 175: 1148–1151, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wuest M, Michel MC. What can maturations studies teach us about adult detrusor overactivity? Neurourol Urodyn 28: 265, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Zderic SA, Duckett JW, Wein AJ, Snyder HM, 3rd, Levin RM. Development factors in the contractile response of the rabbit bladder to both autonomic and non-autonomic agents. Pharmacology 41: 119–123, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Zderic SA, Hypolite J, Duckett JW, Snyder HM, 3rd, Wein AJ, Levin RM. Developmental aspects of bladder contractile function: sensitivity to extracellular calcium. Pharmacology 43: 61–68, 1991 [DOI] [PubMed] [Google Scholar]
- 39. Zderic SA, Sillen U, Liu GH, Snyder H, 3rd, Duckett JW, Wein AJ, Levin RM. Developmental aspects of bladder contractile function: evidence for an intracellular calcium pool. J Urol 150: 623–625, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Zderic SA, Sillen U, Liu GH, Snyder MC, 3rd, Duckett JW, Gong C, Levin RM. Developmental aspects of excitation contraction coupling of rabbit bladder smooth muscle. J Urol 152: 679–681, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Zvara P, Wright AJ, Roach K, Ursiny M, Shapiro B, Dagrosa LM, Nelson MT, Heppner TJ. A non-anesthetized mouse model for recording sensory urinary bladder activity. Front Neurol 1: 127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]