Abstract
Salt and water retention is a hallmark of nephrotic syndrome (NS). In this study, we test for changes in the abundance of urea transporters, aquaporin 2 (AQP2), Na-K-2Cl cotransporter 2 (NKCC2), and Na-Cl cotransporter (NCC), in non-pair-fed and pair-fed nephrotic animals. Doxorubicin-injected male Sprague-Dawley rats (n = 10) were followed in metabolism cages. Urinary excretion of protein, sodium, and urea was measured periodically. Kidney inner medulla (IM), outer medulla, and cortex tissue samples were dissected and analyzed for mRNA and protein abundances. At 3 wk, all doxorubicin-treated rats developed features of NS, with a ninefold increase in urine protein excretion (from 144 ± 21 to 1,107 ± 165 mg/day; P < 0.001) and reduced urinary sodium excretion (from 0.17 to 0.12 meq/day; P < 0.001). Urine osmolalities were reduced in the nephrotic animals (1,057 ± 37, treatment vs. 1,754 ± 131, control). Unlike animals fed ad libitum, UT-A1 protein abundance was unchanged in nephrotic pair-fed rats. Glycosylated AQP2 was reduced in the IM base of both nephrotic groups. Abundances of NKCC2 and NCC were consistently reduced (71 ± 7 and 33 ± 13%, respectively) in both nephrotic pair-fed animals and animals fed ad libitum. In pair-fed nephrotic rats, we observed an increase in the cleaved form of membrane-bound γ-epithelial sodium channel (ENaC). However, α- and β-ENaC subunits were unaltered. NKCC2 and AQP2 mRNA levels were similar in treated vs. control rats. We conclude that dietary protein intake affects the response of medullary transport proteins to NS.
Keywords: UT-A3, nephrosis, focal segmental glomerulosclerosis, doxorubicin, adriamycin
unlike most conditions with acquired tubular dysfunction, nephrotic syndrome (NS) is associated with primary renal salt retention, rather than salt wasting. The mechanisms behind this phenomenon have not been fully elucidated. However, a recently published study suggested that nephrotic urine has the ability to directly activate the epithelial sodium channel (ENaC) at the level of the cortical collecting duct (CCD) (29). Leakage of serine proteases, particularly plasminogen, into the urine of nephrotic rats may cause proteolytic activation of ENaC from the CCD luminal surface. This is the result of activation of plasminogen by in situ urokinase-like proteins in the CCD (29). Consistent with this assumption, salt retention in NS occurs independently of the effects of vasopressin (8) or aldosterone (23).
Urea transporters play a central role in the formation of the corticomedullary osmotic gradient, a key element of renal water homeostasis. Two isomers, UT-A1 and UT-A3, are expressed in the inner medullary (IM) collecting duct. In physiological conditions, UT-A1 and UT-A3 are localized to the apical and basolateral membranes, respectively (4). Previous studies reported reduced IM tissue abundance of the urea transporter and aquaporin 2 (AQP2) in rat models of NS (1, 11, 12). This decrease was postulated to be a compensatory mechanism to salt and water retention. However, only total urea transporter abundance was investigated since different isomers were not known at that time. Moreover, protein abundances were evaluated in whole inner medulla of nephrotic animals fed ad libitum. Doxorubicin-treated nephrotic rats almost always develop marked anorexia, especially in the first week following doxorubicin injection, resulting in significant differences in caloric, protein, and sodium intake between treated and control animals. It is possible that differences in dietary intake may confound some of the previously reported observations regarding urea transporter and aquaporin abundances. In a study by Kim et al. (22), UT-A1 and UT-A3 tissue abundances were reduced in animals fed a low-protein or low-salt diet. In response to a low-protein diet, different changes in UT-A1 were observed in IM tip and base tissue. Similarly, rats fed a low-protein diet manifested reduced AQP2 abundance in the IM (27). Whether changes in urea transporter and aquaporin abundances are biased by changes in dietary intake and whether these changes occur before, during, or follow the associated salt and water retention have not been reported.
Several reports have focused on investigating various sodium transporters in NS-associated salt retention. The abundance of Na-K-2Cl cotransporter 2 (NKCC2) was reduced in outer medulla (OM) of rats with HgCl2-induced (20), puromycin-induced (21), and doxorubicin-induced NS (11). The underlying mechanism behind reduced NKCC2 is not known. It is also not clear whether reduced NKCC2 abundance represents a defect localized to the thick ascending limb of the loop of Henle (with preserved macula densa NKCC2), or a general defect involving macula densa NKCC2. In our view, the former possibility appears more likely since it would tend to reduce glomerular filtration rate through tubuloglomerular feedback, and hence would provide an explanation for the low glomerular filtration rate and balanced sodium delivery to the CCD, both consistently seen in nephrotic animals (15).
Protein abundance of the thiazide-sensitive Na-Cl cotransporter (NCC) was also reduced in HgCl2-induced and puromycin-induced models of NS (20, 21). The whole tissue abundance of ENaC subunits was investigated in various reports. In a model of HgCl2-induced NS, the abundance of α-ENaC was increased in the cortex, while β-ENaC and γ-ENaC were decreased (20). However, other studies in puromycin-induced nephrotic rats showed increased γ-ENaC abundance in the cortex, but no alterations in cortical α-ENaC and β-ENaC (21, 23). In addition, an increase in the active 70-kDa (cleaved) isoform of γ-ENaC was noted in nephrotic rats in some reports (21, 23), but not others (7). More recently, in vitro exposure of ENaC-expressing oocytes to a combination of urokinase plasminogen activator and plasminogen resulted in the appearance of a γ-ENaC cleavage product band suggesting proteolytic activation (29). However, whether similar mechanisms in NS activate ENaC in vivo remains, at this point, untested.
Heart failure and cirrhosis are known to be associated with a state of salt and water retention, similar to that seen in NS. Vasopressin-induced increases in AQP2 activity have been reported in animal models of cirrhosis (9, 11). AQP2 abundance was also shown to be increased in rat models of heart failure (6). In NS, increased sodium reabsorption at the level of the CCD (8, 15), due to abnormal activity of ENaC (29, 30), is coupled with a concurrent defect in sodium reabsorption in the ascending limb of the loop of Henle and distal convoluted tubule, resulting from reduced NKCC2 and NCC (11, 20, 21). This, in turn, is likely to induce a disruption in the corticomedullary osmotic gradient, rendering the medullary collecting duct resistant to the action of vasopressin (1, 12). Previous studies in animals fed ad libitum showed reduced abundance of IM AQP2 and urea transporters (1, 11, 12). In contrast to the expected compensatory response to a defect in water reabsorption, those surprising changes further increase urinary water excretion.
This study investigates the changes in abundance of urea transporter isomers UT-A1 and UT-A3 in the IM collecting duct, AQP2 in medullary collecting duct, and sodium transporters and channels in the thick ascending limb, distal convoluted tubule, and CCD in non-pair-fed and pair-fed nephrotic rats. Changes in protein abundances were evaluated at the time of peak salt retention and were correlated with urine osmolalities, sodium excretion, and urea excretion. We also evaluated changes in transcription of AQP2 and NKCC2 through measurement of messenger RNA levels.
MATERIALS AND METHODS
Animal model.
Emory University Institutional Animal Care and Use Committee approved all the protocols used in this study. Five cohorts of male Sprague-Dawley rats (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; Charles River Laboratories, Wilmington, MA), weighing 229 ± 24 g (controls) and 242 ± 17 g (treated), were anesthetized with isoflurane and received a single femoral vein injection of a sterile solution of doxorubicin (7.5 mg/kg body wt). The non-pair-fed animals were allowed free access to food and water throughout the experimental protocol. For pair feeding, each doxorubicin-treated rat was paired with a weight-matched control rat. Accurate pair feeding was achieved by daily measurement of food intake of each doxorubicin-treated rat, followed by offering the same measured amount over the next 24 h to their matched control. Pair feeding was started immediately following doxorubicin injection and maintained throughout the 3-wk observation period. Regular rodent diet (LabDiet 5001, PMI nutrition) was used for all groups, containing 23.4 g % protein and 0.4 g % sodium. The pair-fed rats were placed in metabolism cages to allow for daily monitoring of water intake and urine output.
Blood and urine analysis.
In the non-pair-fed group of animals, urinary protein excretion was monitored using serial urine dipsticks (Seimens Multistix 9, Tarrytown, NY) twice weekly. Upon establishment of 4+ urine protein (>2,000 mg/dl urine protein), urine quantitative protein assessment was obtained through a spot urine protein-to-creatinine ratio (Pr/Cr). For pair-fed animals, both total daily urine protein excretion and Pr/Cr ratios were evaluated. Urine protein was determined using the Bio-Rad DC protein assay (Hercules, CA). Urine creatinine and serum cholesterol were obtained using reflectance photometry (Reflotron Plus; Roche Diagnostics, Mannheim, Germany). An average Pr/Cr ratio exceeding 120 was used as the primary endpoint indicator. The animals were observed over a median period of 21 days (range 19–33 days) until reaching their primary endpoint, at which time they were killed and tissue analysis was performed. Urine osmolalities were determined using Wescor 5520 Vapor Pressure Osmometer (Wescor, Logan, UT). Urine urea concentration was measured by colorimetric assay using Infinity Urea Reagent from Thermo Scientific (Thermo Fisher Scientific, Chicago, IL). Serum sodium, serum creatinine, and urine creatinine measurements were obtained as a courtesy service of the UT Southwestern O'Brien Kidney Research Center. Serum creatinine was analyzed utilizing a Nova Biomedical blood gas analyzer. Urine sodium measurements were obtained using a sodium-selective electrode (Cole-Parmer).
Protein excretion in pair-fed animals was monitored every 3 to 4 days in urine collected over 24 h. Similarly, 24-h urinary excretion of sodium, urea, and total osmoles was calculated, after adjustment for daily urine volume. Glomerular filtration rate was approximated using creatinine clearance, calculated from the urine creatinine concentration, serum creatinine, and urine flow rate. Fractional excretion of water was calculated as the ratio of serum creatinine to urine creatinine. The relative osmolar contribution of sodium and its associated anion binding was estimated by multiplying the total urine sodium concentration (in meq/l) by two. The relative osmolar contribution of urea was a direct reflection of the urea concentration (mmol/l).
Protein abundances.
Upon establishment of the nephrotic model, urine samples were collected for final analysis and animals were euthanized by decapitation. Blood was collected and serum was saved for laboratory studies; the kidneys were dissected to remove IM tip, IM base, OM, and cortex. Tissue lysate samples of equal total protein concentration were prepared and size separated using SDS-PAGE on 7.5, 10, or 12.5% polyacrylamide gels. Gels were then electroblotted to polyvinylidene difluoride membranes (Immobilon, Millipore, Bedford, MA) and analyzed as previously described (18). Primary antibodies included our polyclonal antibodies to COOH-terminal UT-A1 (detects UT-A1 and UT-A2) (18, 19, 22), NH2-terminal UT-A (detects UT-A1 and UT-A3) (4, 22), AQP2 (18, 19), and NKCC2 (10, 18, 19). Anti-alkaline phosphatase (Rockland, Gilbertsville, PA) was used as a loading control throughout these experiments. All densitometry analyses were reported after correction for the loading controls. Bands were visualized using infrared detection with the LICOR Odyssey protein analysis system (LICOR, Lincoln, NE).
Membrane ENaC analysis.
Plasma membranes were isolated from cortex of pair-fed, untreated and doxorubicin-treated rats. Kidneys from both groups were removed and cortex was dissected. The cortex tissue was finely chopped, washed, and homogenized. Unbroken cells and nuclei were removed by centrifugation at 1,200 g for 15 min to prepare a whole cell lysate. For preparing plasma membranes, the lysate was layered on top of 30% opti-prep (Sigma, St. Louis, MO) and centrifuged at 84,000 g for 30 min. The floating ring containing the rat renal cortical plasma membranes was collected. The whole cell lysate and membranes were resolved on 10% SDS-PAGE and analyzed by Western blotting. The blots were incubated with rabbit anti-ENaC subunit-specific antibodies (14, 16, 17, 24) overnight followed by incubation with secondary goat anti-rabbit lgG, coupled to alkaline phosphatase (Kirkegaard and Perry Laboratories, Gaithersburg, MD). ENaC antibody specificity was verified by preadsorbing the primary ENaC antibodies with or without 1 μg/ml of immunizing peptide for 30 min at room temperature before incubation with the blots overnight. The antigen antibody complex was detected by a chemiluminescent detection system CDP-star (Tropix, Bedford, MA) and Kodak 2000M camera system (Eastman Kodak, Rochester, NY).
RT-PCR.
To measure AQP2 and NKCC2 mRNA by RT-PCR, RNA from IM and OM was extracted using RNAzol RT reagent (Invitrogen, Carlsbad, CA). For reverse transcription, 1 μg of each RNA sample was used with random primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). The expression of AQP2 and NKCC2 mRNA was measured as described (32). PCR was performed with Platinum PCR SuperMix (Invitrogen) using the cycle parameters: 94°C for 2 min and 40 cycles at 94°C for 30 s, 55°C for 30 s, 72°C for 30 s with final extension at 72°C for 10 min. Products were separated in 1% (wt/vol) agarose gel electrophoresis and visualized by ethidium bromide fluorescence. Primers for AQP2 and NKCC2 genes (Invitrogen) were confirmed to cross intron-exon boundaries using Clone Manager Suite 7 software (Sci Ed Software, Durham, NC). They were used to generate amplicons in their linear ranges as follows: AQP2 (NM_012909); forward: TGCATCTTTGCCTCCACCGACGAG, reverse: CATGGAGCAACCGGTGAAATAG; NKCC2 (NM_019134); forward: CCGTCGCCTACAGGTGTT, reverse: CCCTTTGCGAAGAACTGAA. For each sample, 18S rRNA was used as an internal control using QuantumRNA 18S primers (Ambion, Austin, TX). Autoradiographic signals were quantified by densitometry (Bio-Rad) and expressed relative to the corresponding 18S RNA to correct for variations.
Statistical analysis.
The unpaired Student's t-test was used to evaluate differences between mean values of the two groups in the initial three unpaired cohorts of animals. Paired t-test was used to analyze differences between the pair-fed groups of rats. P < 0.05 was considered statistically significant. Densitometry data are presented as percentage change ± SE.
RESULTS
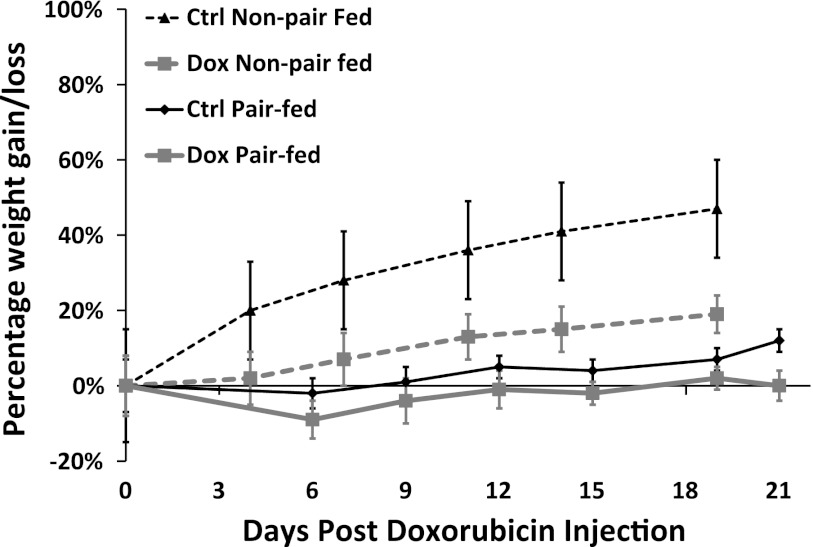
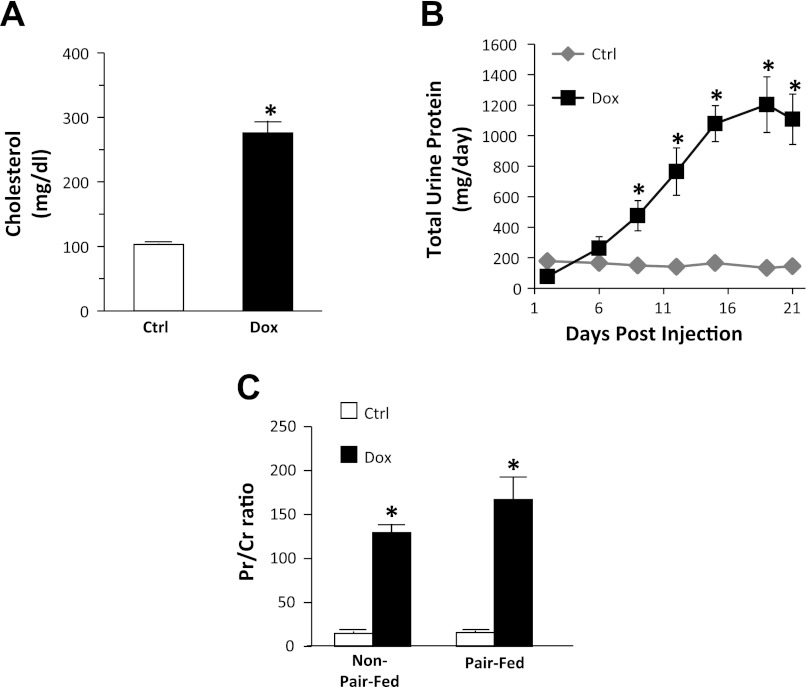
Baseline weight, age, and urine protein were similar between the control and nephrotic groups. Over the 3-wk observation period, weight gain was markedly stunted in non-pair-fed-treated rats compared with their controls (7.7 ± 0.6 g/day, control vs. 2.7 ± 0.5 g/day, treatment, P < 0.01), but only slightly lower in pair-fed-treated rats compared with their control counterparts (1.5 ± 0.7 g/day, control vs. 1.3 ± 0.6 g/day, treatment, P < 0.05; Fig. 1). Although pair-fed control rats showed less overall weight gain than non-pair-fed controls (1.5 ± 0.7 g/day, pair-fed vs. 7.7 ± 0.7 g/day, non-pair-fed, P < 0.01), both groups had similar weight gain in the 2 days preceding the day of tissue analysis (6.8 ± 2.2 g/day, pair-fed vs. 6.6 ± 0.7 g/day non-pair-fed; Fig. 1). All treated rats developed high-grade proteinuria within 2 wk following the doxorubicin injection (Fig. 2B). A Pr/Cr ratio exceeding 120 was used as the endpoint indicator, which corresponded to an eightfold increase in urinary protein excretion. An eightfold cutoff of protein excretion was chosen to closely resemble nephrotic-range proteinuria in humans (13, 28). In addition, this level of proteinuria was associated with the nadir of urinary sodium excretion, heralding renal salt retention, the nephrotic feature of our particular interest. On the date of tissue analysis, both non-pair-fed and pair-fed-treated animals had a similar degree of protein excretion, reflected by an average ninefold increase in urine Pr/Cr ratio (Fig. 2C). Serum cholesterol levels in a subset of treated animals showed an average threefold elevation in the nephrotic group (Fig. 2A). Creatinine clearance was significantly reduced in doxorubicin-treated animals compared with their control counterparts (from 4.76 ± 0.7 to 1.43 ± 0.2 ml/min; P < 0.01).
Fig. 1.
Changes in weight plotted over time in control non-pair-fed rats (interrupted black line), treated non-pair-fed rats (interrupted gray line), pair-fed control rats (solid black line), and pair-fed-treated rats (solid gray line). Data are expressed as mean weight gain/loss (%) ± SE (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10).
Fig. 2.
Establishment of the nephrotic model. A: serum cholesterol levels compared between control (open bar; Ctrl) and doxorubicin (Dox)-treated rats (filled bar). B: total urinary protein excretion in pair-fed rats. C: urine protein-to-creatinine ratio in both non-pair-fed and pair-fed rats. Data are expressed as means ± SE (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; *P < 0.01).
Sodium and urea excretion.
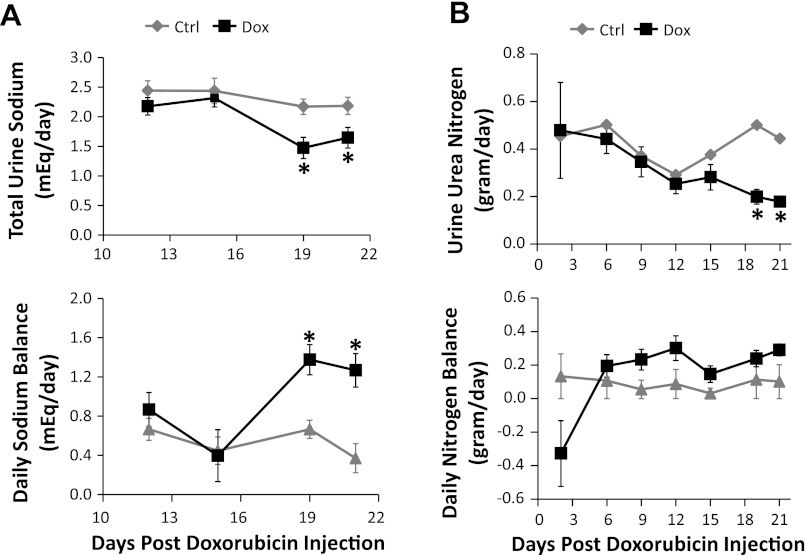
Starting at day 19 following doxorubicin injection, nephrotic animals manifested a marked reduction (32 ± 8%) in urinary sodium excretion along with a more positive sodium balance (Fig. 3A). The majority of treated rats showed visible signs of tissue swelling and significant ascites noted at the time of death. Urine urea excretion became significantly reduced starting at day 19 following doxorubicin injection (60 ± 4%). In the initial 2 days following doxorubicin injection, nephrotic animals developed a transiently negative nitrogen balance, but maintained neutral balance thereafter throughout the observation period (Fig. 3B).
Fig. 3.
Urine sodium and urea excretion. Rats treated with Dox (black lines) are compared with their control pair-fed animals (gray lines). A: urinary sodium excretion (top) and sodium balance (bottom). B: urea nitrogen excretion (top) and daily nitrogen balance (bottom). Daily sodium and nitrogen balance is estimated by taking into account oral intake and urinary excretion (does not include other excretory routes such as stool, sweating, etc.). Data are expressed as means ± SE (n = 10, *P < 0.05).
Urine osmolalities.
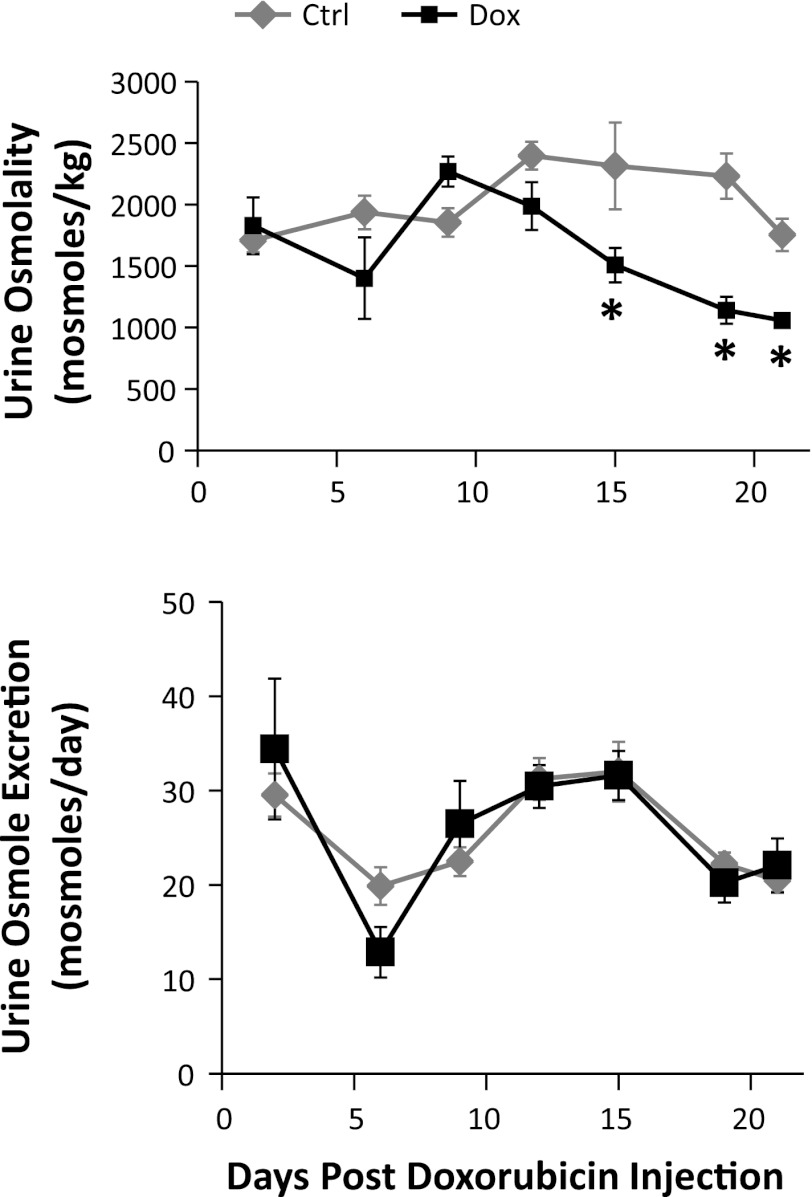
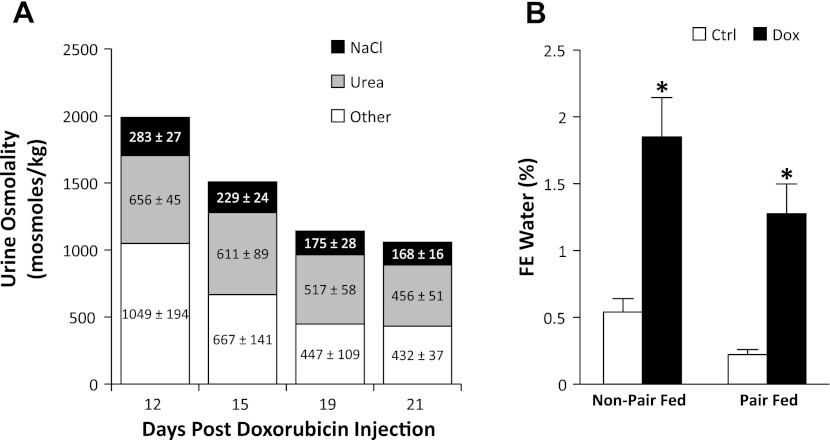
The doxorubicin-treated rats exhibited a progressive reduction in urine osmolality, up to 49 ± 5% on day 19, compared with the control group (Fig. 4). However, total daily excretion of osmoles was similar between the two groups. To further evaluate the decrease in urine osmolality, we estimated the relative contribution of urine sodium (with associated anion binding) and urine urea to the total urine osmolality. The relative contribution of other unidentified osmolytes, which may include organic solutes, potassium, and others, was estimated by exclusion. A proportional reduction in all three components was observed in nephrotic animals (Fig. 5A). This was explained by the increase in urinary water excretion, reflected by a marked increase in the fractional excretion for water in non-pair-fed and pair-fed-treated rats compared with their control counterparts (Fig. 5B). The estimated contribution of urine protein to total osmolality was negligible.
Fig. 4.
Urine osmolalities and total daily urinary excretion of osmoles. Rats treated with Dox (black lines) are compared with their control pair-fed animals (gray lines). Data are expressed as means ± SE (n = 10, *P < 0.05).
Fig. 5.
Relative osmolalities and water excretion. A: relative contribution of sodium (and associated anion binding) and urea to urine osmolalities. Stacked bars represent the total urine osmolality and relative average calculated contribution of urine sodium (and associated anion binding; black) and urine urea (gray). Contribution by other unidentified osmolytes is represented in white. B: fractional excretion of water (calculated as serum Cr/urine Cr) in non-pair-fed and pair-fed animals. Data are expressed as means ± SE (Non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; *P < 0.01).
Urea transporters and AQP2.
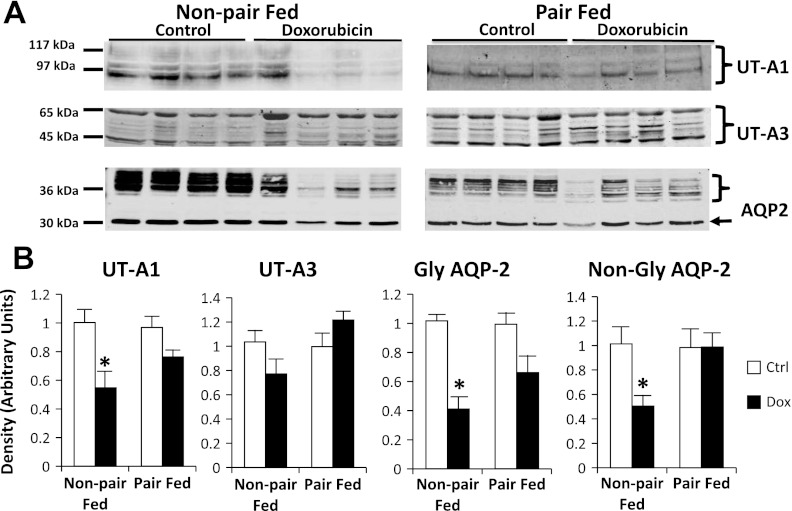
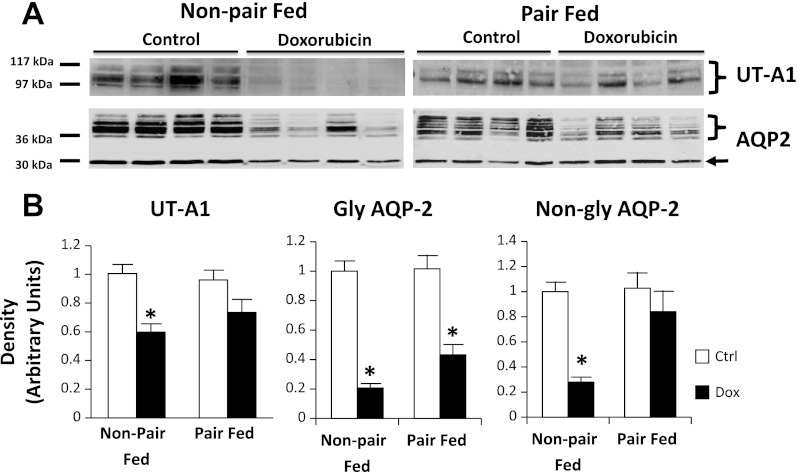
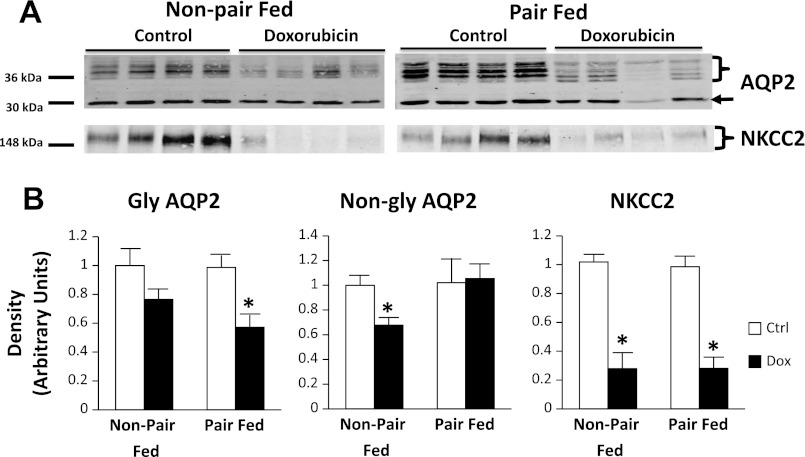
UT-A1 protein abundance was reduced by 44 ± 10% in doxorubicin-treated, non-pair-fed rat IM tip tissue compared with the controls (Fig. 6). However, UT-A1 protein abundance was similar in treated and control pair-fed animals, suggesting a possible nutritional bias affecting UT-A1 protein changes. UT-A3 abundances were similar between the control and doxorubicin-treated groups in non-pair-fed and pair-fed animals. In non-pair-fed, doxorubicin-treated rat IM tip tissue, glycosylated and nonglycosylated AQP2 abundance was decreased by 60 ± 8 and 50 ± 9%, respectively. In pair-fed animals, glycosylated AQP2 reduction in IM tip (33 ± 12%) did not reach statistical significance (Fig. 6). No difference was seen in nonglycosylated AQP2 in IM tip tissue of pair-fed animals. Similarly, UT-A1 abundance was decreased by 41 ± 7% in non-pair-fed-treated rat IM base tissue compared with controls, but similar in pair-fed animals (Fig. 7). As expected, the abundance of UT-A1 was markedly higher in IM tip than base in both control as well as doxorubicin-treated animals (data not shown). Glycosylated AQP2 abundance was reduced in both non-pair-fed and pair-fed-treated rat IM base tissue by 79 ± 3 and 57 ± 7%, respectively. On the other hand, nonglycosylated AQP2 was reduced in non-pair-fed treated animals (72 ± 4%), but not their pair-fed counterparts. In OM tissue, glycosylated AQP2 was reduced by 42 ± 8% in pair-fed nephrotic animals. However, the difference in non-pair-fed rats was not statistically significant. Nonglycosylated AQP2 was reduced by 32 ± 9% in OM of non-pair-fed-treated rats, but not in their pair-fed counterparts (Fig. 8). No appreciable differences were found in membrane localization on immunohistochemical evaluation of phospho-Ser486 UT-A1 or AQP2 in IM tissue of non-pair-fed-treated and control rats (data not shown).
Fig. 6.
UT-A1, UT-A3, and aquaporin 2 (AQP2) abundance in rat inner medullary (IM) tip tissue of the control (Ctrl) group and Dox-treated group. Non-pair-fed as well as pair-fed animals were evaluated. A: representative immunoblots showing abundance of UT-A1, UT-A3, and AQP2 in rat IM tip tissue lysates. The right arrow points to the nonglycosylated (non-gly) AQP2 band. The right brace signifies glycosylated (gly) AQP2 bands. An equal amount of total protein from a different rat tissue sample was loaded into each lane. B: densitometric analysis of Western blots from 3 non-pair-fed and 2 pair-fed cohorts of animals. Data were normalized for the average densitometry of untreated animals in each group and adjusted for alkaline phosphatase as a control protein. Separate densitometric analysis was performed for gly and non-gly AQP2. Data are expressed as means ± SE (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; *P < 0.05).
Fig. 7.
UT-A1 and AQP2 abundance in rat IM base tissue of the Ctrl group and Dox-treated group. Non-pair-fed as well as pair-fed animals were evaluated. A: representative immunoblots showing abundance of UT-A1 and AQP2 in rat IM base tissue lysates. The right arrow points to the non-gly AQP2 band. The right brace signifies gly AQP2 bands. An equal amount of total protein from a different rat tissue sample was loaded into each lane. B: densitometric analysis of Western blots from 3 non-pair-fed and 2 pair-fed cohorts of animals for UT-A1 (left) and AQP2 abundance (right). Separate densitometric analysis was performed for gly and non-gly AQP2. Data were normalized for the average densitometry of untreated animals in each group and adjusted for alkaline phosphatase as a control protein. Data are expressed as means ± SE (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; *P < 0.05).
Fig. 8.
AQP2 and Na-K-2Cl cotransporter 2 (NKCC2) abundance in rat outer medullary (OM) tissue of the Ctrl group and Dox-treated group. Non-pair-fed as well as pair-fed animals were evaluated. A: representative immunoblots showing abundance of AQP2 and NKCC2 in rat OM tissue lysates. The right arrow points to the non-gly AQP2 band. The right brace signifies gly AQP2 bands. An equal amount of total protein from a different rat tissue sample was loaded into each lane. B: densitometric analysis of Western blots from 3 non-pair-fed and 2 pair-fed cohorts of animals for AQP2 (left) and NKCC2 (right). Data were normalized for the average densitometry of untreated animals in each group and adjusted for alkaline phosphatase as a control protein. Separate densitometric analysis was performed for gly and non-gly AQP2. Data are expressed as means ± SE (non-pair-fed n = 18 treated, 12 controls; pair-fed n = 10; *P < 0.05).
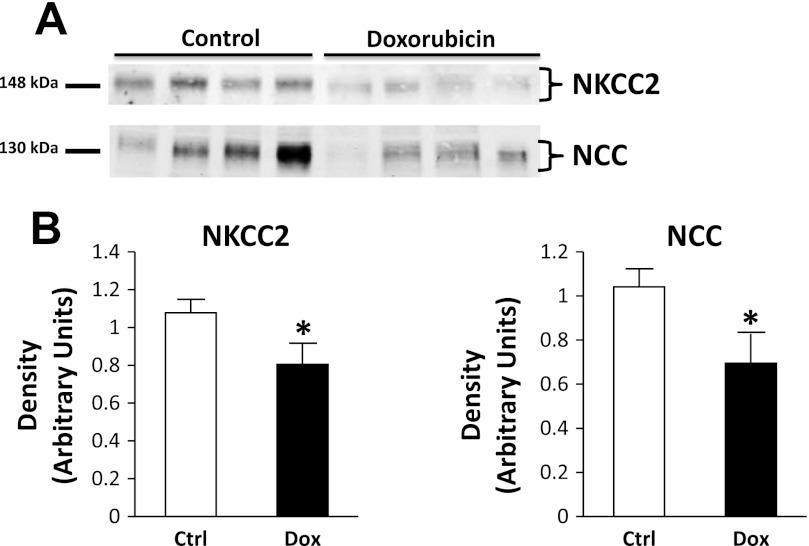
NKCC2 and the thiazide-sensitive cotransporter.
NKCC2 abundance was markedly reduced by 73 ± 5 and 71 ± 7% in OM tissue of nephrotic pair-fed and non-pair-fed rats, respectively (Fig. 8). In cortex tissue of pair-fed animals, a less prominent reduction of NKCC2 was observed (25 ± 11%). NCC was reduced by 33 ± 13% in pair-fed-treated rats (Fig. 9).
Fig. 9.
NKCC2 and Na-Cl cotransporter (NCC) abundance in rat cortex tissue of the Ctrl and Dox-treated pair-fed animals. A: representative immunoblots showing abundance of NKCC2 and NCC in rat cortex tissue lysates. An equal amount of total protein from a different rat tissue sample was loaded into each lane. B: densitometric analysis of Western blots from the 2 pair-fed cohorts of animals for NKCC2 (left) and NCC (right). Data were normalized for the average densitometry of untreated animals in each group and adjusted for alkaline phosphatase as a control protein. Data are expressed as means ± SE (n = 10; *P < 0.05).
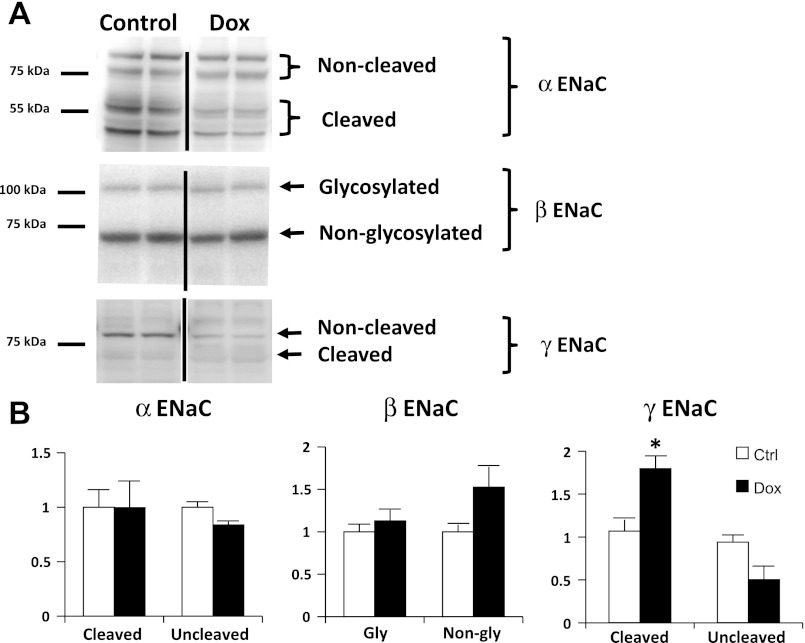
ENaC.
We tested the hypothesis that ENaC hyperactivity contributes to increased sodium retention using isolated plasma membranes from the cortex of untreated and doxorubicin-treated rats. To confirm the specificity of ENaC antibodies in rat cortex tissue, Western blots were obtained with primary ENaC antibodies with and without preadsorption with immunizing peptide (data not shown). We observed no significant increase in either α- or β-subunits. However, an increase in the cleaved form of the γ-subunit was noted (80 ± 15%), suggesting that the increase in ENaC activity may be related to proteolytic γ-ENaC cleavage by an extracellular protease such as prostasin and/or plasmin (Fig. 10).
Fig. 10.
Membrane abundance of epithelial sodium channel (ENaC) subunits in pair-fed nephrotic and Ctrl rats. A: representative immunoblots of membranes from cortex of Dox-treated and Ctrl rats probed with anti-ENaC subunit antibodies. Membranes from rat kidney cortex were prepared from Ctrl rats and rats treated with Dox. Equal amounts of proteins were loaded and resolved on 10% SDS-PAGE. The resolved proteins were transferred onto nitrocellulose membranes and were probed with ENaC subunit antibodies. Lanes 1 and 2 correspond to 2 different rats. B: densitometric analysis of Western blots probed for ENaC subunits. The relative density of cleaved/uncleaved portions of α-ENaC, gly/non-gly β-ENaC, and cleaved/uncleaved portions of γ-ENaC are shown in separate bar graphs. Data are expressed as means ± SE (n = 4; *P < 0.05).
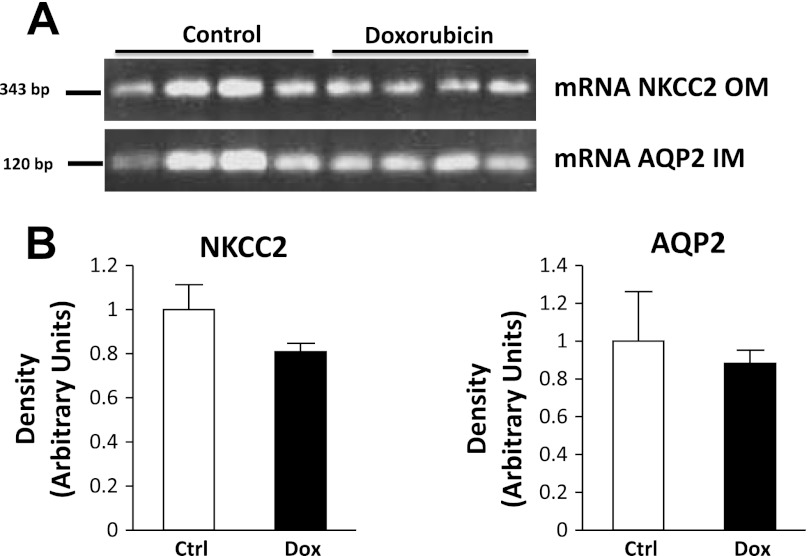
mRNA levels.
mRNA levels of IM AQP2 and OM NKCC2 were similar between treated and untreated pair-fed animals (Fig. 11). This suggests a posttranscriptional mechanism behind the observed changes in protein abundance.
Fig. 11.
RT-PCR analysis of rat IM tissue for AQP2 mRNA and rat OM tissue for NKCC2 mRNA. RT-PCR using AQP2 primers amplified a 122-bp product from rat kidney IM. NKCC2 primers amplified a 343-bp product from rat kidney OM (n = 10).
DISCUSSION
In pair-fed rats with doxorubicin-induced NS, we observed a significant reduction in the abundance of IM glycosylated AQP2, OM NKCC2, cortical NKCC2, and cortical NCC. NS was associated with reduced uncleaved membrane γ-ENaC and increased γ-ENaC cleavage products. Membrane abundance of α- and β-ENaC subunits was unaltered. Reduced UT-A1 abundance was observed in nephrotic rats fed ad libitum, but not in pair-fed nephrotic animals. UT-A3 abundance was not altered in treated animals. NKCC2 and AQP2 mRNA levels were similar in treated and control animals.
Doxorubicin-induced nephropathy is an established model of progressive glomerulosclerosis, prototypical of human focal segmental glomerulosclerosis. A single injection of doxorubicin, an anthracycline antibiotic, predictably induces progressive proteinuria that starts at 5–7 days and peaks at 4–5 wk following the drug administration (2). Doxorubicin is thought to exert an initial cytotoxic phase of podocyte injury, followed by a delayed self-perpetuating progressive phase. The doxorubicin rat model of NS has provided significant contributions to our understanding of this disease. Yet, the model entails certain limitations. The wide animal-to-animal variability in response to doxorubicin is well-documented (26). In addition, different doxorubicin doses and methods of administration may contribute to the wide variability in outcomes between different studies. Furthermore, the possibility that doxorubicin might exert a direct confounding effect on the tubular epithelium cannot be entirely excluded. Nevertheless, the consistency of our findings with previous studies in other models of NS, namely the puromycin-induced and mercury-induced models (1, 11, 12), suggests a general association with NS rather than a direct drug-related effect. Considering the known ability of the tubular epithelium to regenerate, and the short half-life of doxorubicin (20 to 48 h), it also appears unlikely that any direct tubular effects of doxorubicin would persist by the time of our analysis, 3 to 4 wk following drug injection.
The finding of reduced UT-A1 abundance in the non-pair-fed-treated group is consistent with an earlier report (12). In this study, however, we report a likely nutritional bias affecting UT-A1 abundances in nephrotic rats. Reduced caloric intake, along with reduced protein and/or salt intake, appears to confound changes in UT-A1 abundances in treated rats. Consistent with this notion, a study by Kim et al. (22) showed that rats fed a low-salt diet have reduced abundance of IM UT-A1 and UT-A3. The effect of a low-salt diet is most likely mediated by the tonicity enhancer binding protein, which regulates the transcription of both UT-A1 and UT-A3. Similarly, rats fed a low-protein diet develop decreased UT-A1 abundance in IM tip tissue, and to a lesser extent, IM base (22). IM levels of AQP2 were significantly reduced in non-pair-fed-treated animals, and to a lower extent in their pair-fed counterparts. The discrepant responses of AQP2 in the pair-fed and non-pair-fed groups may also be explained by changes in protein intake. In an earlier study, a low-protein diet is associated with reduced AQP2 abundance (27). mRNA tissue levels were similar between the treated and control groups, indicating that the differences in protein abundance are more likely to be mediated via a posttranscriptional mechanism.
In an earlier micropuncture study, nephrotic and control rats demonstrated similar urinary sodium delivery at the level of the connecting tubule (15). This occurs despite a profound defect in sodium reabsorption in the the ascending limb of the loop of Henle and the distal convoluted tubule, resulting from downregulation of the thick ascending limb sodium transporters. Balanced sodium delivery between nephrotic and control animals is probably maintained by a decrease in glomerular filtration of sodium, due to reduced glomerular filtration rate in nephrotic animals. Changes in NKCC2 and NCC appear to play a role in maintaining this balance in distal nephron sodium delivery. Elucidating the underlying mechanisms behind reduced NKCC2 and NCC requires further study. The reduction in NCC abundance may represent a compensatory response to ENaC-induced salt retention. Mechanisms similar to those seen in the known phenomenon of “aldosterone escape” may be involved. Although NS is not consistently associated with elevated aldosterone, ENaC hyperactivity in nephrotic animals results in a similar state of salt retention, simulating hyperaldosteronism. In a rat model of aldosterone escape, NCC protein abundance was reduced, but mRNA levels were not altered, suggesting a posttranscriptional mechanism (31). On the other hand, reduced NKCC2 abundance is not a feature of aldosterone escape, and therefore, it represents a distinctive feature of NS. In this study, we reveal two remarkable observations pertaining to the observed change in NKCC2. First, NKCC2 mRNA levels were similar between the control and doxorubicin-treated group, suggesting a likely posttranscriptional mechanism for reduced protein abundance. Second, NKCC2 abundance was relatively preserved in the cortex compared with the inner stripe of OM, signifying intact macula densa NKCC2, and possibly intact associated chloride-sensing mechanisms. The latter finding may explain, through tubuloglomerular feedback, the reduced glomerular filtration rate observed in nephrotic rats. In this situation, reduced glomerular filtration rate represents an inappropriate attempt to save the animals from excessive salt wasting.
Studies evaluating ENaC subunit abundances in nephrotic models have yielded conflicting results. In one report, utilizing the puromycin aminonucleoside model of NS, the tissue protein abundance of α-ENaC and β-ENaC was increased in the inner stripe of the OM and in the IM but was not altered in the cortex. γ-ENaC abundance was increased in the cortex, inner strip of OM, and IM (21). In another report, the protein abundance of α-ENaC was increased in the cortex/outer stripe of OM and inner stripe of the OM in HgCl2-induced nephrotic rats. The protein abundances of β-ENaC and γ-ENaC were decreased in the cortex/outer stripe of OM but increased in the inner stripe of OM (3). In a third report, the abundance of all ENaC subunits was increased in cortex and outer stripe of OM combined, and inner stripe of OM in adrenalectomized, puromycin-treated nephrotic rats (7). A shift in the electrophoretic migratory pattern of γ-ENaC subunit, with increased whole tissue abundance of the more active 70-kDa isoform, was suggested in two reports (21, 23). However, another study showed complete absence of the 70-kDa isoform in adrenalectomized, puromycin-treated nephrotic rats (7). Different methodological approaches may explain some of those conflicting results. Notably, all previous reports evaluated whole tissue abundance of ENaC subunits rather than membrane fractions. However, ENaC proteolytic activation by luminal extracellular proteases is more likely to be reflected by changes in membrane protein fractions than whole tissue abundance, as reflected in our study. In this study, we measured the membrane abundance of ENaC subunits in the cortex of doxorubicin-treated and control rats. In vitro, nephrotic urine activates the ENaC through proteolytic cleavage of the γ-subunit (29). We observed an increase in the more active γ-cleavage product. This finding is consistent with the proposed hypothesis that salt retention is mediated by proteolytic activation of ENaC by an extracellular protease. The membrane abundance of α-and β-ENaC subunits was unaltered, further supporting this notion. To our knowledge, this constitutes the first in vivo evidence of abnormal proteolytic activation of membrane-bound ENaC in nephrotic animals.
Consistent with earlier reports, we demonstrated a considerable defect in urine concentration in nephrotic rats, manifested by reduced urine osmolality and increased fractional excretion of water. Several factors may contribute to the observed defect in water homeostasis. First, the reduced abundance of NKCC2, a powerful contributor to the corticomedullary osmotic gradient, is likely to result in defective collecting duct water reabsorption. Second, reduced AQP2 abundance is expected to reduce the water permeability of the IM collecting duct, further exacerbating the absorptive defect. Third, rats with doxorubicin-induced NS adapt to the continuous urinary protein losses by reducing overall urea excretion, maintaining a neutral nitrogen balance. However, this adaptation may occur at the expense of reducing the availability of urea, another major participant in the formation of the corticomedullary osmotic gradient, hence reducing urine-concentrating ability. Our observation of reduced urea nitrogen excretion in nephrotic pair-fed rats confirms the findings of an earlier report. Despite equal dietary intake in pair-fed animals, treated rats may develop decreased production of urea as a compensatory response to counteract the excessive urinary protein nitrogen losses. Several human and rat studies attributed this phenomenon to a decrease in amino acid oxidation in nephrotic subjects (5, 6, 25).
In summary, our study reveals a profound reduction in protein abundance of NKCC2 in the OM and, to a lower extent, cortex of non-pair-fed and pair-fed nephrotic rats. A reduction in the uncleaved membrane γ-ENaC was seen in treated rats, coupled with an increase in γ-ENaC membrane cleavage products. NCC and AQP2 abundances were moderately reduced in non-pair-fed and pair-fed nephrotic rats. UT-A1 abundance was reduced in non-pair-fed nephrotic animals, but not their pair-fed counterparts, suggesting a nutritional bias. NKCC2 and AQP2 mRNA levels were unaltered in treated vs. control animals, suggesting a posttranscriptional mechanism behind the decrease in protein abundance. We conclude that these changes in transporter abundances may represent a response to avid reabsorption of sodium at the level of the CCD, coupled with a general disruption of the corticomedullary osmotic gradient.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01DK41707, R37DK037963, and by the UT Southwestern NIH O'Brien Kidney Research Center P30DK079328.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.N.B.M., J.M.S., and J.D.K. conception and design of research; R.N.B.M., B.M., X.H.W., C.F.M., D.C.E., and J.D.K. performed experiments; R.N.B.M., B.M., X.H.W., C.F.M., D.C.E., J.M.S., and J.D.K. analyzed data; R.N.B.M., D.C.E., J.M.S., and J.D.K. interpreted results of experiments; R.N.B.M., B.M., D.C.E., J.M.S., and J.D.K. prepared figures; R.N.B.M. and J.D.K. drafted manuscript; R.N.B.M., B.M., X.H.W., D.C.E., J.M.S., and J.D.K. edited and revised manuscript; R.N.B.M., B.M., X.H.W., C.F.M., D.C.E., J.M.S., and J.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Peter Igarashi, Michel Baum, and Chou-Long Huang from the UT Southwestern O'Brien Center for the generous support, Dr. Robert Hoover at Emory for NCC antibody, and to S. Selvaraj for helping with the creatinine determinations.
REFERENCES
- 1. Apostol E, Ecelbarger C, Terris J, Bradford A, Andrews P, Knepper M. Reduced renal medullary water channel expression in puromycin aminonucleoside-induced nephrotic syndrome. J Am Soc Nephrol 8: 15–24, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Bertani T, Poggi A, Pozzoni R, Delaini F, Sacchi G, Thoua Y, Mecca G, Remuzzi G, Donati MB. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest 46: 16–23, 1982 [PubMed] [Google Scholar]
- 3. Bistrup C, Thiesson HC, Jensen BL, Skott O. Reduced activity of 11beta-hydroxysteroid dehydrogenase type 2 is not responsible for sodium retention in nephrotic rats. Acta Physiol Scan 184: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Choi EJ, Bailey J, May RC, Masud T, Maroni BJ. Metabolic responses to nephrosis: effect of a low-protein diet. Am J Physiol Renal Fluid Electrolyte Physiol 266: F432–F438, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Choi EJ, May RC, Bailey J, Masud T, Dixon A, Maroni BJ. Mechanisms of adaptation to proteinuria in adriamycin nephrosis. Am J Physiol Renal Fluid Electrolyte Physiol 265: F257–F263, 1993 [DOI] [PubMed] [Google Scholar]
- 7. de Seigneux S, Kim SW, Hemmingsen SC, Frokiær J, Nielsen S. Increased expression but not targeting of ENaC in adrenalectomized rats with PAN-induced nephrotic syndrome. Am J Physiol Renal Physiol 291: F208–F217, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Deschenes G, Doucet A. Collecting duct (Na+/K+)-ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J Am Soc Nephrol 11: 604–615, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Doucet A, Favre G, Deschenes G. Molecular mechanism of edema formation in nephrotic syndrome: therapeutic implications. Pediatr Nephrol 22: 1983–1990, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ecelbarger CA, Sands JM, Doran JJ, Cacini W, Kishore BK. Expression of salt and urea transporters in rat kidney during cisplatin-induced polyuria. Kidney Int 60: 2274–2282, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Llama P, Andrews P, Ecelbarger CA, Nielsen S, Knepper MA. Concentrating defect in experimental nephrotic syndrome: altered expression of aquaporins and thick ascending limb Na+ transporters. Kidney Int 54: 170–179, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Llama P, Andrews P, Nielsen S, Ecelbarger CA, Knepper MA. Impaired aquaporin and urea transporter expression in rats with adriamycin-induced nephrotic syndrome. Kidney Int 53: 1244–1253, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol 290: L710–L722, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schor N, Troy JL, Lechene CP, Brenner BM. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest 71: 91–103, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol 285: F303–F309, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kim D, Sands JM, Klein JD. Role of vasopressin in diabetes mellitus-induced changes in medullary transport proteins involved in urine concentration in Brattleboro rats. Am J Physiol Renal Physiol 286: F760–F766, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kim SW, de Seigneux S, Sassen MC, Lee J, Kim J, Knepper MA, Frøkiær J, Nielsen S. Increased apical targeting of renal ENaC subunits and decreased expression of 11βHSD2 in HgCl2-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol 290: F674–F687, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kim SW, Wang W, Nielsen J, Praetorius J, Kwon TH, Knepper MA, Frøkiær J, Nielsen S. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol 286: F922–F935, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Kim YM, Kim WY, Lee HW, Kim J, Kwon HM, Klein JD, Sands JM, Kim D. Urea and NaCl regulate UT-A1 urea transporter in opposing directions via TonEBP pathway during osmotic diuresis. Am J Physiol Renal Physiol 296: F67–F77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lourdel S, Loffing J, Favre G, Paulais M, Nissant A, Fakitsas P, Créminon C, Féraille E, Verrey F, Teulon J, Doucet A, Deschênes G. Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol 16: 3642–3650, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. ENaC degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Maroni BJ, Staffeld C, Young VR, Manatunga A, Tom K. Mechanisms permitting nephrotic patients to achieve nitrogen equilibrium with a protein-restricted diet. J Clin Invest 99: 2479–2487, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Sands JM, Naruse M, Jacobs JD, Wilcox JN, Klein JD. Changes in aquaporin-2 protein contribute to the urine concentrating defect in rats fed a low-protein diet. J Clin Invest 97: 2807–2814, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwab SJ, Christensen RL, Dougherty K, Klahr S. Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med 147: 943–944, 1987 [PubMed] [Google Scholar]
- 29. Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svenningsen P, Uhrenholt TR, Palarasah Y, Skjodt K, Jensen BL, Skott O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol 297: R1733–R1741, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215–222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang XH, Du J, Klein JD, Bailey JL, Mitch WE. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int 76: 751–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]