Abstract
Our recent study in cats revealed that inhibition of bladder overactivity by tibial nerve stimulation (TNS) depends on the activation of opioid receptors. TNS is a minimally invasive treatment for overactive bladder (OAB), but its efficacy is low. Tramadol (an opioid receptor agonist) is effective in treating OAB but elicits significant adverse effects. This study was to determine if a low dose of tramadol (expected to produce fewer adverse effects) can enhance the TNS inhibition of bladder overactivity. Bladder overactivity was induced in α-chloralose-anesthetized cats by an intravesical infusion of 0.25% acetic acid (AA) during repeated cystometrograms (CMGs). TNS (5 Hz) at two to four times the threshold intensity for inducing toe movement was applied during CMGs before and after tramadol (0.3–7 mg/kg iv) to examine the interaction between the two treatments. AA irritation significantly reduced bladder capacity to 24.8 ± 3.3% of the capacity measured during saline infusion. TNS alone reversibly inhibited bladder overactivity and significantly increased bladder capacity to 50–60% of the saline control capacity. Tramadol administered alone in low doses (0.3–1 mg/kg) did not significantly change bladder capacity, whereas larger doses (3–7 mg/kg) increased bladder capacity (50–60%). TNS in combination with tramadol (3–7 mg/kg) completely reversed the effect of AA. Tramadol also unmasked a prolonged (>2 h) TNS inhibition of bladder overactivity that persisted after termination of the stimulation. The results suggest a novel treatment strategy for OAB by combining tibial neuromodulation with a low dose of tramadol, which is minimally invasive with a potentially high efficacy and fewer adverse effects.
Keywords: tibial nerve, overactive bladder, neurotransmitter, stimulation
overactive bladder (OAB) symptoms, including detrusor overactivity, frequency, urgency, and incontinence, have an overall prevalence of 16–17% (12, 27). Currently, antimuscarinic drugs are the primary pharmacological treatment for OAB; however, these agents elicit significant adverse effects and, in turn, a high patient dropout rate (1, 9). Sacral neuromodulation is an alternative treatment option for OAB refractory to pharmacotherapy, but it requires surgery (13, 35). Tibial nerve stimulation (TNS) is also useful in treating OAB and is minimally invasive, but its efficacy is relatively low (10, 19). Our recent studies (28, 31) revealed that TNS can acutely suppress reflex bladder activity in anesthetized cats and also elicit a prolonged increase in bladder capacity that persists after termination of the stimulation. In addition, naloxone (an opioid receptor antagonist) can eliminate TNS-induced inhibition of bladder overactivity, indicating a role of opioid receptors (29).
Drugs that activate opioid receptors are known to suppress bladder activity (2, 8, 11, 15–18, 25). For example, tramadol, an opioid receptor agonist and a serotonin and noradrenaline reuptake inhibitor, is effective in inhibiting normal and overactive bladders in rats (15–18). In normal human subjects, tramadol at the recommended analgesic dosage (150 mg daily) can cause urinary retention (11). It is also effective in treating OAB symptoms at a slightly higher dosage (200 mg daily), which elicits significant adverse effects including nausea, vomiting, dizziness, and constipation (6, 25).
In the present study, which was conducted on acetic acid (AA)-irritated bladders in cats, we determined if activation of opioid receptors with a low dosage of tramadol, which should have fewer adverse effects, enhances the inhibitory effect of TNS on reflex bladder activity. Our results show that a combination of TNS with a low dose of tramadol completely suppressed irritation-induced bladder overactivity, raising the possibility of a new treatment strategy for OAB.
MATERIALS AND METHODS
The Animal Care and Use Committee of the University of Pittsburgh approved all protocols involving the use of animals in this study.
Animal preparation.
Experiments were conducted on 17 adult cats (8 males and 9 females, weight: 2.4–4.0 kg) under α-chloralose anesthesia (65 mg/kg iv). The right carotid artery and ulnar vein were catheterized to monitor blood pressure and administer tramadol, respectively. Heart rate and blood oxygen level were monitored by a pulse oximeter (9847 V, NONIN Medical, Playmouth, MN) with the sensor attached to the tongue. Reduction of the blood oxygen level to ∼85–90% was observed at high doses of tramadol (7–10 mg/kg iv), but artificial respiration was never used in this study. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pump to infuse the bladder with saline or 0.25% AA at a rate of 1–2 ml/min, and the other lumen was connected to a pressure transducer to measure intravesical pressure. A tripolar cuff electrode (NC223pt, MicroProbe, Gaithersburg, MD) was implanted on the left tibial nerve above the ankle.
Stimulation protocol and tramadol administration.
Uniphasic rectangular pulses (0.2-ms pulse width) at 5-Hz frequency were delivered to the tibial nerve via the cuff electrode. The intensity threshold (T) for inducing toe movement was determined by gradually increasing the stimulation intensity. Because our previous study (31) indicated that a stimulus intensity of >2 T was required to inhibit reflex bladder contractions, intensities of 2 or 4 T were used to suppress bladder overactivity induced by 0.25% AA.
In 11 cats, TNS was applied during repeated cystometrograms (CMGs), which consisted of a slow infusion of AA starting with an empty bladder. Bladder capacity is defined as the threshold bladder volume to induce the first large-amplitude (>30 cmH2O), long-duration (>30 s) bladder contraction during a CMG (see the first trace in Fig. 1A). Initially, three to five saline CMGs were performed without TNS to obtain the control bladder capacity and evaluate reproducibility. Another three to five CMGs were then performed during a 30- to 50-min period with an infusion of 0.25% AA to irritate the bladder, activate nociceptive bladder C-fiber afferents, and induce bladder overactivity. After bladder capacity stabilized, four CMGs were performed during AA infusion: 1) control without TNS, 2) during 2-T TNS, 3) during 4-T TNS, and 4) control without TNS (Fig. 1A). Cumulative doses (0.3, 1, 3, and 7 mg/kg iv) of tramadol (Sigma-Aldrich, St. Louis, MO) were then given. Starting 10 min after each dose of tramadol, the four CMGs were repeated during AA infusion. A 5-min rest period was inserted between each CMG to allow for recovery of the micturition reflex. At the end of the experiments (n = 9), a higher dose of tramadol (10 mg/kg iv) was given to examine the maximal inhibitory effect. Naloxone (2 mg/kg iv) was then used to maximally antagonize opioid receptors and to determine if the inhibitory effect induced by combination of TNS and tramadol treatment could be reduced.
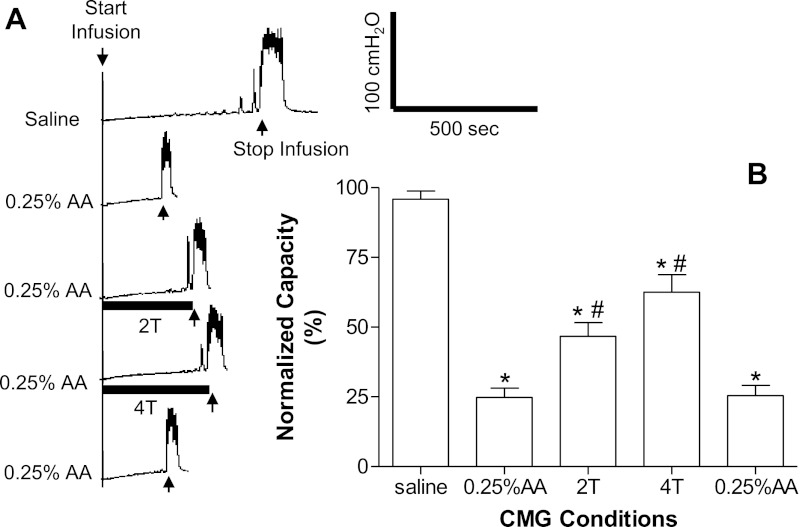
Fig. 1.
Tibial nerve stimulation (TNS) inhibited bladder overactivity induced by bladder irritation using 0.25% acetic acid (AA). A: repeated cystometrograms (CMGs) showing a saline control followed by AA infusion and TNS inhibition and a control after TNS. Stimulation: 0.2-ms pulse width; intensity threshold for inducing toe movement (T) = 0.35 V; infusion rate: 1 ml/min. The solid horizontal bar under the pressure trace indicates stimulation duration. B: summarized results (n = 17 cats). T = 0.35–4.5 V; infusion rate: 1–2 ml/min. *Significantly different from the bladder capacity measured during saline control CMGs; #significantly different from the bladder capacity measured during AA control CMGs.
In another six cats, a similar protocol was used except that after AA irritation of the bladder, saline vehicle was administered intravenously instead of tramadol between each group of four CMGs to evaluate the effect of repeated TNS on bladder capacity. This was followed by a series of CMGs without TNS during which cumulative doses (0.3, 1, 3, and 7 mg/kg) of tramadol were administered to examine the effect of the drug alone on bladder capacity. After administration of the largest dose of tramadol, the four CMG protocol was repeated, followed by an additional five CMGs without stimulation to determine if a poststimulation inhibition was unmasked by tramadol.
Data analysis.
Bladder capacity was measured during each CMG and normalized to the first saline control CMG in each experiment. However, when poststimulation inhibition was evaluated, the bladder capacities measured during the last five CMGs after TNS were normalized to the control CMG performed after 7 mg/kg tramadol. Repeated measurements in the same animal during the same experiment were averaged. Normalized data from different animals are presented as means ± SE. ANOVA followed by Dunnett's multiple-comparison test and Student's t-test were used to detect statistical significance (P < 0.05).
RESULTS
Tibial nerve inhibition of bladder overactivity induced by AA irritation.
Bladder capacities during saline CMGs averaged 9.9 ± 1.1 ml (n = 17 cats). The subsequent infusion of 0.25% AA significantly (P < 0.0001) reduced bladder capacity to 24.8 ± 3.3% of the control capacity (Fig. 1). During AA infusion, bladder capacity remained constant during repeated CMGs (bladder capacity = 26.7 ± 5.9% of control at the end of 2.5–3 h; Fig. 2C).
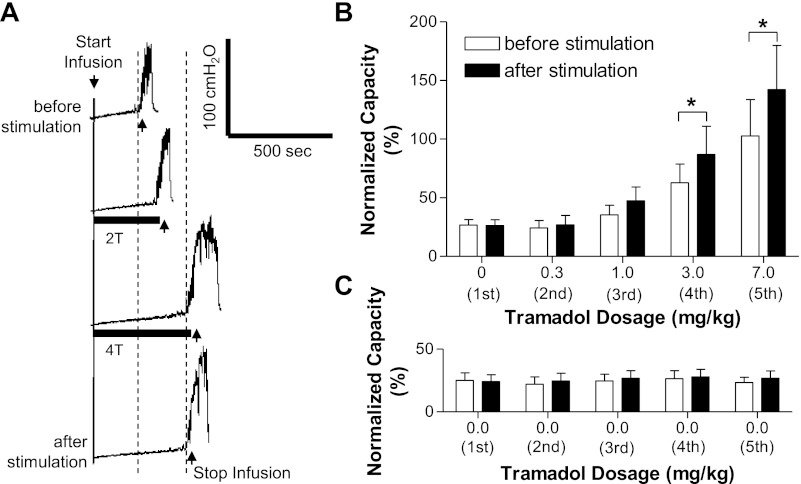
Fig. 2.
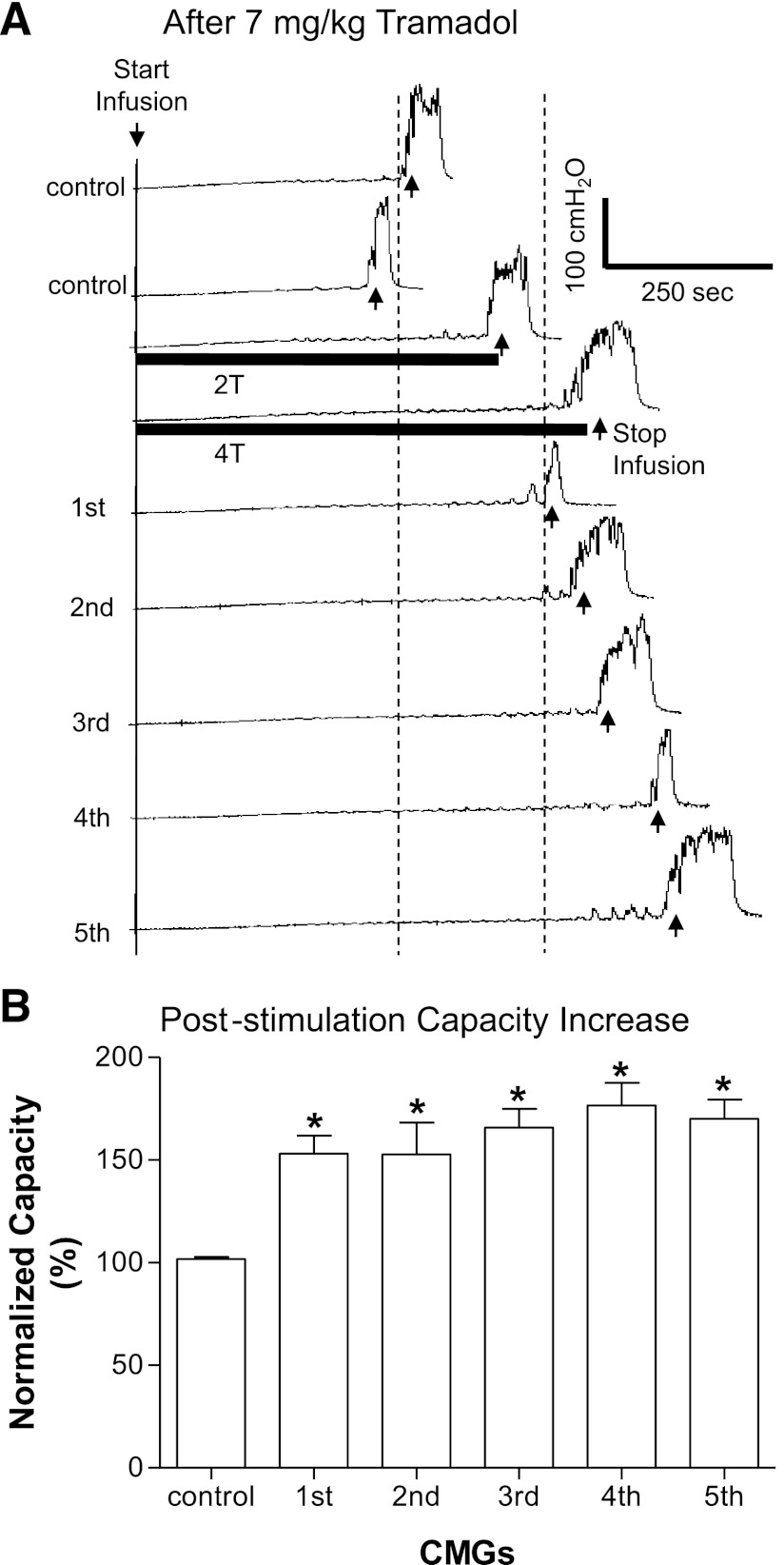
Poststimulation inhibition of AA-induced bladder overactivity emerged after tramadol treatment (A and B) but did not occur before tramadol (C). A: after 3 mg/kg tramadol, the increased bladder capacity induced by 2- and 4-T TNS (T = 1.2 V) applied during CMGs was maintained after the stimulation. B: summarized results from repeated tests of TNS (2 and 4 T) as shown in A. The poststimulation inhibitory effect became significant (*) at a tramadol dosages of 3–7 mg/kg. Stimulation: 0.2-ms pulse width; T = 0.35–4.5 V; n = 11 cats. C: control data obtained from another 6 cats showing that repeated tests of TNS (2 and 4 T) without tramadol treatment did not induce poststimulation inhibitory effects. Stimulation: 0.2-ms pulse width; T = 0.35–1.2 V. 1st through 5th in B and C indicate the repeated tests of TNS (2 and 4 T) as shown in A.
TNS (5 Hz) at 2 or 4 T applied during AA CMGs significantly (P < 0.01, n = 17 cats) increased bladder capacity to 46.7 ± 4.9% and 62.6 ± 6.2%, respectively, of the saline control capacity (Fig. 1) but failed to completely reverse the effect of AA irritation. Bladder capacity returned to the control level within 5 min after the termination of TNS (see last trace and bar in Fig. 1, A and B). Repeated application of TNS at 2 and 4 T during AA infusion CMGs for five times during a period of 2.5–3 h (n = 6 cats) elicited reversible (Fig. 2C) and reproducible increases in bladder capacity (50–60%).
Dose-dependent effect of tramadol on bladder overactivity.
The effect of tramadol alone on AA-irritated bladders in the absence of TNS was examined in six cats by administering increasing doses of the drug at 20-min intervals to construct cumulative dose-response curves (see “no stimulation” curve in Fig. 3). Bladder capacity was not significantly changed at the 0.3 and 1 mg/kg doses, whereas the 3 and 7 mg/kg doses significantly (P < 0.01) reversed the irritant effect of AA, increasing bladder capacity to 50% and 60%, respectively, of the saline control capacity (see no stimulation curve in Fig. 3). The effects of the two largest doses were not significantly different.
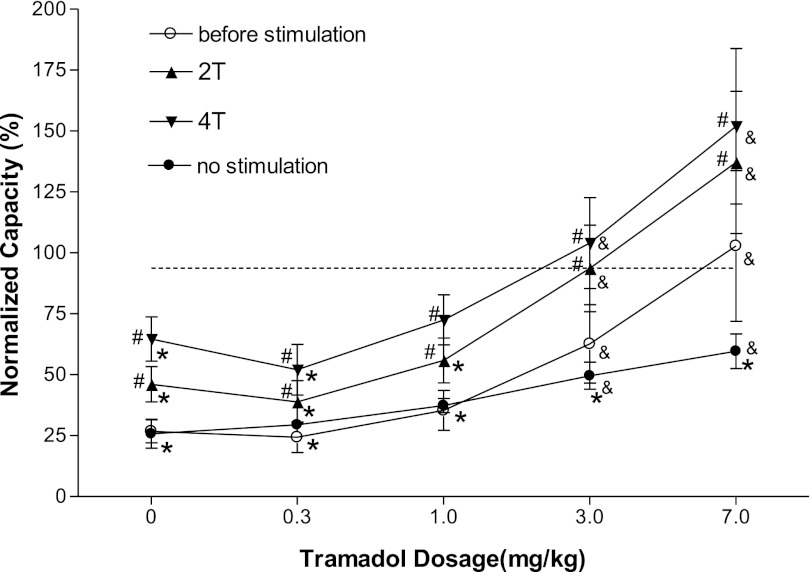
Fig. 3.
Changes in bladder capacity induced by a combination of TNS and/or increasing doses of tramadol administered to each cat during an intravesical infusion of 0.25% AA. The before stimulation curve shows the effect of tramadol injected before each application of TNS at 2 and 4 T in 11 cats. The no stimulation curve shows the effect of tramadol without TNS in 6 cats. The 2T and 4T curves show the effect of TNS after each dose of tramadol. &Significantly different from the bladder capacity measured before tramadol treatment (i.e., at 0 mg/kg) under different conditions (before stimulation, 2T, 4T, or no stimulation curves); *significantly different from the saline control capacity (indicated by the dashed line); #data on the TNS curve are significantly different from those on the before stimulation curve at that dose of tramadol. Stimulation: 0.2-ms pulse width; T = 0.35–4.5 V.
Effect of combined TNS and tramadol treatment on bladder overactivity.
The combined effect of tramadol and TNS was examined by administering increasing cumulative drug doses and then testing TNS at 2 and 4 T after each dose. As the tramadol dose increased above 0.3 mg/kg, bladder capacities measured before each TNS (open bars in Fig. 2B, “before stimulation” curve in Fig. 3, and Fig. 4A) progressively increased. After 3 mg/kg tramadol, bladder capacity was significantly (P < 0.05) larger than control capacity, and after 7 mg/kg, bladder capacity was fully restored to the saline control level (before stimulation curve, Fig. 3). Although these changes in bladder capacity were larger than those induced when tramadol was administered alone in the absence of TNS (compare before stimulation curve with no stimulation curve in Fig. 3), the differences were not statistically significant (P = 0.29).
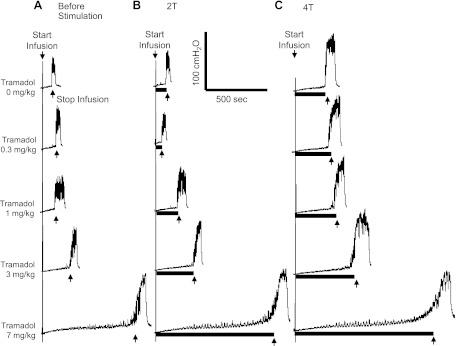
Fig. 4.
Dose-dependent effect of tramadol on bladder overactivity and TNS inhibition during CMGs with an intravesical infusion of 0.25% AA. Tramadol doses on the left apply to A–C. The CMGs at increasing cumulative doses of tramadol were performed in sequence from left to right in A–C and from top to bottom. A: control CMGs before stimulation. B: CMGs during stimulation at 2 T. C: CMGs during stimulation at 4 T. The solid horizontal bar under the pressure trace indicates the stimulation duration. Stimulation: 0.2-ms pulse width; T = 1.2 V; infusion rate: 1 ml/min.
TNS (2 and 4 T) applied after the administration of each tramadol dose significantly (P < 0.05) increased bladder capacity (Figs. 3 and 4, B and C). After 3 mg/kg tramadol, TNS at 2 and 4 T completely reversed the effect of AA irritation (Fig. 3). TNS after 7 mg/kg tramadol increased bladder capacity to 140–150% of the saline control (Figs. 3 and 4). The combined effect of TNS and tramadol at 3–7 mg/kg doses was significantly (P < 0.05) larger than the effect of TNS alone or the combined effect of the lowest dose (0.3 mg/kg) of tramadol and TNS (Fig. 3).
Poststimulation inhibition of bladder overactivity emerged after tramadol treatment.
The increase in bladder capacity induced by TNS during AA CMGs rapidly (within 5 min) and completely reversed after termination of the stimulation (Figs. 1A and 2C). This rapid reversal contrasted with the prolonged increase in bladder capacity (i.e., poststimulation inhibition) that persists for at least 2 h after termination of TNS during saline CMGs (31). To determine if tramadol unmasks poststimulation inhibition in AA-irritated bladders, we examined the recovery of bladder capacity after TNS after a range of tramadol doses (Fig. 2B). Low doses of tramadol (0.3 and 1 mg/kg) did not generate a poststimulation inhibition; however, 3 mg/kg (Fig. 2, A and B) and 7 mg/kg (Figs. 2B and 5) unmasked a significant poststimulation increase in bladder capacity. After 7 mg/kg tramadol, this increase persisted during a series of five subsequent CMGs for a period of at least 1.5–2 h (Fig. 5).
Fig. 5.
Poststimulation inhibition of AA-induced bladder overactivity is long lasting. A: repeated CMG traces showing that after 7 mg/kg tramadol, the increased bladder capacity by 2- and 4-T TNS (T = 0.35 V) was maintained in the following 5 consecutive CMGs. B: summarized results showing a significant (*) increase in bladder capacity during the 5 CMGs that lasted for ∼1.5–2 h. Stimulation: 0.2-ms pulse width; T = 0.35–1.2 V; n = 6 cats.
Effect of naloxone on combined TNS and tramadol treatment.
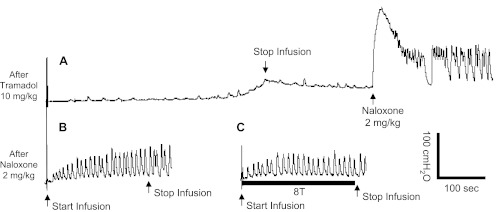
At the end of the experiments, a higher dose (10 mg/kg) of tramadol was administered, which eliminated the micturition reflex in six of nine cats (Fig. 6A). Naloxone (2 mg/kg iv) restored the micturition reflex (Fig. 6A) and significantly reduced bladder capacity to <1 ml (Fig. 6B). After naloxone treatment, TNS did not inhibit bladder activity even at a stimulation intensity of 8 T (Fig. 6C).
Fig. 6.
Effect of naloxone on combined TNS and tramadol treatment. A: the micturition reflex was eliminated after 10 mg/kg iv tramadol but restored by naloxone (2 mg/kg iv). B: control CMG without TNS after naloxone treatment. C: TNS (8 T) was applied during CMG after naloxone treatment. Stimulation: 0.2-ms pulse width; T = 1 V; infusion rate: 1 ml/min.
DISCUSSION
This preclinical study revealed that TNS alone inhibits AA-induced bladder overactivity, but its efficacy was low (50–60% increase in bladder capacity; Fig. 1). Tramadol without TNS also produced an incomplete reversal of AA-induced bladder overactivity, similar to that produced by TNS. However, TNS in combination with tramadol (≥3 mg/kg) normalized the capacity of the irritated bladder (Figs. 3 and 4). Tramadol (3–7 mg/kg) also unmasked a post-TNS inhibition (Figs. 2 and 5) that normalized bladder capacity for a period of at least 1.5–2 h after the stimulation (Fig. 5). These results raise the possibility that neuromodulation in combination with tramadol might be an effective new strategy for treating OAB.
Tramadol is known to produce a number of adverse effects, including nausea, vomiting, dizziness, and constipation (6, 25), which prevent the use of higher doses to achieve greater clinical efficacy. Thus, the dose of tramadol necessary to enhance TNS effects in cats is an important consideration in evaluating the clinical implications of the present results. However, relating the tramadol doses in cats to doses in humans is complicated due to species differences and different routes of administration. When tramadol is used clinically as an analgesic, the recommended oral dose is 50–100 mg every 4–6 h, with a maximum dose of 400 mg/day (6). A recent study (25) has shown that tramadol (100 mg or 1.4 mg/kg for a 70-kg person twice per day) significantly reduced the number of incontinence episodes and produced a 25% increase in bladder capacity in OAB patients. Thus, the human dose is approximately half of the intravenous dose of tramadol (≥3 mg/kg) that increased bladder capacity and facilitated TNS in our present study and less than half of the recommended oral dose (4 mg/kg every 6 h) to treat pain in cats (20, 21). These data indicate that cats are less sensitive to the effects of tramadol. Although our experiments examined the effects of tramadol administered intravenously, this is not likely to be a major impediment to predicting clinical efficacy because oral tramadol is rapidly and almost completely absorbed in cats and intravenous and oral doses of tramadol produce similar plasma concentrations (20). Thus, it seems reasonable to conclude that oral doses of tramadol that produce analgesia in the cat should also be effective in facilitating TNS inhibition of bladder overactivity in the cat. If these data and conclusions are applicable to humans, then analgesic doses of tramadol should also enhance TNS suppression of OAB symptoms.
Tramadol is a complex drug that potentially acts at many sites in the central nervous system and via multiple mechanisms of action (22, 23). Tramadol and its active metabolite are μ-opioid receptor agonists. Tramadol also inhibits reuptake of serotonin and norepinephrine at presynaptic terminals and elicits effects by indirectly activating serotonergic and α2-adrenergic receptors (6, 22, 23). In the cat, the respiratory depression elicited by tramadol (1–4 mg/kg administered intravenously) is significantly reduced by treatment with naloxone, indicating that this effect is dependent on the activation of opioid receptors (32). Reflex bladder activity in the cat is under tonic enkephalinergic inhibitory control mediated by μ- and δ-opioid receptors in the brain and spinal cord, respectively (2, 8, 14, 24). Block of these receptors with naloxone, an opioid receptor antagonist, reduces bladder capacity and induces bladder overactivity in the anesthetized cat (2, 14). Naloxone also suppresses the inhibition of reflex bladder activity induced by TNS (29) or pudendal nerve stimulation (3), indicating an important role of endogenous opioid peptides and opioid receptors in the neuromodulation of bladder function in cats. Our present result (Fig. 6) further showed that naloxone reverses the inhibition of bladder overactivity induced by the combined treatment of tramadol and TNS. These data raise the possibility that the inhibitory effect of tramadol alone on bladder overactivity and the facilitation of the TNS inhibition by tramadol are due to actions at enkephalinergic inhibitory synapses in the brain or spinal cord.
An inhibitory action of tramadol on serotononin and norepinephrine reuptake mechanisms is also a possible contributor to the actions of tramadol on bladder overactivity because naloxone resistant analgesia induced by tramadol has been demonstrated in rodents (22, 23) and some of the naloxone-resistant effects of tramadol on neural pathways in the brain are blocked by α2-adrenergic receptor antagonists or modified by inhibitors of serotonin synthesis (26, 36). Serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine) (33) and serotonergic receptor agonists (7, 30, 34) have prominent inhibitory effects on bladder overactivity in anesthetized cats, indicating a role of endogenous monoamines in the control of the bladder as well as a possible role of these neurotransmitters in the actions of tramadol on bladder reflexes.
The interactions between tramadol and TNS can be separated into two components: 1) enhancement of the transient TNS inhibition by tramadol, which might simply reflect the summation of the two independent inhibitory effects; and 2) the unmasking of TNS poststimulation inhibition. The latter interaction could be defined as potentiation because the effect of the combined treatment is greater than the algebraic summation of the individual treatments.
The failure of TNS to elicit poststimulation inhibition in the AA irritation model before tramadol treatment was unexpected because our previous study (31) in nonirritated bladders in cats showed a TNS poststimulation inhibition of reflex bladder activity that persisted for at least 2 h. Thus, AA irritation may activate a nociceptive mechanism that suppresses the synaptic changes underlying the prolonged TNS poststimulation inhibition. Tramadol that has analgesic properties may block this nociceptive mechanism and unmask the prolonged inhibition.
Another explanation for the absence of TNS poststimulation during AA-irritated CMG is that bladder activity in irritated and nonirritated bladders may depend on different reflex pathways. Bladder distention by saline infusion primarily activates nonnociceptive bladder Aδ-afferent fibers, which induce a supraspinal micturition reflex, whereas AA irritation of the bladder also activates nociceptive bladder C-fiber afferents, which trigger a spinal micturition reflex (4, 5). Therefore, our failure to demonstrate a poststimulation inhibition of the AA-irritated cat bladder suggests that the long-lasting effects of tibial neuromodulation on OAB symptoms in humans might be related to inhibition of the Aδ-fiber-mediated supraspinal micturition pathway and that TNS induced poststimulation inhibition of C-fiber-mediated bladder overactivity can only be induced by TNS when combined with tramadol treatment.
If our results in cats showing additive effects of tramadol and TNS (Fig. 3) translate to humans, then the clinical dosage of tramadol necessary for treating OAB could be significantly reduced when combined with tibial neuromodulation, thereby significantly reducing or eliminating the adverse effects of tramadol. The proposed combined new treatment strategy also provides significant advantages over sacral or tibial neuromodulation alone because sacral neuromodulation, which has a high efficacy in OAB treatment, is invasive, requiring surgery to implant the stimulator and electrode (13, 35). Tibial neuromodulation is minimally invasive, only requiring insertion of a needle electrode at the ankle to stimulate the tibial nerve, but its efficacy is lower than sacral neuromodulation (10, 19). Our results (Figs. 3 and 4) showed that the efficacy of tibial neuromodulation can be significantly increased to completely inhibit bladder overactivity if combined with a low dose of tramadol, potentially yielding a treatment strategy that would be minimally invasive with a high efficacy and fewer adverse effects.
Clinical tibial neuromodulation requires a 30-min treatment once per week for 12 wk followed by maintenance treatments once every 2–3 wk (10, 19), indicating a significant poststimulation inhibitory effect. Our present results (Figs. 2 and 5) showing that tramadol unmasks and/or enhances the TNS poststimulation inhibition in a bladder overactivity model raise the possibility that the clinical use of a combined treatment strategy might reduce the number of treatments required to produce a clinical response and increase the interval between maintenance treatments as well as the maximum efficacy of TNS by expanding the number of central pathways sensitive to neuromodulation. Since tibial neuromodulation and tramadol are both used in clinical practice, a clinical trial to test the combined treatment and to determine the duration of tramadol induced poststimulation inhibition should be feasible.
In summary, this study suggests a novel treatment strategy for OAB by combining tibial neuromodulation with a low dose of tramadol. This strategy is minimally invasive with a potentially high efficacy and fewer adverse effects. If this combined pharmacological-neuromodulation therapy is proven to be successful in clinical trials, it could have a significant impact on the treatment of OAB.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-068566, DK-090006, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. conception and design of research; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; F.Z., A.D.M., P.D.O., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1. Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63, Suppl 3A: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Booth AM, Hisamitsu T, Kawatani M, de Groat WC. Regulation of urinary bladder capacity by endogenous opioid peptides. J Urol 133: 339–342, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol 224: 282–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cats. Am J Physiol Regul Integr Comp Physiol 277: R786–R794, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 43: 879–923, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Gu B, Thor KB, Reiter JP, Dolber PC. Effect of 5-hydroxytryptamine1A serotonin receptor agonists on noxiously stimulated micturition in cats with chronic spinal cord injury. J Urol 177: 2381–2385, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res 298: 51–65, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Lazzeri M, Spinelli M. The challenge of overactive bladder therapy: alternative to antimuscarinic agents. Int Braz J Urol 326: 620–630, 2006 [DOI] [PubMed] [Google Scholar]
- 10. MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, Siegel SW, Tate SB, Feagins BA. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol 183: 234–240, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Meyboom RH, Brodie-Meijer CC, Diemont WL, van Puijenbroek EP. Bladder dysfunction during the use of tramadol. Pharmacoepidemiol Drug Safety 8, Suppl 1): S63–S64, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87: 760–766, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Nakib K, Siegel S. Neuromodulation versus neurotoxin for the treatment of refractory detrusor overactivity: for neuromodulation. Nat Clin Pract Urol 5: 118–119, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int 47, Suppl 1: 19–22, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Oyama T, Homan T, Kyotani J, Oka M. Effect of tramadol on pain-related behaviors and bladder overactivity in rodent cystitis models. Eur J Pharmacol 676: 75–80, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Pandita RK, Pehrson R, Christoph T, Friderichs E, Andersson KE. Actions of tramadol on micturition in awake, freely moving rats. Br J Pharmacol 139: 741–748, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pehrson R, Andersson KE. Tramadol inhibits rat detrusor overactivity caused by dopamine receptor stimulation. J Urol 170: 272–275, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Pehrson R, Stenman E, Andersson KE. Effects of tramadol on rat detrusor overactivity induced by experimental cerebral infarction. Eur Urol 44: 495–499, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Therap 31: 52–59, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Pypendop BH, Siao KT, Ilkiw JE. Effects of tramadol hyhdrochloride on the thermal thershold in cats. Am J Vet Res 70: 1465–1470, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Raffa RB, Friderichs E, Reimann W, Shank RP, Godd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. J Pharmacol Exp Therap 260: 275–285, 1992 [PubMed] [Google Scholar]
- 23. Raffa RB, Friderichs E, Reimann W, Shank RP, Godd EE, Vaught JL, Jacoby HI, Selve N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Therap 267: 331–340, 1993 [PubMed] [Google Scholar]
- 24. Roppolo JR, Booth AM, de Groat WC. The effect of naloxone on the neural control of the urinary bladder of the cat. Brain Res 264: 355–358, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Safarinejad MR, Hosseini SY. Safety and efficacy of tramadol in the treatment of idiopathic detrusor overactivity: a double-blind, placebo-controlled, randomized study. Br J Clin Pharmacol 61: 456–463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Sevcik J, Nieber K, Driessen B, Illes P. Effects of the central analgesic tramadol and it main metabolite, O-desmethyltramadol, on rat locus coeruleus neurons. Br J Pharmacol 110: 169–176, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, Hunt TL, Wein AJ. Prevalence and burden of overactive bladder in the United States. World J Urol 20: 327–336, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Tai C, Chen M, Shen B, Wang J, Roppolo JR, de Groat WC. Irritation induced bladder overactivity is suppressed by tibial nerve stimulation in cats. J Urol 186: 326–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptor in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5 HT1A receptors. Exp Neurol 199: 427–437, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged post stimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tepperma LJ, Nieuwenhuijs D, Olievier CN, Dahan A. Respiratory depression by tramadol in the cat. Anesthesiol 98: 420–427, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural contol of lower urinary tract function in the chloralose-anesthesitized female cat. J Pharmacol Exp Therap 274: 1014–1024, 1995 [PubMed] [Google Scholar]
- 34. Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res 946: 290–297, 2002 [DOI] [PubMed] [Google Scholar]
- 35. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Nijholt AABL, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Yanarates O, Dogrul A, Yildirim V, Sahin A, Sizlan A, Seyrek M, Akgul O, Kozak O, Kurt E, Aypar U. Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-desmethyltramadol, via activation of descending serotonergic pathways. Anesthesiol 112: 696–710, 2010 [DOI] [PubMed] [Google Scholar]