Abstract
Dendritic cells (DCs) protect the respiratory epithelium via induction of innate immune responses and priming of naïve T cells during the initiation of adaptive immunity. Streptococcus pneumoniae, a commonly carried asymptomatic member of the human nasopharyngeal microflora, can cause invasive and inflammatory diseases and the cholesterol-dependent cytotoxin pneumolysin is a major pneumococcal virulence factor implicated in compounding tissue damage and mediating inflammatory responses. While most studies examining the impact of pneumolysin have been based on murine models, we have focused this study on human DC responses. We show that expression of haemolytic pneumolysin inhibits human DC maturation, induction of proinflammatory cytokines and activation of the inflammasome. Furthermore, intracellular production of pneumolysin induces caspase-dependent apoptosis in infected DCs. Similarly, clinical isolates with non-haemolytic pneumolysin were more proinflammatory and caused less apoptosis compared to clonally related strains with active pneumolysin. This study describes a novel role of pneumolysin in the evasion of human DC surveillance that could have a profound clinical impact upon inflammatory disease progression and highlights the need to study human responses to human-specific pathogens.
Keywords: apoptosis, dendritic cells, inflammatory response, pneumococci, pneumolysin
INTRODUCTION
Streptococcus pneumoniae is a commonly carried encapsulated respiratory organism with the potential to cause devastating diseases such as pneumonia, meningitis and sepsis. Innate immune responses to invading pneumococci and their subcomponents play an essential role in disease development. Several pneumococcal virulence factors have been described, of which the polysaccharide capsule is essential for virulence in vivo. Another virulence attribute is the cytotoxin pneumolysin, a cholesterol-dependent pore-forming exotoxin, which also has an important role in pneumococcal pathogenicity. Pneumolysin can induce apoptosis of murine dendritic cells (DCs) (Colino and Snapper, 2003), respiratory epithelial cells (Schmeck et al, 2004; Srivastava et al, 2005) and neuronal cells (Bermpohl et al, 2005; Braun et al, 2002; Mitchell et al, 2004). Pneumolysin can also activate the classical complement pathway (Paton et al, 1984). While the proinflammatory effects of pneumolysin contribute to tissue damage, it is also a focal point of immune responses to pneumococci. Toll-like receptor 4 is involved in host recognition of pneumolysin (Malley et al, 2003), and osmosensing (Ratner et al, 2006) and T cell responses (Kadioglu et al, 2000, 2004) to pneumolysin have been reported. In humans, most of the disease causing isolates express active pneumolysin, but pathogenic serotype 1 isolates producing non-haemolytic pneumolysin are frequently isolated from sterile sources (Jefferies et al, 2007).
DCs are crucial in the host response to microbial pathogens and are widely distributed in tissues throughout the body. They capture antigens by phagocytosis, macropinocytosis and endocytosis (Inaba et al, 1993; Sallusto and Lanzavecchia, 1994). Exposure of phenotypically immature DCs to inflammatory stimuli can convert cells from antigen-capturing to antigen-presenting DCs. The conversion is accompanied by three signals, all of which are required to induce a T cell response. Signal one involves an interaction between peptide-loaded MHC-I and MHC-II molecules on DCs and the T cell receptor. Signal two is mediated by an interaction of B7-2 (CD80 and CD86) co-stimulatory molecules on DCs with the CD28 co-receptor on T cells. Signal three is the production of cytokines by DCs that stimulate the differentiation into effector T cells (TH1, TH2, TH17 or Treg lymphocytes). Once DCs have captured antigen their capacity to acquire additional antigens declines and the activated DCs migrate from the inflammatory site to lymphoid tissues.
DCs can traverse the tight junctions of epithelial surfaces, interact directly with bacteria on the mucosal surface (Rescigno et al, 2001) and control local inflammatory responses. In the lungs DCs are in proximity to alveolar epithelial and capillary endothelial cells and form a network of sentinel cells specialised to sample inhaled bacterial pathogens (Holt, 2000; Holt and Stumbles, 2000). Thus, pneumococci would have to overcome DC-based immunosurveillance in the lungs to promote colonisation of the respiratory tract or cause invasive diseases. However, despite this hypothesis on DC function, a recent study by Winter et al (2007) showed that increasing the murine lung myeloid DCs using FMS-like tyrosine kinase 3 ligand (Flt3L) negatively affected clearance of pneumococci and increased the mortality. Hence, the role of DCs in immunosurveillance remains unclear.
In this study we investigated the effect of pneumococcal pneumolysin production on human DC viability, activation and induction of cytokines involved in inflammation and adaptive immune responses. We show for the first time using human cells that the production of pneumococcal pneumolysin results in a dampening of host immune responses via specific inhibition of human DC-mediated inflammatory responses. In addition, we show that this is in contrast to responses in murine cells.
RESULTS
Capsular and pneumolysin expression affect the uptake and processing of pneumococci by DCs
To examine the interaction of pneumococci with human DCs we infected cells with the wild-type serotype 4 strain T4, and its mutant derivatives deficient in capsule or capsule and pneumolysin production. In Fig 1A the uptake of T4 at a multiplicity of infection (MOI) of 25 was normalised to 1, and fold-changes are reported for comparison. Unencapsulated serotype 4 pneumococci (T4R) were readily taken up by human DCs. Significant uptake of encapsulated strains could be observed after longer infection times indicating that human DCs are capable of taking up encapsulated pneumococci, albeit at a lower efficiency (data not shown). Opsonised conditions (in the presence of antiserum to the serotype 4 capsule) and a higher infection dose also enhanced uptake of the encapsulated strain. The uptake of pneumococci could only be completely inhibited by the addition of both cytochalasin d, to inhibit phagocytosis and endocytosis, and wortmannin, to inhibit macropinocytosis (data not shown). The expression of pneumolysin increased bacterial uptake by approximately 50% (0.76% T4R compared to 0.35% T4RΔply) (Fig 1B) indicating that pneumolysin, directly or indirectly, can induce cell signalling to mediate bacterial uptake. To study the uptake of T4R and T4RΔply in more detail, we fixed infected DCs at different time points post-infection and analysed the intracellular location of bacteria by transmission electron microscopy (TEM). It is thought that in macrophages internalised pneumococci are located within LAMP-containing compartments where they are eventually killed (Gordon et al, 2000). However, to our knowledge, the intracellular fate of whole pneumococci in DCs has not been investigated by microscopy. To compare the intracellular localisation of T4R and T4RΔply during the early stage of infection, we quantified bacteria at 4 h post-infection in the two different types of vacuoles detected in the DCs (Fig 1C–E). Either the bacteria resided in tight vacuoles in close contact with the DC cytosol (Fig 1D, asterisks), or they were located within spacious vacuoles with an electron lucent zone between the phagosomal membrane and the bacterial cell wall (Fig 1E, arrows). Interestingly, the average number of bacteria in spacious vacuoles was considerably higher for T4RΔply (Fig 1C) than for the wild-type strain expressing pneumolysin, which was primarily located in tight vacuoles. At 8 h post-infection (Fig 1F–G) spacious vacuoles were still apparent but the majority of pneumolysin-deficient pneumococci were located within tight vacuoles by this time (Fig 1G), similar to virtually all the pneumolysin-proficient pneumococci (Fig 1F).
Figure 1. Pneumococci are taken up and processed by DCs.
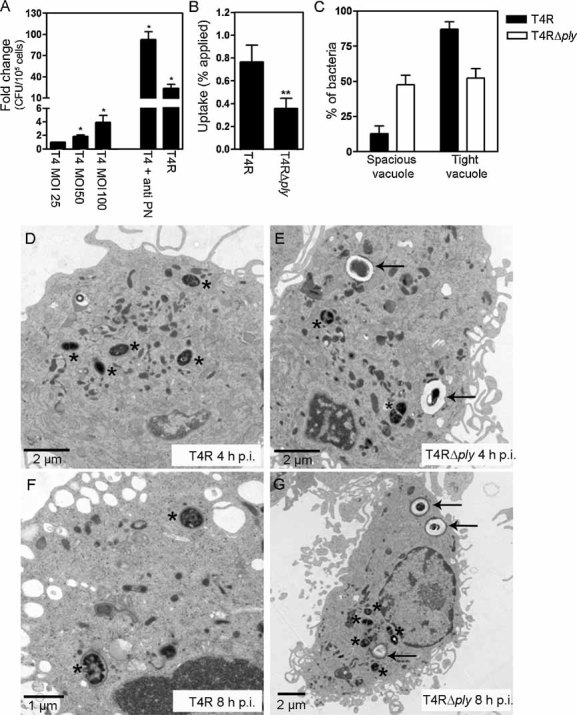
A. DCs were infected with the encapsulated pneumococcal strain T4 (MOI 25, unless otherwise stated) and uptake was monitored by measuring intracellular bacteria by viable count after a 2 h infection period. An increased infection dose and opsonisation using pneumococcal antiserum to serotype 4 (anti-PN) increased the number of intracellular bacteria. The uptake of T4 was also compared to uptake of the unencapsulated mutant T4R (MOI 25). Significant increases compared to uptake of T4 (MOI 25, non-opsonised) are indicated by an asterisk (*). Data represent mean + SE (n = 3).
B. Expression of pneumolysin aided the uptake of pneumococci by DCs. A significant decrease compared to the uptake of T4R is indicated by a double asterisk (**) and data represent mean + SE (n = 10).
C. Quantification of the intracellular location of T4R and T4RΔply revealed that pneumococci reside in two types of vacuoles following uptake. Data represents the mean + standard deviation from two independent experiments.
D. At 4 h post-infection T4R was detected in tight vacuoles in close contact with the cytosol, as indicated by asterisks.
E. In contrast to T4R, after 4 h of infection T4RΔply resided both in spacious (arrows) and tight vacuoles (asterisks).
F, G. After 8 h of infection, both strains of pneumococci were detected primarily in tight vacuoles (asterisks) but spacious vacuoles (arrows) were still observed in T4RΔply infected cells.
Intracellular production of pneumolysin causes apoptosis of infected DCs
As pneumolysin has been implicated in mediating death of various cell types, we examined the impact of pneumolysin expression on the viability of human DCs infected with T4 and mutants deficient in capsule and pneumolysin production. Cells were stained with Annexin V-FITC and PI after infection for 6 and 24 h and cell viability was assessed using flow cytometry. No significant differences were observed in human DC viability after a 6 h infection period (data not shown), whereas after 24 h of infection both T4 and T4R increased the numbers of apoptotic cells compared to uninfected cells (Fig 2A). However, the greatest number of apoptotic cells was observed after infection with unencapsulated T4R showing that host cell cytotoxicity is associated with bacterial uptake, given the greater uptake of unencapsulated strains compared to encapsulated strains (Fig 1A) and since inhibiting uptake of T4R using cytochalasin d and wortmannin also inhibited cell death (Fig 2B). In addition, opsonised uptake of the encapsulated strain also increased DC death (data not shown). Production of pneumolysin promoted DC apoptosis in both the encapsulated and unencapsulated background (Fig 2A). This apoptotic effect of pneumolysin was more pronounced in the unencapsulated background, suggesting that the effect of pneumolysin is dependent on an intracellular location. Hydrogen peroxide has also been shown to mediate host cell death. In human neurons and microglia it is necessary to abolish the expression of both pneumolysin and hydrogen peroxide in order to abrogate cell death, since a lack of either of the factors results in only a partial reduction in apoptosis (Braun et al, 2002). Viability of human DCs was not affected by hydrogen peroxide production in either the presence or absence of pneumolysin expression (data not shown). This resistance is not surprising since human DCs can endogenously produce hydrogen peroxide in response to bacterial products.
Figure 2. Uptake and intracellular pneumolysin production by pneumococci increase the apoptosis of DCs. DCs were infected for 24 h with T4 and mutants in capsule (T4R) and pneumolysin expression (T4Δply and T4RΔply).
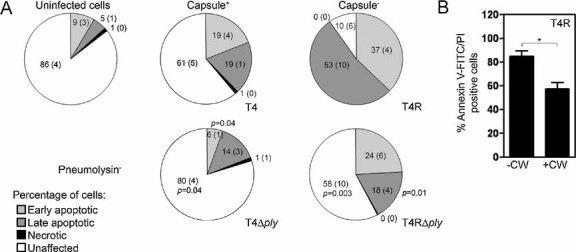
- Statistical analysis of triplicate experiments revealed that T4R induced more apoptosis of human DCs than T4 as monitored by Annexin V-FITC positive cells (early apoptotic), Annexin V and PI positive cells (late-stage apoptotic) and PI positive cells (necrotic). Also, pneumolysin expression increased apoptosis irrespective of capsular expression. Mean (SE) is shown numerically and data is a compilation of three independent experiments. Significant changes compared to the parent strains (T4 and T4R) are indicated by p values. At least 5000 cells per treatment were counted by flow cytometry.
- Inhibition of uptake of T4R with cytochalasin D and wortmannin (CW) decreased cell death. Data represents mean + SE from three independent experiments.
Intracellular pneumolysin expression inhibits DC cytokine production
The production of cytokines by DCs is critical for the development of an appropriate immune response. We investigated the induction of the inflammatory cytokines IL-12 p70 and IL-8 by enzyme-linked immunosorbent assay (ELISA) following infection with live and UV-killed pneumococci at various MOIs and compared the induction of cytokines to cell viability (Fig 3). Lipopolysaccharide (LPS) was used as a positive control and significantly increased the production of IL-12 p70 and IL-8 by DCs (data not shown). A comparison between the cytokine induction by live T4R and T4RΔply revealed that intracellular expression of pneumolysin had a significant down-regulatory effect on human DC production of both IL-12 p70 (Fig 3A) and IL-8 (Fig 3B). The significantly increased amount of IL-12 p70 induced by T4RΔply as compared to T4R could not be solely attributed to the difference in cell viability since at a MOI of 5, a 32-fold change was observed in the level of IL-12 p70 compared to a 3-fold change in viability and at a MOI of 2.5 a 10-fold change in the level of IL-12 p70 was noted compared to a 2-fold change in viability (Fig 3A). The decrease in the amount of IL-8 induced by T4R compared to T4RΔply could however be directly correlated to decreased cell viability, with both parameters showing a 3-fold decrease at a MOI of 5 and a 2-fold decrease at a MOI of 2.5 (Fig 3B). UV-killed pneumococci, with no additional pneumolysin production, did not show the same effect on cytokine production as live bacteria. In this case, UV-killed T4R and T4RΔply induced the same level of both IL-12 p70 (Fig 3C) and IL-8 (Fig 3D) and neither strain caused a decrease in host cell viability. The fine balance regulating the production of cytokines most likely represents signalling via currently unidentified pattern recognition receptors (PRRs) in the spacious vacuole, which we showed to be formed preferentially in the absence of pneumolysin early in infection (Fig 1C).
Figure 3. Pneumolysin expression by live pneumococci inhibits the production of the proinflammatory cytokines IL-8 and IL-12 p70.
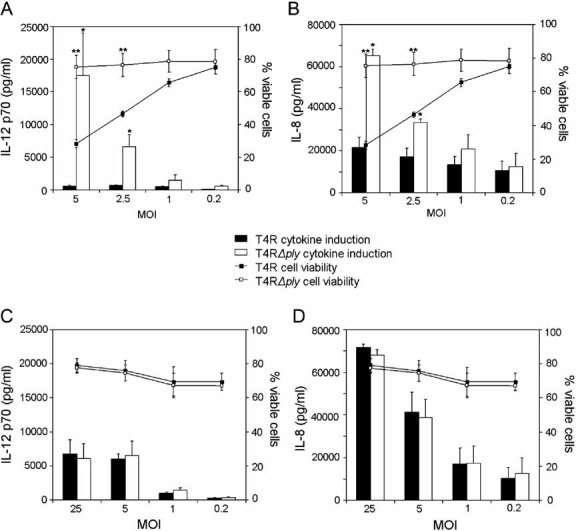
- The effect of pneumolysin expression on the production of IL-12 p70 was examined following infection with live unencapsulated pneumococci at various MOIs. The production of IL-12 p70 was compared to cell viability by analysing the percentage of cells that stained negative for Annexin V and PI with flow cytometry. Data show mean ± SE (n = 3). Significant increases compared to T4R are indicated by an asterisk (*) for cytokines and a double asterisk (**) for cell viability.
- IL-8 production and cell viability was also examined following infection with live unencapsulated pneumococci at various MOIs. Data show mean ± SE (n = 3). Significant increases compared to T4R are indicated by an asterisk (*) for cytokines and a double asterisk (**) for cell viability.
- No effect of pneumolysin following infection with UV-killed pneumococci on the production of IL-12 p70 was observed. Data show mean ± SE (n = 3).
- Pneumolysin did not affect IL-8 or cell viability following infection with UV-killed pneumococci. Data represent mean ± SE (n = 3).
Death of DCs after pneumococcal infection correlates with the activation of multiple caspases by pneumolysin
In the above discussion we reported that apoptosis of human DCs infected with pneumococci was not seen after 6 h of infection but occurred before 24 h post-infection. To determine whether caspases were involved in the induction of apoptosis of human DCs we used a poly-caspase apoptosis stain to examine the expression of active caspases with time. Infection with pneumolysin-proficient pneumococci activated caspases as early as 12 h post-infection (Fig 4A) and at 24 h post-infection, the percentage of cells that expressed caspase 1 (Fig 4B) and caspase-3,7 (Fig 4C) was significantly enhanced after infection with this strain. The induction of caspase-dependent apoptosis of human DCs is likely to have a detrimental role in dampening the adaptive immune response and could be a mechanism whereby bacteria evade local immune responses to promote colonisation.
Figure 4. Pneumolysin expression activates multiple caspases in DCs.
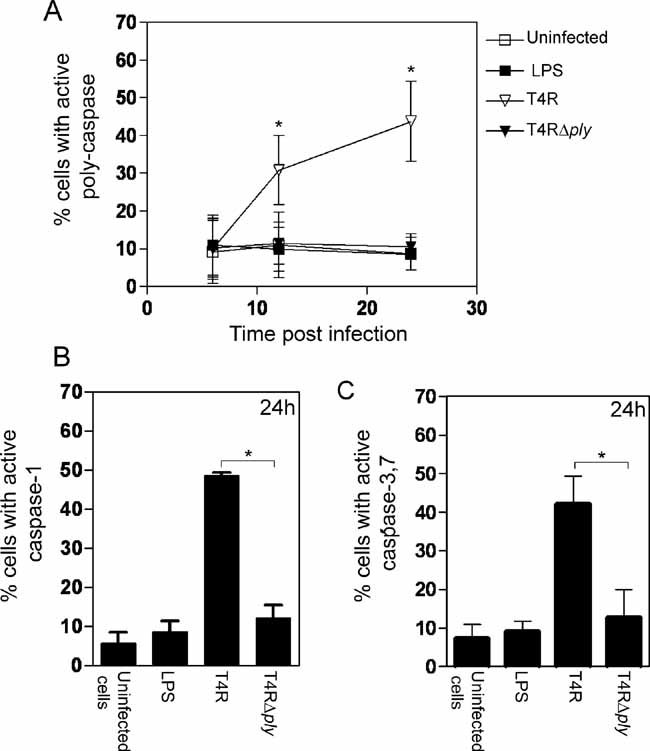
- Pneumolysin-proficient pneumococci significantly activated multiple caspases at 12 and 24 h post-infection. Data represents mean ± SE (n = 3). Significant increases compared to uninfected cells are indicated by an asterisk (*).
- Activation of caspase-1 at 24 h post-infection could also be attributed to pneumolysin expression. Data show mean + SE (n = 3) with significant differences indicated by an asterisk (*). 5000 events per treatment were counted by flow cytometry.
- The activation of caspase 3,7 at 24 h post-infection could be attributed to pneumolysin expression. Data represent mean + SE (n = 3) and significant differences are indicated by an asterisk (*). 5000 events per treatment were counted by flow cytometry.
Pneumolysin-deficient pneumococci induce the production of inflammasome-associated cytokines by infected DCs
The inflammasome is a cytosolic multi-protein platform that allows activation of the precursor of caspase-1, which subsequently cleaves the precursors of interleukin-1β (pro-IL-1β) and IL-18 (pro-IL-18) into the active forms, the secretion of which leads to a potent inflammatory response. To examine the role of pneumolysin in the activation of caspase-1 and the cleavage of the inflammation-associated cytokines IL-1β and IL-18, we infected human DCs with T4R and T4RΔply and monitored the cytokine levels after infection. Above we reported that the late induction (24 h post-infection) of caspase-1 is due to the action of pneumolysin. Unexpectedly, we noted that the pneumolysin-deficient strain (T4RΔply) stimulated an early induction (6 h post-infection) of caspase-1 (Fig 5A), and although this difference was not significant, it was consistently more than the amount of caspase-1 induced by T4R. To determine whether the increased levels of caspase-1 at this early time point could be attributed to activation of the inflammasome, we monitored the levels of the inflammasome-associated cytokine IL-1β in infected human DCs. A higher amount of pro-IL-1β was detected in cells infected with T4RΔply than T4R by Western blotting (Fig 5B). In line with this, mature IL-1β was secreted into the growth medium of cells infected with T4RΔply as early as 6 h post-infection (Fig 5C) and this could be inhibited by the addition of the caspase-1 inhibitor Z-YVAD-FMK. IL-1β was only induced at low levels by T4R at 12 and 24 h post-infection and at 24 h post-infection the caspase-1 inhibitor had no effect. In addition, at 24 h post-infection high amounts of mature IL-18 (379.3 ± 69.4 pg/ml) were secreted into the human DC culture medium after infection with the pneumolysin-deficient strain while no mature IL-18 could be detected in response to the pneumolysin-proficient strain. These results suggest that the induction of caspase-1 during early infection, in the absence of pneumolysin, results in an enhanced cleavage of pro-IL-1β and pro-IL-18.
Figure 5. Pneumolysin-deficient pneumococci induce the inflammasome-associated cytokine IL-1β.
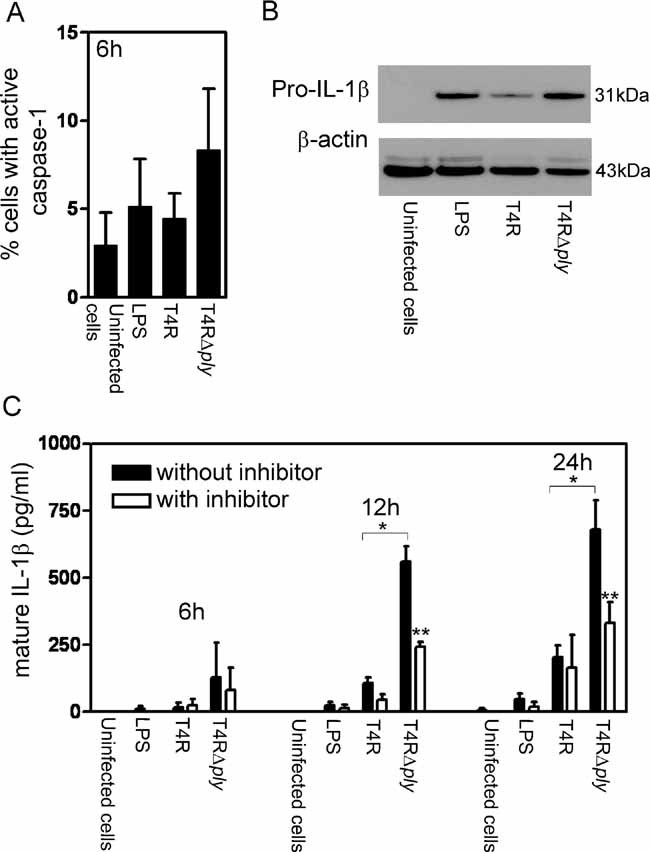
- Pneumolysin-deficient pneumococci activated caspase-1 at 6 h post-infection, albeit at non-significant levels. Data represent the mean + SE (n = 3). At least 5000 events per treatment were counted.
- In the absence of pneumolysin expression an increase in pro-IL-1β could be detected by Western blotting. Data is representative of three independent human DC preparations.
- Pneumolysin-deficient pneumococci enhanced secreted levels of mature IL-1β as early as 6 h post-infection and addition of the caspase-1 inhibitor Z-YVAD-FMK inhibited this secretion. Data represent the mean + SE (n = 3). A single asterisk (*) shows significant increases compared to T4R and a double asterisk (**) indicates significant decreases compared to values obtained without the inhibitor.
Pneumolysin production diminishes DC activation
Coordinated up-regulation of co-stimulatory molecules and translocation of MHC molecules to the cell surface are essential for antigen presentation by DCs and activation of T cells. Since pneumolysin expression was associated with a decreased production of IL-8, IL-12 p70 (Fig 3) and inflammasome activation (Fig 5) by DCs, we examined the impact of pneumolysin on DC activation by monitoring the expression of HLA-DR, CD80 and CD86. DCs were infected with T4R and T4RΔply and the expression of the surface activation markers was examined 24 h post-infection in the viable population of cells using flow cytometry (Fig 6). LPS was used as a positive control of human DC stimulation and was found to significantly increase the expression of CD80 and CD86. In addition, we observed a significant up-regulation of the expression of these activation markers following infection with T4RΔply. Infection with T4R resulted in a significantly lower degree of activation. The lower expression of activation markers following infection with pneumolysin-proficient pneumococci could not be attributed to increased cell death since the level of CD80 and CD86 was measured only on live CD11c+ cells suggesting that human DC activation by pneumococci is inhibited by the production of pneumolysin. Compilation of data from eight donors did not show significant changes in the level of expression of HLA-DR (data not shown). No significant changes were noted in the expression of the isotype control after any treatment (data not shown).
Figure 6. Pneumolysin expression diminishes DC activation.
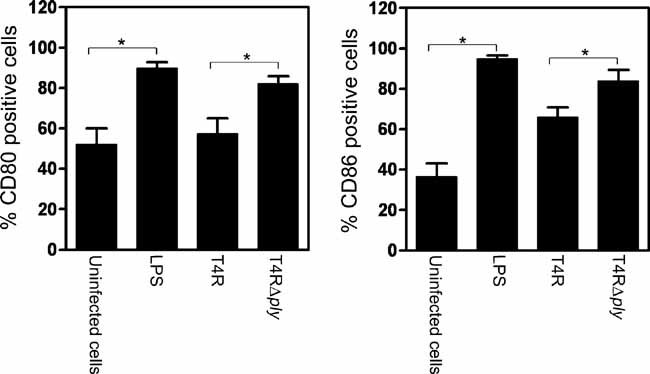
The effect of pneumolysin production on the expression of CD80 and CD86 by viable DCs was examined following infection with unencapsulated pneumococci. Data show the mean (% positive cells) + SE (n = 7). 10000 events per treatment were counted. Significant differences are indicated by an asterisk (*).
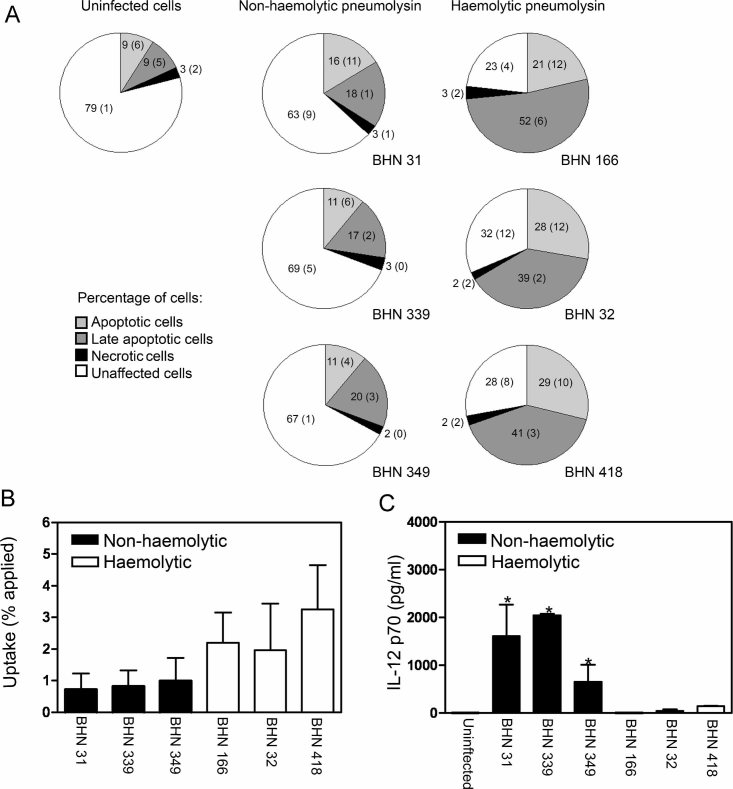
Clonally related serotype 1 isolates differing in the haemolytic activity of pneumolysin mediate divergent responses in DCs
Serotype 1 strains of pneumococci are associated with invasive disease and are rarely found among healthy carriers (Brueggemann and Spratt, 2003). In a recent study by Kirkham et al (2006), a majority of the clinical isolates of serotype 1 tested were shown to express non-haemolytic forms of pneumolysin (Kirkham et al, 2006). All of these isolates were shown by multilocus sequence typing (MLST) to be of ST306, whereas the clonally related ST227 and ST228 isolates and other serotype 1 isolates expressed haemolytic pneumolysin. To investigate the role of pneumolysin in the serotype 1 background we infected human DCs with clinical isolates of ST306, expressing non-haemolytic pneumolysin, and isolates of ST217, ST227 and ST228, expressing haemolytic pneumolysin. We examined cell viability using Annexin V-FITC and PI staining as described above and found that isolates with non-haemolytic pneumolysin (ST306) did not induce significant apoptosis of human DCs while isolates expressing haemolytic pneumolysin showed increased levels of apoptosis (Fig 7A). The production of haemolytic pneumolysin increased the uptake of opsonised pneumococci (Fig 7B) and inhibited the induction of IL-12 p70 (Fig 7C). These results demonstrate that the effects of pneumolysin are independent of serotype, but dependent on the haemolytic activity of the toxin.
Figure 7. DC apoptosis, uptake and inhibition of IL-12 p70 induction following infection with invasive isolates of serotype 1 correlate with haemolytic pneumolysin expression.
- DCs were infected for 24 h with invasive serotype 1 pneumococcal clinical isolates and stained with Annexin V-FITC and PI. Flow cytometry revealed that the isolates of ST217 (BHN 166), ST228 (BHN 32) and ST227 (BHN 418) which express haemolytic pneumolysin induced apoptosis, whereas isolates of ST306 (BHN 31, BHN 339 and BHN 349) which express non-haemolytic pneumolysin did not induce apoptosis. At least 5000 cells per treatment were counted by flow cytometry and data shows mean (SE) numerically (n = 3).
- Strains expressing non-haemolytic pneumolysin showed inhibited opsonised uptake. Data show mean + SE (n = 3).
- Strains expressing non-haemolytic pneumolysin increased IL-12 p70 induction. Data represent mean + SE (n = 3) and significant induction of IL-12 p70 is indicated by an asterisk (*).
Murine DCs vary in the inflammatory response to pneumococci compared to human DCs
The results we have presented thus far are in contrast to the previously reported proinflammatory nature of pneumolysin. Most studies examining the effect of pneumolysin on cells have been conducted in murine systems. In order to examine whether the effects we observed were due to the human-specific response, we compared the response of human monocyte-derived DCs to murine bone marrow-derived DCs (BMDCs) isolated from two mouse strains commonly used in pneumococcal infection models; BALB/c and C57BL/6. Human DCs exhibited a higher uptake of pneumococci compared to murine BMDCs, with 3-fold higher uptake of T4R and 5-fold higher uptake of T4RΔply than BALB/c BMDCs and a 2-fold higher uptake of T4R and 11-fold higher uptake of T4RΔply than C57BL/6 BMDCs (Fig 8A). Since S. pneumoniae is a human-specific pathogen the lower uptake by murine DCs could be due to a failure to induce cell signalling in response to pneumococcal infection or the absence of receptors for pneumococci. Pneumolysin can induce apoptosis in murine BMDCs independent of bacterial internalisation (Colino and Snapper, 2003). We confirmed this finding with murine BMDCs from both BALB/c and C57BL/6 mice showing similar levels of pneumolysin-dependent apoptosis as human DCs (Fig 8B) despite a significantly lower level of uptake (Fig 8A). Previous studies have shown that murine cells induce inflammatory cytokines in response to pneumococci. We compared the induction of the inflammatory cytokines IL-12 p70 and IL-1β in human and murine DCs following infection with T4R and T4RΔply. Human DCs produced significantly higher levels of both IL-12 p70 (Fig 8C) and IL-1β (Fig 8D) in response to the pneumolysin-deficient pneumococci (T4RΔply) compared to C57BL/6 and BALB/c BMDCs. In addition, in human cells, pneumolysin-deficient T4RΔply induced significantly enhanced amounts of IL-12 p70 and IL-1β in comparison to pneumolysin-proficient T4R. Similar results were observed in a serotype 14 background (data not shown) indicating that this response is not serotype specific. However, in BMDCs from both BALB/c and C57BL/6 mice IL-1β could only be induced in significant levels following infection with pneumolysin-proficient T4R (Fig 8D) indicating that in murine cells pneumolysin can stimulate proinflammatory responses, as has been previously reported. These results convincingly demonstrate that human and murine DCs differ in their ability to internalise pneumococci and to secrete certain proinflammatory cytokines in response to pneumococcal infection.
Figure 8. Murine BMDCs respond differently to pneumococci than human DCs.
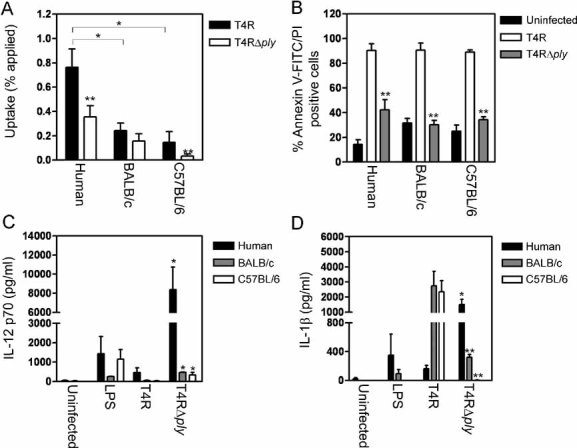
- Human DCs showed enhanced uptake of pneumococci compared to murine BMDCs (BALB/c and C57BL/6). Data represent mean + SE. Significant decreases compared to human DCs are indicated by a single asterisk (*) and significant decreases compared to T4R are indicated by a double asterisk (**). Data comes from several independent experiments (human n = 10, BALB/c n = 4 and C57BL/6 n = 6).
- Murine BMDCs underwent similar apoptosis to human DCs following exposure to pneumolysin-proficient pneumococci, as detected by Annexin V-FITC and PI staining. Data represent mean + SE (n = 3). Significant decreases compared to T4R infected cells are shown with a double asterisk (**). At least 5000 cells per treatment were counted by flow cytometry.
- Human DCs showed increased production of the proinflammatory cytokine IL-12 p70 compared to BMDCs following pneumococcal infection. Data show mean + SE (n = 4). Significant increases compared to T4R infection are indicated by a single asterisk (*).
- Human DCs showed altered production of the proinflammatory cytokine IL-1β compared to BMDCs following pneumococcal infection. Data represent mean + SE (n = 4). Significant increases compared to T4R infection are indicated by a single asterisk (*) and significant decreases (p < 0.05) are indicated by a double asterisk (**).
DISCUSSION
The respiratory epithelium is protected by a variety of different innate and adaptive immune mechanisms, including DCs. In this study, we analysed the interaction between live pneumococcal strains and human DCs in order to investigate the effect of bacterial infection on human DC activation, viability and the induction of cytokines associated with inflammation and the local immune response to infection. The major finding associated with this study is that even at low, but physiologically relevant infection doses, the pneumococcus possesses the capacity to modulate the human DC-mediated immune response using several mechanisms including promotion of pneumolysin-related cell death, inhibition of DC activation and inhibition of inflammasome-associated inflammatory cytokines. The potential mechanisms involved in the response of human DCs to pneumococci are summarised in Fig 9. In addition, this study reports variations in the response to pneumococci between BMDCs from two mouse strains, commonly used in pneumococcal infection models, and human DCs.
Figure 9. Potential mechanisms involved in the human DC response to infection with pneumolysin-proficient and pneumolysin-deficient pneumococci.
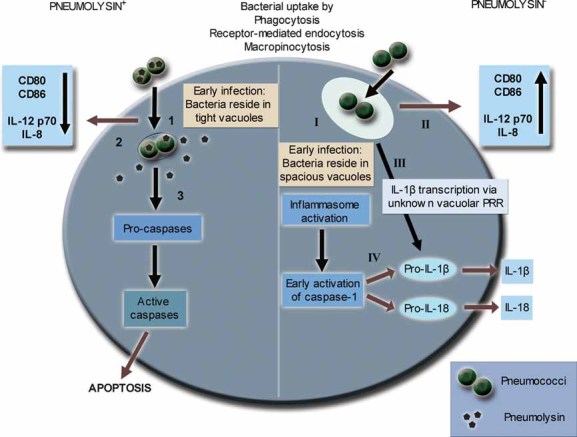
Infection with pneumolysin-proficient pneumococcal strains results in evasion of inflammatory responses (1–3). Following uptake, the pore-forming toxin pneumolysin induces rapid processing of bacteria into tight vacuolar compartments (1). Host sensing of bacterial components leads to an inhibition of cellular activation and cytokine secretion (2) and induction of caspase-dependent apoptosis of the DC (3). Infection of DCs with pneumolysin-deficient strains stimulates strong proinflammatory responses (I–IV). During early infection, bacteria reside in spacious vacuoles (I) and prolonged stimulation of vacuolar receptors leads to an enhanced immune response shown as up-regulation of CD80 and CD86 as well as synthesis of the cytokines IL-8 and IL-12 p70 (II). Signalling via an unknown vacuolar pattern recognition receptor (PRR) contributes to the up-regulation of pro-IL-1β (III). Inflammasome activation is triggered in the cell and early induction of active caspase-1 leads to processing of pro-IL-1β and pro-IL-18 and subsequent release of the mature proinflammatory cytokines IL-1β and IL-18 (IV).
Pneumolysin belongs to a family of cholesterol-dependent cytolysins expressed by a wide range of Gram-positive bacteria (Tweten, 2005). Other members of this family, including listeriolysin, streptolysin O and perfringolysin O, have been shown to facilitate haemolysis and, in the case of listeriolysin, escape of the bacteria from the intracellular vacuole into the cytosol (Gaillard et al, 1986; Portnoy et al, 1988). Recently, it was shown that pneumolysin primarily localises to the cell wall of pneumococci (Price and Camilli, 2009) in 18 different serotypes investigated, including T4. This suggests that bacterial-bound pneumolysin can interact directly with host cell receptors such as those expressed by DCs. In the present study we show that pneumolysin-deficient strains have a decreased uptake by DCs, which may be explained by a reduced interaction with phagocytic receptors. One could speculate that as a result of the impaired uptake there may be a decreased clearance of these strains. Additionally, a postulated mechanism for clearance of pathogens is by apoptosis of infected phagocytes (Dockrell et al, 2003), envisioning another factor which may lead to decreased clearance of pneumolysin-deficient strains.
Here we show that during early infection the production of pneumolysin affects the morphology of the DC vacuole. At 4 h post-infection 90% of pneumolysin producing pneumococci appeared in tight vacuoles, whereas in the absence of pneumolysin production as many as 50% of internalised pneumococci instead appeared in spacious vacuoles. At a later time point (8 h post-infection), the relative percentage of spacious vacuoles decreased, suggesting either that they can mature into tight vacuoles or that it is only the initially internalised bacteria that stimulate spacious vacuoles. It has recently been shown that Listeria monocytogenes, expressing low levels of listeriolysin, stimulates the formation of spacious vacuoles that were found to be non-acidic and non-degradative compartments promoting persistent infection (Birmingham et al, 2008). Furthermore, virulent Salmonella in contrast to attenuated strains stimulates the formation of less hostile spacious phagosomes (Kiama et al, 2006). Regardless of the mechanism, the initial appearance of pneumolysin-deficient pneumococci in spacious vacuoles may increase early signalling to vacuolar receptors and thus lead to the enhanced proinflammatory effects observed in human DCs. We found no evidence for bacterial replication in either of the vacuolar types and observed that the number of viable intracellular bacteria steadily decreased over an 8 h period (data not shown) for both pneumolysin-expressing and non-expressing pneumococci. Hence, internalisation into human DC vacuoles eventually kills the bacteria. We observed numerous lysosomes near the pneumococcal-containing vacuoles, which may fuse to form phagolysosomes and degrade the bacteria. However, further studies are required to investigate the integrity of the vacuolar membrane and the degree of contact with the lysosomes. It would also be important to ascertain whether DCs and macrophages handle S. pneumoniae differently and whether the appearance of pneumolysin-deficient pneumococci in spacious vacuoles is a characteristic of infected human DCs.
We observed that intracellular recognition of bacteria and bacterial components caused caspase-dependent apoptosis of human DCs. Apoptosis of DCs is likely to have a detrimental role by dampening the adaptive immune response and could be a mechanism whereby bacteria evade local immune responses to promote colonisation. Uptake of live pneumolysin-proficient pneumococci also led to decreased production of the cytokines IL-8, IL-12 p70, IL-1β and IL-18. A recent study by Shoma et al (Shoma et al, 2008) showed that pneumolysin is not involved in the pneumococcal induction of IL-12 p40 in murine macrophages. We also observed that non-pneumolysin components induce IL-12 p70 in both murine and human DCs. Furthermore, in the murine system Shoma et al (Shoma et al, 2008) demonstrated that recombinant pneumolysin and pneumolysin-proficient pneumococci could induce IL-1 (α and β) and IL-18. In our study, we confirmed the induction of IL-1β in response to pneumolysin in murine BMDCs from both C57BL/6 and BALB/c mice. Despite this, we also found that human DCs secrete significantly higher levels of cytokines than BALB/c and C57BL/6 BMDCs in response to pneumococcal infection indicating that the proinflammatory nature of pneumococci in murine DCs can not be directly correlated to the human response. However, in our study using human DCs we showed that expression of pneumolysin inhibited the production of IL-1β and IL-18, and consequently that in the human system these cytokines must be stimulated by bacterial components other than pneumolysin. While murine models have been invaluable for studying pneumococcal pathogenesis, the results from our study suggest that care must be taken to directly extrapolate the results from murine cells to the human response.
Serotype 1 isolates commonly cause invasive pneumococcal infections and have a high association with pneumonia (Sjostrom et al, 2006). In a recent study by Kirkham et al, a majority of the clinical isolates of serotype 1 tested were shown to express non-haemolytic forms of pneumolysin (Kirkham et al, 2006). All of these isolates were shown by MLST to be ST306. Our study suggests that expression of pneumolysin is one mechanism by which pneumococci evade the host immune response, by dampening the development of human DC-mediated adaptive immune responses. It is possible that the expression of non-haemolytic pneumolysin could promote the inflammatory reactions associated with pneumonia seen in serotype 1 infections. In recent years there has been an increasing incidence of paediatric empyemas as an inflammatory complication to pneumococcal pneumonia (Byington et al, 2002; Tan et al, 2002) and about 50% of these empyemas have been shown to be caused by serotype 1 (Eltringham et al, 2003; Obando et al, 2008). Interestingly, a majority of serotype 1 empyema isolates were of ST306 (Obando et al, 2008) suggesting that empyemas are frequently caused by pneumococci producing non-haemolytic pneumolysin.
In this study we examined the dynamics of human DC infection by live pneumococci and we show that there is a fine balance between host cell activation and death following pneumococcal uptake. Rather than studying the effects of isolated pneumolysin we looked at the more complex interactions between whole bacteria and the host cell. While pneumolysin is generally regarded to have proinflammatory activities, it has also been shown to inhibit lymphocyte proliferation and cytokine production (Ferrante et al, 1984) and inhibit chemotaxis of neutrophils (Paton and Ferrante, 1983). An interesting finding in our study was that pneumolysin-deficient pneumococci stimulated a much higher cytokine response in DCs compared to the pneumolysin-proficient strain. This could not solely be an effect of more viable cells but may represent signalling via PRRs in the spacious vacuole. Besides drastically reducing the secretion of inflammatory cytokines, infection with pneumolysin-proficient pneumococci also inhibited DC maturation and induced caspase-dependent cell death.
The paper explained
PROBLEM:
DCs are crucial in the host response to microbial pathogens in the respiratory tract. S. pneumoniae is part of the normal flora of the human upper respiratory tract but under certain conditions it can become pathogenic. Pneumolysin, a toxin produced by the pneumococci, has been shown to contribute to tissue damage and inflammatory responses. Despite this, serotype 1 isolates belonging to sequence type 306 express non-haemolytic pneumolysin and are associated with inflammatory infections such as pneumonia and post-pneumonic empyema.
RESULTS:
This study demonstrates mechanisms whereby the expression of haemolytic pneumolysin by pneumococci induces host cell apoptosis and inhibits the development of inflammatory responses by human dendritic cells. In contrast, pneumococci that do not express haemolytic pneumolysin promote strong proinflammatory responses and dendritic cell activation.
IMPACT:
The mechanisms by which pneumococci evade host inflammation may explain the inflammatory reactions associated with non-haemolytic strains causing pneumonia and paediatric empyema.
From these observations we suggest that under physiological conditions pneumolysin-proficient strains have the ability to impair DC functions and thereby evade adaptive immune responses.
It is noteworthy that the haemolytic activity of pneumolysin in not essential for invasive disease, indicating that the non-haemolytic form of the protein could be of some benefit to the pneumococci. The greater proinflammatory response of DCs to clinical isolates with non-haemolytic pneumolysin may drive an excessive lung inflammation as observed in post-pneumonic empyema. This highlights the diversity of pneumococcal virulence factors, even within the same serotype, and warrants further investigation into the role of pneumolysin in disease pathogenesis.
MATERIALS AND METHODS
Bacterial strains
Encapsulated S. pneumoniae of serotype 4 (TIGR4 (T4); ATCC BAA-334) (Tettelin et al, 2001) and an isogenic unencapsulated mutant (T4R) (Fernebro et al, 2004) were used in these experiments. Mutants deficient in pneumolysin expression in both the encapsulated and unencapsulated background were constructed by insertion of an erythromycin cassette into the pneumolysin (ply) gene. Clinical invasive isolates of serotype 1 were also used in this study. These isolates included strains expressing haemolytic pneumolysin: BHN 32 (ST228), BHN 166 (ST217) and BHN 418 (ST227) and strains belonging to ST306 that expressed non-haemolytic pneumolysin (BHN 339, BHN 349 and BHN 31). For infection experiments pneumococci were grown overnight on blood agar plates at 37 °C and 5% CO2. Colonies were inoculated into C + Y medium and grown to OD620 = 0.5. Dilutions were made to obtain the desired concentration of bacteria and viable counting was performed to retrospectively confirm bacterial numbers. Serotype 1 bacterial strains were assessed for haemolysis using a 2% v/v human blood suspension in phosphate buffered saline (PBS) containing 1 mM dithiothreitol, and the pneumolysin gene was sequenced, as previously described (Walker et al, 1987).
Human DC culture
Monocytes were purified from buffy coats of healthy donors (Karolinska University Hospital) using a RosetteSep™ monocyte purification kit (Stem Cell Technologies) and Ficoll-Hypaque Plus (Amersham Biosciences) gradient centrifugation. DCs were derived in RPMI 1640 containing 10% FCS and 2 mM l-glutamine supplemented with GM-CSF (75 ng/mL) and IL-4 (75 ng/mL). Cells were given fresh media and cytokines in the ratio of 1:1 on day 4 and cultured until day 6. The DC phenotype was assessed by examination of CD11c and CD1a expression using allophycocyanin (APC) conjugated mouse anti-human CD11c and fluorescein isothiocyanate (FITC) conjugated mouse anti-human CD1a (BD Pharmingen).
Murine DC culture
Primary bone marrow-derived DCs were obtained from the bone marrow of 6–10 week old C57BL/6 and BALB/c mice. Cells were cultivated in RPMI 1640 supplemented with 10% FCS, l-glutamine (20 µM), sodium pyruvate (10 µM), Hepes (0.01 M), 2-mercaptoethanol (50 µM), non-essential amino acids (1×) and recombinant murine GM-CSF (4 ng/ml) in the presence of penicillin (100 U/ml) and streptomycin (0.1 mg/ml). Cells were given fresh media and cytokines in the ratio of 1:1 on day 3, and cultured until day 5. On day 5, cells were given fresh media without antibiotics and cultured until day 6. The BMDC phenotype was assessed by examination of CD11c using FITC anti-mouse CD11c (BD Pharmingen).
Infection procedure
In infection experiments, S. pneumoniae was added to DCs at a MOI of 10, unless otherwise stated. At 2 h post-infection gentamicin (100 µg/ml) was added to kill extracellular bacteria and was maintained in the cell culture medium for up to 24 h.
Uptake assay
Human and murine DCs (1 × 105/well) were infected with bacteria, and at specific time points (2, 3 and 4 h) after infection gentamicin (200 µg/ml) was added to cultures for 1 h to kill extracellular bacteria. Cells were washed two times with PBS, lysed with 0.1% saponin (Sigma) in PBS for 5 min and the number of viable internalised bacteria were enumerated by viable count on blood agar plates. For opsonised uptake the pneumococci were incubated in the presence pneumococcal antiserum for serotype 1 or 4 (Statens Serum Institut, Denmark) for the duration of the uptake period. In control treatments the uptake of bacteria was inhibited by pre-incubation of the cells with cytochalasin d (Sigma; 0.5 µM) and wortmannin (Sigma; 100 µM) for 15 min prior to infection and for the duration of the infection period. The percentage of uptake was calculated by dividing the number of bacteria isolated from DCs following a 2 h infection by the initial total number of bacteria applied to the cells.
TEM
DCs were infected with T4R and T4RΔply (MOI 10) and at 2 h post-infection gentamicin was added at a final concentration of 100 µg/ml. At two time points (4 and 8 h post-infection), cells were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer containing 0.1 M sucrose and 3 mM CaCl2 at room temperature for 20 min. After fixation, cells were rinsed in 0.15 M sodium cacodylate buffer containing 3 mM CaCl2 (pH 7.4) and centrifuged. The pellets were then post-fixed in 2% osmium tetroxide in 0.07 M sodium cacodylate buffer containing 1.5 mM CaCl2 (pH 7.4) at 4 °C for 2 h, dehydrated in ethanol followed by acetone and embedded in LX-112 (Ladd, Burlington, Vermont, USA). Sections were contrasted with uranyl acetate followed by lead citrate and examined under a Leo 906 transmission electron microscope at 80 kV. Digital images were taken with a Morada digital camera (Soft Imaging System, GmbH, Münster, Germany). A blinded evaluation of the intracellular location of the bacteria was performed.
Assessment of DC viability and caspase activity
The influence of pneumococcal infection on DC viability was determined using the Annexin V-FITC Apoptosis Detection kit I (BD Pharmingen). Briefly, cells were infected as described previously and 6 and 24 h after infection the number of apoptotic and necrotic cells were determined by staining cells with Annexin V-FITC and propidium iodide, according to the manufacturer's instructions. Cells were assessed by flow cytometry. In control treatments bacterial uptake was inhibited using cytochalasin d and wortmannin as described above. For the measurement of caspase activity, the Image-iT LIVE green Poly Caspases Detection Kit (Invitrogen), as well as the caspase-1 and caspase-3,7 caroboxyfluorescein FLICA apoptosis detection kit (Immunochemistry Technologies) were used. Caspase activation was detected by flow cytometry. Caspase-1 induction was inhibited by the addition of the caspase-1 inhibitor Z-YVAD-FMK (MBL) at 10 mM for the duration of the infection.
Quantification of cytokine production by infected DCs
For cytokine assessment infected culture supernatants were harvested after infection and used directly for the measurement of IL-12 p70 and IL-8 by ELISA or frozen at −20 °C for the measurement of IL-1β and IL-18. Concentrations of IL-12 p70 and IL-8 were measured using OptEIA™ ELISA kits (BD Biosciences). The level of IL-1β was measured with a Human IL-1β ELISA Ready-SET-Go! Kit (eBioscience) and IL-18 was measured with a Human IL-18 ELISA kit (MBL). Murine IL-12 p70 and IL-1β were measured using OptEIA™ ELISA kits (BD Biosciences).
Western blotting
Levels of pro-IL-1β were measured by Western blotting. Briefly, cell lysates were prepared from cells infected for 24 h by washing cells once with PBS and lysing with RIPA buffer containing 1× protease inhibitors (Roche) on ice for 15 min. Cell debris were removed by centrifugation. Samples were diluted with lysis buffer and approximately 30 µg was loaded on to a 16% Tris-Glycine gel (Invitrogen). Proteins were transferred to PVDF and blocked with 5% skim milk powder in PBS containing 5% Tween-20. Pro-IL-1β was detected using rabbit anti-IL-1β (H-153). As a loading control a mouse-anti-β-actin (ACTBD11B7) antibody was used. Primary antibodies were used at a 1:200 dilution and secondary anti-mouse HRP (sc-2005) and anti-rabbit HRP (sc-2004) antibodies were used at a 1:1,000 dilution. All antibodies were from Santa Cruz Biotechnology. Detection was carried out using the Amersham™ ECL Plus Western blotting detection system (GE Healthcare Life Sciences).
Analysis of DC activation markers
Flow cytometric analysis was performed to examine the expression of the surface maturation markers CD80, CD86 and HLA-DR in S. pneumoniae infected DCs. The antibodies used for these analyses were: FITC-conjugated anti-human CD80 (L307.4), R-phycoerythrin (R-PE)-conjugated anti-human CD86 (IT2.2) and PE-Cy5-conjugated anti-human HLA-DR (G46-6). All antibodies were from BD Pharmingen. DCs were infected as described above, and phenotypic maturation was determined 24 h after incubation with pneumococci. Isotype controls were used in all the studies.
Statistical analysis
Statistical significance was determined by using Student's t-test in Excel. Results that are shown graphically represent mean + SE, unless otherwise specified. Values of p < 0.05 were considered statistically significant in all experiments.
Acknowledgments
We thank Kjell Hultenby for the TEM analysis, and Sönke Andres for assistance in scoring the TEM pictures. This work was supported by grants from the Swedish Research Council, the Åke Wiberg Foundation, Lars Hiertas Minne Fond, Torsten and Ragnar Söderbergs Foundation, Swedish Royal Academy of Sciences, Swedish Foundation for Strategic Research and Karolinska Institutet (KID faculty funds).
The authors declare that they have no conflict of interest.
Author contributions
ML, SN, BHN and LP conceived and designed the experiments. ML, AF, BA and LP performed the experiments. ML, SN, BHN and LP analysed the data. ML, SN, BHN and LP wrote the paper.
References
- Bermpohl D, Halle A, Freyer D, Dagand E, Braun JS, Bechmann I, Schroder NW, Weber JR. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest. 2005;115:1607–1615. doi: 10.1172/JCI23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann AB, Spratt BG. Geographic distribution and clonal diversity of Streptococcus pneumoniae serotype 1 isolates. J Clin Microbiol. 2003;41:4966–4970. doi: 10.1128/JCM.41.11.4966-4970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington CL, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, Kaplan S, Carroll KC, Daly JA, Christenson JC, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34:434–440. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol. 2003;171:2354–2365. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- Eltringham G, Kearns A, Freeman R, Clark J, Spencer D, Eastham K, Harwood J, Leeming J. Culture-negative childhood empyema is usually due to penicillin-sensitive Streptococcus pneumoniae capsular serotype 1. J Clin Microbiol. 2003;41:521–522. doi: 10.1128/JCM.41.1.521-522.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernebro J, Andersson I, Sublett J, Morfeldt E, Novak R, Tuomanen E, Normark S, Normark BH. Capsular expression in Streptococcus pneumoniae negatively affects spontaneous and antibiotic-induced lysis and contributes to antibiotic tolerance. J Infect Dis. 2004;189:328–338. doi: 10.1086/380564. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Rowan-Kelly B, Paton JC. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984;46:585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard JL, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun. 2000;68:2286–2293. doi: 10.1128/iai.68.4.2286-2293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG. Antigen presentation in the lung. Am J Respir Crit Care Med. 2000;162:S151–S156. doi: 10.1164/ajrccm.162.supplement_3.15tac2. [DOI] [PubMed] [Google Scholar]
- Holt PG, Stumbles PA. Characterization of dendritic cell populations in the respiratory tract. J Aerosol Med. 2000;13:361–367. doi: 10.1089/jam.2000.13.361. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci USA. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies JM, Johnston CH, Kirkham LA, Cowan GJ, Ross KS, Smith A, Clarke SC, Brueggemann AB, George RC, Pichon B, et al. Presence of nonhemolytic pneumolysin in serotypes of Streptococcus pneumoniae associated with disease outbreaks. J Infect Dis. 2007;196:936–944. doi: 10.1086/520091. [DOI] [PubMed] [Google Scholar]
- Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun. 2004;72:2689–2697. doi: 10.1128/IAI.72.5.2689-2697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68:492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiama SG, Dreher D, Cochand L, Kok M, Obregon C, Nicod L, Gehr P. Host cell responses of Salmonella typhimurium infected human dendritic cells. Immunol Cell Biol. 2006;84:475–481. doi: 10.1111/j.1440-1711.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- Kirkham LA, Jefferies JM, Kerr AR, Jing Y, Clarke SC, Smith A, Mitchell TJ. Identification of invasive serotype 1 pneumococcal isolates that express nonhemolytic pneumolysin. J Clin Microbiol. 2006;44:151–159. doi: 10.1128/JCM.44.1.151-159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L, Smith SH, Braun JS, Herzog KH, Weber JR, Tuomanen EI. Dual phases of apoptosis in pneumococcal meningitis. J Infect Dis. 2004;190:2039–2046. doi: 10.1086/425520. [DOI] [PubMed] [Google Scholar]
- Obando I, Munoz-Almagro C, Arroyo LA, Tarrago D, Sanchez-Tatay D, Moreno-Perez D, Dhillon SS, Esteva C, Hernandez-Bou S, Garcia-Garcia JJ, et al. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis. 2008;14:1390–1397. doi: 10.3201/eid1409.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JC, Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983;41:1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KE, Camilli A. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J Bacteriol. 2009;191:2163–2168. doi: 10.1128/JB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, Weiser JN. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem. 2006;281:12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeck B, Gross R, N'Guessan PD, Hocke AC, Hammerschmidt S, Mitchell TJ, Rosseau S, Suttorp N, Hippenstiel S. Streptococcus pneumoniae-induced caspase 6-dependent apoptosis in lung epithelium. Infect Immun. 2004;72:4940–4947. doi: 10.1128/IAI.72.9.4940-4947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoma S, Tsuchiya K, Kawamura I, Nomura T, Hara H, Uchiyama R, Daim S, Mitsuyama M. Critical involvement of pneumolysin in production of interleukin-1alpha and caspase-1-dependent cytokines in infection with Streptococcus pneumoniae in vitro: a novel function of pneumolysin in caspase-1 activation. Infect Immun. 2008;76:1547–1557. doi: 10.1128/IAI.01269-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom K, Spindler C, Ortqvist A, Kalin M, Sandgren A, Kuhlmann-Berenzon S, Henriques-Normark B. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis. 2006;42:451–459. doi: 10.1086/499242. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73:6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TQ, Mason EO, Jr, Wald ER, Barson WJ, Schutze GE, Bradley JS, Givner LB, Yogev R, Kim KS, Kaplan SL. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002;110:1–6. doi: 10.1542/peds.110.1.1. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Allen RL, Falmagne P, Johnson MK, Boulnois GJ. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Taut K, Langer F, Mack M, Briles DE, Paton JC, Maus R, Srivastava M, Welte T, Maus UA. FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. J Immunol. 2007;179:3099–3108. doi: 10.4049/jimmunol.179.5.3099. [DOI] [PubMed] [Google Scholar]