Abstract
The participation of the HH pathway in colon cancer has remained controversial. In this issue, Varnat et al (2009) demonstrate that HH signalling is essential for human colon cancer growth, recurrence, metastases and stem cell expansion. This closeup by Gulino et al discusses the paper and its implications.
Keywords: colorectal cancer, hedgehog signalling
Colorectal cancer (CC) is a common cancer worldwide, but despite the overall therapeutic improvements, there is still a high disease-related mortality (∼33%). In order to predict which patients would benefit from a specific treatment, there is a great need to improve our knowledge of the molecular mechanisms leading to tumour formation and progression.
In this issue of EMBO Molecular Medicine, Varnat et al demonstrate the essential role of the HEDGEHOG-GLI (HH-GLI) signalling pathway in human colorectal cancer (CC) and its stem cells, making this pathway a therapeutic target in colon carcinogenesis.
The adult intestinal epithelium is made of repeated units with one crypt of Lieberkühn and an associated flat absorptive surface in the case of the colon, or a villus in the case of the small intestine (Fig 1). The crypt is the proliferative compartment that harbours, at its base, actively dividing multipotent stem cells (reviewed in Humphries & Wright, 2008). Transit amplifying cells derived from the proliferative compartments, migrate towards the top of the crypt and differentiate into four mature epithelial intestinal lineages: one absorptive (the enterocytes) and three secretory types (Goblet cells, enteroendocrine cells and Paneth cells). The human intestinal epithelium renews itself every 5 days, following apoptosis and exfoliation of the differentiated epithelial cells. Colon epithelial stem cell proliferation and cell lineage differentiation must be tightly regulated; unbalanced cell proliferation and reduced differentiation rate of the epithelial colon cells lead to cancer transformation (Humphries & Wright, 2008).
Figure 1. The fate of intestinal stem cells in colon differentiation and cancer.
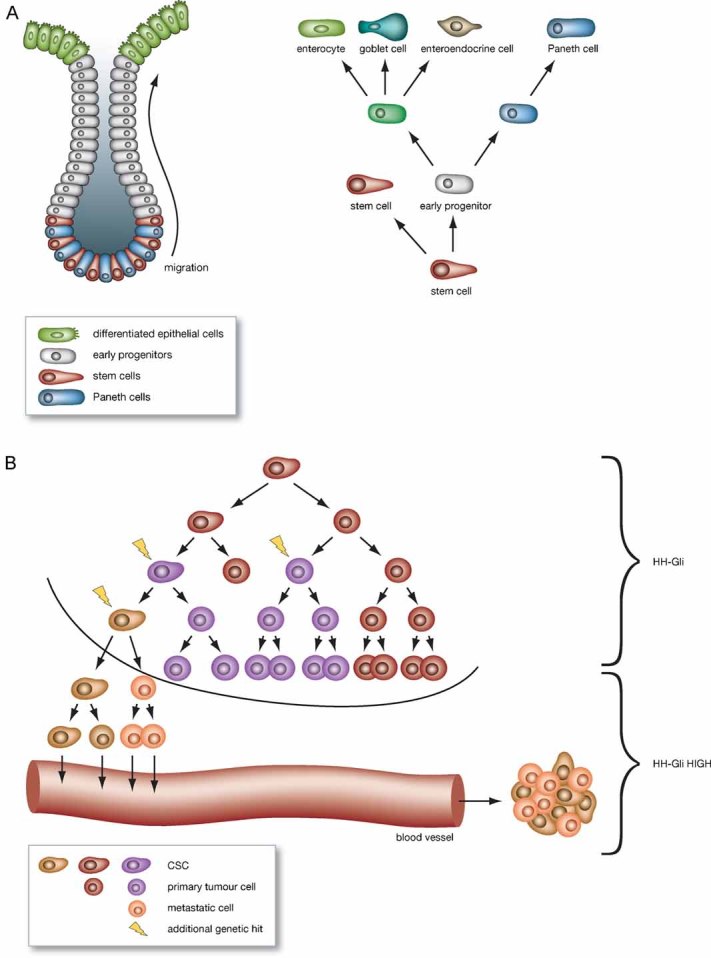
- Stem cells reside at the bottom of the intestinal crypt (on the left), just above or intermingled with the Paneth cells, where they divide asymmetrically and give rise to a new stem cell and an epithelial progenitor (early progenitor) which will in turn generate the various differentiated cell types of the mature colon epithelium (on the right).
- HH in colon cancer: CSCs exhibit active HH-GLI signalling, which is also present in the bulk tumour. HH-GLI signalling further increases in metastatic cells and such an increase correlates with the number of CSCs present in the tumour mass.
In CC, as in most tumour types, the traditional model of tumourigenesis, based on the concept that cancer originates from any cell which, through stochastic genetic events, acquires uncontrolled proliferation and reduced apoptosis, had been challenged a few years ago by the cancer stem cell (CSC) model (Visvader & Lindeman, 2008). This hypothesis suggests that colon tumours are generated and maintained by a subset of undifferentiated cells (the CSCs), able to self-renew and differentiate into the bulk tumour population. In favour of this hypothesis, researchers have identified specific human CC initiating cell population(s) within the tumour mass, that express particular stem cell markers as CD133 (reviewed in Ricci-Vitiani et al, 2008). Interestingly, these cells exhibit most of the properties of the multipotent stem cells residing in the intestinal crypt (Fig 1) (Humphries & Wright, 2008) that we discussed above. As in other cancer types, early progenitor or stem cells seem to be the target of oncogenic transformation and therefore, the identification of the molecular pathways that are involved in the maintenance and proliferation of this stem cell compartment, is critical for the development of appropriate therapeutic strategies. Activation of the HH pathway is an important event in many types of human cancer as well as in the maintenance and proliferation of stem cells (Varjosalo & Taipale, 2008). In this issue of EMBO Molecular Medicine, Varnat et al have explored the role and relevance of HH signalling in the tumour epithelia of CCs and in its CD133+ CSCs.
» …the role of the HH pathway in CC has remained controversial… «
HH signalling transduction requires binding of HH to the transmembrane receptor Patched (Ptch). This leads to the release of Ptch-mediated repression of Smo and consequent activation of the downstream signalling cascade that culminates in the activation of the Gli family of transcription factors (Varjosalo & Taipale, 2008). Previous studies have proposed the existence of an epithelial to mesenchymal (paracrine) signalling in colon cancer (reviewed in Theunissen & de Sauvage, 2009). However, the role of the HH pathway in CC has remained controversial until now as the analysis of different models of CC, human CC derived cell lines, mouse models or sporadic tumour samples have provided contradictory and conflicting data. Varnat et al now report the expression of GLI1, PTCH1 and sonic hedgehog (SHH) in primary local and metastatic human CC samples. Interestingly, they do not observe expression of Gli1 protein in the stroma, indicating that the stroma does not have an active HH-GLI signalling. It thus appears that in human CCs HH-GLI signalling is operative among epithelial cells (autocrine), while the previously described paracrine signalling may reflect the nature of the mouse model used (reviewed in Theunissen & de Sauvage, 2009). Incidentally, this is yet another example of how cautiously mouse models need to be used to immediately translate the pathological mechanisms of tumourigenesis and the therapeutic approach into humans.
» Varnat et al report an increase in HH-GLI activity which parallels progression of the tumours. «
Importantly, Varnat et al report an increase in HH-GLI activity which parallels progression of the tumours (Fig 1). In addition, GLI1 levels are enriched in CD133+ cells from patients with advanced/metastatic cancers. The authors go on to demonstrate that repression of HH-Gli signalling decreases proliferation and enhances apoptosis of epithelial cells and inhibition of SMOOTHENED (Smo) in these cells (through RNAi or cyclopamine treatment) abrogates tumour growth. Notably, cyclopamine treatment prolonged for 20 days after tumour regression, leads to an apparent complete remission from the disease in mouse, indicating that HH-Gli signalling controls CC recurrence and metastatic growth.
Accordingly, the authors report that HH-GLI activity is required for the survival of CC stem cells in vivo and its levels modulate the rate of CD133+ stem cells and drive the whole tumour growth in vitro and in vivo. Of note, Varnat et al developed a novel ‘red/green’ competition assay, to test stem cell behaviour in vivo. By using RFP (as internal control), and green fluorescent protein (GFP)/shSMOH or /shPtch1 expressing lentivectors to transduce CD133+ cells, they show that survival and expansion of the GFP+ population in vivo depends on the levels of HH-GLI activity.
Overall, the findings described by Varnat et al clearly establish a key role of HH-GLI in colon tumourigenesis through a direct action on CSCs but also on the tumour bulk. The discrepancies of this study with previously published ones (cited in Varnat et al, 2009) may be explained by the fact that established human cell lines, rather than primary tumours, have been mostly studied in the past or by the lack of single-cell resolution data on the localization of HH-GLI components. Furthermore, fundamental differences in HH signalling in mouse and human models needs to be taken in account.
» …for the use of HH pathway antagonists in the control of human CCs and their metastases.«
The data by Varnat et al, raise a number of issues that will need to be addressed in the future. Additional studies are now required to detail the role of HH signalling in the adult human colon stem cell ‘niche’, as well as elucidating the cross-talk between HH and other pathways, namely WNT and NOTCH that control colon epithelial cell proliferation, differentiation and tumourigenesis. As emphasized above, the molecular characterization of CC cells is crucial for the development of new therapeutic strategies. The role of HH signalling in CC described in this paper, opens new therapeutic perspectives. The data provide a clear and solid basis for the use of HH pathway antagonists in the control of human CCs and their metastases.
Acknowledgments
Authors' research is supported by Telethon Grant GGP07118, Associazione Italiana per la Ricerca sul Cancro, Ministry of University and Research, Ministry of Health and Pasteur Institute/Cenci-Bolognetti Foundation.
The authors declare that they have no conflict of interest.
References
- Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pagliuca A, Palio E, Zeuner A, De Maria R. Colon cancer stem cells. Gut. 2008;57:538–548. doi: 10.1136/gut.2007.127837. [DOI] [PubMed] [Google Scholar]
- Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–6010. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. Embo Mol Med. 2009;1 doi: 10.1002/emmm.200900039. DOI: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]


