Abstract
We have reported previously that ETS family transcription factor PU.1 is expressed in mature adipocytes of white adipose tissue. PU.1 expression is increased greatly in mouse models of genetic or diet-induced obesity. Here, we show that PU.1 expression is increased only in visceral but not subcutaneous adipose tissues of obese mice, and the adipocytes are responsible for this increase in PU.1 expression. To further address PU.1's physiological function in mature adipocytes, PU.1 was knocked down in 3T3-L1 cells using retroviral-mediated expression of PU.1-targeting shRNA. Consistent with previous findings that PU.1 regulates its target genes, such as NADPH oxidase subunits and proinflammatory cytokines in myeloid cells, the mRNA levels of proinflammatory cytokines (TNFα, IL-1β, and IL-6) and cytosolic components of NADPH oxidase (p47phox and p40phox) were downregulated significantly in PU.1-silenced adipocytes. NADPH oxidase is a main source for reactive oxygen species (ROS) generation. Indeed, silencing PU.1 suppressed NADPH oxidase activity and attenuated ROS in basal or hydrogen peroxide-treated adipocytes. Silencing PU.1 in adipocytes suppressed JNK1 activation and IRS-1 phosphorylation at Ser307. Consequently, PU.1 knockdown improved insulin signaling and increased glucose uptake in basal and insulin-stimulated conditions. Furthermore, knocking down PU.1 suppressed basal lipolysis but activated stimulated lipolysis. Collectively, these findings indicate that obesity induces PU.1 expression in adipocytes to upregulate the production of ROS and proinflammatory cytokines, both of which lead to JNK1 activation, insulin resistance, and dysregulation of lipolysis. Therefore, PU.1 might be a mediator for obesity-induced adipose inflammation and insulin resistance.
Keywords: reactive oxygen species, nicotinamide adenine dinucleotide phosphate oxidase, tumor necrosis factor-α, interleukin-1β, c-Jun NH2-terminal kinase, lipolysis
obesity induces profound changes in adipose tissue such as macrophage infiltration, oxidative stress, and secretion of proinflammatory cytokines, leading to the onset of insulin resistance, cardiovascular diseases, and type 2 diabetes (6, 31). However, the underlying mechanism is not fully understood. One hallmark of obesity-associated adipose tissue is the increased production of a variety of proinflammatory cytokines, such as TNFα, IL-1β, and IL-6 (21, 26, 59). Increased macrophage population in adipose tissue is thought to be responsible for this increase (73). But adipocytes also contribute to the production of these cytokines (21, 26, 59). Dysregulated production of these factors is involved in the pathogenesis of obesity-associated metabolic syndrome and type 2 diabetes (16). TNFα plays a key role in mediating insulin resistance in rodent models of obesity (19, 21). Multiple mechanisms may contribute to the metabolic effects of TNFα, including the downregulation of genes involved in insulin action and negative regulation of insulin-sensitizing nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) and changes of lipolysis (3). TNFα also activates kinases such as JNK to serine phosphorylate insulin receptor substrate (IRS) to inhibit insulin signaling (20, 62). IL-1β and IL-6 were found to regulate the expression of IRS-1 and glucose transporter 4 (GLUT4) and directly impair insulin action in adipocytes (26, 59). Thus, regulation of adipose production of proinflammatory cytokines is one viable approach to restore insulin sensitivity and treatment of obesity-related type 2 diabetes.
Oxidative stress is also linked closely to metabolic syndrome. The metabolic abnormalities associated with obesity, such as hyperglycemia, dyslipidemia, and elevated free fatty acid, cause excess reactive oxygen species (ROS) production (2, 5, 25). ROS impairs glucose uptake in muscle and fat (41, 61) and decreases insulin secretion in pancreatic β-cells (43). Antagonizing ROS by various methods ameliorates insulin resistance (22). Oxidative stress plays a causative role in insulin resistance and the progression of type 2 diabetes in animal models as well as human patients (6, 22, 31). Increased oxidative stress is a common pathway implicated in different models of insulin resistance (22). NADPH oxidase is a major source of ROS production in diabetic/hyperglycemic conditions (24, 31). The NADPH oxidase consists of cytosolic components (p40phox, p47phox, p67phox, and the small G protein Rac2) as well as plasma membrane oxidase subunits (p22phox and gp91phox) (1). Assembly of these components in the membrane is required for its activation. NADPH oxidase was found originally in phagocytic cells (1, 4), but recent studies showed that this enzyme also exists in nonphagocytic cells, including adipocytes (30, 42). It was observed that expression levels of NADPH oxidase components in adipose tissue are upregulated in obese mice (9, 44). Interestingly, the increase in ROS production in adipose tissue of high-fat diet-induced obese and insulin-resistant mice is mediated partially by NADPH oxidase (66). Furthermore, p47phox deficiency protects mice against high-fat diet-induced adipose tissue macrophage infiltration, adipose expression of TNFα and IL-6, and systemic insulin resistance (76).
PU.1 transcription factor belongs to the ETS family of proteins (29, 38, 40). It was identified originally as the oncogene Spi-1 (13), and its expression was detected in granulocytes, macrophages, and B lymphocytes (23). PU.1-deficient mice die early with defective development of macrophages, granulocytes, and B lymphocytes (45, 64). PU.1 also plays an important role in osteoclast development since mice lacking PU.1 develop osteoporosis (68). Reduction of PU.1 expression or ablation of PU.1 in adult mice resulted in the development of myeloid leukemia (46, 58).
Our recent study demonstrated that PU.1 is also expressed in adipocytes but not the stromal-vascular cells in the white adipose tissue (70). Similarly, PU.1 protein is not expressed in 3T3-L1 preadipocytes but is gradually upregulated at the late stage of adipocyte differentiation. Furthermore, constitutive expression of PU.1 in 3T3-L1 cells inhibits adipocyte differentiation through the inhibition of CCAAT/enhancer-binding protein (C/EBP) family transcription factors. Remarkably, we found that PU.1 mRNA and protein levels are upregulated in the white adipose tissue of mouse models of genetic obesity, such as ob/ob and agouti Avy, as well as in mice fed a high-fat diet. Consistent with our finding, the increase in PU.1 mRNA levels in obese adipose tissue is also observed in microarray and RT-PCR studies (9, 53). It has been reported that PU.1 is involved in the transcription regulation of NADPH oxidase components. The PU.1 consensus-binding sequence GAGGAA is present in the promoters of p40phox (34), p47phox (4), p67phox (33), and gp91phox (69) and is important for their expression in myeloid cells. Moreover, PU.1 is also involved in transactivation of various proinflammatory cytokine genes in macrophages and mast cells, including TNFα (8), IL-1β (72), and IL-6 (54). Therefore, obesity-induced elevation of adipose PU.1 levels might be responsible for the increased expression of NADPH oxidase and proinflammatory cytokines.
In this study, we explored the function of PU.1 in the mature adipocytes by silencing PU.1 in 3T3-L1 adipocytes with stable expression of PU.1 targeting short-hairpin RNA (shRNA). The expression of NADPH oxidase components and proinflammatory cytokine genes in these cells was examined. We further investigated the consequences of silencing PU.1 on adipocyte ROS production, insulin signaling, and lipolysis.
EXPERIMENTAL PROCEDURES
Animals.
C57BL/6 mice and agouti (Avy) mice were purchased from The Jackson Laboratory. Mice were fed a standard chow diet (Lab Diet 5053; Purina Mills). All procedures used in the animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Mice were maintained under conditions of controlled temperature (∼24°C) and illumination (12:12-h light-dark cycle, 6 AM to 6 PM) with free access to water.
Adipose tissue fractionation.
Gonadal fat pads from Avy and lean control mice were minced in Krebs-Ringer phosphate buffer and digested with 1 mg/ml collagenase type I (Worthington Biochemical) at 37°C for 1 h, as described previously (70). Digested tissue was filtered through a nylon mesh and centrifuged at 500 rpm for 10 min. The top layer (adipocyte fraction) was collected. The remaining was centrifuged again at 1,500 rpm for 10 min, and the pellet (stromal vascular fraction) was collected. Proteins were extracted from both fractions for Western blot analysis.
Plasmids and antibodies.
The DNA cassettes used to generate shRNA against PU.1 (shPU.1) and scrambled mRNA (shScr) in this experiment were accgctggagctcagctggatgttcaagagacatccagctgagctccagctttttc and accggaagaacggcctcactatttcaagagaatagtgaggccgttcttcctttttc. The 19nt sense and reverse complementary targeting sequences were underlined. Double-stranded oligos were inserted into the EcoRI/XhoI site of MSCV-LMP vector (Thermo Scientific, Lafayette, CO), which contains GFP as a marker for retroviral integration and puromycin resistance gene for selection of stable integrants using puromycin.
Establishment of PU.1 knockdown 3T3-L1 cells.
Retroviral-mediated PU.1 shRNA knockdown 3T3-L1 cells were generated as follows. 293T cells were transfected with 20 μg of MSCV-LMP plasmids expressing PU.1 shRNA (shPU.1) or scrambled shRNA (shScr) using lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in 10-cm plates. Medium was changed on the 2nd day. Two days later, packaged retroviral particles in the supernatant were collected and filtrated through 0.2-μm filters. Viral suspension (3 ml) was then mixed with 1 ml of culture medium containing 16 μg of polybrene to infect 3T3-L1 cells in 10-cm plates. Three hours after infection, medium was changed to fresh DMEM. Forty-eight hours after infection, 2 μg/ml puromycin was added to the medium to select for infected cells for 4 days. Selection was continued with 4 μg/ml puromycin for another 4 days. Medium was changed every other day.
Adipocyte differentiation.
3T3-L1 preadipocytes were maintained in high-glucose (4.5 g/l, 25 mM) DMEM with 10% calf serum and 1% penicillin and streptomycin. To induce differentiation, 2 days after cells reached confluence, they were treated for 2 days with DMEM containing 10% cosmic calf serum (CCS), 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methyl xanthine (IBMX), and 5 μg/ml (872 nM) insulin. In all experiments investigating the function of PU.1 in adipocytes, 1 μM rosiglitazone (Fisher Scientific, Atlanta, GA) was included in the first 2 days of differentiation. The cells were then maintained in DMEM with 10% CCS and 5 μg/ml insulin for another 4–5 days. During the differentiation process, media were changed every other day. For some specified experiments, low-glucose DMEM (1 g/l, 5.5 mM) with 10% CCS and 5 μg/ml insulin was used starting from day 4 of differentiation. For H2O2 treatment experiment, fully differentiated adipocytes were treated with 100 μM H2O2 overnight.
Oil Red O staining.
Differentiated 3T3-L1 adipocytes on day 7 were washed in phosphate-buffered saline (PBS; pH 7.4) three times, and cells were fixed in 3.7% formaldehyde for 10 min. Then cells were washed with 60% isopropanol, followed by staining with Oil Red O working solution for 30 min. Oil Red O working solution was prepared by diluting a stock solution [0.5 g of Oil Red O (Sigma, St. Louis, MO) in 200 ml of isopropanol] with water [60:40 (vol/vol)], followed by filtration. After staining, plates were washed twice with PBS and photographed.
Real-time PCR.
Total RNA of cells was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA), following the manufacturer's instructions. The cDNA was synthesized using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). Real-time PCR was performed on a LightCycler using the FastStart DNA Master SYBR Green (Roche Diagnostics, Indianapolis, IN) according to the protocol provided by the manufacturer. Sequences of primers used for real-time PCR were as follows: PU.1 forward 5′-CCTCAGTCACCAGGTTTCCTACA-3′ and PU.1 reverse 5′-CTCTCACCCTCCTCCTCATCTG-3′; gp91phox forward 5′-TTGGGTCAGCACTGGCTCTG-3′ and gp91phox reverse 5′-TGGCGGTGTGCAGTGCTATC-3′; p22phox forward 5′-GTCCACCATGGAGCGATGTG-3′ and p22phox reverse 5′-CAATGGCCAAGCAGACGGTC-3′; p47phox forward 5′-GATGTTCCCCATTGAGGCCG-3′ and p47phox reverse 5′-GTTTCAGGTCATCAGGCCGC-3′; p40phox forward 5′-GCCGCTATCGCCAGTTCTAC-3′ and p40phox reverse 5′-GCAGGCTCAGGAGGTTCTTC-3′; TNFα forward 5′-CCCTCACACTCAGATCATCTTCT-3′ and TNFα reverse 5′-GCTACGACGTGGGCTACAG-3′; IL-1β forward 5′-GACCTTCCAGGATGAGGACA-3′ and IL-1β reverse 5′-AGCTCATATGGGTCCGACAG-3′; IL-6 forward 5′-ACAACCACGGCCTTCCCTACTT-3′ and IL-6 reverse 5′-CAGGATTTCCCAGCGAACATGTG-3′; Nox4 forward 5′-TTGCCTGGAAGAACCCAAGT-3′ and Nox4 reverse 5′-TCCGCACAATAAAGGCACAA-3′; 18S ribosomal RNA forward 5′-AACGAGACTCTGGCATGCTAACTAG-3′ and 18S ribosomal RNA reverse 5′-CGCCACTTGTCCCTCTAAGAA-3′. The expression levels of genes of interest were normalized by the levels of 18S RNA.
Western blot analysis.
Cells were lysed in lysis buffer (50 mM Tris, 50 mM KCl, 20 mM NaF, 1 mM Na3VO4, 10 mM EDTA, 1% NP-40, 1 mM PMSF, and 5 μg/ml leupeptin, pH 8.0). Protein concentration was determined with BCA protein assay kit (Pierce, Rockford, IL). Twenty micrograms of protein of each sample was separated by SDS-PAGE and electrotransferred to nitrocellulose membrane for immunoblot analysis. The following antibodies were used: anti-PU.1 (sc-352, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), anti-α-tubulin (T5168, 1:100,000; Sigma), anti-actin (sc-1616, 1:1,000; Santa Cruz Biotechnology), anti-PPARγ (sc-7196, sc-7273, 1:500; Santa Cruz Biotechnology), anti-C/EBPα (sc-61, 1:500; Santa Cruz Biotechnology), anti-GLUT4 (2299, 1:1,000; Cell Signaling Technology, Danvers, MA), anti-GLUT1 (07-1401, 1:1,000; Millpore, Billerica, MA), anti-phospho-JNK1 (Tyr183/185) (sc-6254, 1:1,000; Santa Cruz Biotechnology), anti-JNK1 (sc-571, 1:1,000; Santa Cruz Biotechnology), anti-phospho-Akt (4058S, 1:1,000, Ser472/473; Cell Signaling Technology), anti-Akt (9272, 1:1,000; Cell Signaling Technology), anti-phospho-IRS-1 (Ser307, sc-33956, 1:1,000; Santa Cruz Biotechnology), anti-phospho-IRS-1 (Tyr989, sc-17200, 1:1,000; Santa Cruz Biotechnology), anti-IRS-1 (06-248, 1:1,000; Millipore), horseradish peroxidase-conjugated anti-mouse (170-6516, 1:30,000; Bio-Rad, Richmond, CA), and anti-rabbit (170–6515, 1:30,000; Bio-Rad). The SuperSignal West Pico Chemiluminescent kit (Pierce) was used as substrate.
Assessment of ROS levels in 3T3-L1 adipocytes.
ROS production was detected by 2′,7′-dichlorofluorescin diacetate (DCFDA) assay (65) and nitroblue tetrazolium (NBT) assay (56). Briefly, differentiated cells were washed with Krebs-Ringer buffer and then incubated in the dark with the fluorescent probe 10 μM DCFDA (Sigma) in Krebs-Ringer buffer buffer for 45 min at 37°C. The fluorescence was analyzed in an HTS 7000 Bio Assay Fluorescent Plate Reader (PerkinElmer Life Sciences, Waltham, MA) at an excitation wavelength of 485 nm and emission at 530 nm. For NBT assay, differentiated 3T3-L1 adipocytes were incubated in PBS containing 0.2% NBT (Sigma) for 90 min. NBT is reduced by ROS to a dark blue, insoluble form called formazan. Then, formazan was dissolved in 50% acetic acid and the absorbance determined at 560 nm.
Measurement of NADPH oxidase activity.
NADPH oxidase activity was measured using the lucigenin-enhanced chemiluminescence method, following previously described procedures, with minor modifications (14, 63). To prepare cell homogenates, the fully differentiated adipocytes were washed three times with ice-cold PBS and scraped on ice in lysis buffer containing 20 mM K-phosphate buffer (pH 7.0), 1 mM EGTA, 1 mM PMSF, 10 g/ml aprotinin, and 5 g/ml leupeptin, followed by brief sonication on ice. The cell homogenate was used immediately. The assay was performed using a Sirius single tube luminometer (Titertech-Berthold Instruments, Huntsville, AL) by adding 300 μl of reaction buffer [50 mM K-phosphate buffer (pH 7.0) containing 1 mM EGTA, 150 mM sucrose, 5 M lucigenin, and 100 M NADPH] into 20 μl of the homogenate in the presence or absence of 200 μM apocynin. Photon emission in response to superoxide generation was measured every 60 s with a 5-s signal integration time for 16 min. The activity is expressed as the difference of the NADPH oxidase activity in the presence or absence of apocynin in relative light units per milligram of protein. The protein concentration was measured using the BCA protein assay (Pierce).
Measurement of 2-deoxy-d-[1,2-3H]glucose uptake.
Assay of 2-deoxy-d-[1-3H]glucose uptake was performed as described previously (57). Briefly, the cells were serum-starved overnight. After being washed three times with warm Krebs-Ringer-HEPES (KRH) buffer (pH 7.4), cells were treated with or without 100 nM insulin in KRH buffer for 15 min, followed by addition of KRH buffer containing 1.1 mM 2-deoxy-d-glucose with 0.4 μCi of 2-deoxy-d-[3H]glucose/ml with or without 100 nM insulin for 10 min at 37°C. Glucose transport was terminated with three washes of ice-cold KRH buffer, and cells in each plate were harvested in 1 ml of 0.1% SDS solution. A 0.25-ml aliquot of each sample was mixed with 5 ml of scintillation fluid for the determination of radioactivity by liquid scintillation counting, and a 20-μl aliquot was used for measurement of protein using BCA assay.
Lipolysis assay.
3T3-L1 cells were fully differentiated for 8 days. Before glycerol release was assayed, adipocytes were serum-starved in DMEM with 0.5% fatty acid-free BSA for 4 h. The cells were then incubated in DMEM with 2% BSA and 100 nM insulin for 2 h. Afterward, fresh medium containing insulin and 10 μM isoproterenol was added. One hour later, glycerol content in the medium was measured by the glycerol assay kit (Sigma-Aldrich), following the manufacturer's instructions. Briefly, a 10-μl supernatant of culture was incubated with 200 μl of assay reagent for 5 min at 37°C. The absorbance was measured by spectrometer at 540 nm.
Statistics.
The data are represented as means ± SE. Statistical significance was determined using the two-tailed Student t-test. P < 0.05 was considered to be statistically significant.
RESULTS
PU.1 is expressed in the adipocytes of obese mice.
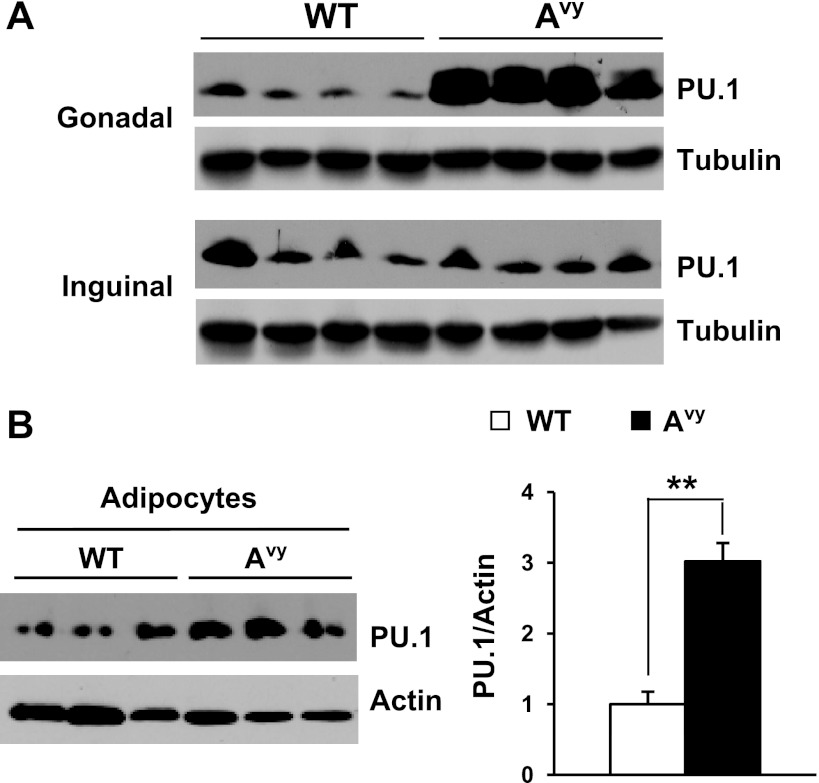
We have reported that PU.1 expression in the adipose tissue is increased greatly in visceral fat of various obese mouse models (70). But the expression of PU.1 in different adipose depots in obese mice is unknown. We examined PU.1 protein levels in visceral (paraovarian) and subcutaneous (inguinal) fat depots of lean control or obese agouti Avy mice. As we have reported previously, obesity increased the amount of PU.1 proteins in the visceral paraovarian adipose tissues. Interestingly, in the inguinal adipose tissues of these mice, PU.1 expression was unaltered (Fig. 1A). We further determined the cellular origin of the elevated PU.1 expression by obtaining adipocyte and stromal vascular fractions from paraovarian adipose tissues from lean control or obese agouti Avy mice. We found that PU.1 protein levels in the adipocyte fraction from obese mice were increased significantly (Fig. 1B), whereas PU.1 expression in the stromal vascular fraction was not increased (data not shown), indicating that mature adipocytes are responsible for the elevation of PU.1 expression in the visceral fat of obese mice. The above data suggest that PU.1 might regulate adipocyte function and mediate obesity-associated insulin resistance.
Fig. 1.
Obesity induces PU.1 expression in adipose tissue. A: PU.1 levels in paraovarian and inguinal adipose tissues from Avy and control mice were detected by Western blot analysis. B: adipocyte and stromal vascular cell fractions were prepared from gonadal white adipose tissues of wild-type (WT) and obese Avy mice (n = 7). PU.1 expression was detected by Western blot analysis. **P < 0.01.
PU.1 knockdown promotes adipocyte differentiation.
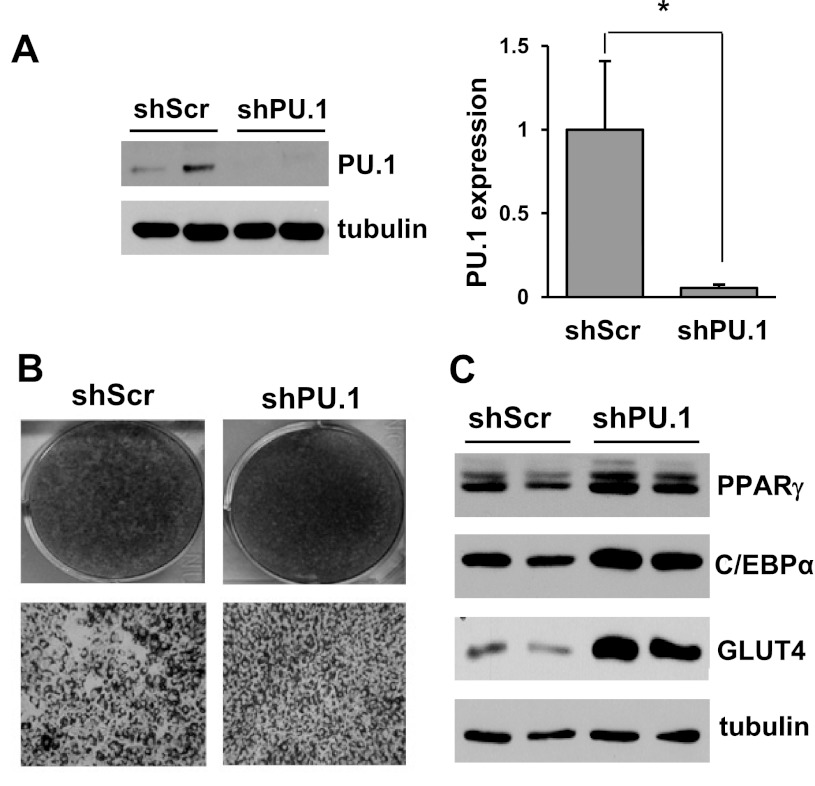
To investigate the physiological function of PU.1 in mature adipocytes, we generated stable 3T3-L1 preadipocytes expressing a retrovirus-mediated shRNA targeting PU.1. To confirm the downregulation of PU.1, these cells were differentiated into adipocytes, and the PU.1 protein level was examined by Western blot analysis. As shown in Fig. 2A, compared with the control adipocytes expressing a scrambled shRNA, PU.1 shRNA-expressing adipocytes exhibit a 90% decreased PU.1 protein amount. Previously, we reported that overexpression of PU.1 suppresses adipogenesis (70). Here, downregulation of PU.1 expression enhanced adipocyte differentiation when the 3T3-L1 preadipocytes were differentiated under conventional conditions with exposure to a mixture of IBMX, dexamethasone, and insulin for 2 days and then insulin alone for another 4 days. Adipocytes with PU.1 knockdown accumulated more lipid droplets, indicated by Oil Red O staining (Fig. 2B). Cells with their PU.1 expression silenced also had higher expression of adipocyte differentiation markers such as PPARγ, C/EBPα, and GLUT4 (Fig. 2C). The differences in adipocyte differentiation status and the expression of adipocyte-associated genes influence adipocyte function, preventing us from analyzing the functional consequences of PU.1 downregulation in adipocytes under this condition. Instead, we differentiated preadipocytes with the addition of PPARγ agonist rosiglitazone (1 μM) to the conventional hormone mixture on the first 2 days of differentiation. Under this condition, the adipocytes expressing PU.1-targeting shRNA achieved equal differentiation as the adipocytes expressing a scrambled control shRNA, as indicated by either comparable Oil Red O staining (data not shown) or equal levels of expression of several differentiation markers (Fig. 3A). In the subsequent experiments, adipocytes were always differentiated with rosiglitazone treatment.
Fig. 2.
PU.1 knockdown promotes 3T3-L1 adipocyte differentiation. A: 3T3-L1 cells expressing shPU.1 or shScr were generated. PU.1 protein levels in these cells were measured by Western blot analysis and normalized to α-tubulin (n = 6). B: 3T3-L1 cells with retroviral-mediated expression of shPU.1 or shScr were differentiated in high-glucose (25 mM) DMEM. Lipid accumulation in cells was visualized by Oil Red O staining. C: the expression of differentiation markers [peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT/enhancer-binding protein-α (C/EBPα), and glucose transporter 4 (GLUT4)] in shPU.1 and shScr cells was detected by Western blot analyses. *P < 0.05. shPU.1, 3T3-L1 cells with stable expression of PU.1 targeting short-hairpin RNA (shRNA); shScr, 3T3-L1 cells with stable expression of PU.1 targeting scrambled shRNA.
Fig. 3.
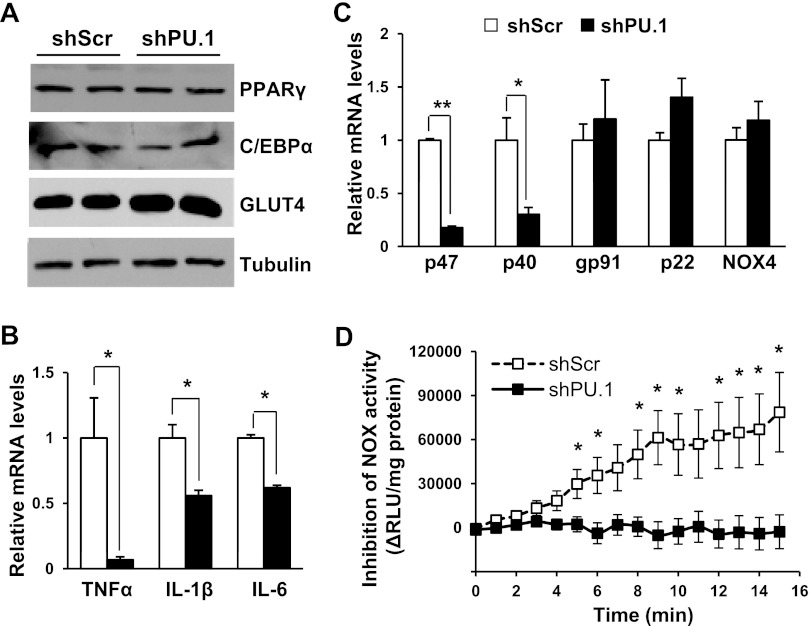
Effects of PU.1 knockdown on transcription levels of inflammatory cytokines and NADPH oxidase. A: 3T3-L1 cells expressing shPU.1 or shScr were differentiated in the presence of rosiglitazone. The expression of differentiation markers (PPARγ, C/EBPα, and GLUT4) in cells was detected by Western blot analyses. B: transcription levels of inflammatory cytokines were detected using real-time RT-PCR method. Data are means ± SE (n = 6). C: mRNA levels of components of NADPH oxidase were measured using real-time RT-PCR method. Data are means ± SE (n = 6). D: NADPH oxidase activity in cell homogenates was measured. Data are means ± SE (n = 6). *P < 0.05; **P < 0.01. RLU, relative luminescence unit.
Silencing PU.1 represses the transcription of proinflammatory cytokines and NADPH oxidase subunits.
To address whether PU.1 regulates the expression of its target genes in adipocytes, quantitative RT-PCR experiments were performed to determine the transcription levels of proinflammatory cytokines. TNFα mRNA level was reduced >90% in PU.1-silenced adipocytes. Additionally, the transcription levels of IL-1β and IL-6 were also significantly repressed (Fig. 3B). Next, we examined the expression of NADPH oxidase components in differentiated adipocytes. Compared with control cells, PU.1 knockdown adipocytes had greatly decreased mRNA expression of the cytosolic components p47phox and p40phox (Fig. 3C), whereas the transcriptional levels of membrane-bound gp91phox (Nox2), Nox4, and p22phox were unaltered. Then, we measured NADPH oxidase enzymatic activity in these cells, using lucigenin-enhanced chemiluminescent superoxide detection (14, 63). We found that PU.1 knockdown abolished NADPH oxidase-mediated ROS production (Fig. 3D). The above results reveal that PU.1 controls the transcription of proinflammatory cytokines and NADPH oxidase subunits and regulates NADPH oxidase activity in adipocytes.
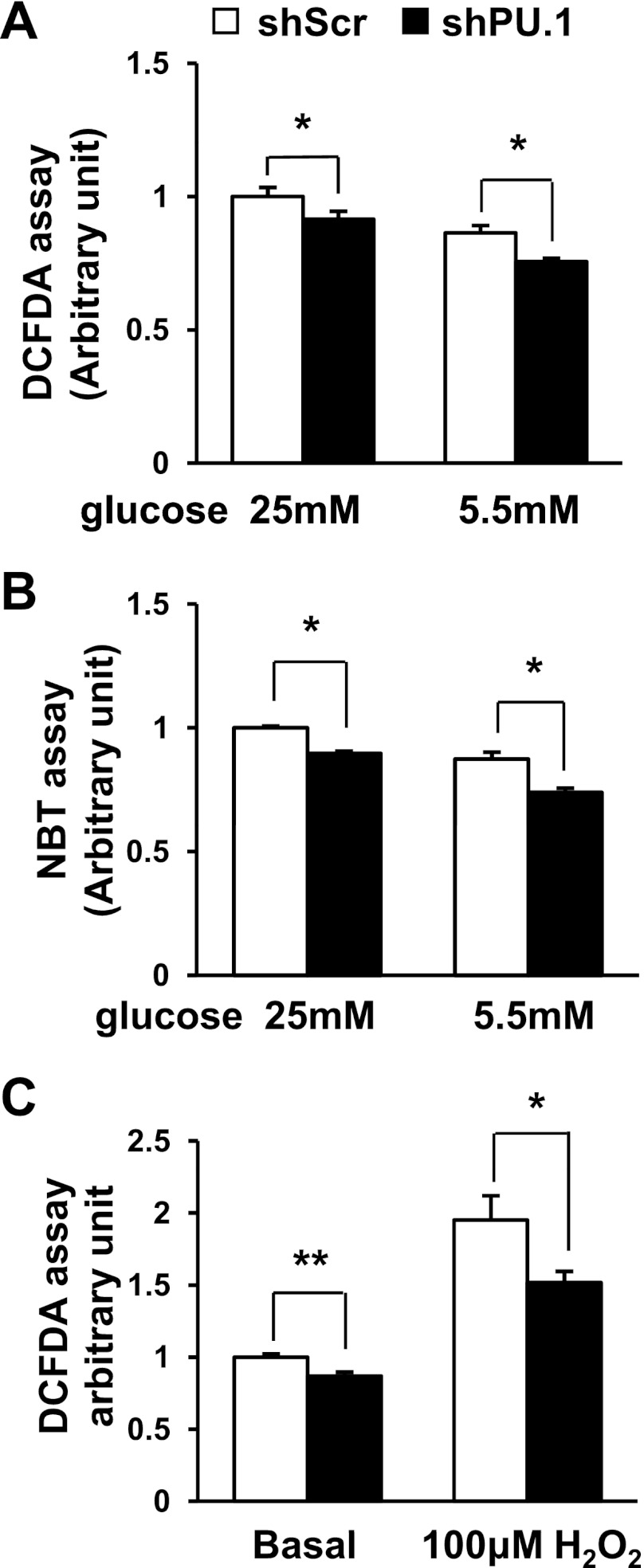
To confirm whether the suppression of NADPH oxidase activity affects intracellular ROS levels in these cells, we stained the cells with the ROS-sensitive fluorescent dye DCFDA (65). As expected, silencing PU.1 in adipocytes downregulated ROS generation (Fig. 4A). It was reported that 3T3-L1 adipocytes differentiated in high-glucose medium are insulin resistant with excess ROS production (35). Therefore, we also differentiated 3T3-L1 cells in low-glucose medium (5.5 mM, glucose), which is close to the normal physiological glucose level. Lowering the glucose level decreased the ROS levels in both PU.1 knockdown and control adipocytes. However, suppression of PU.1 expression further reduced the ROS production in adipocytes (Fig. 4A). Because DCFDA staining reflects the cellular levels of hydrogen peroxide, hydroxyl radicals, and peroxynitrite, we also performed NBT staining, which reacts mainly with superoxide. We obtained a similar result as with DCFDA staining (Fig. 4B). It was known that obesity and insulin resistance are associated with adipose tissue oxidative stress (9). To investigate the role PU.1 plays in stressed adipocytes, we challenged PU.1 knockdown adipocytes with H2O2. We found that H2O2 treatment increased cellular ROS levels significantly in adipocytes. However, silencing PU.1 significantly attenuated adipocyte levels of ROS (Fig. 4C).
Fig. 4.
PU.1 regulates adipocyte reactive oxygen species (ROS) production. 3T3-L1 adipocytes expressing shPU.1 or shScr were differentiated in media containing high (25 mM) or low glucose (5.5 mM). ROS levels in these cells were determined with 2′,7′-dichlorofluorescin diacetate (DCFDA) staining (A) or nitroblue tetrazolium (NBT) staining (B). Data are means ± SE (n = 6). C: adipocytes were differentiated in high-glucose-containing (25 mM) medium. The cells were treated with 100 μM H2O2 overnight. ROS levels were determined with DCFDA assay. Data are means ± SE (n = 6). *P < 0.05; **P < 0.01.
PU.1 knockdown suppresses JNK activation in 3T3-L1 adipocytes.
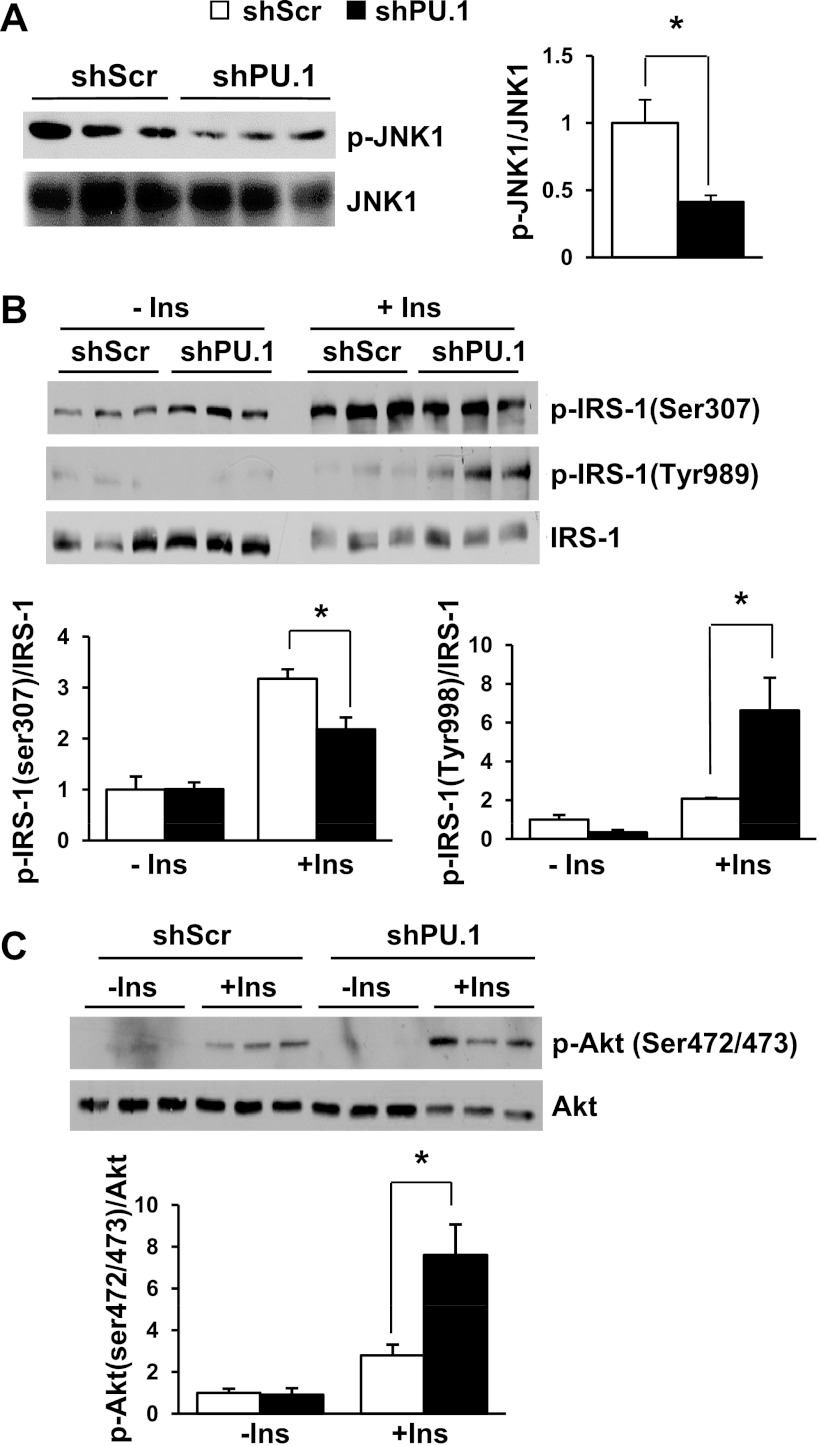
It was reported that elevation of inflammatory cytokines, such as TNFα, and oxidative stress activate JNK, which is a key mediator for insulin resistance (18, 62). The reduced production of inflammatory cytokine and ROS in PU.1 knockdown cells should lead to suppressed JNK activation. Analysis of JNK phosphorylation levels in PU.1 knockdown adipocytes confirmed that JNK1 phosphorylation was decreased significantly (Fig. 5A).
Fig. 5.
Regulation of JNK1 and insulin (Ins) signaling in PU.1 knockdown adipocytes. A: the levels of phosphorylated (p) JNK1 and total JNK1 were detected by Western blot analysis. B: 3T3-L1 adipocytes expressing shPU.1 or shScr were serum starved overnight and then stimulated with 100 nM insulin for 15 min. Immunoblot analysis of p-IRS-1 (Ser307 and Tyr989) and total IRS-1 was performed. C: immunoblot analysis of p-Akt (Ser472/473) and total Akt in the same cells as Fig. 4A. *P < 0.05.
PU.1 knockdown increases insulin signaling and enhances insulin-mediated glucose uptake in 3T3-L1 adipocytes.
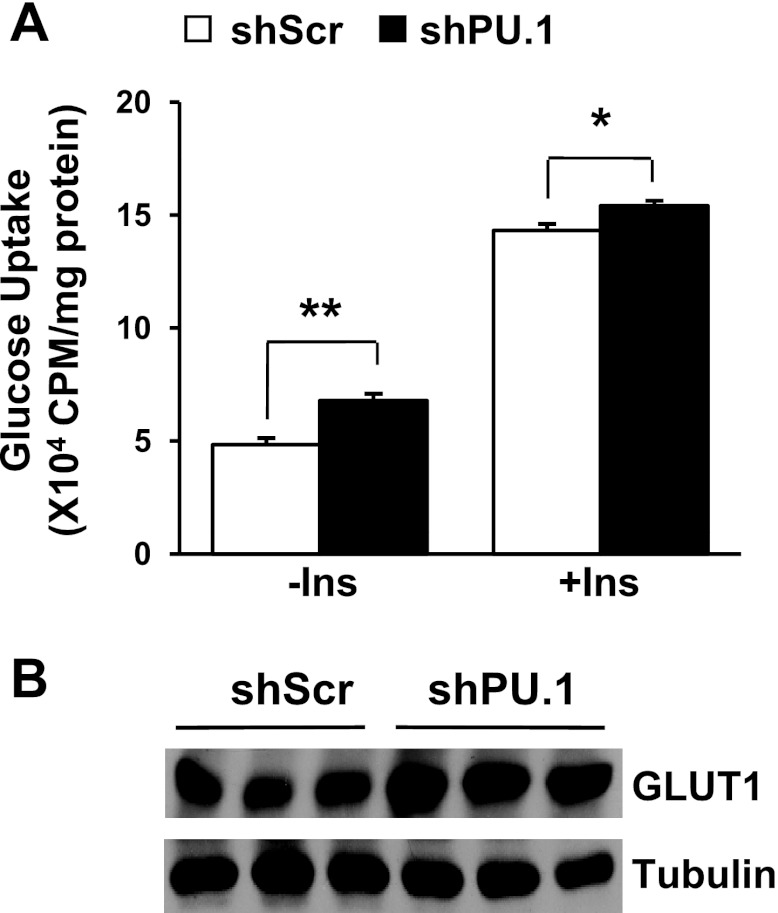
It is well documented that oxidative stress and proinflammatory cytokines such as TNFα and IL-1β cause insulin resistance and repress glucose uptake in 3T3-L1 adipocytes (60, 61). One of the underlying mechanisms is the increased inhibitory serine phosphorylation on IRS proteins, such as the phosphorylation of IRS-1 at Ser307, a known target of JNK (62). Therefore, we decided to examine insulin signaling in these cells. To this end, adipocytes with or without PU.1 knockdown were serum starved and then stimulated with insulin, and the insulin-signaling pathway components were assessed. As expected, IRS-1 Ser307 phosphorylation was decreased in PU.1 knockdown adipocytes (Fig. 5B). Consequently, suppression of PU.1 expression in adipocytes resulted in a significant increase in insulin-stimulated IRS-1 Tyr989 and Akt Ser472/473 phosphorylation (Fig. 5, B and C). When the differentiated adipocytes were treated with H2O2, silencing PU.1 still increased Akt Ser472/473 phosphorylation at both basal and insulin-stimulated conditions (data not shown). We next investigated the effect of PU.1 on glucose uptake using 2-deoxy-d-[1,2-3H]glucose. We found that silencing PU.1 increased both the basal and insulin-stimulated glucose uptake (Fig. 6A). We examined the expression levels of GLUT1 and found that it was increased in cells with PU.1 knockdown (Fig. 6B), offering an explanation for the increase of glucose uptake (55). All of these results indicate that PU.1 is likely to play an inhibitory role in insulin signaling and glucose uptake in adipocytes.
Fig. 6.
Regulation of glucose uptake in PU.1 knockdown adipocytes. A: both basal and 100 nM insulin-stimulated 2-deoxy-d-[1-3H]glucose uptake in 3T3-L1 adipocytes expressing shPU.1 or shScr were determined. Data are means ± SE (n = 9). B: the protein levels of GLUT1 in these cells were detected by Western blot analysis. *P < 0.05; **P < 0.01.
PU.1 knockdown regulates adipocyte lipolysis.
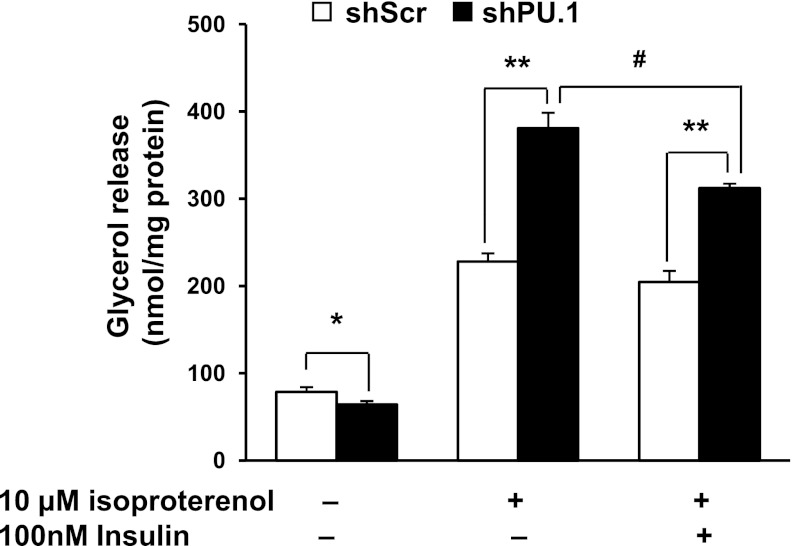
Lipolysis, the hydrolysis of lipids to release free fatty acids and glycerol, has been implicated in the development of insulin resistance (36, 71). Both TNFα (3, 15, 48) and ROS (37, 50) were reported to increase basal lipolysis and decrease stimulated lipolysis. To investigate whether PU.1 knockdown influences lipolysis, we measured glycerol released into the culture supernatant of differentiated adipocytes with the stable expression of scrambled or PU.1-targeting shRNA. We found that silencing PU.1 in adipocytes reduced the release of glycerol (Fig. 7). Activation of adrenergic signaling by isoproterenol treatment induced adipocyte lipolysis as reported (12). PU.1 knockdown cells had a significantly higher level of isoproterenol-stimulated lipolysis than that of the control cells (Fig. 7). When control adipocytes were treated with insulin for 2 h, on top of 1 h of isoproterenol stimulation, the glycerol release was slightly decreased (not statistically significant). However, in adipocytes with PU.1 knockdown, insulin treatment suppressed stimulated lipolysis significantly (Fig. 7). These results indicate that PU.1 activates basal lipolysis but inhibits stimulated lipolysis in adipocytes. In addition, downregulation of PU.1 sensitizes adipocytes to insulin's inhibition of lipolysis.
Fig. 7.
PU.1 regulates adipocyte lipolysis. 3T3-L1 adipocytes expressing shPU.1 or shScr were serum starved for 4 h and then treated with insulin (100 nM) for 2 h, followed by addition of isoproterenol (10 μM). One hour later, the supernatant was harvested and free glycerol content determined. Data are means ± SE (n = 6). *P < 0.05; **P < 0.01 between control and PU.1 knockdown cells; #P < 0.01 between samples with and without insulin treatment.
DISCUSSION
We have found previously that PU.1 expression in the adipose tissue is increased greatly in the visceral adipose tissue of various obese mouse models (70). In the present study, we further determined that the increase in PU.1 expression occurred only in visceral but not subcutaneous adipose tissues (Fig. 1A). Adipocytes appeared to be responsible for the elevated expression of PU.1 in the adipose tissues of obese mice (Fig. 1B). A progressive increase in visceral adiposity is a prominent risk factor for insulin resistance and type 2 diabetes (47). Conversely, removal of visceral fat has been shown to protect aging rats against insulin resistance and glucose intolerance (10). The visceral adipose depot-specific increase in PU.1 expression in obese mice is in line with the notion that PU.1 plays a role in obesity-induced insulin resistance.
It was reported previously that in adipose tissue of genetically obese mice the mRNA levels of NADPH oxidase subunits, including p40phox, p47phox, p22phox, and gp91phox, are increased significantly (9). In this study, we found that PU.1 knockdown in adipocytes downregulated two cytosolic components of NADPH oxidase, p47phox and p40phox, whereas the expression of p22phox, gp91phox (Nox2), and Nox4 was not changed (Fig. 3C). The p47phox is the major subunit responsible for translocating the cytosolic complex to the cellular membrane for NADPH oxidase activation (17). The p40phox subunit shares structure similarity with p47phox and may compensate for p47phox deficiency in maintaining basal ROS production (7). Suppression of p47phox and p40phox was shown to impair NADPH oxidase activation and reduce ROS production (1). Our results confirmed that PU.1 knockdown leads to a significant suppression of NADPH oxidase activity (Fig. 3D). As a result, the ROS level in PU.1-silencing adipocytes was decreased (Fig. 4, A and B). The decreasing of ROS might also be due to upregulation of oxidative defense (9). Although we cannot completely rule out the involvement of detoxification enzymes, we detected no change of MnSOD protein level in PU.1 knockdown cells (data not shown). Furthermore, H2O2 treatment elevated adipocyte ROS levels, whereas silencing PU.1 dampened ROS generation significantly (Fig. 4C). Because oxidative stress is associated with obesity (9) and insulin resistance (22), PU.1 is likely to meditate obesity-associated adipose oxidative stress.
Obesity also increases adipocyte production of proinflammatory cytokines such as TNFα, IL-1β, and IL-6, which contribute to insulin resistance (21, 26, 59). It is reported that PU.1 binds to the promoter and regulates the transcription of TNFα, IL-1β, and IL-6 in bone marrow-derived dendritic cells and mast cells (8, 49, 59). We found that PU.1 also regulated the transcription of these proinflammatory cytokines in adipocytes since silencing PU.1 represses the expression of these proinflammatory factors (Fig. 3B). The secretion of proinflammatory cytokines may recruit macrophage infiltration and increase adipose inflammation (73, 75). All three cytokines can inhibit adipocyte differentiation and the expression of adipocyte-associated genes such as GLUT4 (26, 59). These cytokines also negatively regulate insulin signaling via multiple mechanisms, including the decrease in IRS-1 expression (39, 67) and the increase in IRS-1 serine phosphorylation (20). It was documented that TNFα activates JNK and IRS-1 serine phosphorylation (62). Obesity-associated oxidative stress also contributes to insulin resistance. Oxidative stress leads to the activation of multiple serine kinases, including JNK (6). In this study, we found that PU.1's ability to regulate both inflammatory cytokines and ROS resulted in decreased phosphorylation of JNK1 (Fig. 5A) and IRS-1 phosphorylation at Ser307 (Fig. 5B) in adipocytes with PU.1 knockdown. Therefore, JNK1 might be a key mediator of PU.1's regulation of insulin sensitivity. In PU.1 knockdown adipocytes, insulin signaling in terms of IRS-1 Tyr989 and Akt Ser472/473 phosphorylation was upregulated (Fig. 5, B and C). Even when adipocytes were challenged with oxidants (H2O2), silencing PU.1 improved insulin sensitivity (data not shown). Furthermore, both basal and insulin-stimulated glucose uptake were also increased in PU.1 knockdown cells (Fig. 6A). Elevated GLUT1 expression and increased insulin signaling contribute to this phenomenon.
Adipocyte lipolysis plays an important role in meeting the energy needs of the body. However, excess of free fatty acids may also cause lipotoxicity and induce insulin resistance. We found that PU.1 activates basal adipocyte lipolysis but inhibits stimulated lipolysis (Fig. 7). As PU.1 knockdown increases insulin signaling it also sensitizes adipocytes to insulin-induced suppression of lipolysis. The mechanism underlying PU.1's regulation of lipolysis could also be through the changes in ROS and TNFα. It was documented that H2O2 treatment increases basal lipolysis but suppresses β-adrenergic-stimulated lipolysis in adipocytes (37, 50) through the activation of cAMP hydrolysis at the surface of lipoid droplets by H2O2 (51, 52). Additionally, TNFα also causes an increase in basal lipolysis and a decrease in stimulated lipolysis through downregulation of perilipin and cell death-inducing DFFA-like effector C (3, 15, 48). Therefore, PU.1 might regulate lipolysis through the elevation of both ROS and TNFα. It is interesting to note that obesity is associated with increased basal lipolysis and decreased catecholamine-stimulated lipolysis in both mouse models (11, 28) and humans (27, 32, 74). Since PU.1 expression level is increased in adipocytes under the condition of obesity, PU.1 may contribute to this pattern of lipolysis in obese individuals.
In summary, PU.1 expression in abdominal adipocytes is induced by obesity. Silencing PU.1 suppresses the expression of PU.1 target genes, including proinflammatory cytokines and NADPH oxidase subunits. This leads to a block of NADPH oxidase activity, which results in decreased ROS production. The downregulation of proinflammatory cytokines and ROS represses JNK1 to improve adipocyte insulin signaling. The low level of proinflammatory cytokine and ROS also suppress the basal lipolysis. Therefore, targeting PU.1 in adipocytes may suppress the production of both proinflammatory cytokines and ROS to achieve therapeutic benefits against obesity-induced insulin resistance and metabolic syndrome.
GRANTS
This work was supported by a US Department of Agriculture/Agricultural Research Service (USDA/ARS) grant (6250-51000-049) and a National Institute of Diabetes and Digestive and Kidney Diseases grant (DK-075978) to Q. Tong. This study was also supported by USDA/ARS Grant 6250-51000-055 and American Heart Association Grant 12IRG9230004 to Y. Sun.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.L., W.P., K.C., F.W., and J.G. performed the experiments; L.L., W.P., K.C., F.W., and J.G. analyzed the data; L.L. and Q.T. interpreted the results of the experiments; L.L. and K.C. prepared the figures; L.L. drafted the manuscript; L.L., Y.S., and Q.T. edited and revised the manuscript; L.L. and Q.T. approved the final version of the manuscript; Q.T. did the conception and design of the research.
ACKNOWLEDGMENTS
We thank Dr. Robert Waterland for providing Avy and control mice.
REFERENCES
- 1. Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 2. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32, Suppl 3: 14–23, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dusi S, Donini M, Lissandrini D, Mazzi P, Bianca VD, Rossi F. Mechanisms of expression of NADPH oxidase components in human cultured monocytes: role of cytokines and transcriptional regulators involved. Eur J Immunol 31: 929–938, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23: 599–622, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Fan LM, Teng L, Li JM. Knockout of p47 phox uncovers a critical role of p40 phox in reactive oxygen species production in microvascular endothelial cells. Arterioscler Thromb Vasc Biol 29: 1651–1656, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukai T, Nishiyama C, Kanada S, Nakano N, Hara M, Tokura T, Ikeda S, Ogawa H, Okumura K. Involvement of PU.1 in the transcriptional regulation of TNF-alpha. Biochem Biophys Res Commun 388: 102–106, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gabriely I, Barzilai N. Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep 3: 201–206, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol 298: C961–C971, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goebl MK. The PU.1 transcription factor is the product of the putative oncogene Spi-1. Cell 61: 1165–1166, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121: 2102–2110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care 5: 551–559, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Heyworth PG, Curnutte JT, Nauseef WM, Volpp BD, Pearson DW, Rosen H, Clark RA. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest 87: 352–356, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665–668, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Hromas R, Orazi A, Neiman RS, Maki R, Van Beveran C, Moore J, Klemsz M. Hematopoietic lineage- and stage-restricted expression of the ETS oncogene family member PU.1. Blood 82: 2998–3004, 1993 [PubMed] [Google Scholar]
- 24. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–S232, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148: 241–251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest 83: 1168–1173, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kinney BP, Qiao L, Levaugh JM, Shao J. B56alpha/protein phosphatase 2A inhibits adipose lipolysis in high-fat diet-induced obese mice. Endocrinology 151: 3624–3632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klemsz MJ, McKercher SR, Celada A, Van Beveren C, Maki RA. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell 61: 113–124, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem 272: 10135–10143, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Kuroki T, Isshiki K, King GL. Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol 14: S216–S220, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res 40: 2059–2066, 1999 [PubMed] [Google Scholar]
- 33. Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Li SL, Valente AJ, Qiang M, Schlegel W, Gamez M, Clark RA. Multiple PU.1 sites cooperate in the regulation of p40(phox) transcription during granulocytic differentiation of myeloid cells. Blood 99: 4578–4587, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 280: 4617–4626, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis 19: 146–152, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Little SA, de Haen C. Effects of hydrogen peroxide on basal and hormone-stimulated lipolysis in perifused rat fat cells in relation to the mechanism of action of insulin. J Biol Chem 255: 10888–10895, 1980 [PubMed] [Google Scholar]
- 38. Lloberas J, Soler C, Celada A. The key role of PU.1/SPI-1 in B cells, myeloid cells and macrophages. Immunol Today 20: 184–189, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab 292: E166–E174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macleod K, Leprince D, Stehelin D. The ets gene family. Trends Biochem Sci 17: 251–256, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes 50: 404–410, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem 276: 48662–48669, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Matsuoka T, Kajimoto Y, Watada H, Kaneto H, Kishimoto M, Umayahara Y, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest 99: 144–150, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 57: 1071–1077, 2008 [DOI] [PubMed] [Google Scholar]
- 45. McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J 15: 5647–5658, 1996 [PMC free article] [PubMed] [Google Scholar]
- 46. Metcalf D, Dakic A, Mifsud S, Di Rago L, Wu L, Nutt S. Inactivation of PU.1 in adult mice leads to the development of myeloid leukemia. Proc Natl Acad Sci USA 103: 1486–1491, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 283: E1135–E1143, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 11: 212–217, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Mouri F, Tsukada J, Mizobe T, Higashi T, Yoshida Y, Minami Y, Izumi H, Kominato Y, Kohno K, Tanaka Y. Intracellular HMGB1 transactivates the human IL1B gene promoter through association with an Ets transcription factor PU.1. Eur J Haematol 80: 10–19, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Mukherjee SP. Mediation of the antilipolytic and lipogenic effects of insulin in adipocytes by intracellular accumulation of hydrogen peroxide. Biochem Pharmacol 29: 1239–1246, 1980 [DOI] [PubMed] [Google Scholar]
- 51. Müller G, Over S, Wied S, Frick W. Association of (c)AMP-degrading glycosylphosphatidylinositol-anchored proteins with lipid droplets is induced by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes. Biochemistry 47: 1274–1287, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Müller G, Wied S, Jung C, Over S. Hydrogen peroxide-induced translocation of glycolipid-anchored (c)AMP-hydrolases to lipid droplets mediates inhibition of lipolysis in rat adipocytes. Br J Pharmacol 154: 901–913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci USA 97: 11371–11376, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishiyama C, Nishiyama M, Ito T, Masaki S, Masuoka N, Yamane H, Kitamura T, Ogawa H, Okumura K. Functional analysis of PU.1 domains in monocyte-specific gene regulation. FEBS Lett 561: 63–68, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O'Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J Biol Chem 276: 9149–9157, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes 52: 1457–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Pekala P, Kawakami M, Vine W, Lane MD, Cerami A. Studies of insulin resistance in adipocytes induced by macrophage mediator. J Exp Med 157: 1360–1365, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y, Akashi K, Fiering S, Tenen DG. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet 36: 624–630, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278: 45777–45784, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51: 3176–3188, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 47: 1562–1569, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293: C584–C596, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265: 1573–1577, 1994 [DOI] [PubMed] [Google Scholar]
- 65. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280: 13560–13567, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Talior I, Tennenbaum T, Kuroki T, Eldar-Finkelman H. PKC-δ-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol Endocrinol Metab 288: E405–E411, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 14: 222–231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tondravi MM, McKercher SR, Anderson K, Erdmann JM, Quiroz M, Maki R, Teitelbaum SL. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386: 81–84, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Voo KS, Skalnik DG. Elf-1 and PU.1 induce expression of gp91(phox) via a promoter element mutated in a subset of chronic granulomatous disease patients. Blood 93: 3512–3520, 1999 [PubMed] [Google Scholar]
- 70. Wang F, Tong Q. Transcription factor PU.1 is expressed in white adipose and inhibits adipocyte differentiation. Am J Physiol Cell Physiol 295: C213–C220, 2008 [DOI] [PubMed] [Google Scholar]
- 71. Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab 95: 117–126, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Wara-aswapati N, Yang Z, Waterman WR, Koyama Y, Tetradis S, Choy BK, Webb AC, Auron PE. Cytomegalovirus IE2 protein stimulates interleukin 1beta gene transcription via tethering to Spi-1/PU.1. Mol Cell Biol 19: 6803–6814, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wolfe RR, Peters EJ, Klein S, Holland OB, Rosenblatt J, Gary H., Jr Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol Endocrinol Metab 252: E189–E196, 1987 [DOI] [PubMed] [Google Scholar]
- 75. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol 30: 2518–2527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]