Abstract
Glucose is an essential nutrient for mammalian cells. Emerging evidence suggests that glucose within the oocyte regulates meiotic maturation. However, it remains controversial as to whether, and if so how, glucose enters oocytes within cumulus-oocyte complexes (COCs). We used a fluorescent glucose derivative (6-NBDG) to trace glucose transport within live mouse COCs and employed inhibitors of glucose transporters (GLUTs) and gap junction proteins to examine their distinct roles in glucose uptake by cumulus cells and the oocyte. We showed that fluorescent glucose enters both cumulus-enclosed and denuded oocytes. Treating COCs with GLUT inhibitors leads to simultaneous decreases in glucose uptake in cumulus cells and the surrounded oocyte but no effect on denuded oocytes. Pharmacological blockade of of gap junctions between the oocyte and cumulus cells significantly inhibited fluorescent glucose transport to oocytes. Moreover, we find that both in vivo hyperglycemic environment and in vitro high-glucose culture increase free glucose levels in oocytes via gap junctional channels. These findings reveal an intercellular pathway for glucose transport into oocytes: glucose is taken up by cumulus cells via the GLUT system and then transferred into the oocyte through gap junctions. This intercellular pathway may partly mediate the effects of high-glucose condition on oocyte quality.
Keywords: reproduction, cumulus cell, gap junction
glucose is an essential nutrient for mammalian cells. It is employed as an energy source via glycolysis and the tricarboxylic acid (TCA) cycle. Glucose can also be metabolized via the pentose phosphate pathway (PPP) to produce NADPH and ribose 5-phosphate for nucleotide biosynthesis. In addition, glucose can be used for the synthesis of amino acids, O-linked glycosylation, and production of substrates for extracellular matrices (45). Glucose enters cells either by an active process via sodium-coupled glucose transporters (SGLTs) or through facilitative glucose transporters (GLUTs). Fourteen paralogous members of the GLUT gene family have been identified, including GLUT1–12, the H+-coupled myoinositol transporter, and GLUT14 (24).
In mammalian antral follicles of the ovary, the oocyte is surrounded by numerous layers of granulosa cells (also termed cumulus cells) forming the cumulus-oocyte complex (COC). Oocytes are coupled to these companion somatic cells throughout oogenesis by specific membrane specializations known as gap junctions (1). Gap junctions consist of an array of intercellular channels that allow direct sharing of small molecules between the interconnected cells. Each intercellular channel consists of two hemichannels (connexons) that are docked end to end, with each cell contributing one connexon (27). Passage of ions, metabolites, amino acid, and signaling molecules etc., from granulosa cells to oocytes via gap junctions provides a physical basis for their metabolic cooperation (43).
A tenet of COC energy metabolism established 40 years ago is that the resident oocyte has limited capacity to utilize glucose (2) and that glucose first needs to be converted to pyruvate by follicle cells to ensure long-term viability of the oocyte and successful meiotic maturation (15, 18, 19). However, measurable glucose metabolism has been detected in denuded oocytes from numerous species (4, 5, 16, 35, 37, 39, 41, 49), although to a lesser extent than in COCs. Importantly, glucose has been demonstrated to be able to influence oocyte maturation in the absence of cumulus cells, as evidenced by findings that iodoacetate, a glycolysis inhibitor, partially reverses the meiosis-arresting action of hypoxanthine on denuded oocytes (13). Furthermore, active pentose shunt enzymes have been found in oocytes and unfertilized ova (3, 7, 20, 32), and small molecule manipulation of the PPP affects meiotic resumption and the developmental potential of oocytes (10, 14, 16, 22), indicating the involvement of glucose in oocyte maturation via the PPP.
Despite the suggested importance of glucose, there is so far no direct experimental evidence delineating how glucose is transported into the COC. In the present study, by using 6-NBDG ({6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-6-deoxyglucose}), a nonmetabolizable fluorescent glucose derivative, to directly report glucose transfer and accumulation in live mouse COCs, we describe an intercellular pathway for glucose transport into oocytes whereby glucose is taken up in cumulus cells via GLUTs and then transferred into the oocyte through gap junctions. Additionally, we show that a high-glucose environment induces free glucose accumulation in oocytes by this pathway.
MATERIALS AND METHODS
All mouse studies were approved by the Animal Studies Committee at Washington University School of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Collection of COCs.
Female ICR mice (Taconic Farms, Hudson, NY; 20–24 days old) were superovulated with 10 IU pregnant mare's serum gonadotropin (PMSG; Sigma, St. Louis, MO) by intraperitoneal injection. Forty-eight hours later, the ovaries were removed and transferred to M2 medium (Sigma). Large antral follicles were punctured with sterile acupuncture needles to release COCs. Only COCs that consisted of an oocyte surrounded by at least two intact layers of cumulus cells were selected for further experiment. In some experiments, denuded oocytes were obtained by mouth pipetting the COCs repeatedly to remove cumulus cells.
To collect ovulated metaphase II (MII) oocytes, mice received an injection of 10 IU human chorionic gonadotropin (hCG) 2 days after PMSG priming. Oocytes were recovered from oviduct ampullae 13.5 h after hCG, and cumulus cells were removed by incubating briefly in 1 mg/ml hyaluronidase.
Evaluation of glucose transport in COCs.
6-NBDG (Molecular Probes, Eugene, OR), a fluorescent glucose analog, was used to report glucose transport. In brief, COCs or denuded oocytes (DO) were incubated in M2 medium containing 200 μM NBDG, with the concentration chosen as the capable of giving an adequate signal-to-noise ratio. Following three rapid washes, live cells were immediately imaged at 488 nm by fluorescence microscope (Zeiss Axioskop, Gottingen, Germany). Fluorescence signal was quantified using NIH Image J software and then was calculated as the average intensity after background subtraction.
Glucose competition assay.
To test the competitive cellular uptake of 6-NBDG, COCs were incubated for 3 min at room temperature in Krebs-Ringer bicarbonate (KRB) buffer containing 200 μM NBDG in the presence of indicated concentrations of d-glucose. Glucose concentrations were chosen according to previously published reports (15, 29). Fluorescence intensity was randomly measured in regions of interest strictly limited to cumulus cell area. KRB was (in mM) 129 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.0 CaCl2, 5.0 NaHCO3, and 10 HEPES, pH 7.4, supplemented with 0.1% bovine serum albumin.
Pharmacological inhibition of glucose transporters and gap junctions in COCs.
The gap junction inhibitor carbenoxolone (CBX; Sigma) was initially solubilized in water to make a stock of 100 mM. The glucose transporter (GLUT) inhibitor cytochalasin B (CB; Sigma) was initially solubilized in 100% ethanol to make a stock of 100 mM. To check the effects of gap junction inhibition on glucose transport in COCs, COCs were preincubated for 30 min in M2 medium with and without 100 μM CBX and then moved to M2 medium containing NBDG with and without CBX for another 5-min culture at 37°C. To examine the role of GLUTs in glucose uptake of COC and DO, COCs and DOs were preincubated for 30 min in M2 medium with and without 100 μM CB and then added NBDG to M2 medium for another 5-min culture at 37°C. After three washes, cells were imaged for quantification of 6-NBDG uptake.
Generation of diabetic mice.
To generate a type 1 diabetic model, female B6SJLF1 mice (Jackson Laboratories, Bar Harbor ME; 20–24 days old) received a single injection of streptozotocin at a dose of 190 mg/kg. Four days after injection, a tail blood sample was measured for glucose concentrations. If glucose levels were greater than 300 mg/dl, the animal was selected for use as a diabetic model. A few age-matched control mice were randomly selected.
Enzymatic measurement of free glucose in oocytes.
To determine whether a high-glucose environment leads to free glucose accumulation in oocytes, two experimental models were employed. In the first, type 1 diabetic mice (see above) were used as an in vivo high-glucose environment model. Immature GV: (germinal vesicles) and ovulated MII oocytes were collected from control and diabetic mice, and glucose levels were measured. In the second, an in vitro high-glucose environment was established by culturing COCs from normal mice in M2 medium with 50 mM d-glucose for 60 min. This concentration was chosen because it is the upper range of blood glucose level in type 1 diabetic mice. In some experiments, to evaluate the roles of glucose transporters and gap junctions under this condition, COCs were incubated in M2 medium containing 100 μM CB and/or CBX for 30 min prior to exposure to high-glucose medium; 50 mM l-glucose was included as an osmotic pressure control.
The detailed analytic procedure to measure free glucose has been described in Chi et al. (7, 33). Briefly, single oocytes freed of cumulus cells were frozen on a glass slide. After being freeze-dried overnight, samples were extracted in nanoliter volumes under oil. A glucose assay was designed to link reactions ending with NADP/NADPH, which then were enzymatically amplified in a cycling reaction, and a byproduct (6-phosphogluconate) of the amplification step was measured in a fluorometric assay. Glucose levels are expressed as millimoles per kilogram wet weight (mM/Kw) based on the wet weight of 160 pg per oocyte. Absolute glucose concentrations can be calculated in picomoles by multiplying by 0.16.
Statistical analyses.
Data are presented as means ± SE. Group differences were evaluated using Student's t-test (GraphPad Prism 5, San Diego, CA). P < 0.05 was considered to be statistically significant.
RESULTS
Time course of 6-NBDG uptake in COCs.
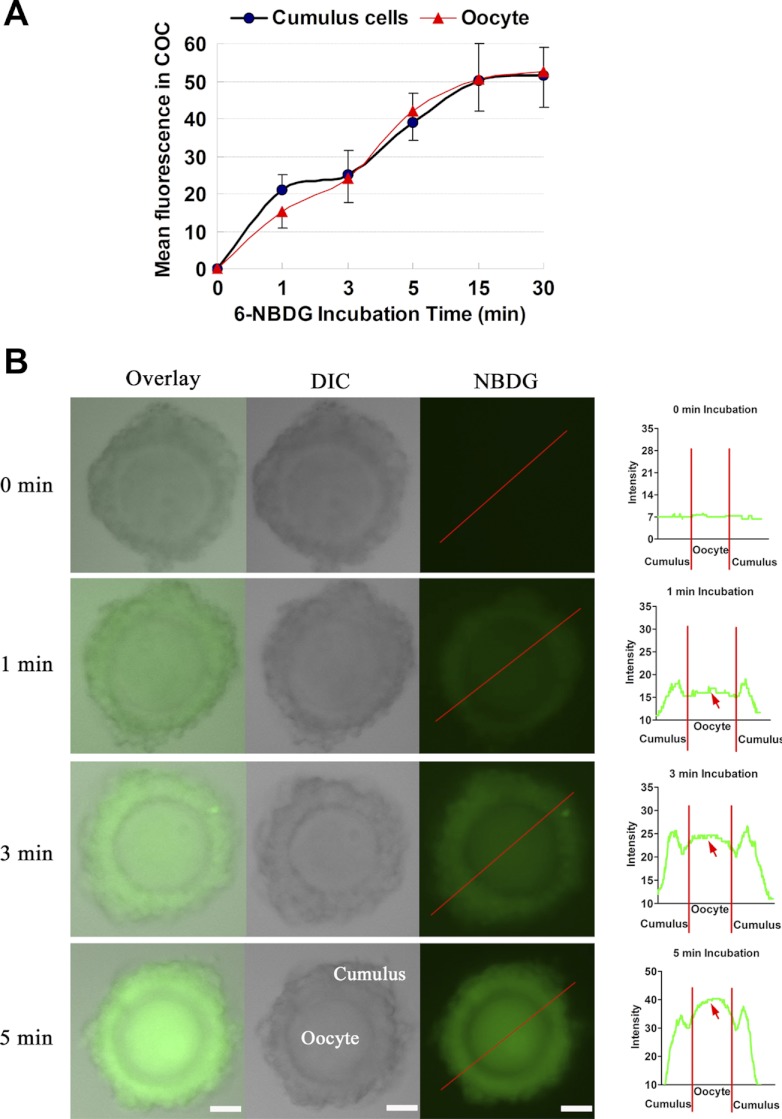
To measure glucose uptake, a variety of radiolabeled tracers have been used effectively. However, the spatial and temporal resolution of these methods is not high, and they require cell destruction. On the other hand, given that the intimate interconnection between cumulus cells and the oocyte could be critical for glucose uptake by the oocyte, it is important to include an examination of the live COC as a whole (as opposed to the isolated oocyte) (8). Hence, instead of using an isotope, we here employed a fluorescent glucose derivative (6-NBDG) to directly visualize glucose transport in live mouse COCs. The time-dependent uptake of 6-NBDG in COCs is shown in Fig. 1A. For both cumulus cells and oocytes, an initial rapid NBDG uptake was observed over the first 5 min, followed by a slower uptake phase that appeared to reach maximum accumulation after 15 min. Interestingly, we always found that the NBDG fluorescence in oocytes was apparently lower than that in surrounding cumulus cells at the 1-min incubation. With increasing culture time up to 3–5 min, similar fluorescence intensity was observed between them, evidenced by plot profiles (Fig. 1B; red arrows). This initial observation suggests that glucose probably was first taken up by cumulus cells and then progressed inward to the oocyte through gap junctional channels. The following experiments were designed to test this assumption.
Fig. 1.
Uptake of the nonmetabolizable fluorescent glucose derivative 6-NBDG by mouse cumulus-oocyte complex (COC). COCs were incubated in medium containing 6-NBDG for different time periods and then were imaged for fluorescence quantification. A: time course of 6-NBDG uptake in cumulus cells and oocytes within live COCs. B: representative fluorescent images of COCs exposed to 6-NBDG for indicated time periods. Right: graphs are fluorescence intensity profiles of 6-NBDG in COCs. Lines were drawn through the complexes, and pixel intensities were quantified along the lines. Red arrows indicate 6-NBDG uptake in oocytes. DIC, differential interference contrast microscopy. Data are means ± SE of 30–40 COCs from ≥2 independent experiments. Scale bars, 30 μm.
Glucose transporters mediate 6-NBDG uptake by cumulus cells.
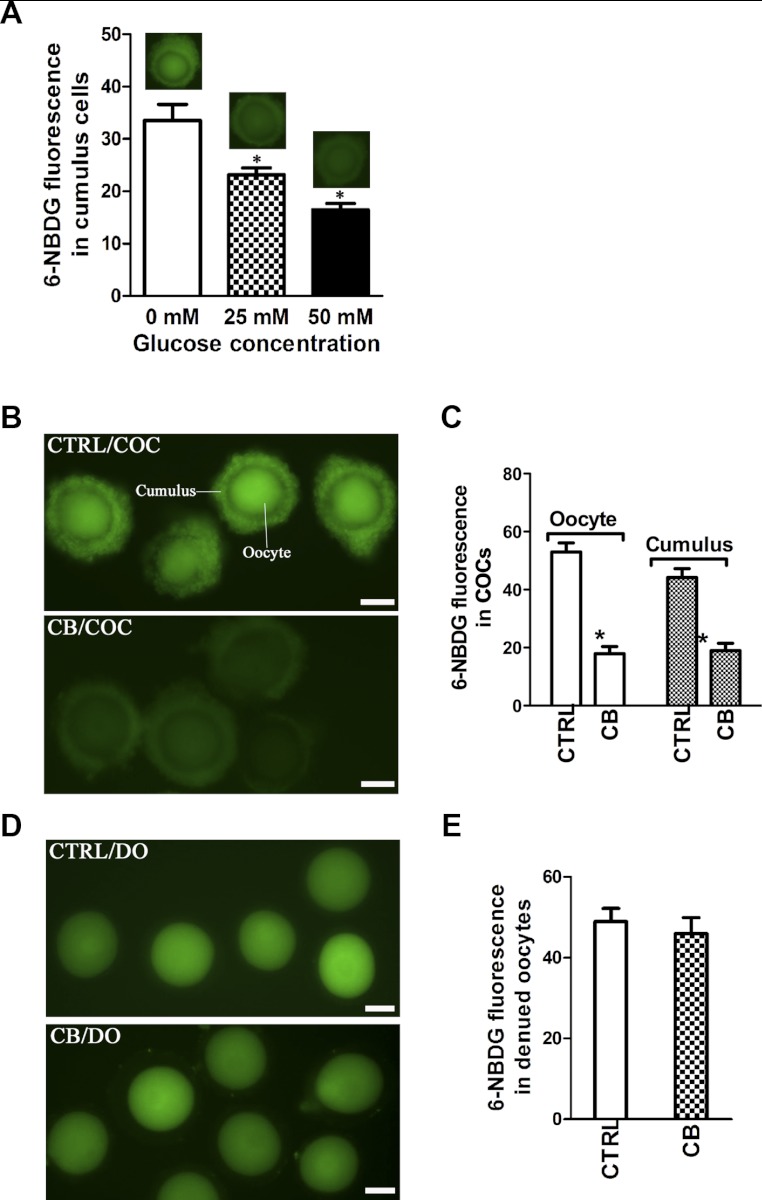
To determine whether 6-NBDG uptake by cumulus cells is mediated via glucose-specific transport system and not via passive diffusion, we performed d-glucose competition assays. 6-NBDG fluorescence was measured in cumulus cells within live COCs in the absence or presence of various concentrations of d-glucose. As shown in Fig. 2A, 6-NBDG uptake was reduced by d-glucose in a dose response manner, inhibited by 34 ± 5.3 and 51 ± 6.8% in the presence of 25 and 50 mM d-glucose, respectively. To further confirm that 6-NBDG is transported to cumulus cells through GLUTs, we examined the effect of CB, an antagonist of GLUTs (17, 34, 52), on 6-NBDG uptake in live COCs. As shown in Fig. 2, B and C, the fluorescence intensity in cumulus cells was significantly decreased upon CB treatment (17.87 ± 2.52 vs. 52.99 ± 3.12 control, P < 0.05), suggesting GLUTs-mediated 6-NBDG uptake in cumulus cells. Meanwhile, CB treatment also resulted in reduced 6-NBDG fluorescence in the cumulus-enclosed oocytes (18.94 ± 2.56 vs. 44.15 ± 3.10 control, P < 0.05). This result indicates that 6-NBDG perhaps 1) entered the oocyte via GLUTs directly or 2) moved from cumulus cells to the oocyte or 3) that the two pathways worked in combination. To clarify this, cumulus cells were removed from COCs, and DO were treated with or without CB prior to 6-NBDG incubation. Significantly, 6-NBDG accumulated in denuded oocytes, but GLUTs inhibition with CB was unable to block this entry (48.94 ± 3.28 vs. 45.95 ± 3.99 control, P > 0.05; Fig. 2, D and E). These data suggest that in mouse COCs glucose enters the oocyte largely through the connections between oocyte and cumulus cells not via resident oocyte GLUTs.
Fig. 2.
Glucose transporters (GLUTs) mediate 6-NBDG uptake by cumulus cells. A: image-based analysis of glucose competition by fluorescence microscopy. COCs were incubated with 6-NBDG for 3 min in the absence or presence of various concentrations of d-glucose. Data are means ± SD of 50 COCs. B and C: effects of GLUT inhibitor cytochalasin B (CB) on 6-NBDG uptake in COCs. Representative fluorescent images of COCs incubated in the absence or presence of CB (B), and quantifications of fluorescence intensity (C) are shown. Data are means ± SE (n = 120 COCs for control and 130 for CB treatment pooled from 3 replicates). D and E: effects of CB on 6-NBDG uptake in denuded oocytes (DO). Representative fluorescent images of DOs incubated in the absence or presence of CB (D) and quantifications of fluorescence intensity (E) are shown. For CB and carbenoxolone(CBX) treatment experiments, COCs were incubated with 6-NBDG for 5 min. Data are means ± SE (n = 115 DOs for control and 120 for CB treatment pooled from 3 replicates). *P < 0.05 vs. controls. Scale bars, 50 μm.
Gap junctions are critical for 6-NBDG transport to oocytes.
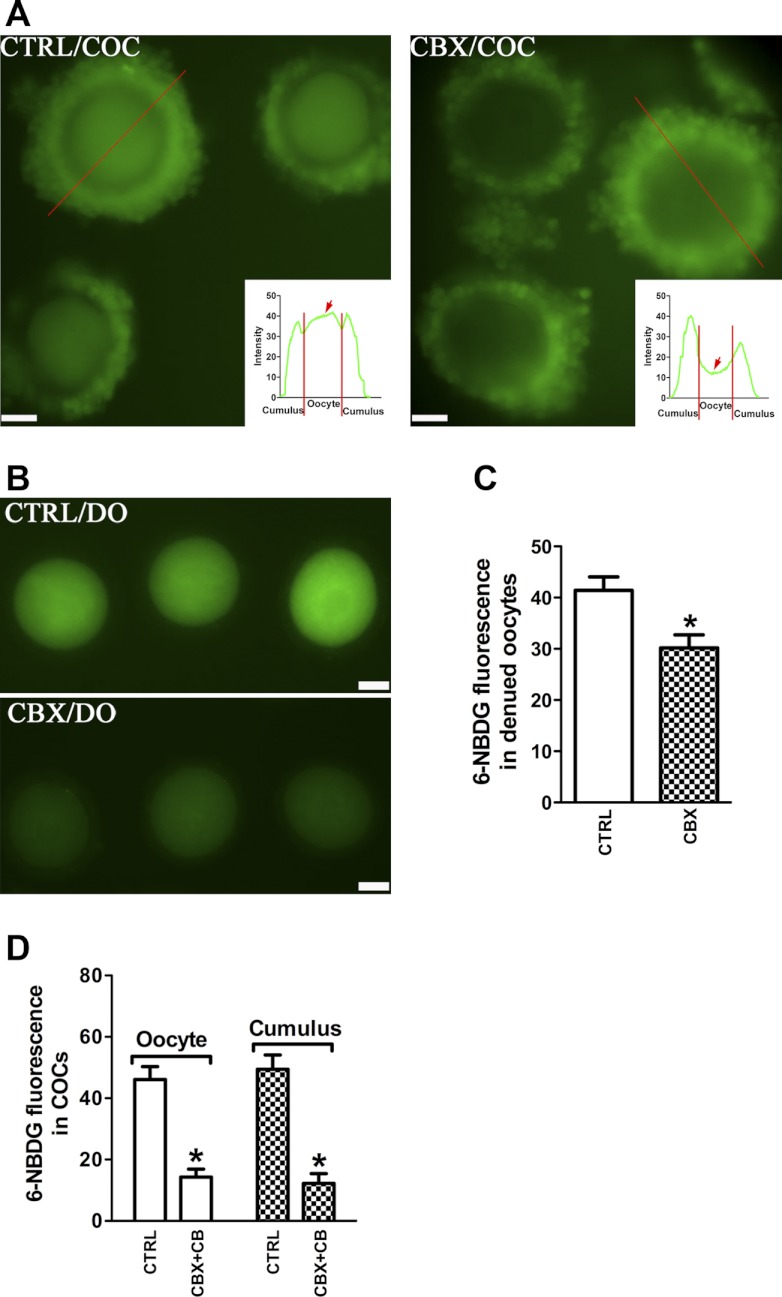
Oocytes are coupled to the surrounding cumulus cells by gap junctions that permit the passage of small molecules between the two compartments. We reasoned that gap junctions might provide a route by which glucose enters oocytes. Therefore, we next examined the effect of gap junction inhibition on 6-NBDG trafficking in COCs. COCs were treated with or without CBX, a gap junction blocker (42), prior to NBDG exposure; representative images are shown in Fig. 3A. Compared with the control group, CBX markedly blocked 6-NBDG accumulation in the oocyte, within the COC (red arrows in insets), with no effect on cumulus cells. CBX is also known as a blocker of gap junction hemichannels (46) containing one connexon unit. CBX treatment of denuded oocytes also reduced the 6-NBDG uptake (30.20 ± 2.54 vs. 41.43 ± 2.60 control, P < 0.05; Fig. 3B), suggesting the existence of functional gap junction hemichannels in denuded oocytes. Moreover, treating COCs with CBX and CB simultaneously abolished the 6-NBDG uptake of both oocyte and cumulus cells (oocyte: 14.32 ± 2.55 vs. 46.12 ± 4.2 control; cumulus: 12.22 ± 3.21 vs. 49.41 ± 4.72 control; P < 0.05; Fig. 3C). Collectively, these data suggest that glucose taken up by cumulus cells via GLUTs can be further transferred to oocytes through gap junctions.
Fig. 3.
Gap junction-dependent 6-NBDG transport to the cumulus enclosed-oocyte. A: effects of gap junction inhibitor carbenoxolone (CBX) on 6-NBDG uptake into COCs. Representative fluorescent images of COCs incubated in the absence or presence of CBX are shown (n = 80 for control group; n = 95 for CBX group). Insets: line scans drawn through the COCs and showing relative fluorescence intensity in cumulus cells and oocytes (red arrows) along its length. B and C: effects of CBX on 6-NBDG uptake in DOs. Representative fluorescent images of DOs incubated in the absence or presence of CBX (B) and quantifications of fluorescence intensity (C) are shown. Data are means ± SE (n = 68 DOs for control and 76 for CBX treatment pooled from 3 replicates). D: effects of CBX + CB on 6-NBDG uptake in COCs. Fluorescence intensity was quantified in oocytes and cumulus cells. Data are means ± SE (n = 84 COCs for control and 80 for CBX+CB treatment pooled from 3 replicates). *P < 0.05 vs. controls. Scale bars, 30 μm.
High-glucose environment induces glucose accumulation in oocytes.
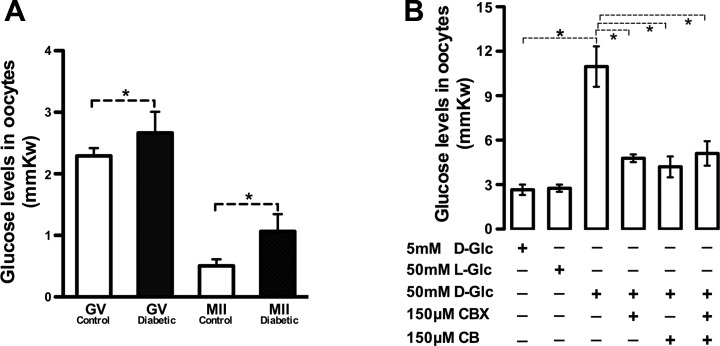
To determine whether high-glucose conditions affect glucose levels in oocytes, we tested type 1 diabetic mice, which is an in vivo model of hyperglycemic milieu (blood glucose levels: 6.7 ± 0.8 mM control vs. 33.5 ± 10.7 mM diabetic). Immature GV and ovulated MII oocytes were isolated from control and diabetic mice, respectively, and then intracellular free glucose was measured enzymatically in individual oocytes. Notably, glucose content among oocytes from diabetic mice was consistently higher at both stages than that from control mice (GV: 2.67 ± 0.07 vs. 2.29 ± 0.02 control; MII: 1.07 ± 0.06 vs. 0.51 ± 0.02 control; P < 0.05; Fig. 4A). Moreover, in control oocytes, the glucose levels were reduced from GV to MII stage (2.29 ± 0.02 GV vs. 0.51 ± 0.02 MII), indicating that free glucose was metabolized during this transition. Second, we tested whether in vitro exposure of COCs to high glucose also increases glucose content in oocytes. COCs from normal mice were cultured in medium with different concentrations of d-glucose, and then single enclosed oocytes were obtained for glucose measurement. Consistent with the in vivo data, an approximately fourfold increase in glucose content was detected in oocytes exposed to 50 mM d-glucose compared with those exposed to 5 mM d-glucose (10.97 ± 1.36 vs. 2.66 ± 0.35, P < 0.05; Fig. 4B). Furthermore, disruption of gap junctions and/or GLUTs significantly blocked this enhancement; 50 mM l-glucose incubation was included as an osmotic control. Taken together, these results indicate that a high-glucose environment can increase glucose accumulation in oocytes through a GLUTs- and gap junctional channel-dependent mechanism.
Fig. 4.
High-glucose environment increases glucose accumulation in oocytes. A: glucose levels were enzymatically measured in immature GV and ovulated metaphase II (MII) oocytes collected from control and diabetic mice, respectively. B: COCs were in vitro cultured in medium containing different concentrations of glucose with or without GLUT inhibitors and gap junctions, as described in materials and methods. Glucose levels were measured in DOs following removal from the COC. Data are means ± SE (n = 30 COCs pooled from 3 replicates for each group). *P < 0.05.
DISCUSSION
In the present study, by imaging fluorescent glucose in live COCs, we present evidence for an intercellular pathway that transports glucose into oocytes: cumulus cells take up glucose via glucose transporters and glucose in cumulus cells can be further transferred into oocytes via gap junctions. Furthermore, we show that through this pathway a high-glucose environment induces elevated free glucose in the oocyte.
Glucose transport pathway in COCs.
A long-established dogma of COC energy metabolism is that the oocyte is unable to use glucose as a sole energy substrate in the absence of cumulus cells and must be supplied with pyruvate (2). However, the presence of hexokinase (48), glucose-6-phosphate dehydrogenase (32), the pentose phosphate pathway (PPP) (16, 49), as well as GLUTs (36), in oocytes suggests a possible involvement of glucose metabolism in some oocyte functions. Remarkably, it is still controversial as to how and even whether glucose can enter oocytes. For example, some research groups have showed that denuded mouse and human oocytes can take up and metabolize the radiolabeled glucose (6, 12, 16, 30, 50). To the contrary, other reports suggested an inability of denuded oocytes to take up and metabolize glucose (41, 44, 54). Nevertheless, deoxyglucose (DG) and DG-6-P were detected in oocytes within COCs, leading Saito et al. (41) to propose that there may be transport into oocytes from cumulus cells.
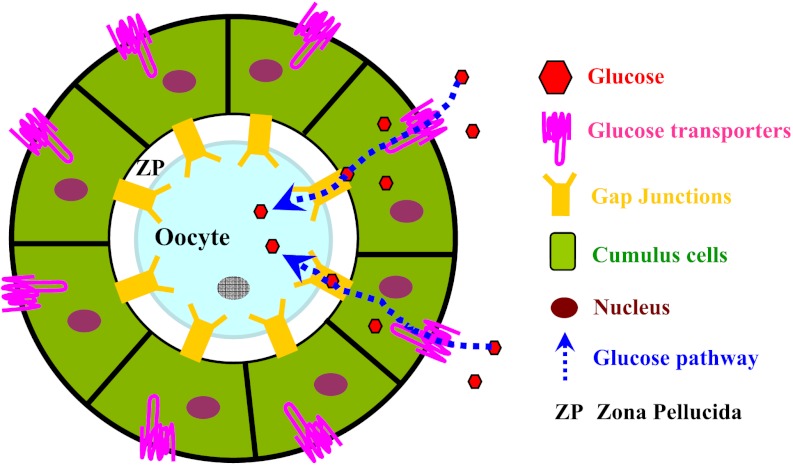
To clarify past findings, we used 6-NBDG to directly visualize glucose uptake in live COCs and employed inhibitors of GLUTs and gap junctions to examine the role of these two processes in glucose transport into the oocyte. First, our study provides direct evidence that fluorescent glucose can enter both cumulus-enclosed mouse oocytes and denuded oocytes (Fig. 2). A similar phenomenon was also reported in bovine COCs by Sutton-McDowell et al. (45). Second, we observed that inhibition of GLUTs leads to decreased glucose uptake in cumulus cells as well as in the enveloped oocyte (Fig. 2B). In support of these data, we have identified three GLUTs (GLUT1, -8, and -12) expressed in mouse cumulus cells by quantitative RT-PCR (36a). In addition, despite the presence of GLUT1, -7, and -9 in mouse oocyte (36), CB treatment has no apparent effect on 6-NBDG incorporation to denuded oocytes (Fig. 2D), indicating that the GLUT system may play only a minor role in oocyte glucose uptake. Compared with the radiolabeled glucose tracers, the 6-NBDG method has better spatial and temporal resolution but is less quantitative and sensitive. Hence, on the basis of this study we cannot completely rule out the possibility that some glucose directly enters the oocyte via GLUTs. Third, we show experimentally for the first time that disruption of gap junctions between oocyte and cumulus cells dramatically blocked glucose transport into the oocyte (Fig. 3). Gap junctions are specialized structures occurring at points of very close cell-cell contact, directly connecting the cytoplasm of two cells, allowing various molecules and ions to pass freely between the cells. One gap junction channel is composed of two connexons (or hemichannels), which connect across the intercellular space (31). Interestingly, CBX as a blocker of hemichannels (25) reduces the 6-NBDG uptake in denuded oocytes (Fig. 3B), implying that the connexons in denuded mouse oocytes are partly functional. It should be pointed out that simple 6-NBDG diffusion into oocytes perhaps occurred, because the weak fluorescence can still be detected in COCs treated with both inhibitors (Figs. 2 and 3). Figure 5 presents a model describing the pathway for intercellular transport of glucose into the oocyte, where glucose is taken up by cumulus cells via GLUTs and then transported into the oocyte through gap junctions.
Fig. 5.
Model for the intercellular glucose transport pathway in mouse COC. Schematic diagram illustrating that glucose is taken up by cumulus cells via GLUTs and then transported into the oocyte through gap junctions. See text for details.
Potential functions of free glucose in oocytes.
We find that high-glucose exposure, both in vivo and in vitro, results in elevated glucose levels in oocytes within COCs through a GLUTs- and gap junction-dependent mechanism (Fig. 4). Furthermore, we show that free glucose is metabolized in the transition from GV oocytes to the MII stage (Fig. 4A) for energy generation, biosynthesis, or conversion into a nonfree form (e.g., glycogen). These findings raise two important questions: what are the potential functions of the free glucose in the oocyte; and do high (or low) glucose levels impact on oocyte quality? Below, we speculate on some possibilities.
Emerging data suggest that glucose in the oocyte may be utilized primarily by the pentose shunt, which is more active than the glycolytic pathway and TCA cycle (3, 7, 47). Furthermore, use of PPP stimulators such as phenazine ethosulfate and pyrroline-5-carboxylate leads to a dose-dependent increase in germinal vesicle breakdown and glucose consumption in the mouse oocyte (14, 16). Similarly, manipulation of the PPP altered the developmental potential of porcine and bovine oocytes (10, 22). In particular, two metabolic intermediates of PPP, NADPH as a key regulator for glutathione reduction and phosphoribosyl pyrophosphate (PRPP) as a substrate for de novo purine synthesis, have been demonstrated to be important for meiotic regulation within oocytes (14, 45). These data indicate that glucose participates in modulating oocyte maturation via the PPP. In addition to being an energy source, glucose also has important regulatory functions, controlling stress resistance, growth, and development in bacteria, yeasts, plants, and animals (38). Several glucose-sensing pathways have been well documented in yeast. One of them employs the cyclic AMP (cAMP) as a second messenger to regulate cell signaling (26). It is interesting to note that cAMP plays a crucial role in meiotic resumption of the oocyte (11). Therefore, free glucose in oocytes may exert effects on oocyte maturation by cAMP pathway.
Both the in vivo hyperglycemic environment and in vitro high-glucose culture have been reported to adversely affect the developmental competence of oocytes (9, 15, 21, 28, 51). Glucose accumulation in oocytes perhaps partly mediates such effects. For instance, mitochondrial dysfunction resulting from high glucose has been reported in various cell types (40, 53). Low glucose is necessary to protect the oocyte against oxidative stress under high-oxygen conditions (21). High glucose may inhibit enzymes responsible for GSH synthesis, thus impairing the oocyte's ability to reduce reactive oxygen species (ROS) (28). Consistent with this notion, altered mitochondria properties and raised ROS generation were found in oocytes from diabetic and obese mice (23, 51). Regardless, more work is necessary to determine the exact functions of free glucose in oocyte.
GRANTS
This work was supported by National Institutes of Health Grants GM-085150 (T. Schedl) and HD-40390 (K. H. Moley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.W. and K.H.M. conception and design of research; Q.W. and M.M.-Y.C. performed experiments; Q.W., M.M.-Y.C., and T.S. analyzed data; Q.W., M.M.-Y.C., T.S., and K.H.M. interpreted results of experiments; Q.W. prepared figures; Q.W. drafted manuscript; Q.W., T.S., and K.H.M. edited and revised manuscript; K.H.M. approved final version of manuscript.
REFERENCES
- 1. Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 71: 680–686, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA 58: 560–567, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinster RL. Glucose 6-phosphate-dehydrogenase activity in the preimplantation mouse embryo. Biochem J 101: 161–163, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinster RL. Hexokinase activity in the preimplantation mouse embryo. Enzymologia 34: 304–308, 1968 [PubMed] [Google Scholar]
- 5. Brinster RL. Oxidation of pyruvate and glucose by oocytes of the mouse and rhesus monkey. J Reprod Fertil 24: 187–191, 1971 [DOI] [PubMed] [Google Scholar]
- 6. Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol 90: 144–153, 1982 [DOI] [PubMed] [Google Scholar]
- 7. Chi MM, Manchester JK, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biol Reprod 39: 295–307, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Clark AR, Stokes YM, Thompson JG. Estimation of glucose uptake by ovarian follicular cells. Ann Biomed Eng 39: 2654–2667, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biol Reprod 67: 220–231, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Comizzoli P, Urner F, Sakkas D, Renard JP. Up-regulation of glucose metabolism during male pronucleus formation determines the early onset of the S phase in bovine zygotes. Biol Reprod 68: 1934–1940, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Conti M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann Endocrinol (Paris) 71: 189–190, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Dan-Goor M, Sasson S, Davarashvili A, Almagor M. Expression of glucose transporter and glucose uptake in human oocytes and preimplantation embryos. Hum Reprod 12: 2508–2510, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Downs SM. The influence of glucose, cumulus cells, and metabolic coupling on ATP levels and meiotic control in the isolated mouse oocyte. Dev Biol 167: 502–512, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Downs SM, Humpherson PG, Leese HJ. Meiotic induction in cumulus cell-enclosed mouse oocytes: involvement of the pentose phosphate pathway. Biol Reprod 58: 1084–1094, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Downs SM, Mastropolo AM. The participation of energy substrates in the control of meiotic maturation in murine oocytes. Dev Biol 162: 154–168, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod 60: 1446–1452, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Ebstensen RD, Plagemann PG. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci USA 69: 1430–1434, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eppig JJ. Analysis of mouse oogenesis in vitro. Oocyte isolation and the utilization of exogenous energy sources by growing oocytes. J Exp Zool 198: 375–382, 1976 [DOI] [PubMed] [Google Scholar]
- 19. Fagbohun CF, Downs SM. Requirement for glucose in ligand-stimulated meiotic maturation of cumulus cell-enclosed mouse oocytes. J Reprod Fertil 96: 681–697, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Ferrandi B, Cremonesti F, Geiger R, Consiglio AL, Carnevali A, Porcelli F. Quantitative cytochemical study of some enzymatic activities in preovulatory bovine oocytes after in vitro maturation. Acta Histochem 95: 89–96, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto S, Minami N, Yamada M, Imai H. Excessive concentration of glucose during in vitro maturation impairs the developmental competence of bovine oocytes after in vitro fertilization: relevance to intracellular reactive oxygen species and glutathione contents. Mol Reprod Dev 56: 520–526, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Herrick JR, Brad AM, Krisher RL. Chemical manipulation of glucose metabolism in porcine oocytes: effects on nuclear and cytoplasmic maturation in vitro. Reproduction 131: 289–298, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 5: e10074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282: E974–E976, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry 33: 181–198, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Kaniak A, Xue Z, Macool D, Kim JH, Johnston M. Regulatory network connecting two glucose signal transduction pathways in Saccharomyces cerevisiae. Eukaryot Cell 3: 221–231, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol 88: 399–413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krisher RL. The effect of oocyte quality on development. J Anim Sci 82, E-Suppl: E14–E23, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lee HY, Lee JJ, Park J, Park SB. Development of fluorescent glucose bioprobes and their application on real-time and quantitative monitoring of glucose uptake in living cells. Chemistry 17: 143–150, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 72: 9–13, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Mangia F, Epstein CJ. Biochemical studies of growing mouse oocytes: preparation of oocytes and analysis of glucose-6-phosphate dehydrogenase and lactate dehydrogenase activities. Dev Biol 45: 211–220, 1975 [DOI] [PubMed] [Google Scholar]
- 33. Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol Endocrinol Metab 275: E38–E47, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 132: 1039–1048, 2008 [DOI] [PubMed] [Google Scholar]
- 35. O'Brien JK, Dwarte D, Ryan JP, Maxwell WM, Evans G. Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reprod Fertil Dev 8: 1029–1037, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Purcell SH, Moley KH. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol Metab 20: 483–489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a. Purcell SH, Chi MM, Moley KH. Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology 153: 2444–2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rieger D, Loskutoff NM. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J Reprod Fertil 100: 257–262, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Rushmer RA, Brinster RL. Carbon dioxide production from pyruvate and glucose by bovine oocytes. Exp Cell Res 82: 252–254, 1973 [DOI] [PubMed] [Google Scholar]
- 40. Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16: 1738–1748, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Saito T, Hiroi M, Kato T. Development of glucose utilization studied in single oocytes and preimplantation embryos from mice. Biol Reprod 50: 266–270, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147: 2280–2286, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 27: 32–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugiura K, Eppig JJ. Society for Reproductive Biology Founders' Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 17: 667–674, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139: 685–695, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science 312: 924–927, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Tsutsumi O, Satoh K, Taketani Y, Kato T. Determination of enzyme activities of energy metabolism in the maturing rat oocyte. Mol Reprod Dev 33: 333–337, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Tsutsumi O, Yano T, Satoh K, Mizuno M, Kato T. Studies of hexokinase activity in human and mouse oocyte. Am J Obstet Gynecol 162: 1301–1304, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Urner F, Sakkas D. Characterization of glycolysis and pentose phosphate pathway activity during sperm entry into the mouse oocyte. Biol Reprod 60: 973–978, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Urner F, Sakkas D. Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod 55: 917–922, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 23: 1603–1612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamada K, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nature Protocols 2: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zuelke KA, Brackett BG. Increased glutamine metabolism in bovine cumulus cell-enclosed and denuded oocytes after in vitro maturation with luteinizing hormone. Biol Reprod 48: 815–820, 1993 [DOI] [PubMed] [Google Scholar]