Abstract
The triple-tracer (TT) dilution technique has been proposed to be the gold standard method to measure postprandial glucose appearance. However, validation against an independent standard has been missing. We addressed this issue and also validated the simpler dual-tracer (DT) technique. Sixteen young subjects with type 1 diabetes (age 19.5 ± 3.8 yr, BMI 23.4 ± 1.5 kg/m2, HbA1c 8.7 ± 1.7%, diabetes duration 9.0 ± 6.9 yr, total daily insulin 0.9 ± 0.2 U·kg−1·day−1, mean ± SD) received a variable intravenous 20% dextrose infusion enriched with [U-13C]glucose over 8 h to achieve postprandial-resembling glucose excursions while intravenous insulin was administered to achieve postprandial-resembling levels of plasma insulin. Primed [6,6-2H2]glucose was infused in a manner that mimicked the expected endogenous glucose production and [U-13C; 1,2,3,4,5,6,6-2H7]glucose was infused in a manner that mimicked the expected glucose appearance from a standard meal. Plasma glucose enrichment was measured by gas chromatography-mass spectrometry. The intravenous dextrose infusion served as an independent standard and was reconstructed using the TT and DT techniques with the two-compartment Radziuk/Mari model and an advanced stochastic computational method. The difference between the infused and reconstructed dextrose profile was similar for the two methods (root mean square error 6.6 ± 1.9 vs. 8.0 ± 3.5 μmol·kg−1·min−1, TT vs. DT, P = NS, paired t-test). The TT technique was more accurate in recovering the overall dextrose infusion (100 ± 9 and 92 ± 12%; P = 0.02). The root mean square error associated with the mean dextrose infusion profile was 2.5 and 3.3 μmol·kg−1·min−1 for the TT and DT techniques, respectively. We conclude that the TT and DT techniques combined with the advanced computational method can measure accurately exogenous glucose appearance. The TT technique tends to outperform slightly the DT technique, but the latter benefits from reduced experimental and computational complexity.
Keywords: meal tolerance test, triple-tracer technique, dual-tracer technique, glucose flux, glucose tracer, Radziuk/Mari model
assessments of postprandial glucose turnover facilitate understanding of carbohydrate physiology in health and diseases (7, 22, 30). In the postprandial state, endogenous glucose production (EGP) and glucose disposal (Rd) vary with time together with the appearance of glucose originating from the meal (Ra meal). Direct measurements of postprandial turnover are generally not possible, and indirect assessments using the tracer dilution methodology are hindered by the nonsteady state of the glucoregulatory system (1) and ill-conditioning (small measurement errors propagate into large estimation errors) of the computational problem (12).
The dual-tracer (DT) technique is the original method to assess postprandial glucose fluxes (29) and has been adopted in a range of studies (19, 21, 22, 30). The DT technique uses one glucose tracer infused intravenously to measure EGP while a second glucose tracer mixed with the meal allows oral glucose appearance to be determined (35). Infusing the intravenous tracer in a manner that mimics the expected EGP pattern minimizes the change over time in the specific activity or the tracer-to-tracee ratio (TTR) and improves accuracy of EGP estimates (3, 18).
The triple-tracer (TT) technique utilizes a third tracer, which is infused in a manner that mimics the expected glucose appearance from the meal (1). The TT technique has been proposed to be the gold standard to measure postprandial glucose appearance (1). The TT technique provides a model-independent estimate of Ra meal if TTR of the meal tracer over the meal-mimicking tracer does not change over time. However, for practical reasons, variations in the TTR are inevitable, and errors of unknown magnitude arise resulting from model misspecification and ill conditioning.
Validation of the TT and DT methods against an independent standard is part of our research to measure gut absorption rates of meals containing complex carbohydrates in subjects with type 1 diabetes (T1D) (6). In the present study, we assessed the accuracy of the TT and DT techniques by contrasting the administered rate of intravenous dextrose against the reconstructed rate. The delivery pattern of intravenous dextrose mimicked a meal-derived glucose appearance serving as an independent standard. We used an advanced computational approach based on population stochastic modeling to reduce the effect of ill conditioning.
MATERIALS AND METHODS
Subjects and experimental protocol.
Sixteen young subjects with T1D (age 19.5 ± 3.8 yr, BMI 23.4 ± 1.5 kg/m2, HbA1c 8.7 ± 1.7%, diabetes duration 9.0 ± 6.9 yr, total daily insulin 0.9 ± 0.2 U·kg−1·day−1, mean ± SD) participated in the study performed at the Wellcome Trust Clinical Research Facility, Addenbrooke's Hospital, Cambridge, UK. The study was approved by the local Ethics Committee and subjects provided written informed consent.
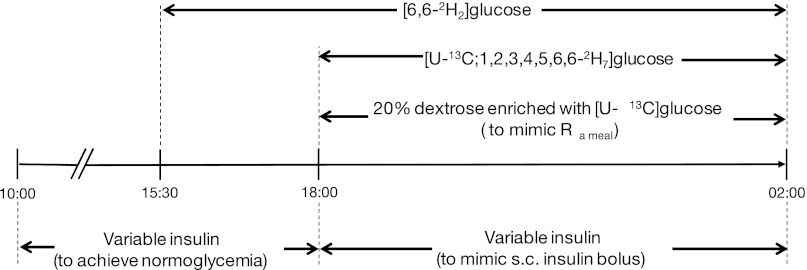
Figure 1 shows the study design. Subjects arrived at the clinical facility after breakfast. Long intermediate-acting insulin was withdrawn 12 h before admission and replaced with short-acting insulin. From 1000 to 1800 during the stabilization period, subjects fasted and received variable intravenous insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark) titrated every 15 min to achieve plasma glucose at 6.0 mmol/l.
Fig. 1.
Experimental study protocol.
A primed constant continuous intravenous infusion of [6,6-2H2]glucose (6 mg/kg prime, 0.06 mg·kg−1·min−1 continuous infusion; Cambridge Isotope Laboratories, Andover, MA) was given for 150 min from 1530. From 1800, the infusion rate was altered in a manner that mimicked the expected postprandial-like suppression of the EGP [6,6-2H2]glucose was infused at a rate of 70, 60, 50, 35, 45, 50, 55, 65, and 70% (100% represents the rate of 0.06 mg·kg−1·min−1) at 0, 10, 20, 30, 220, 240, 280, 300, and 400 min after 1800.
From 1800 until 0200, a variable intravenous insulin infusion was given to achieve plasma insulin concentrations comparable to that resulting from a subcutaneous bolus of rapid-acting insulin analog. The total amount of variable insulin corresponded to an insulin bolus associated with a meal containing 120 g of carbohydrates. The calculations utilized individualized insulin-to-carbohydrate ratios. Starting at 1800, a variable intravenous 20% dextrose infusion (the independent standard mimicking appearance of exogenous glucose from a standard meal) enriched with [U-13C]glucose (to represent the tracer that would normally be added to the meal; 13 mg/g, Cambridge Isotope Laboratories) was infused over 8 h to replicate post-meal plasma glucose excursions. Postprandial glucose patterns were taken from a previous study in young subjects with T1D who consumed a standard meal containing 120 g of carbohydrates (6). An adaptive model-predictive controller (gMPC, version 1.0.2; University of Cambridge, Cambridge, UK) was used to adjust the dextrose infusion utilizing information about subject's total daily insulin dose, the predetermined intravenous insulin delivery, and the desirable plasma glucose profile.
Starting at 1800, [U-13C; 1,2,3,4,5,6,6-2H7]glucose (meal-mimicking tracer; Cambridge Isotope Laboratories) was infused in a manner that mimicked the expected glucose appearance from a standard meal; [U-13C; 1,2,3,4,5,6,6-2H7]glucose was infused at 20, 40, 55, 70, 90, 100, 90, 70, 50, 40, 30, 20, 10, 5, and 0% of 0.1 mg·kg−1·min−1 at 0, 10, 20, 30, 40, 50, 90, 120, 150, 180, 195, 225, 270, 330, and 405 min after 1800.
From 1000, venous blood samples were taken every 10–15 min for the determination of plasma glucose. Samples were taken at 1500, 1515, and 1530 to determine background glucose enrichments. From 1700, venous blood samples were taken every 10–30 min for the determination of plasma insulin, [U-13C]glucose, [6,6-2H2]glucose, and [U-13C; 1,2,3,4,5,6,6-2H7]glucose. The samples were immediately centrifuged and separated. Plasma glucose was measured immediately while other samples were stored at −80°C until assayed.
Assays.
Plasma glucose was measured using a YSI2300 STAT Plus Analyser (YSI, Lynchford House, Farnborough, UK). Plasma insulin was measured using an immunochemiluminometric assay (Invitron, Monmouth, UK) with an intra-assay coefficient of variation of 4.7% and an interassay coefficient of variation of 7.2–8.1%. Plasma samples were derivatized to form the methoxime-trimethylsilyl derivative for gas chromatography mass-spectrometry analysis (Agilent 5975C inert XL EI/CI MSD; Agilent Technologies, Wokingham, UK) to measure ions m/z 319.2 (M + 0), 321.2 (M + 2), 322.2 (M + 3), 323.2 (M + 4), 324.2 (M + 5), and 327.2 (M + 8) (26).
Data analysis.
TTRs were calculated using a variation of a method described previously (12, 14). In brief, ions m/z M+0, M+2, M+4, and M+8 were used to calculate TTRs of [6,6-2H2]glucose, [U-13C]glucose, and [U-13C; 1,2,3,4,5,6,6-2H7]glucose corrected for recycled glucose using ions m/z M+0, M+3, and M+5. The calculations accounted for spectra overlap (23). It was assumed that [U-13C; 1,2,3,4,5,6,6-2H7]glucose is recycled equally into glucose molecules with m/z of M+4 and M+5 and that [U-13C]glucose is recycled equally into glucose molecules with m/z of M+2 and M+3 (15, 16). A system of algebraic equations was solved analytically, and the solution was reduced and validated using Mathematica (Wolfram Research, Champaign, IL). The endogenous glucose concentration was calculated using a model-independent method as described previously (1).
A stochastic modeling approach was used to reconstruct the exogenous dextrose infusions (Ra exo) and to calculate EGP and Rd. The DT method utilized the plasma concentration of unlabeled glucose, [6,6-2H2]glucose, and [U-13C]glucose. The TT methods utilized additionally the plasma concentration of [U-13C; 1,2,3,4,5,6,6-2H7]glucose. A hierarchical Bayes model was created implementing the Radziuk/Mari two-compartment model of glucose kinetics (18, 22). The method used Bayesian inference with a regularizing prior that assumes smooth glucose fluxes (12), with individualized smoothness levels drawn from a population distribution (27). The method has been described previously (9), and additional details are given in the appendixes.
Statistical analysis.
Values are presented as means ± SD or median (interquartile range) as appropriate. The difference between the actual and reconstructed dextrose infusion rates was determined using the root mean square error (RMSE).
where is the actual dextrose infusion rate and Ra exo,i is its estimate at time i. The overall dextrose recovery was calculated. The EGP and Rd profiles were summarized using the partial area under the curve (AUC) from time 0 to 60 min and from time 0 to 480 min. A paired t-test was used to contrast differences between the TT and DT methods. P < 0.05 was considered statistically significant.
RESULTS
Plasma glucose and plasma insulin.
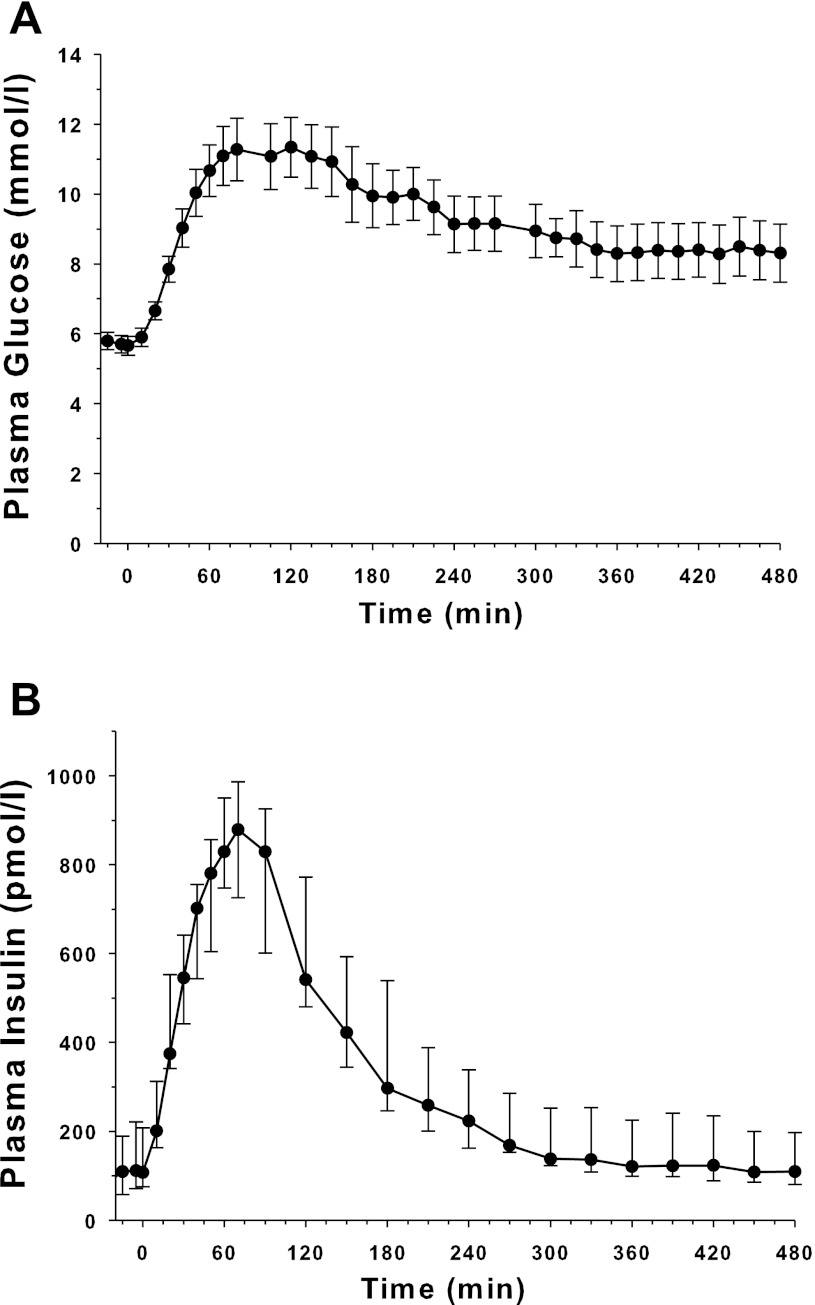
Plasma glucose and plasma insulin concentrations are shown in Fig. 2. The plasma glucose concentration before the start of dextrose infusion averaged 5.7 ± 1.1 mmol/l and peaked at 11.3 ± 3.7 mmol/l after 120 min, and then decreased slowly to around 8.0 mmol/l by the end of the experiment. The concentration of plasma insulin was 96 (57–197) pmol/l before the start of the dextrose infusion and peaked at 879 (726–986) pmol/l after 70 min. The plasma insulin concentration returned to basal level by 360 min.
Fig. 2.
Concentration of plasma glucose (top; mean ± SE) and plasma insulin [bottom; median (IQR)] during the time course of the experiment (n = 16).
TTRs.
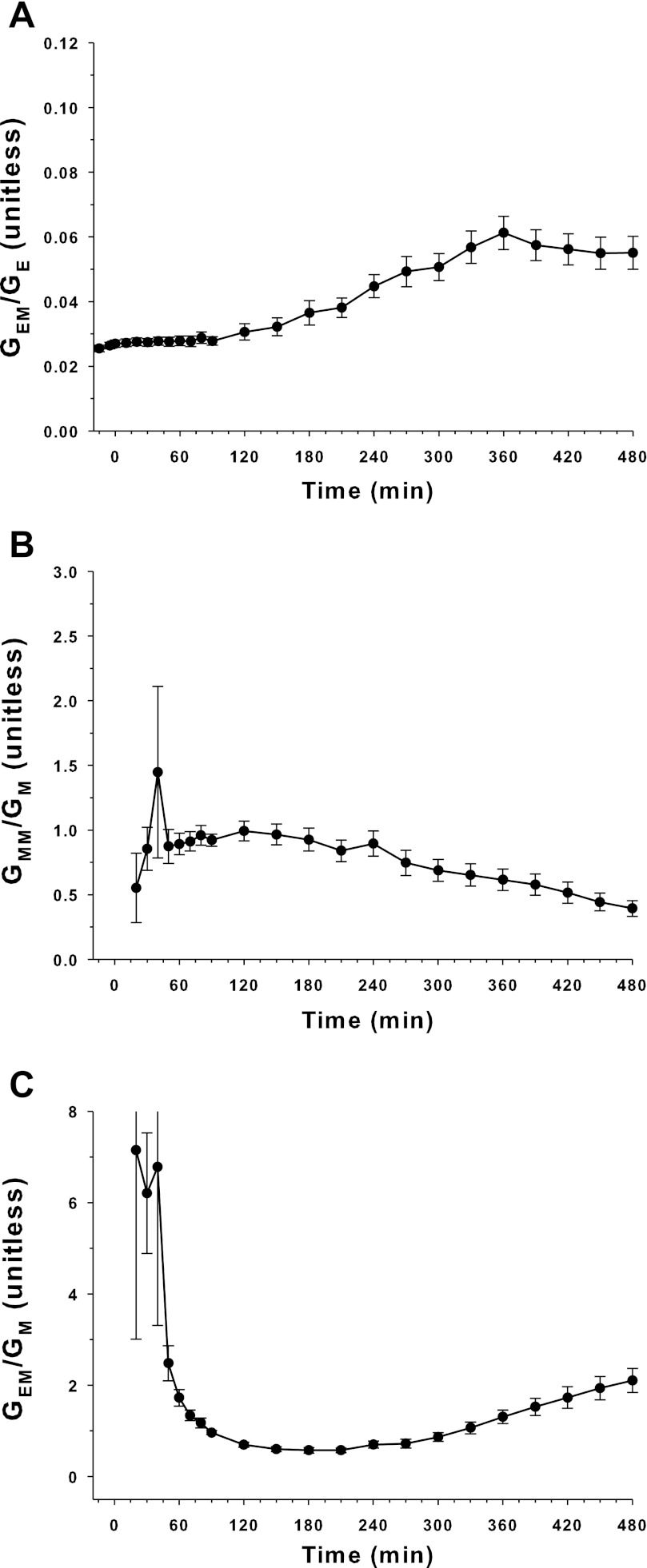
The TTRs are shown in Fig. 3. Due to the infusion pattern of [6,6-2H2]glucose and [U-13C; 1,2,3,4,5,6,6-2H7]glucose, the changes in the ratios [6,6-2H2]glucose over endogenous glucose and [U-13C; 1,2,3,4,5,6,6-2H7]glucose over [U-13C]glucose were reduced, minimizing the model-dependent error when estimating EGP and Ra exo by the TT method.
Fig. 3.
Tracer-to-tracee ratios (TTRs) used by triple-tracer and dual-tracer techniques. Top: TTR of GEM/GE ([6,6-2H2]glucose over endogenous glucose). Middle: TTR of GMM/GM ([U-13C; 1,2,3,4,5,6,6-2H7]glucose over [U-13C]glucose). Bottom: TTR of GEM/GM ([6,6-2H2]glucose over [U-13C]glucose) (means ± SE; n = 16).
The ratio [6,6-2H2]glucose over [U-13C]glucose is shown in Fig. 3. The [6,6-2H2]glucose was infused in a manner that mimicked the expected EGP, and this led to marked changes in this particular ratio, potentially leading to a model-dependent error in the estimation of Ra exo by the DT method.
Reconstructing dextrose infusion.
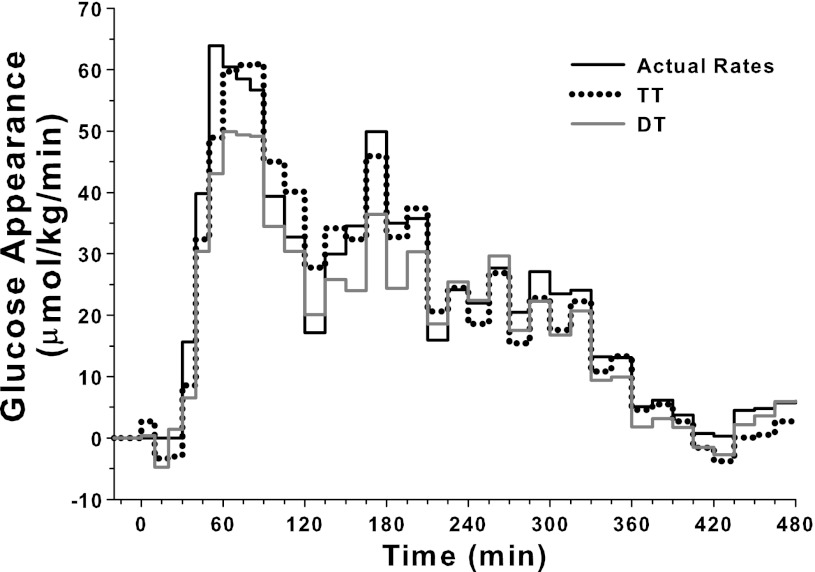
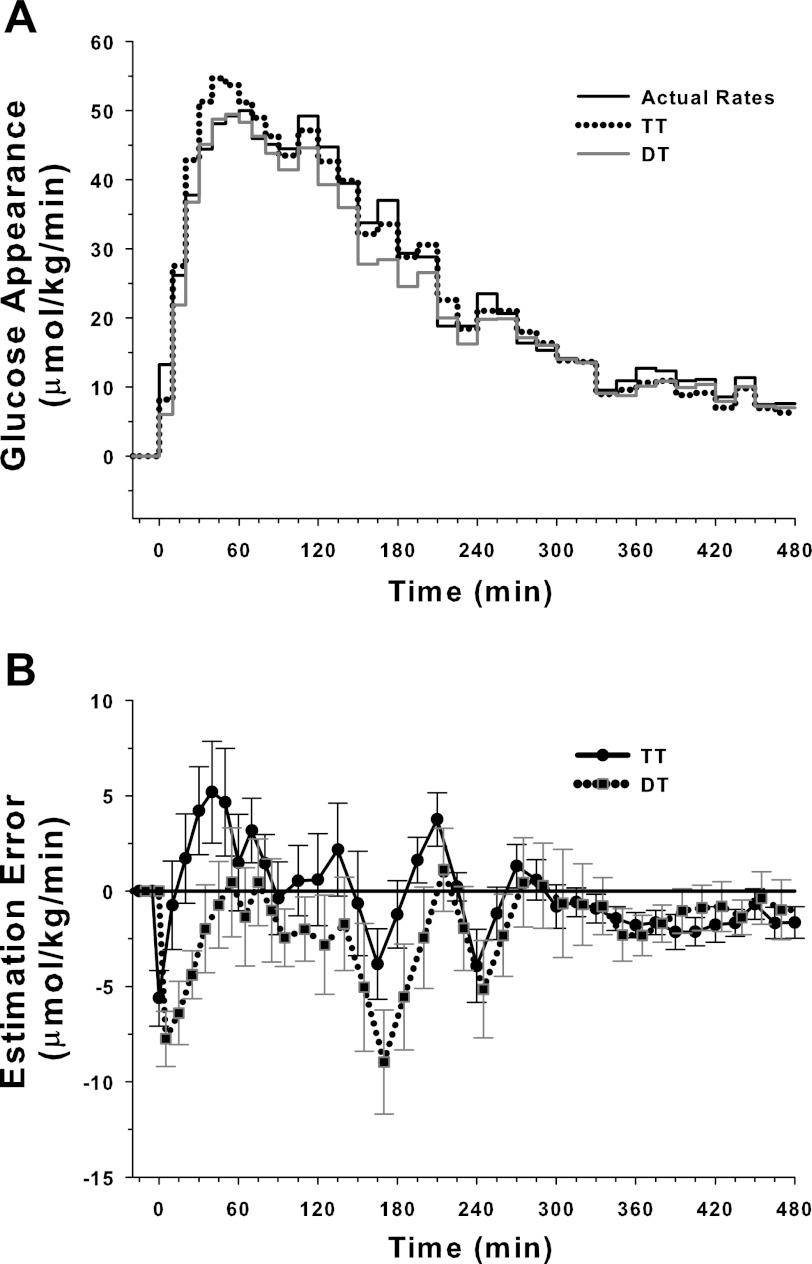
Figure 4 shows a sample dextrose infusion profile and its reconstructed profiles by the TT and DT techniques.
Fig. 4.
Sample dextrose infusion profile and reconstructed infusion profiles estimated by triple-tracer (TT) and dual-tracer (DT) methods.
The difference between individual actual and individual reconstructed dextrose infusion rates assessed by RMSE is shown in Table 1. The RMSEs associated with the TT and DT techniques were comparable (P = NS). The DT technique slightly underestimated the overall glucose appearance compared with the TT method (P = 0.02). The TT method provided 100% recovery.
Table 1.
RSME of individual and mean dextrose profiles and percentage of dextrose recovery as calculated by triple-tracer and dual-tracer methods
| Triple Tracer | Dual Tracer | P Value | |
|---|---|---|---|
| RMSE associated with individual dextrose profiles, μmol•kg−1•min−1 | 6.6 ± 1.9 | 8.0 ± 3.5 | NS |
| RMSE associated with the mean dextrose profile, μmol•kg−1•min−1 | 2.5 | 3.3 | |
| Percentage of recovered dextrose infusion* | 100 ± 9 | 92 ± 12 | 0.02 |
Values are means ± SD; n = 16. RMSE, root mean square error; NS, not significant.
Difference between actual and estimated total dextrose delivery.
The mean dextrose infusion and its reconstructed patterns are shown in Fig. 5. The calculations indicate nearly identical mean profiles obtained with the two methods, documenting the ability of the two methods to reliably estimate exogenous glucose appearance. The RMSE associated with the mean dextrose infusion profile is shown in Table 1.
Fig. 5.
Top: mean dextrose infusion profile and mean reconstructed profiles calculated by TT and DT methods. Bottom: differences between actual and reconstructed profiles associated with TT and DT methods (means ± SE; n = 16).
Estimation errors over time are shown in Fig. 5. The DT technique tended to underestimate the early portion of the dextrose infusion rate. This could be ameliorated by higher sampling frequency. The estimation error of the DT method tended to be less than zero, in concordance with the observation that the overall dextrose recovery was slightly underestimated.
EGP and glucose disposal.
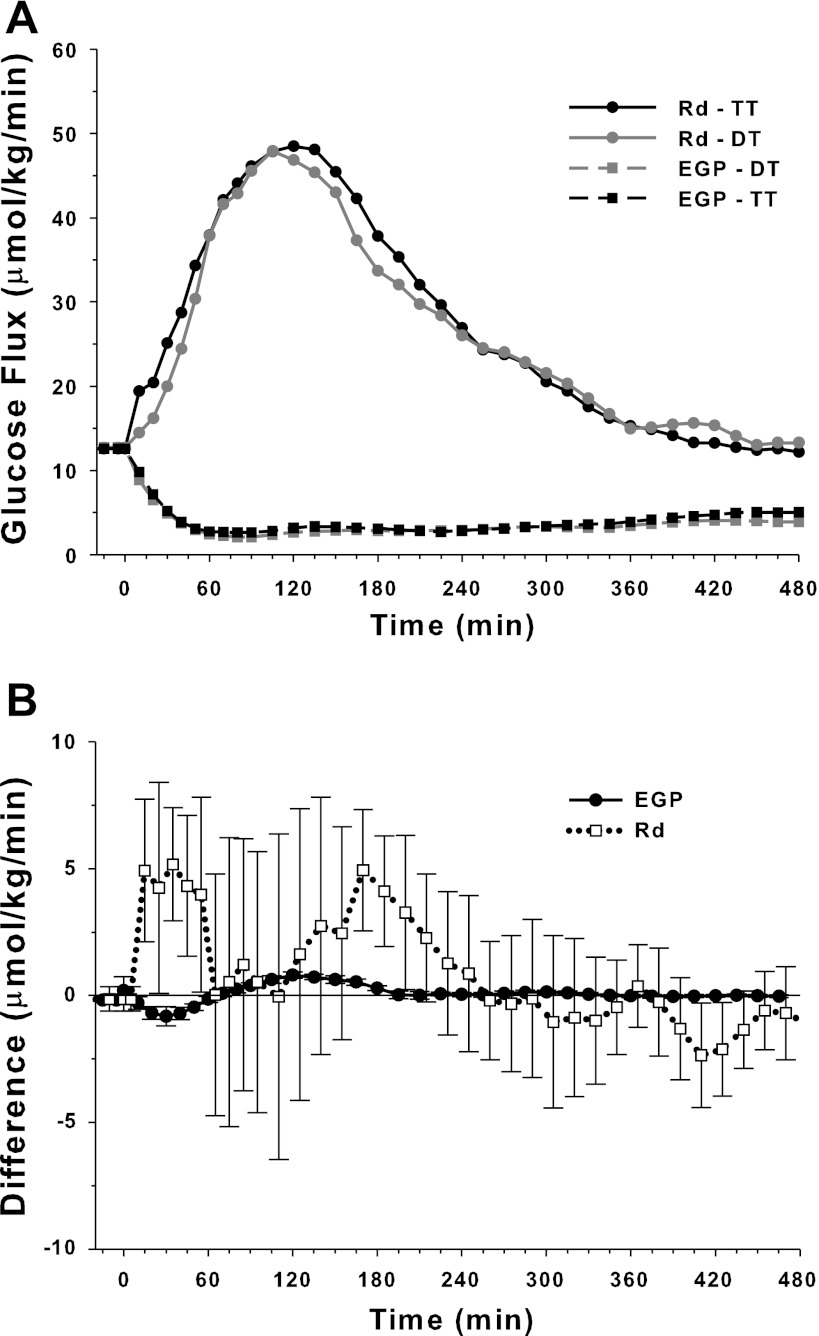
Figure 6 documents a nearly identical EGP and Rd obtained with the two methods. The plot of differences shown in Fig. 6 suggests that, on an individual basis, EGP estimates by the two methods were nearly identical as documented by the low variability of the differences at each time point. Differences in individual estimates of Rd occurred. The TT technique provided slightly higher estimates of Rd at 30 and 180 min.
Fig. 6.
Top: mean endogenous glucose production (EGP) and Rd profiles calculated by TT and DT methods. Bottom: differences between EGP and Rd profiles calculated by te DT and TT methods (means ± SE; n = 16).
Table 2 shows partial AUCs associated with the EGP and Rd profiles. AUCs of EGP were statistically lower with the DT technique, but the difference lacked clinical significance.
Table 2.
AUCs for the first 60 and 480 min of EGP and Rd profiles as estimated by dual-tracer and triple-tracer methods
| Triple Tracer | Dual Tracer | P Value | |
|---|---|---|---|
| AUC0–480 Rd, mmol/kg per 480 min | 13.0 ± 2.9 | 12.6 ± 2.9 | NS |
| AUC0–480 EGP, mmol/kg per 480 min | 1.9 ± 0.8 | 1.8 ± 0.8 | <0.001 |
| AUC0–60 Rd, mmol/kg per 60 min | 1.5 ± 0.5 | 1.3 ± 0.2 | NS |
| AUC0–60 EGP, mmol/kg per 60 min | 0.4 ± 0.2 | 0.4 ± 0.2 | <0.001 |
Values are means ± SD; n = 16. AUC area under the curve; EGP, endogenous glucose production.
Fractional clearance rate.
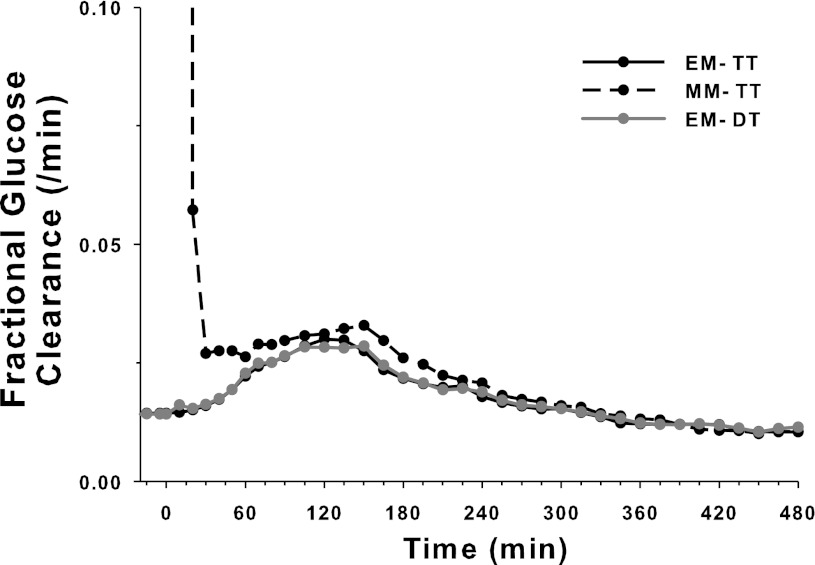
As expected, the fractional clearance rate obtained by the two methods with the use of [6,6-2H2]glucose were virtually superimposable (Fig. 7). The two methods are fitting the same [6,6-2H2]glucose concentration. Minor differences in the fractional glucose clearance resulted from the way the parameter estimation process was set up utilizing simultaneous fit to all glucose species (see Appendix A). We also confirm that the TT and DT methods were equivalent when estimating EGP. The time course of the factional clearance rate calculated with [U-13C; 1,2,3,4,5,6,6-2H7]glucose by the TT method was slightly higher during the initial study period. This likely reflects a high relative measurement error associated with low concentrations of [U-13C; 1,2,3,4,5,6,6-2H7]glucose at the start of the study. This overestimation is linked to a higher [U-13C]glucose turnover calculated by the TT method contributing to a slightly higher overall Rd (Fig. 6).
Fig. 7.
Mean fractional clearance rates of EM and MM glucose tracers estimated by the TT method and the mean fractional clearance rate of the EM glucose tracer estimated by the DT method (n = 16).
DISCUSSION
The present study suggests that the TT and DT techniques provide accurate estimates of exogenous glucose appearance. The mean dextrose infusion was accurately reconstructed. The total amount of delivered dextrose was well recovered by the TT technique, whereas the DT technique underestimated the amount by 8%.
We adopted a unique validation method mimicking postprandial conditions and utilizing an independent standard represented by the intravenous dextrose infusion. A concomitant intravenous insulin infusion achieved typical postprandial insulin excursions in subjects with T1D. We focused on T1D because accurate estimation of gut absorption is required for optimum timing and amount of prandial insulin (2, 25). Optimal prandial insulin dosing is important to minimize postprandial glucose excursions, which are important determinants of overall glucose control in T1D (24). Examining T1D avoids the need to consider endogenous insulin secretion, facilitating a simpler characterization of the relationship between plasma insulin and glucose excursions. In combination with mathematical modeling, estimates of gut absorption can be used to explore, in silico (34), alternative insulin dosing strategies including closed-loop systems (5, 10, 11, 13, 33). Given the common underlying computational principles and experimental procedures, it is reasonable to assume applicability of present results to other populations.
On theoretical grounds, the model misspecification error is the only source for differences between the DT and TT techniques. If the Radziuk/Mari model provided an accurate description of glucose kinetics, the two methods would return identical results. In the early portion of the experiment, when dextrose delivery was rapidly increasing, the DT method underestimated the dextrose infusion. The overall amount of dextrose was slightly underestimated by the DT method, in concordance with results obtained by others (1, 32); otherwise, results provided by the two techniques were comparable. The present study thus suggests that the two-compartment Radziuk/Mari model provides a reasonable approximation of glucose kinetics during meal-like experimental scenarios. This is supported by the observation that the fractional turnover rates were comparable apart from the initial part of the study. We argue that the DT technique is the preferred approach, given the reduced cost and reduced experimental and analytic complexity, unless highly accurate measurements of the early postprandial period are required.
The DT approach is equally suitable to estimate EGP as is the TT approach. From a physiological viewpoint, the results were comparable between the two techniques despite the statistical significance in partial AUCs. Indirectly, the present study also validates estimates of EGP. Given the comparability of the computational approach to estimate EGP and dextrose infusion, it is reasonable to assume validity for estimates of EGP, given that the dextrose infusion was accurately reconstructed. The main difference is that EGP originated endogenously but the dextrose infusion exogenously, but this should not affect the validity of the calculations.
The infusion pattern of EGP-mimicking tracer was designed to minimize changes over time of the TTR GEM/GE but led to considerable fluctuations in the ratio GEM/GM (Fig. 3), possibly accentuating model misspecification errors by the DT method. Despite glucose fluxes calculated by the DT and TT methods being similar, they indicate that the model misspecification error may have been mitigated by the use of the novel computational method. Further studies are warranted to determine whether the amount of exogenous glucose would be less underestimated by the DT method if the infusion of the EGP-mimicking tracer was kept constant, as is the case in many (21, 30), but not all (20), DT studies. We suggest that the infusion pattern of EGP-mimicking tracer should be designed to match the research question. Studies focusing on measuring EGP may adopt a pattern similar to that adopted in the present study.
The low initial concentrations of the meal-mimicking tracer and the meal tracer at the start of the experiment caused inaccurate estimation of the initial portion of the fractional clearance rate k01,MM (Fig. 7). However, unlike the traditional calculation approach using an analytic solution and the fractional clearance rate (1, 18), our approach avoids the use of fractional clearance rate, shown here for illustrative purposes, and utilizes model fit to measured concentrations. This renders the calculations more robust and less prone to ill conditioning. As the measurement errors associated with the meal tracer and the meal-mimicking tracer at the early portion of the experiment are large compared with actual measurements, the initial part of the dextrose infusion estimate will be less dependent on the measured concentrations and will more reflect the assumption of “smooth glucose fluxes”. This is a drawback of the TT study design; the DT approach is not affected by similar considerations.
The novel computational approach may have increased the accuracy of the calculations. The approach assumed that glucose fluxes were smooth to limit the effect of ill conditioning (12, 31). The extent of smoothing was shared among individuals by assuming a population distribution from which individual smoothing estimates were drawn. This “pulled” individual smoothing factors closer to their mean value (27) and avoided aberrations and oscillations, which can be present when data are processed separately. Additionally, this novel approach used an appropriate model of the measurement error. The traditional method (1), although computationally simpler, transforms data, which leads to transformations of the measurement error. This is of particular concern at the initial part of the study when concentrations of meal tracer are low and the associated relative measurement errors are high. This was addressed previously by excluding the initial portion of the data from calculations (1), but all data can be utilized with the present approach.
The computational approach estimates all glucose fluxes simultaneously by fitting measurements of all glucose species in all subjects within a single parameter estimation run (Appendix A). This facilitates interaction between fits to different glucose species. This also explains why the DT and TT methods provide slightly different estimates of endogenous glucose production and fractional clearance rate k01EM while fitting identical EGP-mimicking tracer concentrations. The TT method will additionally be fitting the meal-mimicking tracer concentration, and this will propagate slightly into estimates of EGP and the fractional clearance rate.
In conclusion, our results suggest that triple-tracer and dual-tracer techniques combined with advanced computational methods can measure accurately and reliably glucose appearance during postprandial-like conditions. The triple-tracer technique tends to outperform slightly the dual-tracer technique, but the latter benefits from reduced experimental and analytic complexity.
GRANTS
This work was supported by Juvenile Diabetes Research Foundation (nos. 22-2006-1113, 22-2007-1801, 22-2009-801, 22-2009-802), Diabetes UK (BDA07/0003549), the National Institute of Diabetes and Digestive and Kidney Diseases (1R01 DK-085621), Medical Research Council Centre for Obesity and Related metabolic Diseases, and the National Institute for Health Research Cambridge Biomedical Research Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.H., M.N., N.J., M.U., and R.H. analyzed data; A.H., D.E., and R.H. interpreted results of experiments; A.H. and R.H. prepared figures; A.H. and R.H. drafted manuscript; A.H., D.E., M.W., M.L.E., D.D., and R.H. edited and revised manuscript; A.H., D.E., J.A., J.H., K.K., M.N., C.A., M.W., N.J., M.U., M.L.E., D.D., and R.H. approved final version of manuscript; D.E., C.A., M.L.E., D.D., and R.H. conception and design of research; D.E., J.A., J.H., K.K., M.N., M.W., and R.H. performed experiments.
ACKNOWLEDGMENTS
Angie Watts provided laboratory support. The Diabetes Research Network Laboratory Wales (Dr. Steve Luzio) measured plasma insulin. Tomas Hovorka devised TTR calculations and validated the reduced formulae. We acknowledge support by the staff at the Addenbrooke's Wellcome Trust Clinical Research Facility. We are grateful to study volunteers for their participation.
APPENDIX A: CALCULATION OF GLUCOSE FLUXES
Nomenclature
- EGP
Endogenous glucose production
- Ra exo
Appearance of unlabeled glucose due to exogenous infusion of dextrose
- Rd
Disposal of unlabeled glucose
- RdE
Disposal of unlabeled glucose originating endogenously
- RdM
Disposal of unlabeled glucose originating exogenously
- E
Glucose originating endogenously
- EM
Meal tracer (glucose tracer included with the dextrose)
- MM
Meal-mimicking tracer
- T
Total glucose (native and all tracers)
The computational method utilizes the Bayes theory (4) to calculate the posterior density of unknown fluxes and parameters f(u|y,v) defined as:
where f(u) is the prior density function, f(y,v|u) is the likelihood function and f(y,v) is the joint probability acting as a normalizing factor.
Prior and Likelihood Functions
The prior and the likelihood functions with the TT method adopting the Radziuk/Mari model are described below. The prior and the likelihood functions for the DT method can be obtained using a similar approach. In what follows, Ra exo represents the dextrose infusion, RdE represents the disposal of endogenous glucose, and RdM represents the disposal of glucose originating from the dextrose infusion.
Let n be the number of subjects and m be the number of samples taken in each subject. The prior distribution of the unknown glucose flux f, f = EGP, Ra exo, RdE, and RdM, is defined as random walk:
| (A1) |
where j = 1, . . . , n, i = 0, . . . , m − 1, N(a,b) is the normal distribution with mean a and precision (i.e., inverse of the variance) b. The flux f is assumed to be piecewise constant for Ra exo, and piecewise linear for EGP, RdE, and RdM. The random walk enforces temporal smoothing (4) with an extent of smoothing determined by the precision of the random walk εji, corrected for nonequidistant sampling schedules and sampled from a log-normal distribution to assure positivity:
where t1,i=0, . . . , m−1, are the sampling times and Γ(a,b) is the gamma distribution with parameters a and b, ε is the mean precision of the population distribution and τ is the precision of the population distribution representing inter-patient variability. Gamma distributions are typically used to define noninformative priors for precisions (4). A log-normal population distribution is used to ensure positivity of the precisions. The values of the fluxes at time 0 are defined, assuming steady-state conditions of glucose appearance and disposal and noting that exogenous glucose infusion commences at time 0, as:
where EGP and τ2 are the mean and the precision of the normal population distribution of the EGP at time 0. EGP is drawn from a vague normal distribution characterized by a low precision and thus a large variance (variance is an inverse of precision). The initial conditions of glucose masses are defined, assuming steady-state conditions, as follows:
where QE1(0), QE2(0), QM1(0),QM2(0), QMM1(0), QMM2(0), QEM1(0), and QEM2(0) are glucose masses of different glucose species, QE1 and τ3 are the mean and the precision of the normal population distribution of the initial mass of endogenous glucose and are drawn from vague prior distributions.
The time courses of glucose masses of different species as calculated by the Radziuk/Mari model are related to the measurements of tracer and total plasma glucose concentrations , , , and as follows:
where eEM(i), eM(i), eMM(i), and eT(i) are the measurement errors and TTRdex is the tracer-to-tracee ratio of the tracer in the dextrose.
The method estimates individual parameters such as vertices of random walks RW(i) for each of the four time-varying profiles EGP, Ra exo, RdE, and RdM, one “smoothness” parameter ε per random walk (all together four smoothness parameters), EGP at time 0 EGP(0) and the mass of glucose in the accessible compartment at time 0 QE1(0), and the population parameters EGP,QE1,ε,, τ, τ2, and τ3. In total there are 147 parameters per individual and 6 population parameters. Individual parameters from all subjects and population parameters are estimated simultaneously during a single parameter estimation run (in total, 2,358 parameters) using all available measurements of the total and labeled glucose concentrations.
Implementation Details
We use the Markov chain Monte Carlo (MCMC) method (8) to obtain the posterior distributions of the fluxes. The MCMC was implemented using WinBUGS version 1.4 (17, 28), extended by the WBDiff package version 1.9.4 (MRC Biostatistics Unit, Cambridge, UK). The latter is used to solve numerically the differential equations defining the Radziuk/Mari model. WinBUGS is a public domain package facilitating Bayesian estimation using MCMC methods. An outline of MCMC principles is provided below.
For the purposes of parameter estimation, measurement errors were assumed to be normally distributed with zero mean. The measurement errors associated with the total glucose and [6,6-2H2]glucose were assumed to be multiplicative with coefficients of variation (CV) of 2 and 5%, respectively. The measurement errors associated with [U-13C]glucose and [U-13C; 1,2,3,4,5,6,6-2H7]glucose were assumed to be multiplicative with 5% CV if tracer concentrations were greater than 0.015 mmol/l and 0.01 mmol/l, respectively, and additive with standard deviations of 0.0075 and 0.005 mmol/l, respectively, otherwise. The additive measurement errors were determined empirically and expressed that, at low tracer concentrations, occurring at the early portion of the experiment after the start of tracer infusions, the measurement instrumentation has precision independent of the measured value and that instrumentation detection limits apply.
APPENDIX B: RADZIUK/MARI MODEL SETUP WITH THE TRIPLE-TRACER METHOD
The kinetics of glucose species S, S = E, EM, M, and MM are described by the following differential equations.
where Q1,S(t) and Q2,S(t) are glucose masses in the accessible and nonaccessible compartments, respectively, GS(t) is the plasma glucose concentration, V is the distribution volume of glucose in the accessible compartment, uS(t) is the glucose appearance rate, and k12 and k21 are fractional turnover rates. The parameters of the Radziuk/Mari model were identical across all subjects, and glucose species and were assigned standard values k12 = 0.07 min−1, k21 = 0.05 min−1, and V = 160 ml/kg (12).
The fractional clearance k01,S(t) is defined as:
| A2 |
where RdM(t) is the rate of disposal of meal glucose, and RdM(t) is the rate of disposal of endogenous glucose. The use of two fractional clearance rates instead of a single rate is the main feature of the TT method. Whereas the DT method assumes identical k01 across all species, the TT method assumes one k01 to describe kinetics of the M-tracer and the MM-tracer and another k01 to describe kinetics of the EM-tracer and endogenous glucose. The difference between estimates of the two fractional clearance rates by the TT method is an indicator of model misspecification. However, at the early part of the experiment, the fractional clearance rate associated with the M-tracer and the MM-tracer is usually poorly estimated due to low inaccurate tracer concentrations invalidating meaningful comparison of the two fractional rates during this specific period.
The total unlabeled glucose disposal Rd(t) is equal to summation of RdM(t) and RdE(t). The glucose appearance rate is defined as follows:
where uEM(t) and uMM(t) are the known rates of the intravenously infused tracers.
APPENDIX C: BASIC DESCRIPTION OF MONTE CARLO MARKOV CHAIN METHODS
MCMC methods are a class of algorithms that allow sampling from complex intractable posterior Bayesian distributions. The MCMC methods create a Markov chain that, under fairly general conditions, converges to the posterior distribution (8). The Metropolis-Hasting algorithm is a generally applicable schema to generate the Markov chain, although faster approaches such as Gibbs sampler exist (8). Basic steps of the Metropolis-Hasting algorithm applied to present computational problem are given below.
represent the measurements of total and labeled glucose concentrations, U represents the vector of individual parameters such as RW(i), ε, EGP(0), and QE1(0), and P represents the vector of population parameters EGP,QE1, ε, τ, τ2, and τ3. The MCMC is used to obtain the joint posterior distribution of all unobserved stochastic variables U and P conditional on the observed data Y, i.e., p(U,P|Y). The steps are as follows:
1. Define a Markov chain θ(i), i = 1,2,..., as θ(i) = [ UiPi].
2. Initialize the Markov chain θ(1) by setting suitable initial values for each of the stochastic variables, e.g., glucose fluxes, smoothing parameters, initial individual EGP values, population means, etc.
3. Propose a new state of the Markov chain θ̄(i+1) that depends only on the previous state θ(i).
4. Solve numerically the differential equations of the Radziuk/Mari model with accompanying initial conditions and individual glucose fluxes as defined in the proposed state θ̄(i+1).
5. The proposed state θ̄(i+1) is accepted, i.e., θ(i+1) = θ̄(i+1), if α, drawn from the standard uniform distribution, satisfies
where f is known as a proposal or jumping density (8, 17, 28). Note that P(Y|θ(i+1)) and P(Y|θ¯(i+1)) are calculated from the Bayesian likelihood function, and P(θ¯(i+1)) and P(θ(i+1)) are calculated from the Bayesian prior distribution. If the proposed new state is rejected, then the next state is the same as the current state, i.e., θ(i+1) = θ(i).
Go to Step 3.
After a sufficient number of samples, the Markov chain converges to the joint posterior distribution of the stochastic parameters, and all subsequent samples are considered as posterior realizations of the distribution. Medians of posterior distributions are then used to infer point estimates of unknown parameters. In the present study, the Fisherian approach was utilized to calculate population mean (or median as appropriate) of glucose fluxes across the individuals.
REFERENCES
- 1. Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza R, Cobelli C. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 284: E55–E69, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Chase HP, Saib SZ, MacKenzie T, Hansen MM, Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabetic Med 19: 317–321, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cobelli C, Mari A, Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol Endocrinol Metab 252: E679–E689, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Congdon P. Bayesian Statistical Modelling. Chichester, UK; Hoboken, NJ: Wiley & Sons, 2006 [Google Scholar]
- 5. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A Bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2: 27ra27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elleri D, Allen JM, Harris J, Haidar A, Kumareswaran K, Leelarthna L, Nodale M, Wilinska ME, Weston J, Acerini CL, Jackson N, Umpleby AM, Evans ML, Dunger DB, Hovorka R. Glucose appearance of large evening meals with low and high glycaemic loads in type 1 diabetes. Diabetologia 54, Suppl 1: S54, 2011 [Google Scholar]
- 7. Ferrannini E, Bjorkman O, Reichard GA, Jr, Pilo A, Olsson M, Wahren J, DeFronzo RA. The disposal of an oral glucose load in healthy subjects A quantitative study. Diabetes 34: 580–588, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Gilks WR, Richardson S, Spiegelhalter DJ. Markov Chain Monte Carlo in Practice. Boca Raton, FL: Chapman & Hall, 1998 [Google Scholar]
- 9. Haidar A, Potocka E, Boulet B, Umpleby AM, Hovorka R. Estimating postprandial glucose fluxes using hierarchical Bayes modelling. Computer Methods Programs Biomed. 2012. February 22 [Epub ahead of print] PMID: 22364961 [DOI] [PubMed] [Google Scholar]
- 10. Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 7: 385–395, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 375: 743–751, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Hovorka R, Jayatillake H, Rogatsky E, Tomuta V, Hovorka T, Stein DT. Calculating glucose fluxes during meal tolerance test: a new computational approach. Am J Physiol Endocrinol Metab 293: E610–E619, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, Kollman C, Nodale M, Murphy HR, Dunger DB, Amiel SA, Heller SR, Wilinska ME, Evans ML. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 342: d1855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab 282: E992–E1007, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kalderon B, Korman SH, Gutman A, Lapidot A. Glucose recycling and production in glycogenosis type I and III: stable isotope technique study. Am J Physiol Endocrinol Metab 257: E346–E353, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Katz J, Lee WN, Wals PA, Bergner EA. Studies of glycogen synthesis and the Krebs cycle by mass isotopomer analysis with [U-13C]glucose in rats. J Biol Chem 264: 12994–13004, 1989 [PubMed] [Google Scholar]
- 17. Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10: 325–337, 2000 [Google Scholar]
- 18. Mari A. Estimation of the rate of appearance in the nonsteady state with a two-compartment model. Am J Physiol Endocrinol Metab 263: E400–E415, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Pennant ME, Bluck LJ, Marcovecchio ML, Salgin B, Hovorka R, Dunger DB. Insulin administration and rate of glucose appearance in people with type 1 diabetes. Diabetes Care 31: 2183–2187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potocka E, Hovorka R, Baughman RA, Umpleby M, Diaz MLM, Chen R, Boss AH, Richardson PC. AFRESA (TM) suppresses endogenous glucose production earlier than a rapid-acting analog (Lispro) and inhaled exubera. Diabetes 58, Suppl 1: A61, 2009 [Google Scholar]
- 21. Rabasa-Lhoret R, Burelle Y, Ducros F, Bourque J, Lavoie C, Massicotte D, Peronnet F, Chiasson JL. Use of an alpha-glucosidase inhibitor to maintain glucose homoeostasis during postprandial exercise in intensively treated Type 1 diabetic subjects. Diabetes Med 18: 739–744, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Radziuk J, McDonald TJ, Rubenstein D, Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism 27: 657–669, 1978 [DOI] [PubMed] [Google Scholar]
- 23. Rosenblatt J, Chinkes D, Wolfe M, Wolfe RR. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol Endocrinol Metab 263: E584–E596, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Scavone G, Manto A, Pitocco D, Gagliardi L, Caputo S, Mancini L, Zaccardi F, Ghirlanda G. Effect of carbohydrate counting and medical nutritional therapy on glycaemic control in Type 1 diabetic subjects: a pilot study. Diabetic Med 27: 477–479, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Schernthaner G, Wein W, Shnawa N, Bates PC, Birkett Preprandial vs MA. postprandial insulin lispro-a comparative crossover trial in patients with Type 1 diabetes. Diabetic Med 21: 279–284, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Shojaee-Moradie F, Jackson NC, Jones RH, Mallet AI, Hovorka R, Umpleby AM. Quantitative measurement of 3-O-methyl-d-glucose by gas chromatography mass spectrometry as a measure of glucose transport in vivo. J Mass Spectrom 31: 961–966, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Spiegelhalter D, Thomas A. Graphical modeling for complex stochastic systems: the BUGS project. IEEE Intelligent Syst Appl 13: 14–15, 1998 [Google Scholar]
- 28. Spiegelhalter D, Thomas A, Best N, Lunn D. WinBugs User Manual. Cambridge, UK: Medical Research Council Biostatistics Unit, 2003 [Google Scholar]
- 29. Steele R, Bjerknes C, Rathgeb I, Altszuler N. Glucose uptake and production during the oral glucose tolerance test. Diabetes 17: 415–421, 1968 [DOI] [PubMed] [Google Scholar]
- 30. Thorburn A, Litchfield A, Fabris S, Proietto J. Abnormal transient rise in hepatic glucose production after oral glucose in non-insulin-dependent diabetic subjects. Diabetes Res Clin Pract 28: 127–135, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Tikhonov AN, Goncharsky A, Stepanov VV, Yagola AG. Numerical Methods for the Solution of Ill-Posed Problems. Dordrecht; Boston, MA: Kluwer Academic, 1995 [Google Scholar]
- 32. Toffolo G, Basu R, Dalla Man C, Rizza R, Cobelli C. Assessment of postprandial glucose metabolism: conventional dual- vs. triple-tracer method. Am J Physiol Endocrinol Metab 291: E800–E806, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 31: 934–939, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R. Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabet Sci Technol 4: 132–144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolfe R. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992 [Google Scholar]