Abstract
Inflammatory cell infiltration in the liver is a hallmark of nonalcoholic steatohepatitis (NASH). The chemokine-chemokine receptor interaction induces inflammatory cell recruitment. CC-chemokine receptor (CCR)2 is expressed on hepatic macrophages and hepatic stellate cells. This study aims to investigate the therapeutic potential of CCR2 to NASH. Twenty-two weeks on a choline-deficient amino acid-defined (CDAA) diet induced steatosis, inflammatory cell infiltration, and liver fibrosis with increased CCR2 and monocyte chemoattractant protein (MCP)-1 expression in the wild-type livers. The infiltrated macrophages expressed CD68, CCR2, and a marker of bone marrow-derived monocytes, Ly6C. CCR2−/− mice had less steatosis, inflammatory cell infiltration, and fibrosis, and hepatic macrophages expressing CD68 and Ly6C were decreased. Toll-like receptor (TLR)4−/−, TLR9−/−, and MyD88−/− mice had reduced hepatic macrophage infiltration with decreased MCP-1 and CCR2 expression because TLR signaling is a potent inducer of MCP-1. To assess the role of Kupffer cells at the onset of NASH, Kupffer cells were depleted by liposomal clodronate. The Kupffer cell depletion ameliorated steatohepatitis with a decrease in the MCP-1 expression and recruitment of Ly6C-expressing macrophages at the onset of NASH. Finally, to test the therapeutic potential of targeting CCR2, a CCR2 inhibitor was administered to mice on a CDAA diet. The pharmaceutical inhibition of CCR2 prevented infiltration of the Ly6C-positive macrophages, resulting in an inhibition of liver inflammation and fibrosis. We concluded that CCR2 and Kupffer cells contribute to the progression of NASH by recruiting bone marrow-derived monocytes.
Keywords: CC-chemokine receptor, Kupffer cells, Toll-like receptors
nonalcoholic fatty liver disease (NAFLD) is now a common disease worldwide. Simple steatosis has been considered a benign liver disease associated with obesity. However, ∼30% of patients with NAFLD progress to nonalcoholic steatohepatitis (NASH) and its complications including insulin resistance, hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (5, 27, 40). The pathology of NASH is characterized by hepatocyte steatosis associated with liver inflammation and hepatocyte damage, which is a distinct feature from simple steatosis (5, 22). A variety of liver cells such as hepatocytes, hepatic macrophages, and hepatic stellate cells (HSCs) are involved in the pathogenesis of NASH (5). Among these cells, hepatic macrophages consisting of resident Kupffer cells and recruited bone marrow (BM)-derived macrophages are the major cells that produce inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen species (ROS) in NASH (29, 31). These inflammatory mediators further stimulate hepatocytes and HSCs to induce hepatocyte steatosis and fibrosis, respectively.
Among the inflammatory mediators, chemokines play pivotal roles in the recruitment of a variety of cells including immune cells to the sites of inflammation through interaction with chemokine receptors (4). Monocyte chemoattractant protein (MCP)-1, also known as CCL2, is a potent chemoattractive mediator produced from hepatic macrophages and HSCs. A principle receptor for MCP-1 is C-C motif chemokine receptor 2 (CCR2) that is expressed on monocyte-lineage cells including recruited and resident hepatic macrophages and HSCs (13, 26, 32). We and others demonstrated that CCR2 is important for the recruitment of BM-derived macrophages and HSCs in liver fibrosis induced by bile duct ligation and treatment with carbon tetrachloride (13, 23, 32). MCP-1 and CCR2 are also crucial for the development of hepatosteatosis, insulin resistance, and obesity in mice fed a high-fat diet (HFD) (12, 39). Recent studies demonstrated that the recruited BM-derived macrophages, but not resident Kupffer cells, predominantly express CCR2 and the inflammatory phenotype that promote liver steatosis in mice fed a HFD, suggesting the importance of the recruited macrophages in the development of NAFLD (15, 26). However, HFD-induced hepatic steatosis is not accompanied by profound liver inflammation and fibrosis. Clinically, NASH is more important than simple steatosis in patients. Our present study aims to investigate the role of CCR2 and recruited macrophages in the development of NASH.
Here we demonstrate the critical role of CCR2 in diet-induced NASH. In CCR2−/− mice, the recruitment of BM-derived macrophages and HSC activation was blunted. We also found that the initial MCP-1 production and subsequent macrophage recruitment are dependent on Toll-like receptor (TLR) signaling and resident Kupffer cells. Finally, we tested the pharmacological inhibition of CCR2 for the prevention of early and late stages of NASH.
MATERIALS AND METHODS
Animals, diet, and reagents.
Wild-type (WT) C57BL/6 mice and CCR2−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). TLR4−/−, TLR9−/−, and MyD88−/− mice (1, 7, 8) backcrossed at least 10 generations onto the C57BL/6 background were gifts from Dr. Akira (Osaka University, Osaka, Japan). These null mice exhibited similar hepatic phenotypes and hepatic lipid contents when fed standard laboratory chow (24). Male mice were divided into two groups at 8-wk old: choline-supplemented l-amino acid-defined diet (CSAA) (cat. no. 518754; Dyets, Bethlehem, PA) and choline-deficient l-amino acid-defined diet (cat. no. 518753, Dyets). These diets were continued for 22 wk without any interruption. In the experiments of Kupffer cell depletion, we used a short protocol: 2-wk CDAA diet feeding to examine the early events of NASH. Kupffer cell-depleted mice were generated by the intravenous injection of liposomal clodronate (38). For experiments with the chemical inhibitor of CCR2, we used both 2-wk and 22-wk CDAA diet protocols. For the 2-wk short protocol, a CCR2 antagonist (RS102895; 10 mg/kg per day; Sigma, St. Louis, MO) was administered in the drinking water for 2 wk. The other group was treated with a CCR2 antagonist during the final 4 wk of total 22 wk of CDAA diet feeding. The mice received humane care according to US National Institutes of Health recommendations outlined in the Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the University of California San Diego and Akita University Institutional Animal Care and Use Committees.
Histological examination.
Hematoxylin and eosin (H-E), Oil Red O, and Sirius Red staining were performed as previously described (24). NAFLD activity score was determined according to the published criteria (16). Immunohistochemical staining for α-smooth muscle actin (Dako Cytomation, Kyoto, Japan), F4/80 (eBioScience, San Diego, CA), and Ly6C (Abcam, Cambridge, MA) were performed. For CCR2 staining, Envision+ system (Dako Cytomation) was used according to the manufacturer's instructions. Briefly, deparaffinized sections were heated in citrate buffer (Dako Cytomation) to accomplish antigen retrieval. Endogenous peroxidase was blocked with 3% H2O2 in H2O. Anti-CCR2 antibody (Abcam) was applied as the primary antibody followed by the application of horseradish peroxidase-conjugated dextran-polymer prepared to detect the primary antibody. Peroxidase activity was visualized by diaminobenzidine with hematoxylin (Wako, Osaka, Japan) used as counterstain. F4/80-, CCR2-, and Ly6C-positive cells were counted on 10 high-power (×200) fields per slide.
Quantitative real-time PCR analysis.
Extracted RNA from livers and cells was subjected to reverse transcription and subsequent PCR reaction using ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster city, CA). Genes were normalized to 18S RNA as an internal control. Primers used are shown in Table 1.
Table 1.
Sequence of primers used for real-time quantitative PCR
| Gene | Forward | Reverse |
|---|---|---|
| 18S | AGTCCCTGCCCTTTGTACACA | CGATCCGAGGGCCTCACTA |
| CCR2 | AGCACATGTGGTGAATCCAA | TGCCATCATAAAGGAGCCA |
| CD68 | ACCGCCATGTAGTCCAGGTA | ATCCCCACCTGTCTCTCTCA |
| Collagen α1(I) | TAGGCCATTGTGTATGCAGC | ACATGTTCAGCTTTGTGGACC |
| Collagen α1(IV) | CACATTTTCCACAGCCAGAG | GTCTGGCTTCTGCTGCTCTT |
| F4/80 | CATAAGCTGGGCAAGTGGTA | GGATGTACAGATGGGGGATG |
| IL-1β | GGTCAAAGGTTTGGAAGCAG | TGTGAAATGCCACCTTTTGA |
| MCP-1 | ATTGGGATCATCTTGCTGGT | CCTGCTGTTCACAGTTGCC |
| PAI-1 | GCCAGGGTTGCACTAAACAT | GCCTCCTCATCCTGCCTAA |
| TGF-β1 | GTGGAAATCAACGGGATCAG | ACTTCCAACCCAGGTCCTTC |
| TIMP-1 | AGGTGGTCTCGTTGATTTCT | GTAAGGCCTGTAGCTGTGCC |
| TNF-α | AGGGTCTGGGCCATAGAACT | CCACCACGCTCTTCTGTCTAC |
CCR2, C-C motif chemokine receptor; MCP, monocyte chemoattractant protein; PAI-1, plasminogen activator inhibitor-1; TIMP-1, tissue inhibitor of metalloproteinase-1.
Lipid isolation and measurement.
Triglyceride, total cholesterol, and free fatty acid contents were measured using Triglyceride Reagent Set (Pointe Scientific, Cantom, MI), Cholesterol E (Wako Chemicals, Richmond, VA), free fatty acid, half micro test (Roche, Mannheim, Germany) as previously reported (24).
Assessment of insulin resistance.
A homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as [immunoreactive insulin (μU/ml) × FBS (mg/dl)/405].
Cell isolation and treatment.
Kupffer cells were isolated from mice as previously described (24). After cell attachment, Kupffer cells were serum starved for 16 h, followed by treatment with 100 ng/ml LPS, 5 μg/ml CpG-oligodeoxynucleotide (ODN) (ODN1826: 5′-tccatgacgttcctgacgtt-3′) or 5 μg/ml Non-CpG-ODN (5′-tccatgagcttcctgagctt-3′).
Statistical analysis.
Differences between two groups were compared using Mann-Whitney U-test. Differences between multiple groups were compared using one-way ANOVA (Dr. SPSS II); P < 0.05 was considered significant.
RESULTS
A CDAA diet induces NASH and fibrosis along with the recruitment of hepatic macrophages.
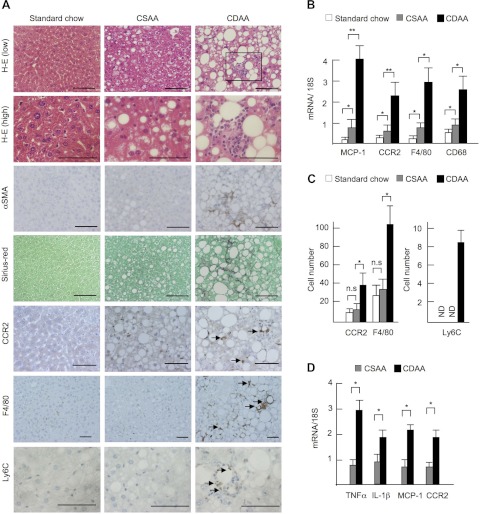
Two control diets were used in the present study, an isocaloric CSAA diet that induces simple steatosis and a low-calorie standard chow diet. Although the CSAA diet caused mild steatosis, both control diets induced neither liver inflammation nor fibrosis (Fig. 1A). Consistent with previous reports (17, 25), a 22-wk CDAA diet induced severe steatosis, inflammatory cell infiltration, hepatocyte ballooning, HSC activation, and fine pericellular fibrosis (Fig. 1A). We found that the mRNA of MCP-1, a potent chemoattractive mediator that recruits macrophages, and its receptor, CCR2, was upregulated in the NASH livers (Fig. 1B). Consistently, the numbers of CCR2-expressing cells were increased after the CDAA diet feeding (Fig. 1, A and C). Increased hepatic macrophage infiltration after CDAA diet feeding was demonstrated by the increased number of F4/80-positive cells and hepatic mRNA levels of F4/80 and an activated macrophage marker CD68 (Fig. 1, A–C). The numbers of cells that express Ly6C, a marker for BM-derived circulating peripheral blood monocytes, were significantly increased in the livers of CDAA diet-fed mice, whereas few Ly6C-positive cells were seen in the livers of CSAA diet- or standard chow-fed mice (Fig. 1, A and C) (2, 13, 14). Moreover, hepatic macrophages isolated from CDAA diet-fed mice expressed inflammatory genes including TNF-α, IL-1β, MCP-1, and CCR2 (Fig. 1D). These results suggest that infiltrated hepatic macrophages express inflammatory phenotypes with the expression of MCP-1 and CCR2 in the CDAA diet-induced NASH livers.
Fig. 1.
Choline-deficient amino acid-defined (CDAA) diet-induced steatohepatitis shows increased infiltration of hepatic macrophages. Wild-type (WT) mice were fed standard chow (n = 5), control choline-sufficient amino acid-defined (CSAA) diet (n = 5) or CDAA diet (n = 10) for 22 wk. A: liver sections were stained with hematoxylin and eosin (H-E), α-smooth muscle actin (SMA), Sirius red, C-C motif chemokine receptor 2 (CCR2), F4/80, and Ly6C. WT mice on the CDAA diet show severe steatosis, inflammatory cell infiltration, and fibrosis. The boxed area in H-E (low) (inflammatory foci) is magnified in H-E (high). F4/80-positive cells are seen in inflammatory foci (arrow) in addition to hepatic parenchyma. CCR2- and Ly6C-positive cells are predominantly seen in inflammatory foci. Original magnification, ×200 for F4/80, ×400 for H-E (low), α-SMA, Sirius red, and CCR2, ×600 for H-E (high) and Ly6C. Bar = 100 μm. B: hepatic mRNA expression of monocyte chemoattractant protein (MCP)-1, CCR2, F4/80, and CD68 was determined by quantitative real-time PCR. C: numbers of CCR2-, F4/80- and Ly6C-positive cells are shown. D: mRNA expression of TNF-α, IL-1β, MCP-1, and CCR2 in hepatic macrophages was determined by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. ND; not detected. Data represent means ± SD, *P < 0.05; **P < 0.01.
Loss of CCR2 inhibits the development of NASH, fibrosis, and insulin resistance.
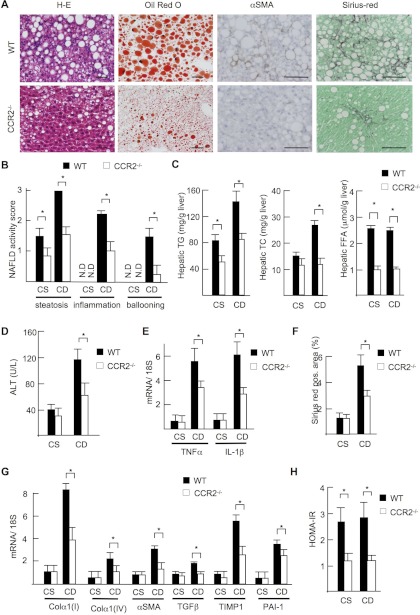
To investigate the role of CCR2 in NASH, WT and CCR2−/− mice were fed with CDAA diet for 22 wk. In contrast to WT mice, CCR2−/− mice exhibited less steatosis, inflammatory cell infiltration, and hepatocyte ballooning (Fig. 2A). The NAFLD activity score was significantly lower in CCR2−/− mice than in WT mice (total score, WT vs. CCR2−/− mice = 6.5 vs. 4.0, P < 0.05) (Fig. 2B). Decreases in hepatic lipid content of triglyceride, total cholesterol, free fatty acids, and serum alanine transaminase (ALT) levels were also seen in CCR2−/− mice after CDAA diet feeding (Fig. 2, C and D). Hepatic gene expression of inflammatory cytokines including TNF-α and IL-1β were also significantly lower in CCR2−/− mice than in WT mice (Fig. 2E). Liver fibrosis, HSC activation, and the expression of fibrogenic genes including collagen α1(I), collagen α1(IV), tissue inhibitor of metalloproteinase-1 (TIMP-1), and plasminogen activator inhibitor-1 (PAI-1) were significantly suppressed in CCR2−/− livers (Fig. 2, A, F, and G). These findings indicate that CCR2 is important for the progression of steatohepatitis and fibrosis induced by the CDAA diet.
Fig. 2.
Steatohepatitis is attenuated in CCR2−/− mice. WT and CCR2−/− mice were fed the CSAA diet (CS, n = 5) or the CDAA diet (CD, n = 10) for 22 wk. A: H-E, Oil Red O, α-SMA, and Sirius red staining are shown. Steatosis, inflammatory cell infiltration, and fibrosis were attenuated in CCR2−/− mice fed the CDAA diet. Original magnification, ×200 for H-E and Oil Red O staining, ×400 for α-SMA and Sirius red staining. Bar = 100 μm. B: nonalcoholic fatty liver disease (NAFLD) activity score. C: hepatic triglyceride (TG) and total cholesterol (TC) and free fatty acid (FFA) content. D: serum alanine transferase (ALT) levels. E: hepatic mRNA expression of inflammatory cytokines. F: Sirius red-positive area was measured on 10 low-power (x100) fields/slide and quantified with the use of NIH imaging software. G: hepatic mRNA expression of fibrogenic genes was measured by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. TIMP, tissue inhibitor of metalloproteinase; PAI, plasminogen activator inhibitor. H: insulin resistance was assessed by a homeostasis model assessment of insulin resistance (HOMA-IR). Data represent means ± SD, *P < 0.05.
CDAA diet feeding for 22 wk induces obesity and insulin resistance in WT mice (25). Table 2 is a summary of metabolic markers in mice fed standard chow, isocaloric control CSAA, and CDAA diets. In CCR2−/− mice, body, liver and visceral fat weights were significantly lower than those in WT mice fed the CDAA diet. Moreover, CCR2 deficiency improved insulin resistance as assessed by HOMA-IR (Fig. 2H).
Table 2.
Body/liver/fat weight and lipid level at 22 wk after the CSAA and CDAA diet feeding
| CSAA |
CDAA |
|||
|---|---|---|---|---|
| WT mice (n = 5) | CCR2−/− mice (n = 5) | WT mice (n = 10) | CCR2−/− mice (n = 10) | |
| Body weight, g, start, wk = 0 | 22.0 ± 1.0 | 22.8 ± 0.9 | 22.5 ± 1.1 | 22.3 ± 1.1 |
| Body weight, g, end, wk = 22 | 39.4 ± 1.1 | 32.4 ± 2.3* | 39.6 ± 2.0 | 30.6 ± 2.7*† |
| Liver weight, g | 2.07 ± 0.03 | 1.25 ± 0.10* | 2.55 ± 0.23* | 1.73 ± 0.32†‡ |
| Liver weight, % | 5.26 ± 0.13 | 3.88 ± 0.15* | 6.41 ± 0.29* | 5.65 ± 0.70†‡ |
| Epididymal fat, g | 2.33 ± 0.15 | 1.08 ± 0.40* | 2.35 ± 0.30 | 1.11 ± 0.39*† |
| Epididymal fat, % | 5.91 ± 0.36 | 3.30 ± 1.08* | 5.94 ± 0.73 | 3.57 ± 1.01*† |
| Plasma | ||||
| Triglyceride, mg/dl | 32.6 ± 7.8 | 30.0 ± 6.4 | 47.6 ± 9.1* | 37.8 ± 9.5 |
| Total cholesterol, mg/dl | 154.0 ± 16.7 | 135.8 ± 16.6 | 86.6 ± 21.6* | 73.4 ± 7.9*‡ |
| Free fatty acid, mM | 0.47 ± 0.12 | 0.32 ± 0.71 | 0.49 ± 0.15 | 0.34 ± 0.93† |
| HOMA-IR | 2.74 ± 0.50 | 1.28 ± 0.27* | 2.86 ± 0.58 | 1.25 ± 0.17*† |
Values are means ± SD.
Significantly different from wild-type (WT)-choline-supplemented amino acid-defined (CSAA), P < 0.05.
Significantly different from WT-choline-deficient amino acid-defined (CDAA), P < 0.05.
Significantly different from CCR2−/−-CSAA, P < 0.05. HOMA-IR, homeostasis model assessment of insulin resistance.
Reduction of the recruitment of hepatic macrophages in CCR2−/− mice.
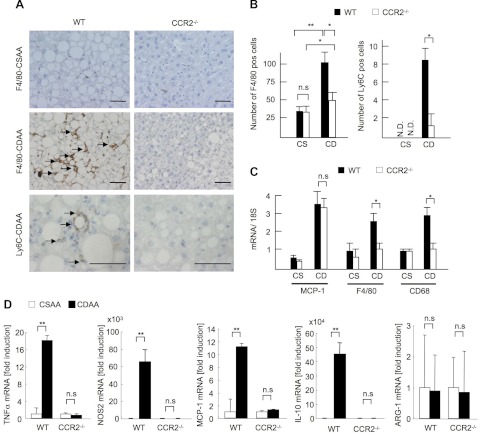
Because the recruitment of hepatic macrophages was increased in WT mice on the CDAA diet, we examined macrophage infiltration in CCR2−/− livers. Infiltration of hepatic macrophages was significantly suppressed in CCR2−/− livers compared with WT livers as assessed by counting F4/80-positive cells and measuring hepatic mRNA levels of F4/80 and CD68 (Fig. 3, A–C). Moreover, Ly6C-positive cells were decreased in CCR2−/− mice (Fig. 3, A and B). We then assessed the phenotypes of hepatic macrophages. WT macrophages isolated from mice on CDAA diet expressed high levels of inflammatory markers TNF-α, inducible nitric oxide synthase (iNOS), and MCP-1, as well as anti-inflammatory IL-10 compared with cells from control mice on CSAA diet (Fig. 3D). Inflammatory gene expression was similar in CCR2−/− macrophages from mice fed CDAA and control CSAA diets (Fig. 3D). We did not find any changes in M2 marker Arg-1 in CCR2−/− macrophages with or without CDAA diet treatment (Fig. 3D). These findings suggest that BM-derived Ly6C-positive macrophages are recruited to the liver through CCR2, and these recruited macrophages express inflammatory phenotypes responsible for the NASH development.
Fig. 3.
CCR2 deficiency inhibits infiltration of inflammatory macrophages. WT and CCR2−/− mice were fed the CSAA diet (CS, n = 5) or the CDAA diet (CD, n = 10) for 22 wk. A: immunohistochemical staining for F4/80 and Ly6C. Macrophage infiltration is suppressed in CCR2−/− mice. Arrows indicate inflammatory foci. Original magnification, ×200 for F4/80, ×600 for Ly6C. Bar = 100 μm. B: numbers of F4/80- and Ly6C-positive cells. C: hepatic mRNA expression of MCP-1, F4/80, and CD68 was measured by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. Data represent means ± SD, *P < 0.05. D: mRNA expressions of TNF-α, nitric oxide synthase (NOS)2, MCP-1, IL-10, and arginase (ARG)-1 in hepatic macrophages were determined by quantitative real-time PCR. Genes were normalized to the internal control, 18S RNA. Data represent means ± SD, **P < 0.01.
TLR signaling induces MCP-1 production and macrophage infiltration in the liver.
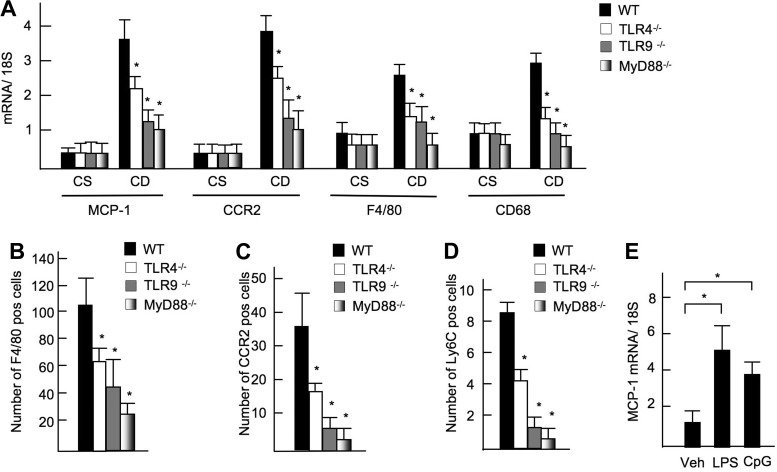
Because MCP-1 is a downstream target of TLR signaling, we measured hepatic expression of MCP-1 in TLR mutated mice, in which NASH was diminished after the CDAA diet feeding (24, 30). Hepatic mRNA expression of MCP-1 was suppressed in TLR4−/−, TLR9−/−, and MyD88−/− mice compared with WT mice (Fig. 4A). As a result of decreased MCP-1 expression, the recruitment of CCR2-positive cells was significantly inhibited in TLR4−/−, TLR9−/−, and MyD88−/− mice (Fig. 4B). Because infiltrated macrophages express CCR2 (Fig. 1D), hepatic macrophages expressing F4/80 and CD68 were significantly decreased in TLR4−/−, TLR9−/−, and MyD88−/− mice (Fig. 4B). CCR2-expressing cells and BM-derived recruited Ly6C-expressing cells were also decreased in these mutant mice (Fig. 4, C and D). Furthermore, TLR4 ligand LPS and TLR9 ligand increased mRNA expression of MCP-1 in primary cultured WT-Kupffer cells (Fig. 4E). These results suggest that the TLR/MyD88 signaling induces MCP-1 production that recruits BM-derived macrophages to the site of liver inflammation through CCR2 in NASH.
Fig. 4.
Reduced MCP-1 expression and macrophage recruitment in Toll-like receptor (TLR)4−/−, TLR9−/−, and MyD88−/− mice. WT, TLR4−/−, TLR9−/−-, and MyD88−/− mice were fed the CDAA diet for 22 wk. A: hepatic mRNA expression of MCP-1, CCR2, F4/80, and CD68 in TLR mutant mice (n = 7–8). Gene expressions were determined by quantitative real-time PCR and normalized to 18S RNA as an internal. B–D: numbers of F4/80- (B), CCR2- (C), and Ly6C-positive cells (D) in TLR4−/−, TLR9−/−, and MyD88−/− mice. E: Kupffer cells were isolated from WT mice, followed by treatment with vehicle (Veh), 100 ng/ml LPS, or 5 μg/ml CpG-oligodeoxynucleotide (CpG). MCP-1 mRNA levels in Kupffer cells were examined by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. Data represent means ± SD, *P < 0.05.
A critical role of Kupffer cells in the early stage of NASH.
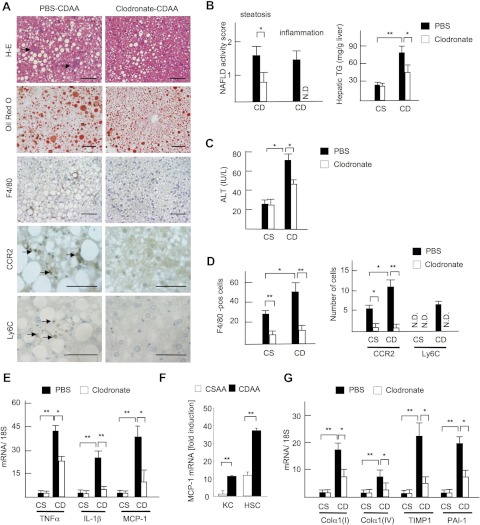
To gain insights into the role of the liver resident macrophage Kupffer cell at the onset of NASH, mice were subjected to Kupffer cell depletion and a short protocol of 2-wk CDAA diet feeding. Intravenous injection of liposomal clodronate successfully depleted Kupffer cells without affecting other liver cells such as hepatocytes, endothelial cells, and HSCs (24). Liposomal PBS was used as a control for liposomal clodronate. In the PBS group, moderate degrees of steatosis and the elevation of serum ALT levels were observed after the 2-wk CDAA diet feeding (Fig. 5, A and B). Infiltration of inflammatory cells including F4/80-positive macrophages was also increased in PBS-liposome-treated CDAA diet-fed mice (Fig. 5, A–C). In contrast, the clodronate treatment significantly suppressed hepatic steatosis and inflammatory cell infiltration and decreased hepatic triglyceride content and serum ALT levels at the point of 2 wk of CDAA diet feeding (Fig. 5, A–C). The distribution of F4/80-, CCR2-, and Ly6C-positive cells was also inhibited in Kupffer cell-depleted mice (Fig. 5, A and D). Moreover, Kupffer cell depletion led to decreased hepatic mRNA expression of inflammatory cytokines including TNF-α, IL-1β, and MCP-1 (Fig. 5E). Because both HSCs and hepatic macrophages express MCP-1 (Fig. 5F), Kupffer cells and HSCs are major sources of MCP-1 that recruit Ly6C-positive BM-derived monocytes to the liver through CCR2 in the initial stage of NASH. Although collagen deposition was not present after the 2-wk CDAA diet feeding, the expressions of fibrogenic genes including collagen α1(I), collagen α1(IV), TIMP-1, and PAI-1 were upregulated in PBS-liposome-treated mice, whereas these genes were suppressed in Kupffer cell-depleted mice (Fig. 5G). These results suggest that Kupffer cells and recruited Ly6C-positive macrophages contribute to HSC activation and fibrogenesis in the early events of NASH.
Fig. 5.
Kupffer cell depletion delays the progression of steatohepatitis. Mice were treated with liposomal PBS (closed bar) or clodronate (open bar), and fed on the CSAA (CS) and CDAA (CD) diets for 2 wk. A: H-E, Oil red O staining, and immunohistochemical staining for F4/80, CCR2, and Ly6C. Steatosis and inflammatory cell infiltration (arrows) are attenuated by clodronate liposome injection. Bar = 100 μm. B: NAFLD activity score and hepatic TG content are shown. Hepatocyte ballooning is not seen in 2-wk CDAA diet. C: serum ALT levels. D: numbers of F4/80-, CCR2-, and Ly6C-positive cells were counted. E and G: hepatic mRNA expression of TNF-α, IL-1β, MCP-1 (E), and fibrogenic factors (G) was determined by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. Data represent means ± SD, *P < 0.05; **P < 0.01. F: MCP-1 mRNA expression in hepatic macrophages (KC) and hepatic stellate cells (HSC) was determined by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. Data represent means ± SD, **P < 0.01.
The pharmaceutical inhibition of CCR2 ameliorates NASH phenotypes in the early and the late stages.
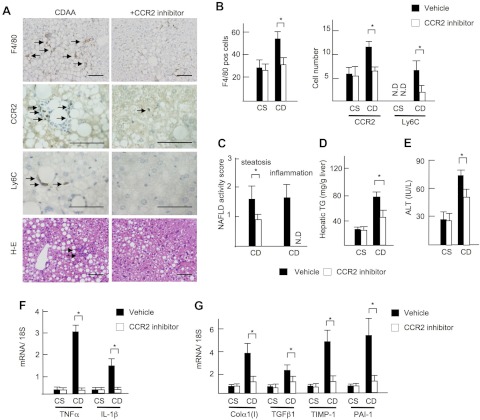
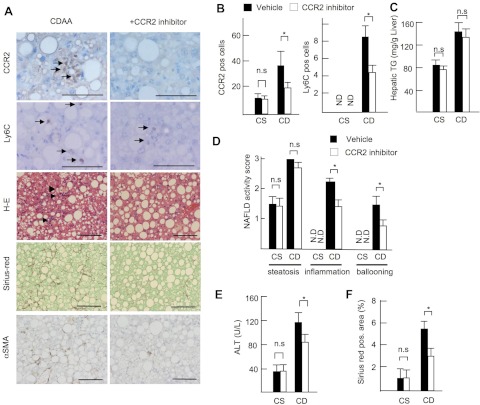
Finally, we investigated the potential of CCR2 as a therapeutic target for the treatment of NASH using a pharmaceutical CCR2 inhibitor (RS102895; 10 mg/kg per day; Sigma). To test the therapeutic effect of this drug at the early stage of NASH, we initially tested a short protocol of 2-wk CDAA diet feeding model. The CCR2 inhibitor significantly decreased the number of F4/80-, CCR2-, and Ly6C-positive cells in the liver (Fig. 6, A and B). Consistent with impaired macrophage recruitment, the CCR2 inhibitor-treated mice showed less hepatic steatosis with decreased hepatic triglyceride content and serum ALT levels (Fig. 6, A and C–E). In addition, hepatic mRNA expression of inflammatory cytokines TNF-α and IL-1β and fibrogenic genes was significantly suppressed by the CCR2 inhibitor (Fig. 6, F and G). Subsequently, we examined the benefit of the CCR2 inhibitor to the existing NASH. The CCR2 inhibitor was administered to the mice in the last 4 wk of the total 22 wk of CDAA diet feeding. The CCR2 inhibitor decreased infiltration of inflammatory cells including CCR2- and Ly6C-positive cells (Fig. 7, A, B, and D). The CCR2 inhibitor suppressed the grades of inflammation, liver injury, and fibrosis but not steatosis on the existing NAFLD (Fig. 7, A–F). These results indicated that, whereas the CCR2 inhibitor prevents hepatic steatosis, inflammation, and fibrosis in early NASH, it inhibits liver inflammation and fibrosis, but not steatosis, in the existing NASH.
Fig. 6.
Pharmaceutical inhibition of CCR2 prevents early steatohepatitis. Mice were treated with vehicle (closed bar) and CCR2 inhibitor (open bar) and fed on the CSAA (CS) and CDAA (CD) diets for 2 wk. A: immunohistochemical staining for F4/80, CCR2, Ly6C, and H-E staining. Arrows show inflammatory foci. Original magnification, ×200 for F4/80 and H-E staining, ×600 for CCR2 and Ly6C. Bar = 100 μm. B: numbers of F4/80-, CCR2-, and Ly6C-positive cells. C: NAFLD activity score. D: hepatic TG content. Steatosis and inflammatory cell infiltration are suppressed by a CCR2 inhibitor. E: serum ALT levels. F: hepatic mRNA levels of TNF-α and IL-1β were determined by quantitative real-time PCR. G: hepatic mRNA expression of fibrogenic factors was determined by quantitative real-time PCR. Genes were normalized to 18S RNA as an internal control. Data represent means ± SD, *P < 0.05.
Fig. 7.
CCR2 inhibitor attenuates existing liver inflammation and fibrosis. Mice were treated with vehicle (closed bar) and CCR2 inhibitor (open bar) and fed on the CSAA (CS) and CDAA (CD) diets in the last 4 wk of total 22 wk of CDAA diet feeding. A: immunohistochemical staining for CCR2, Ly6C, α-SMA, H-E, and Sirius red staining. Arrows show inflammatory foci. Original magnification, ×200 for H-E and Sirius red staining and staining for α-SMA, ×600 for CCR2 and Ly6C. Bar = 100 μm. B: numbers of CCR2- and Ly6C-positive cells. C: NAFLD activity score. D: hepatic TG content. Inflammatory cell infiltration and fibrosis are suppressed by a CCR2 inhibitor. E: serum ALT levels. F: Sirius red-positive area. Data represent means ± SD, *P < 0.05.
DISCUSSION
With sustained inflammation, NASH may progress to cirrhosis and hepatocellular carcinoma. Thus control of liver inflammation may be a potential strategy for the therapy of NASH. Here, we demonstrated that genetic and pharmaceutical inactivation of CCR2 inhibited diet-induced NASH and fibrosis. Expression of CCR2 and its ligand MCP-1 was significantly upregulated after CDAA diet, suggesting an important role of the MCP-1-CCR2 interaction in NASH (Fig. 1). Hepatic macrophages are the key cells inducing liver inflammation and HSC activation, and infiltration of hepatic macrophages was increased in WT mice on the CDAA diet. Particularly, the hepatic macrophages expressing Ly6C, which are derived from circulating peripheral blood monocytes or BM cells, were increased (2, 13, 14, 18). This event was blunted in CCR2−/− mice (Figs. 2 and 3), suggesting that CCR2 mediates the recruitment of BM-derived Ly6C-positive monocytes. We also found that Kupffer cell depletion by liposomal clodronate prevented early NASH with decreased MCP-1 expression and recruitment of Ly6C-expressing macrophages (Fig. 5). Furthermore, CCR2−/− mice had less HSC activation and fibrosis in diet-induced NASH (Fig. 2), which is consistent with our previous report that demonstrated a critical role of CCR2 in liver fibrosis (32). TLR signaling is a potent inducer of MCP-1 in hepatic macrophages and HSCs (17, 24, 33). In fact, induction of MCP-1 and CCR2 and macrophage recruitment was suppressed in mice deficient in TLR4, TLR9, and MyD88 (Fig. 4). Finally, we demonstrated that a pharmacological CCR2 inhibitor inhibits the early and late features of NASH including fibrosis (Fig. 6).
HFD is often used to study metabolic disease, which develops hepatic steatosis, obesity, and insulin resistance. The importance of MCP-1 and CCR2 in HFD-induced hepatosteatosis has been reported (12, 39). In the livers of HFD-fed mice, BM-derived macrophages, but not resident Kupffer cells, express CCR2 and a proinflammatory phenotype that promote hepatic steatosis (15, 26). Whereas HFD treatment induces hepatic steatosis, it does not induce liver inflammation and fibrosis, which are both clinically important features of NASH livers. Methionine and choline-deficient (MCD) diet model is widely used to study NASH that induces strong liver steatosis and inflammation without evident fibrosis and extrahepatic features of metabolic syndrome, such as obesity and insulin resistance. Lately, it has been reported that pharmacological inhibition of MCP-1 and genetic modification of CCR2 ameliorated a mouse model of NASH induced by MCD diet (3). In the present study, we used CDAA diet that induces NASH accompanied by fine fibrosis, weight gain, and insulin resistance, which are relevant features of human NASH compared with HFD- or MCD-induced NAFLD. Thus we aimed to investigate the role of CCR2 and recruited macrophages in NASH and fibrosis using a CDAA diet model. Moreover, we gained insight into the role of CCR2 and resident Kupffer cells on the recruitment of Ly6C-positive BM-derived macrophages in CDAA diet-induced NASH and fibrosis. Our data demonstrated that Kupffer cell depletion inhibited the initial recruitment of BM-derived Ly6C expressing macrophages at the onset of NASH, suggesting that the resident Kupffer cells are important for recruitment of proinflammatory BM-derived macrophages by production of inflammatory cytokines and chemokines including MCP-1.
Hepatic macrophages are classified into two major populations, liver-resident macrophages (Kupffer cells) and recruited macrophages derived from BM/circulating peripheral blood monocytes (15). On the other hand, macrophages can be roughly divided into two phenotypes, proinflammatory macrophages so called M1 type and anti-inflammatory M2 type macrophages (21). Both recruited macrophages and Kupffer cells are the primary cells that produce inflammatory cytokines. Recruited macrophages derived from extrahepatic monocytes preferentially express Ly6C with inflammatory phenotype (13, 15, 26). Our data demonstrated that macrophages of WT mice on a CDAA diet express high levels of TNF-α, iNOS, and MCP-1, but cells of CCR2−/− mice do not (Fig. 3D). This suggests that BM-derived macrophages recruited through CCR2 express an inflammatory phenotype, whereas resident macrophages (Kupffer cells) are less inflammatory, with lower CCR2 expression, which corroborates the reports from Klein et al. (15, 26). We did not find any association between CCR2 expression and M2 polarization. Moreover, our previous study demonstrated that the Ly6C-negative resident Kupffer cells express an anti-inflammatory phenotype with the expression of CX3CR1 (2), which further suggests that resident Kupffer cells have less inflammatory properties. These findings implicated that Ly6C-positive BM-derived hepatic macrophages are proinflammatory (M1 type), and resident Kupffer cells have dual phenotypes, capable of both the promotion (M1 type) and the inhibition (M2 type) of inflammation.
Kupffer cell-depleted mice exhibited a significant reduction of MCP-1 expression, suggesting a large contribution of hepatic macrophages for MCP-1 production in the liver. Intriguingly, both hepatic macrophages and HSCs are the major sources of MCP-1 in the liver (17, 30, 33), and HSCs express more MCP-1 than hepatic macrophages (Fig. 5F). We suggest two mechanisms by which MCP-1 expression was blunted by Kupffer cell depletion despite the higher expression of MCP-1 by HSCs compared with hepatic macrophages. First, the number of hepatic macrophages is much higher than that of HSCs, and thus the total amount of MCP-1 produced in hepatic macrophages is higher those in HSCs; second, mediators secreted by hepatic macrophages are indispensable factors for MCP-1 production from HSCs. Thus the deletion of Kupffer cells results in suppression of MCP-1 production in HSCs to produce MCP-1.
MCP-1 is produced in hepatic macrophages through TLR4 and TLR9 signaling and in HSCs via TLR4 (17, 24, 33). Because MyD88 is a common adaptor molecule for TLR4 and TLR9, it is conceivable that MCP-1 is produced from hepatic macrophages and HSCs through TLR4, TLR9, and MyD88 in murine models of NASH (Fig. 4). Subsequently, the MCP-1 binding to CCR2 on hepatic macrophages and possibly HSCs promotes NASH and NASH-related fibrosis. Notably, the hepatic recruitment of Ly6C-expressing BM-derived monocytes was abolished in CCR2−/− mice after CDAA diet feeding. These findings strongly suggest that the recruitment of Ly6C-positive cells in NASH is dependent on CCR2.
Hepatic steatosis and fibrosis are promoted by inflammatory cytokines including TNF-α and IL-1β that are produced mainly by hepatic macrophages (20, 24, 34, 36). These cytokines induce lipid accumulation through the expression of lipogenesis-related genes including peroxisome proliferator-activated receptor-γ and disacylglycerol acyltransferase 2 in hepatocytes (20, 24, 34). TNF-α and IL-1β also induce the expression of the fibrogenic factor TIMP-1 in HSCs (24, 36). The expression of TNF-α and IL-1β was suppressed in TLR4−/− mice, TLR9−/− mice, and MyD88−/− mice in which steatosis and fibrosis were attenuated after MCD or CDAA diet feeding (24, 30). Besides the direct effects of inflammatory cytokines on lipogenesis and fibrogenesis, TNF-α and IL-1β can induce insulin resistance (10, 11). Importantly, insulin resistance is further associated with the development of steatosis and liver fibrosis (19, 24, 28). We found that insulin sensitivity was improved in CCR2−/− mice with decreased expression of inflammatory cytokines after CDAA diet feeding. Our studies also showed that insulin resistance is improved in TLR4−/− mice, TLR9−/− mice, and MyD88−/− mice fed CDAA diet (K. Miura and E. Seki, unpublished observations) (24), which is consistent with previous reports that the HFD-induced insulin resistance is attenuated in CCR2−/−, TLR4−/−, and MyD88−/− mice (9, 37, 39). This further suggests that inflammatory cytokines derived from hepatic macrophages may regulate steatosis and liver fibrosis directly and indirectly through insulin resistance.
Evidence is accumulating that CCR2 antagonists are effective in experimental disease models including rheumatoid arthritis, multiple sclerosis, hypertension, and renal fibrosis (6). A recent study demonstrated the impact of MCP-1 inhibitor on MCD diet-induced NASH (3). Our study investigated a CCR2 inhibitor as a potential agent for the treatment of NASH. The CCR2 inhibitor prevented CDAA diet-induced NASH, including hepatic steatosis, macrophage infiltration, inflammation, and fibrosis. This is suggested to result from the suppression of recruitment of Ly6C-positive monocytes, Kupffer cells, and HSCs by CCR2 inhibition. Although we did not find M2 polarization in CCR2−/− macrophages, it has been reported that a CCR2 inhibitor can shift M1 type macrophages to M2 phenotypes (35). This further suggests that the CCR2 inhibitor, not only suppresses the recruitment of macrophages and HSCs, but also induces phenotypical changes in macrophages from M1 to M2, resulting in the prevention of hepatic steatosis, inflammation, and insulin resistance.
In summary, the accumulation of proinflammatory macrophages expressing Ly6C, resident Kupffer cells, and HSCs contributes to hepatic steatosis, inflammation, fibrosis, and insulin resistance. These features commonly observed in human NASH were ameliorated by genetic depletion or pharmaceutical inhibition of CCR2. Therefore, CCR2 is an interesting potential target for the treatment of NASH.
GRANTS
This study is supported by a Liver Scholar Award from the AASLD/ ALF, the pilot grant from the UCSD Digestive Diseases Research Development Center (DK080506), the research grant from ABMRF, NIH grant R01AA02172 and R01DK085252 (all to E. Seki), Takeda Science Foundation (K. Miura), The Mochida Memorial Foundation for Medical and Pharmaceutical Research (K. Miura), Mishima Kaiun Memorial Foundation (K. Miura), and by JSPS [Grant-in-Aid for Scientific Research (C)] (K. Miura).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.M. and E.S. conception and design of research; K.M. and L.Y. performed experiments; K.M., L.Y., and E.S. analyzed data; K.M., L.Y., N.v.R., H.O., and E.S. interpreted results of experiments; K.M., L.Y., and E.S. prepared figures; K.M. and E.S. drafted manuscript; K.M., H.O., and E.S. edited and revised manuscript; E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Shizuo Akira (Osaka University, Suita, Japan) for the generous gift of TLR4−/−, TLR9−/−, and MyD88−/− mice, and Rie Seki, Karin Diggle, Jingyi Isabelle Song (Department of Medicine, UCSD, La Jolla, CA), and Yukie Komatsu (Akita University Graduate School of Medicine) for excellent technical assistance.
Contact information for K. Miura: Dept. of Gastroenterology; Akita Univ. Graduate School of Medicine, 1-1-1 Hondo Akita-shi, Akita, Japan 010-8543 (e-mail: miura116@doc.med.akita-u.ac.jp).
REFERENCES
- 1. Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9: 143– 150, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52: 1390– 1400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61: 416– 426, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354: 610– 621, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 300: G697– G702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313– 326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740– 745, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749– 3752, 1999 [PubMed] [Google Scholar]
- 9. Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One 5: e12537, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87– 91, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148: 241– 251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494– 1505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50: 261– 274, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F. The fractalkine receptor CXCR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 52: 1769– 1782, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 110: 4077– 4085, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313– 1321, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, Schnabl B, Seki E, Brenner DA. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology 137: 1467– 1477; e1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733– 6743, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Lo L, McLennan SV, Williams PF, Bonner J, Chowdhury S, McCaughan GW, Gorrell MD, Yue DK, Twigg SM. Diabetes is a progression factor for hepatic fibrosis in a high fat fed mouse obesity model of non-alcoholic steatohepatitis. J Hepatol 55: 435– 444, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology 48: 770– 781, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677– 686, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14: 72– 81, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Renia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174: 1766– 1775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139: 323– 334; e327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterol Res Pract 2010: 362847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59: 916– 925, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol 26, Suppl 1: 153– 162, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y, Nakanuma Y, Kaneko S. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology 132: 282– 293, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol 13: 777– 784, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 47: 571– 579, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology 130: 1886– 1900, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology 50: 185– 197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324– 1332, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Muller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology 51: 511– 522, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb 17: 219– 228, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 55: 415– 424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986– 1998, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83– 93, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115– 124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124– 131, 2011 [DOI] [PubMed] [Google Scholar]