Abstract
Eosinophilic esophagitis (EoE) is an emerging chronic esophageal disease. Despite the increasing diagnosis of EoE globally, the causes of EoE and other esophageal eosinophilic disorders are not clearly understood. EoE pathology includes accumulation of inflammatory cells (e.g., eosinophils, mast cells), characteristic endoscopic features (e.g., furrows, the formation of fine concentric mucosal rings, exudates), and functional impairments (e.g., esophageal stricture, dysmotility). We hypothesized that the esophageal structural pathology and functional impairments of EoE develop as a consequence of the effector functions of the accumulated inflammatory cells. We analyzed eosinophils (anti-major basic protein immunostaining), esophageal stricture (X-ray barium swallowing), and esophageal motility (isometric force) in two established transgenic murine models of EoE (CD2-IL-5 and rtTA-CC10-IL-13) and a novel eosinophil-deficient model (ΔdblGATA/CD2-IL-5). Herein, we show the following: 1) CD2-IL-5 and doxycycline (DOX)-induced rtTA-CC10-IL-13 mice have chronic eosinophilic and mast cell esophageal inflammation; 2) eosinophilic esophageal inflammation promotes esophageal stricture in both transgenic murine models; 3) the eosinophil-deficient ΔdblGATA/CD-2-IL-5 mice were protected from the induction of stricture, whereas the eosinophil-competent CD2-IL-5 mice develop esophageal stricture; 4) esophageal stricture is not reversible in DOX-induced rtTA-CC10-IL-13 mice (8 wk DOX followed by 8 wk no-DOX); and 5) IL-5 transgene-induced (CD2-IL-5) EoE evidences esophageal dysmotility (relaxation and contraction) that is independent of the eosinophilic esophageal inflammation: CD2-IL-5 and ΔdblGATA/CD2-IL-5 mice have comparable esophageal dysmotility. Collectively, our present study directly implicates chronic eosinophilic inflammation in the development of the esophageal structural impairments of experimental EoE.
Keywords: eosinophils, esophagus, interleukin, mast cells, motility, stricture
eosinophilic esophagitis (EoE) is an emerging chronic disease characterized by esophageal mucosal eosinophilia that exhibits a lack of response to acid repression (4, 32) and whose prevalence is rising throughout the world (18). Patients with EoE typically suffer from dysphagia, food impaction (29, 37, 39), and esophageal dysmotility and stricture (29, 37, 39); however, the etiology of the esophageal pathology and dysfunction in EoE is unclear. Radiographic and endoscopic studies of patients with EoE have shown many structural pathologies, including small-caliber esophagi, strictures, mucosal rings, ulcerations, whitish papules, and polyps (3, 14, 15, 34, 40, 42). Esophageal stricture is an abnormal narrowing of the esophagus and may be associated with esophageal rings, mucosal inflammation, or tissue remodeling, such as collagen deposition and esophageal fibrosis (13, 14, 25, 34, 42). Diseases that evidence esophageal stricture can be grouped into two categories: 1) intrinsic diseases that narrow the esophageal lumen through inflammation, fibrosis, and neoplasia; 2) extrinsic diseases that compromise the esophageal lumen by direct invasion, such as lymph node enlargement or neoplasia. We and others (23, 43) have previously established murine models of EoE in which transgenic IL-5 (CD-IL-5) or IL-13 (rtTA-CC10-IL-13) overexpression promotes esophageal eosinophilia, mastocytosis, and tissue remodeling. Other studies have shown that eosinophil and mast cell activation and degranulation promote tissue damage, fibrosis, epithelial cell hyperplasia (14, 19, 34), and notably, muscle cell dysfunction (1, 11, 38). We therefore hypothesize that the chronic esophageal inflammation of EoE causes esophageal stricture and dysmotility. To dissect the role of eosinophils in the development of esophageal stricture and dysmotility in EoE, we bred a novel model of mice (ΔdblGATA/CD2-IL-5) that is both deficient in the eosinophil lineages (ΔdblGATA) (41) and overexpresses transgenic IL-5 under the control of the T cell-specific CD2 promoter (CD2-IL-5). The previously established CD2-IL-5 and rtTA-CC10-IL-13 murine models of EoE overexpress transgenic IL-5 and IL-13, respectively, and have high levels of esophageal eosinophilia (19, 20, 43). Herein, we provide the evidence that chronic esophageal inflammation of IL-5- and IL-13-induced EoE has a critical role in development of esophageal stricture.
MATERIALS AND METHODS
Mice.
The wild-type (Balb/c), CD2-IL-5 transgenic (20), rtTA-CC10-IL-13 transgenic (43), and ΔdblGATA/CD2-IL-5 transgenic mice (developed in our laboratory) were used to understand the development of esophageal functional abnormalities in experimental EoE. All mice were maintained in a barrier facility, and the animals were handled under Institutional Animal Care and Use Committee-approved protocols. There was no serologic evidence of pathogens in sentinel mice maintained with the colony.
Generation of GATA-1 gene-deficient (ΔdblGATA), IL-5 transgenic mice.
The CD2-IL-5 transgenic mice were crossed with ΔdblGATA mice, and their F1 generation was further mated to generate ΔdblGATA/CD2-IL-5 transgenic mice. The F2 progeny were screened for the IL-5 transgene and ΔdblGATA. The generated ΔdblGATA/CD2-IL-5 transgenic mice are composed of a mixed background; therefore, experiments were performed with age- and gender-matched littermate controls.
IL-5 and IL-13 protein analysis in the esophagus.
Cytokine protein levels of esophageal homogenate were determined using a DuoSet ELISA set (R&D Systems, Minneapolis, MN) as per the manufacturer's protocol. Briefly, the homogenate was applied to cytokine-specific monoclonal antibody-precoated, 96-well ELISA plates after blocking nonspecific protein binding with 10% FBS. The plates were incubated for 2 h at room temperature and washed with PBS-0.05% Tween-20. Biotinylated cytokine-specific monoclonal antibody was applied to each well followed by streptavidin-horseradish peroxidase conjugate reagent. Finally, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (BD Biosciences Pharmingen) was added to each well, and the color was developed in the dark at room temperature. The reaction was stopped using 2N-H2SO4; the optical density was immediately read at 450 nm. The cytokine concentration of each sample was calculated by using a standard curve.
Analysis of esophageal eosinophils and mast cells in the murine models of EoE.
The 5-μm esophageal paraffin tissue sections of wild-type, CD2-IL-5 transgenic, and ΔdblGATA/CD2-IL-5 transgenic mice were immunostained with antiserum against mouse eosinophil major basic protein (anti-MBP) as described (17, 21). In brief, endogenous peroxide in the tissue was quenched with 0.3% hydrogen peroxide in methanol followed by nonspecific protein blocking with normal goat serum. Tissue sections were then incubated with rat anti-MBP (1:2,000) primary antibody overnight at 4°C, followed by a 1:200 dilution of biotinylated anti-rat IgG secondary antibody and avidin-peroxidase complex (Vector Laboratories, Burlingame, CA) for 30 min each. These slides were further developed with nickel diaminobenzidine-cobalt chloride solution to form a black precipitate and counterstained with hematoxylin. Negative controls included replacing the primary antibody with normal rat serum. Furthermore, the esophageal paraffin tissue sections of mice were deparaffinized, stained with hexazonized new fuchsin (Sigma Aldrich) with 4% sodium nitrate in naphthol-AS-D chloroacetate (Sigma Aldrich), and washed in PBS solution for 30 min, and counterstained with hematoxylin. Nonhexazonized new fuchsin was used as a negative control. Eosinophils and mast cells were quantified by counting the anti-MBP-positive immunostained cells and chloroacetate estrase-stained cells in the epithelial mucosa and lamina propria; combined cell numbers in each esophageal tissue section were quantified with the assistance of digital morphometry using the Metamorph Imaging System (Universal Imaging, West Chester, PA) (eosinophils/mm2 or mast cells/mm2) as described previously (20, 22).
Examination for esophageal stricture following barium swallowing in murine model of EoE.
5- to 6-mo-old wild-type, CD2-IL-5 transgenic, and ΔdblGATA/CD2-IL-5 transgenic mice were orally given the barium suspension with a 5-ml syringe; this was done slowly to allow for swallowing of the barium suspension. After administration of ∼4–5 ml of suspension, wild-type and transgenic mice were examined by X-ray analysis for presence of esophageal stricture; the complete mouse esophagus and gastrointestinal tract were examined. The barium swallowing technique was validated by performing the technique in different age groups of wild-type and transgenic mice. Our observation indicated that the younger transgenic mice (4–6 wk old) do not show esophageal stricture; stricture is only detected in the older mice. This indicates that stricture is progressive in chronic esophageal inflammation.
Analysis of the contractility of the esophageal circular and longitudinal muscle layer in experimental EoE.
Contractility of esophageal circular and longitudinal muscles in CD2-IL-5 transgenic mice, along with respective wild-type mice, was examined as per the protocol developed by us in Dr. Richard Paul's laboratory in the University of Cincinnati College of Medicine's Division of Physiology. In brief, the mouse's esophagus was removed, and a circular, 1-mm esophageal segment was taken for studies of contractile function in circular muscle. The rest of the esophagus was used for testing contractility of the longitudinal muscles. From the time of the dissection, the esophagus was maintained in physiological salt solution (PSS). PSS contained (mmol/l) 118 NaCl, 4.73 KCl, 1.2 MgSO4, 0.026 EDTA, 1.2 NaH2PO4, 2.5 CaCl2, 5.5 glucose, was buffered with 25 NaHCO3, and had a pH of 7.4 when bubbled with 95% O2/5% CO2 at 37°C. The esophageal ring and longitudinal segment were attached to the Differential Capacitor Force Transducer (Harvard Apparatus, Holliston, MA) so that isometric force in the circumferential and longitudinal directions could be measured. Experiments were conducted at optimal tension for force production, which was established by adjusting the length of the esophagi to a point where maximum isometric contractions were observed. Basal measurements were taken after 1 h of equilibration following exposure of the esophagi to at least two stimulation/relaxation cycles with 15 mM KCl to insure reproducible contractions. Concentration-isometric force relations were generated in response to cumulative additions of carbamylcholine (carbachol, 1 nM to 10 μM) at 5-min intervals. Concentration-relaxation relations were undertaken at 80% of the maximum isometric force as determined from the concentration-force relation. This is to avoid saturated concentrations of the agonist and to compare all esophagi at the same level of activation. Relaxation was measured at 5-min intervals with cumulative addition of isoproterenol (1 nM to 10 μM) . Measurements were sampled at 100 Hz using a model MP100 Data Acquisition System (Biopac Systems, Goleta, CA). Data analysis was completed using the Biopac AcqKnowledge (Biopac Systems) software program. Force was normalized by dividing by the cross-section area, which was estimated as wet weight/length for longitudinal preparations or 2*(wet weight/circumference) for rings. Concentration-response relations were fitted with a logistic function using Origin software, and differences in parameters of transgenic and wild-type mice were analyzed by t-test. Data were expressed as means ± standard deviation (SD).
Analysis of the relaxation of the esophageal circular and longitudinal muscle layer in experimental EoE.
Relaxation of esophageal circular and longitudinal muscles following IL-5 transgene-induced esophageal inflammation was examined in the mice. Circular and longitudinal muscle relaxation was examined following a similar experimental protocol as the one described for the contractility experiment. Briefly, the basal measurement was sampled for 100 s following exposure of the esophageal segments to two cycles of stimulation with 15 mM KCl. The peak contraction force response was generated in response to CCh (1 μM), and the subsequent concentration-dependent relaxation of muscles was generated by using cumulative addition of isoproterenol (1 nM to 1 μM) to determine the relaxation force of the circular and longitudinal muscle layers of control and inflamed esophagi. The data were analyzed using the companion Biopac Acqknowledge software.
Statistical analysis.
The nonparametric Mann-Whitney U-test was employed for comparison of data between two groups, and the Kruskal-Wallis test was used for comparison of more than two groups. The concentration-isometric force and -relaxation data was fitted to a logistic equation using Origen software. Differences in the maximum forces and ED50 values were tested using Student's t-test. Parametric data were compared using t-tests or ANOVA. Values are reported as means ± SD or means ± SE. P values < 0.05 were considered statistically significant.
RESULTS
CD2-IL-5 and rtTA-CC10-IL-13 transgenic mice show induced levels of esophageal eosinophils and mast cells.
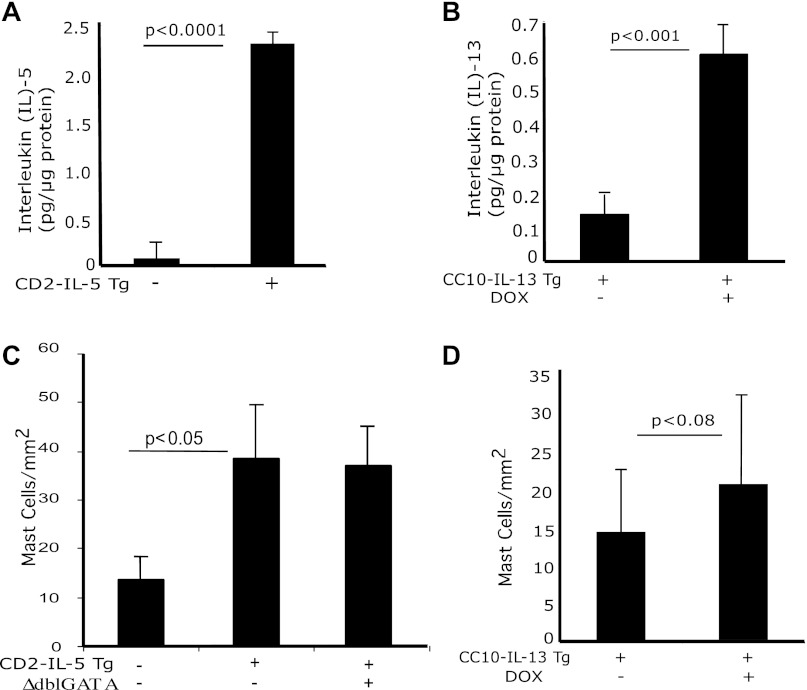
We hypothesized that transgenic IL-5- or IL-13-induced chronic esophageal eosinophil and mast cell inflammation in mice lead to the development of esophageal stricture and peristaltic dysfunction. Accordingly, we first analyzed the IL-5 and IL-13 protein and eosinophil levels in the esophagus of wild-type, CD2-IL-5, no-doxycycline (no-DOX) and DOX-exposed rtTA-CC10-IL-13 transgenic mice. We observed an approximately tenfold increase in IL-5 protein (Fig. 1A) in the esophagus of the CD2-IL-5 mice compared with wild-type mice and an approximately sixfold increase in IL-13 protein in DOX-induced rtTA-CC10-IL-13 mice compared with no-DOX mice, respectively (Fig. 1B). The number of eosinophils observed in the esophagus of wild-type and CD2-IL-5 mice was 1.92 ± 1.9/mm2 and 121.3 ± 21.4 (means ± SD), respectively, and of 6-wk no-DOX and DOX-exposed rtTA-CC-10-IL-13 mice was 2.1 ± 1.8/mm2 and 76.3 ± 19.2/mm2 (means ± SD), respectively. Interestingly, no esophageal eosinophilia was detected in ΔdblGATA/CD2-IL-5 mice. Furthermore, we also examined mast cell numbers in wild-type, CD2-IL-5, no-DOX and DOX-exposed rtTA-CC10-IL-13, and ΔdblGATA/CD2-IL-5 mice. We observed an approximately twofold increase in mast cell number in CD2-IL-5 and ΔdblGATA/CD2-IL-5 mice compared with wild-type mice (Fig. 1C), indicating that the IL-5-induced increase in mast cells is independent of eosinophilia as the number of mast cells in CD2-IL-5 (has eosinophilia) and ΔdblGATA/CD2-IL-5 (lacks eosinophilia) was comparable. Of note, the number of mast cells in no-DOX and DOX-exposed rtTA-CC10-IL-13 mice was also found comparable (Fig. 1D), supporting that the increase in esophageal mast cells in these EoE murine models is independent of the presence of esophageal eosinophilia.
Fig. 1.
IL-5, IL-13, and mast cell induction in the esophagus. Protein levels of IL-5 and IL-13 from esophageal tissue of wild-type (WT), CD2-IL-5, and uninduced and doxycycline (DOX)-induced rtTA-CC10-IL-13 mice were measured by performing ELISA. Levels of IL-5 in 12-wk-old WT and in CD2-IL-5 mice and of IL-13 in 8-wk-old WT and in rtTA-CC-10-IL-13 mice are shown (A and B). The level of mast cells in the esophagus of WT, CD2-IL-5, ΔdblGATA/CD2-IL-5, and DOX- and no-DOX-treated rtTA-CC10-IL-13 mice were quantified by performing morphometric analysis following chloroacetate tissue staining (C and D). The results are the summary of 3 independent experiments, reported as means ± SE with n = 6 mice for each group. The statistical P values are provided in each figure.
Esophageal stricture develops in transgenic IL-5-induced chronic inflammation.
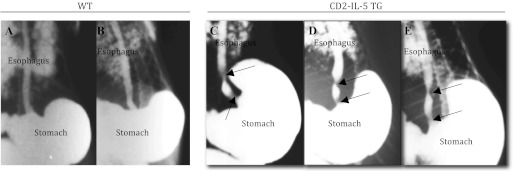
Esophageal stricture is reported in a number of esophageal diseases including EoE (33, 40). Therefore, we tested the hypothesis that chronic eosinophilic and mast cell inflammation in the esophagus leads to esophageal stricture. To detect the presence or absence of esophageal stricture, barium-swallowing experiments were performed in 5- to 6-mo-old wild-type and CD2-IL-5 mice. Esophageal stricture was present in all examined CD2-IL-5 mice. Notably, no esophageal stricture was observed in any of the age- and sex-matched wild-type mice. The X-ray photographs of the barium-filled esophagi of wild-type mice (Fig. 2, A and B) and CD2-IL-5 mice (Fig. 2, C–E) are shown. In addition, we performed morphometric analysis in formalin-fixed, hematoxylin and eosin-stained cross-sections of these same wild-type and CD2-IL-5 mice to measure for lumen narrowing. We observed a reduction in lumen area in the esophagi of CD2-IL-5 mice compared with wild-type mice, with lumens measuring 4.8 ± 0.9 × 104 μm2 in wild-type mice compared with 2.6 ± 0.7 × 104 μm2 in CD2-IL-5 mice (means ± SD, n = 6, P < 0.01).
Fig. 2.
Esophageal stricture in IL-5-induced esophageal inflammation. The WT and CD2-IL-5 mice were given 10% barium sulfate solution orally via a 5-ml syringe. Mice were allowed to slowly swallow ∼3–4 ml of the solution and then anesthetized with isoflurane. X-ray analysis was performed on the whole body. Radiological barium-swallowing experiments indicated esophageal stricture in CD2-IL-5 mice develops in response to IL-5-induced chronic inflammation in the esophagus. Representative radiophotographs of WT (A and B) and CD2-IL-5 (C–E) mice are shown. Esophageal stricture is marked by the arrows for the CD2-IL-5 mice; no stricture is visible in WT mice. For WT and CD2-IL5 mice, n = 8–10/group. TG, transgenic.
Esophageal stricture does not develop in eosinophil-deficient CD2-IL-5 mice.
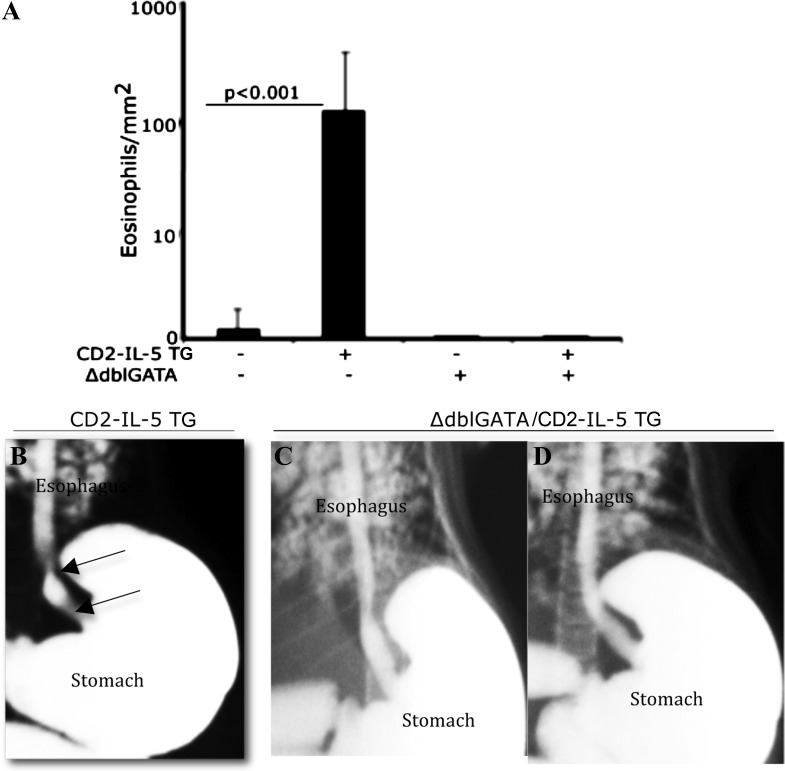
To test whether the development of esophageal stricture was dependent on transgenic IL-5-induced esophageal eosinophilia, we generated ΔdblGATA/CD2-IL-5 transgenic mice in our laboratory by cross-breeding CD2-IL-5 mice with ΔdblGATA mice. The 5- to 6-mo-old ΔdblGATA/CD2-IL-5 mice lacked the esophageal eosinophilia (Fig. 3A) and esophageal stricture that was observed in the CD2-IL-5 mice (Fig. 3, B–D). The lumen area in the esophagus of 5- to 6-mo-old ΔdblGATA/CD2-IL-5 mice was greater than the lumen area of CD2-IL-5 mice. The lumens measuring 5.1 ± 1.2 × 104 μm2 in ΔdblGATA/CD2-IL-5 mice compared with 3.1 ± 1.2 × 104 μm2 in CD2-IL-5 mice (means ± SD, n = 6, P < 0.01).
Fig. 3.
GATA-1 deficiency in CD2 promoter-driven IL-5 transgenic (TG) mice prevents esophageal eosinophilia and stricture. Esophageal eosinophils in wild-type (WT) and CD2-IL-5 (IL-5 TG), ΔdblGATA, and ΔdblGATA/CD2-IL-5 TG mice were examined. The morphometric analysis indicated an increase in the number of esophageal eosinophils in CD2-IL-5 mice compared with WT, ΔdblGATA (no IL-5 transgene), and ΔdblGATA/CD2-IL-5 mice (A). Both ΔdblGATA and ΔdblGATA/CD2-IL-5 showed no esophageal eosinophils. Furthermore, barium-swallowing experiments showed that ΔdblGATA/CD2-IL-5 TG mice are protected from the development of esophageal stricture. Representative X-ray radiophotographs of CD-IL-5 (B, esophageal stricture is marked by the arrows) and ΔdblGATA/CD2-IL-5 (C and D) mice are shown. Data are expressed as means ± SE, n = 10 mice/group.
Esophageal stricture is not reversible in experimental EoE.
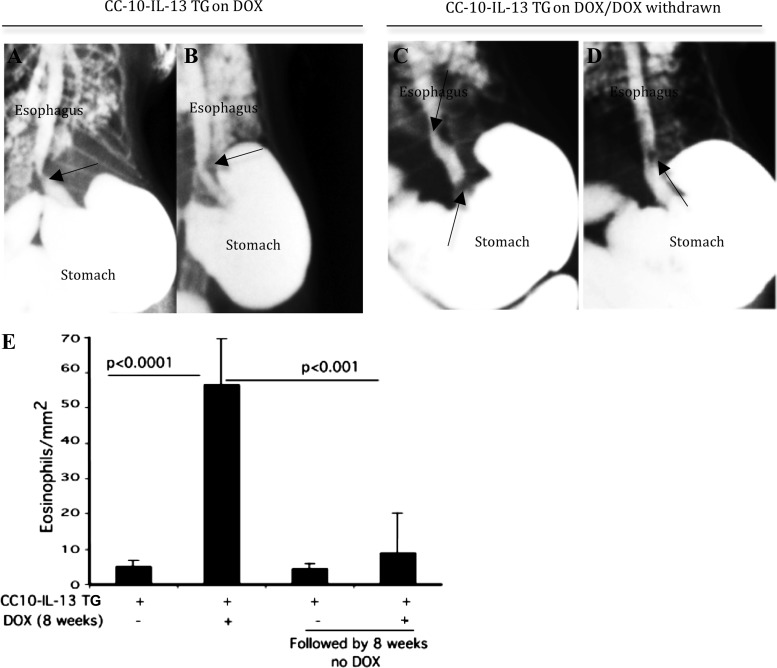
To determine whether esophageal stricture is reversible following reduced eosinophilic inflammation, we employed a DOX-regulated transgenic IL-13-induced murine model of EoE, rtTA-CC10-IL-13. As previously reported, these mice develop esophageal fibrosis after DOX food exposure (43). rtTA-CC10-IL-13 mice examined for esophageal stricture and eosinophilia after 8 wk of DOX food evidenced esophageal stricture and eosinophilia (Fig. 4, A, B, and E). rtTA-CC10-IL-13 mice given DOX food for 8 wk and subsequently switched to a regimen of no-DOX for another 8 wk showed comparable esophageal stricture (Fig. 4, C and D), but significantly reduced esophageal eosinophilia (Fig. 4E), compared with the rtTA-CC10-IL-13 mice assessed immediately after 8 wk of DOX food.
Fig. 4.
Esophageal stricture is irreversible even after reducing the eosinophilic inflammation in mice. Esophageal eosinophilic inflammation was induced in DOX-regulated IL-13 TG mice (rtTA-CC10-IL-13) following an 8-wk regimen of DOX food. One group of mice was examined for the development of esophageal stricture after the 8-wk DOX food regimen (representative radiophotographs; A and B). The second group of mice was switched to normal (no-DOX) food for 8 wk and then examined for stricture (representative radiophotographs; C and D). Both groups of mice showed esophageal stricture (arrows). A high degree of esophageal eosinophilia is shown in the 8-wk DOX-exposed group of rtTA-CC10-IL-13 mice compared with the significantly reduced esophageal eosinophilia in the DOX withdrawn (after 8 wk) group of rtTA-CC10-IL-13 mice (E). The 16-wk rTA-CC10-IL-13 mice with no-DOX food show a baseline eosinophilia that is not statistically different from that of the 8 wk on DOX followed by 8 wk no-DOX food given rTA-CC10-IL-13 mice (E). Data are shown as means ± SD, n = 8 mice/group.
IL-5-induced EoE has impaired longitudinal esophageal contractility.
It has been shown that expression of the IL-5 transgene in the esophagus promotes fibrotic responses in the esophagus (23). Therefore, we investigated whether the esophageal remodeling induced by the chronic esophageal inflammation affects esophageal motility. We examined the esophageal circular and longitudinal muscle contraction activity of CD2-IL-5 mice in response to different concentrations of carbachol, which induces contraction of smooth muscle. Our analysis indicated that the esophagus of CD2-IL-5 mice developed more force in response to increased carbachol concentration compared with wild-type mice (Fig. 5, A–B). The concentration-force relation for the longitudinal muscle is shown in Fig. 5B. The maximum CD2-IL-5 isometric force of 5.45 ± 0.15 mN/mm2 was significantly (P < 0.005) greater than that of the wild-type, 3.90 ± 0.37; however, the sensitivity in response to carbachol was not statistically significant (CD2-IL-5 = 0.40 ± 0.04 μM; wild-type = 0.60 ± 0.20 μM). Esophageal circular muscle contractility in response to increased carbachol concentration was qualitatively similar to the longitudinal preparation. These data indicate that the chronic esophageal inflammation of CD2-IL-5 mice affects the contractility of the esophageal smooth muscles.
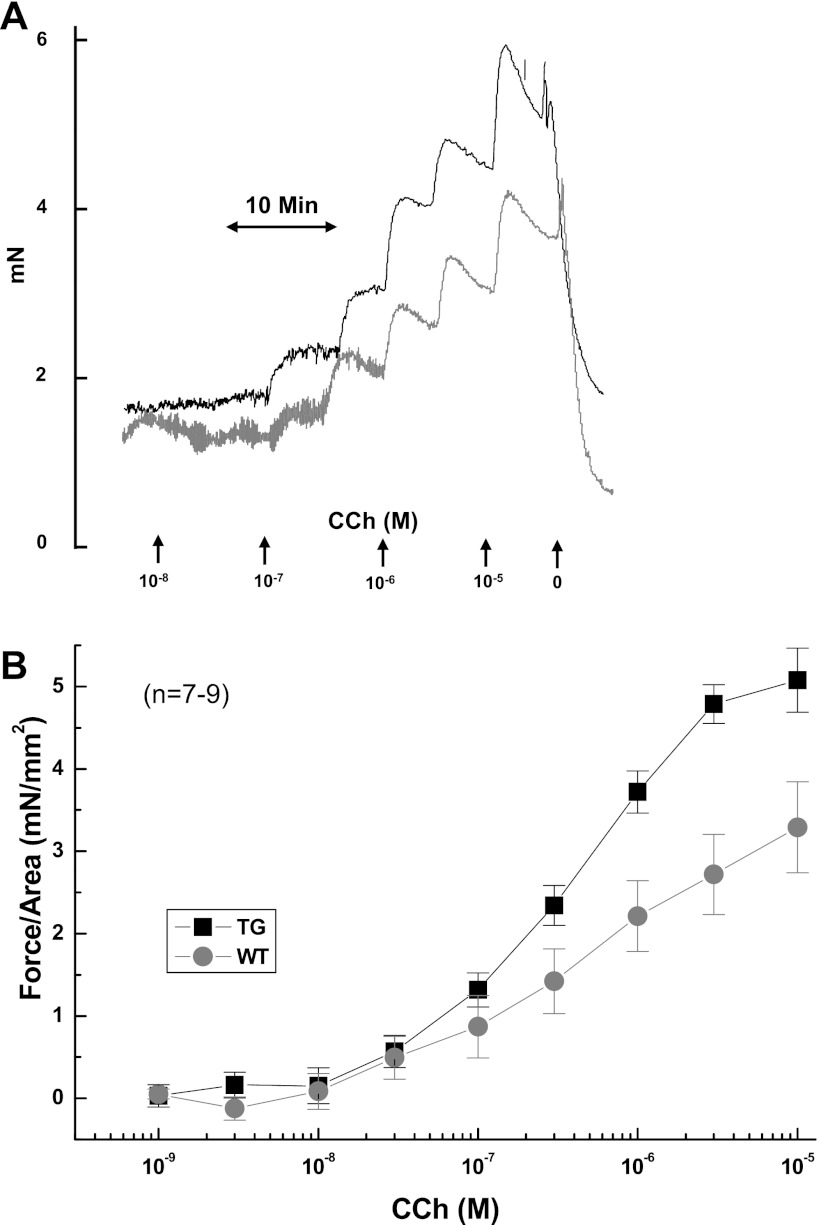
Fig. 5.
Carbachol (CCh)-induced esophageal muscle contraction impairment in a murine model of eosinophilic esophagitis. A representative kinetic tracing of the dose-dependent contraction of esophageal muscles from wild-type (WT) (gray line) and CD2-IL-5 (TG; black line) mice in response to increasing concentrations of carbachol is shown (A). The concentration-dependent, carbachol-induced esophageal muscle contraction of esophageal longitudinal muscles from WT (gray circles with line) and CD2-IL-5 (TG, black squares with line) mice was measured by adding increasing concentrations of carbachol, as indicated in the figure (B). Data are given as means ± SE, n = 6 mice/group, P < 0.001.
IL-5-induced EoE has altered esophageal muscle relaxation.
We examined the effects of chronic inflammation in relaxation of esophageal smooth muscle. Longitudinal preparations of esophageal muscles were precontracted with carbachol to achieve 80% of the maximal response. The relaxation of isometric force in esophageal smooth muscle from wild-type and CD2-IL-5 mice was measured in response to cumulative additions of isoproterenol. Both CD2-IL-5 and wild-type muscles responded in a concentration-dependent manner and were completely relaxed in response to 10−6 M isoproterenol (Fig. 6A). The concentration dependence of the isoproterenol-induced relaxation of smooth muscles from the CD2-IL-5 and wild-type mice is shown in Fig. 6B. In Fig. 6B, the initial force of the IL-5 transgenic mice was greater than the wild-type mice, consistent with Fig. 5B. The sensitivity of the CD2-IL-5 mice was lower, as expressed in the ED50 of the response (CD2-IL-5 = 1.68 × 10−8 ± 0.11 × 10−8 M vs. wild-type = 0.94 × 10−8 ± 0.06 × 10−8 M; P <0.001). These data indicate that chronic inflammation decreases the isoproterenol sensitivity of CD2-IL-5 mice compared with wild-type mice. Relaxation of the esophageal circular muscle of CD2-IL-5 mice was also more with isoproterenol than that of wild-type mice (data not shown).
Fig. 6.
Isoproterenol (ISO)-induced esophageal muscle relaxation impairment in a murine model of eosinophilic esophagitis. A representative kinetic tracing of the dose-dependent relaxation of carbachol-precontracted (105 M) esophageal smooth muscles from WT (gray line) and CD2-IL-5 (TG; black line) mice in response to increasing concentrations of isoproterenol is shown (A). The isoproterenol-induced relaxation of carbachol-precontracted esophageal muscles from WT (gray circles with line) and CD2-IL-5 (TG, black squares with line) mice was measured by adding increasing concentrations of isoproterenol, as indicated in the figure (B). Data are given as means ± SE, n = 6 mice/group, P < 0.001.
Esophageal motility dysfunction is independent of eosinophilic inflammation.
To determine whether esophageal muscle contractility and relaxation is dependent on transgenic IL-5-induced esophageal eosinophilic inflammation, we examined carbachol-induced esophageal muscle contractility and isoproterenol-induced relaxation of carbachol-precontracted muscles in wild-type, CD2-IL-5, and ΔdblGATA/CD2-IL-5 mice. Notably, both CD2-IL-5 and ΔdblGATA/CD2-IL-5 mice developed more force in response to carbachol and were more sensitive to isoproterenol-induced relaxation than wild-type mice (Fig. 7, A and B). Although the ΔdblGATA/CD2-IL-5 transgenic mice lacked eosinophilic esophageal inflammation, the alterations in carbachol-induced esophageal longitudinal muscle contractility and isoproterenol-induced relaxation and in sensitivity to carbachol and isoproterenol were comparable to those of CD2-IL-5 mice. Notably, we also observed comparable carbachol-induced esophageal muscle contractility and isoproterenol-induced relaxation between uninduced (no esophageal eosinophilia) and induced (6-wk DOX; high degree of eosinophilia) rtTA-CC10-IL-13 mice (data not shown), indicating independence of esophageal motility dysfunction from eosinophilic inflammation in two transgenic EoE models.
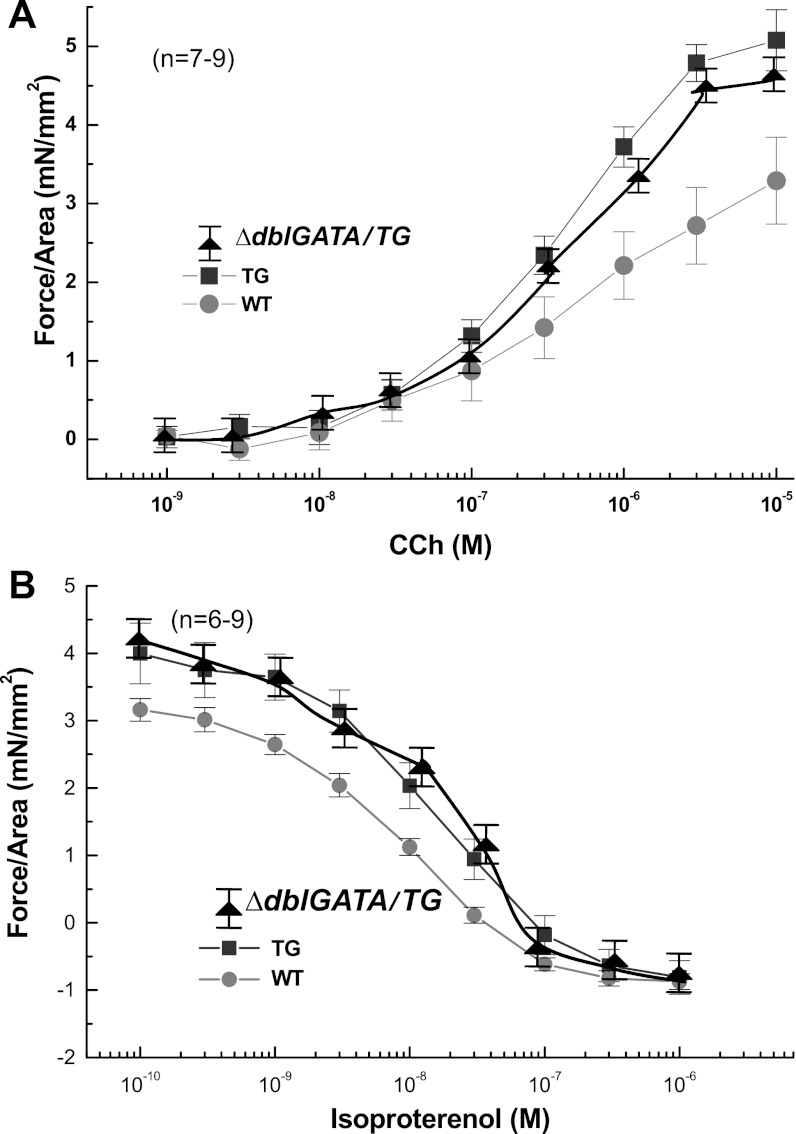
Fig. 7.
Esophageal motility impairment is independent of eosinophilic inflammation. The concentration-dependent, carbachol-induced contraction of esophageal longitudinal muscle and isoproterenol-induced relaxation of carbachol-precontracted (105 M) esophageal longitudinal muscle contraction was measured in WT, CD2-IL-5, and ΔdblGATA/CD2-IL-5 mice by adding increasing concentrations of carbachol or isoproterenol, as indicated (A and B). Esophageal muscle contraction and relaxation in CD2-IL-5 mice is impaired compared with WT mice; comparable esophageal motility impairment is shown between CD2-IL-5 TG and ΔdblGATA/CD2-IL-5 TG mice (A and B). Data are given as means ± SE, n = 6 mice/group, P < 0.001.
DISCUSSION
EoE is a chronic inflammatory disease associated with marked eosinophil and mast cell accumulation in the esophageal mucosa (24, 30, 31). Previous reports indicate that macrophages, lymphocytes, and granulocytes possess the ability to stimulate fibrogenesis (6, 7, 16, 27, 35), and we recently reported esophageal remodeling in experimental and human EoE (23). Several clinical reports also indicate that esophageal dysmotility and stricture develop in patients in a variety of esophageal disorders including EoE (3, 14, 15, 34, 40, 42). Herein, we show that esophageal stricture develops in chronic EoE and is dependent on eosinophilic inflammation; however, esophageal motility dysfunction is independent of esophageal eosinophilic inflammation in experimental EoE.
We show that esophageal stricture is associated with a large influx of eosinophils in the esophagus in murine models of EoE. We have previously shown that IL-5 and IL-13 have central roles in regulating esophageal eosinophilia (19, 20); therefore, we first examined CD2-IL-5 and rtTA-CC10-IL-13 mice for the development of esophageal stricture. We show that both CD2-IL-5 transgenic and DOX-treated rtTA-CC10-IL-13 mice induce esophageal IL-5 and IL-13. We also show that CD2-IL-5 transgenic mice have an increased number of mast cells in the esophagus that is independent of the recruitment of eosinophils. The eosinophil-deficient ΔdblGATA/CD2-IL-5 transgenic mice show a comparable number of mast cells with the CD2-IL-5 transgenic mice, indicating that mast cells may not have an important role in promoting esophageal stricture and that IL-5- and IL-13-mediated eosinophilia is only responsible for promoting esophageal stricture in mice. Eosinophil granules contain a crystalloid core composed of MBP-1 and MBP-2 and a matrix composed of eosinophil cationic protein, eosinophil-derived neurotoxin, and eosinophil peroxidase (5). These toxic mediators are capable of promoting scarring in the tissue and accelerating the remodeling process. We earlier showed that esophageal remodeling develops in these transgenic mice (23). Additionally, the data obtained from the eosinophil-deficient ΔdblGATA/CD2-IL-5 mice support our theory that chronic esophageal eosinophilic inflammation is needed to promote esophageal stricture. Of note, the ΔdblGATA/CD2-IL-5 mice do not develop stricture in the esophagus. Additionally, we show that CD2-IL-5 transgenic and ΔdblGATA/CD2-IL-5 mice have a comparable number of mast cells; therefore, it may be possible that mast cells may not have a role in the development of esophageal stricture. Furthermore, to determine whether esophageal stricture is a reversible process following reduction of esophageal eosinophilic inflammation, we used DOX-inducible IL-13 overexpression mice (rtTA-CC10-IL-13). Our data revealed that 8 wk of DOX exposure promotes IL-13-induced esophageal eosinophilia and esophageal stricture but that the esophageal stricture is not reversed following an 8-wk cessation of DOX food even though the eosinophilia diminishes within this time period. Taken together, these data denote that eosinophils and their mediators have a significant role in inducing stricture; however, these results also emphasize that once the stricture has developed, it is not completely reversible even if esophageal eosinophilia is reduced in the mice. Furthermore, isometric force in the CD2-IL-5 mice was greater than that in the wild-type mice. Since these force values were normalized to a calculated cross-sectional area, this may suggest an increase in force-generating capacity due to increasing cellularity or hypertrophy within the esophageal wall. Relaxation to isoproterenol, on the other hand, was less sensitive in CD2-IL-5 mice. Potentially, both could be responses to inflammation. At this stage, speculation is premature, as there is little data in the literature to this effect.
Furthermore, our data indicate that chronic esophageal inflammation induced by local IL-5 expression in the esophagus leads to altered esophageal motility in the CD2-IL-5 transgenic mice. It has been shown that the eosinophilic protein MBP directly increases smooth muscle reactivity by causing dysfunction of vagal muscarinic M2 receptors (10). In addition, MBP also triggers degranulation of mast cells and basophils (26). The clinical evidence is emerging that esophageal mast cell numbers increase in human EoE and possibly are also involved in the pathogenesis of the disease (2, 8, 36). These mast cells also contain a number of toxic proteins that may alter the esophageal pathophysiology, including esophageal motility dysfunction. Notably, our present data indicate that motility dysfunction is not dependent on eosinophilic inflammation, as carbachol-induced esophageal muscle contractile and isoproterenol-induced relaxation properties were comparable between CD2-IL-5 and ΔdblGATA/CD2-IL-5 transgenic mice. Therefore, the possibility is that mast cells may be involved in the development of esophageal motility dysfunction in experimental EoE. The comparable mast cell induction in the esophagus of CD2-IL-5 and ΔdblGATA/CD2-IL-5 transgenic mice support the possible role of mast cells in the development of motility dysfunction in the esophagus.
In conclusion, we show that esophageal stricture develops in EoE and is dependent on chronic eosinophilic inflammation. Second, IL-13 transgene-induced stricture is an irreversible process. Third, we show that both local IL-5- and IL-13-induced inflammation promote longitudinal esophageal muscle motility dysfunction that is independent of eosinophilic inflammation but that may be dependent on mast cells, as these cells are also induced in EoE and possess highly toxic granules. Taken together, these studies are the first to directly implicate eosinophils in promoting esophageal stricture in experimental EoE and, thus, will potentially provide critical insight in the development of human esophageal pathophysiological disorders such as EoE.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-DK-067255 (to A. Mishra), R01-AI-080581 (to A. Mishra), and the Digestive Health Center Grant DK-078392.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.M. and R.J.P. performed experiments; P.M., P.R., M.R., R.J.P., and A.M. approved the final version of the manuscript; P.R. analyzed data; P.R. and M.R. prepared figures; M.R. and R.J.P. edited and revised the manuscript; R.J.P. and A.M. drafted the manuscript; A.M. contributed the conception and design of the research; A.M. interpreted results of the experiments.
ACKNOWLEDGMENTS
We acknowledge Dr. Marc Rothenberg, Allergy and Immunology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, for supplying the CD2-IL-5 and rtTA-CC-10-IL-13 transgenic mice for these experiments. The authors also thank Shawna Hottinger for editorial assistance.
REFERENCES
- 1. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 38: 109–116, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa'ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 116: 536–547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis: It's not just kid's stuff. Gastrointest Endosc 56: 260–270, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133: 1342–1363, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 39: 177–253, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med 44: 85–101, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 42: 22–26, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest 91: 1314–1318, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Justinich CJ, Ricci A, Jr, Kalafus DA, Treem WR, Hyams JS, Kreutzer DL. Activated eosinophils in esophagitis in children: a transmission electron microscopic study. J Pediatr Gastroenterol Nutr 25: 194–198, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr 44: 20–26, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Liacouras CA, Markowitz JE. Predictors of early recurrence of benign esophageal strictures: what about eosinophilic esophagitis? Am J Gastroenterol 99: 182–183, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Liacouras CA, Ruchelli E. Eosinophilic esophagitis. Curr Opin Pediatr 16: 560–566, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr 26: 380–385, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Martin LB, Kita H, Leiferman KM, Gleich GJ. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol 109: 207–215, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, Wert SE, Rothenberg ME. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA 95: 6273–6278, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mishra A. Mechanism of eosinophilic esophagitis. Immunol Allergy Clin North Am 29: 29–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107: 83–90, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol 168: 2464–2469, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 103: 1719–1727, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 125: 1419–1427, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology 134: 204–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noel RJ, Putnam PE, Collins MH, Assa'ad AH, Guajardo JR, Jameson SC, Rothenberg ME. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol 2: 568–575, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Nostrant TT. Esophageal dilatation. Dig Dis 13: 337–355, 1995 [DOI] [PubMed] [Google Scholar]
- 26. O'Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med 157: 1981–1991, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol 34: 1534–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 48: 152–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potter JW, Saeian K, Staff D, Massey BT, Komorowski RA, Shaker R, Hogan WJ. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc 59: 355–361, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Rothenberg ME. Eosinophilia. N Engl J Med 338: 1592–1600, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 113: 11–28, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol 108: 891–894, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Ruigomez A, Alberto Garcia Rodriguez L, Wallander MA, Johansson S, Eklund S. Esophageal stricture: incidence, treatment patterns, and recurrence rate. Am J Gastroenterol 101: 2685–2692, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Sant'Anna AM, Rolland S, Fournet JC, Yazbeck S, Drouin E. Eosinophilic esophagitis in children: symptoms, histology and pH probe results. J Pediatr Gastroenterol Nutr 39: 373–377, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 64: 405–430, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol 108: 954–961, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 125: 1660–1669, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Tottrup A, Fredens K, Funch-Jensen P, Aggestrup S, Dahl R. Eosinophil infiltration in primary esophageal achalasia. A possible pathogenic role. Dig Dis Sci 34: 1894–1899, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Vasilopoulos S, Murphy P, Auerbach A, Massey BT, Shaker R, Stewart E, Komorowski RA, Hogan WJ. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc 55: 99–106, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Vasilopoulos S, Shaker R. Defiant dysphagia: small-caliber esophagus and refractory benign esophageal strictures. Curr Gastroenterol Rep 3: 225–230, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 195: 1387–1395, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zimmerman SL, Levine MS, Rubesin SE, Mitre MC, Furth EE, Laufer I, Katzka DA. Idiopathic eosinophilic esophagitis in adults: the ringed esophagus. Radiology 236: 159–165, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, Rothenberg ME. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2-inhibited pathway. J Immunol 185: 660–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]