Abstract
Il-10-deficient mice develop colitis associated with exaggerated Th1/Th17 responses and are a valuable model of inflammatory bowel disease. Mkp-1 is a major negative regulator of MAPKs, and its expression is enhanced by IL-10. To understand the role of Mkp-1 in the regulation of intestinal mucosal immune responses, we studied the effect of Mkp-1 deletion on the pathogenesis of colitis in Il-10−/− mice. We found that knockout of Mkp-1 on an Il-10−/− background accelerated the development of colitis. Compared with Il-10−/− mice, colitis not only appeared earlier but also was more severe in Il-10−/−/Mkp-1−/− mice. Il-10−/− mice exhibited a mild intestinal inflammation in the specific pathogen-free environment, and rectal prolapse rarely appeared before 6 mo of age. In contrast, the majority of Il-10−/−/Mkp-1−/− mice developed severe colitis rapidly and presented with rectal prolapse after only 2–3 mo. The colon of Il-10−/−/Mkp-1−/− mice showed diffuse transmural chronic inflammation and mucosal hyperplasia, with significantly more proliferating crypt epithelial cells than those of Il-10−/− mice. In addition to the severe colitis, Il-10−/−/Mkp-1−/− mice also developed conjunctivitis and blepharitis. The colon of Il-10−/−/Mkp-1−/− mice contained significantly higher levels of proinflammatory cytokines and exhibited greater MAPK activities than did the colon of Il-10−/− mice. Splenocytes and lymphocytes from Il-10−/−/Mkp-1−/− mice produced higher levels of Th1 cytokines ex vivo upon activation than did cells from Il-10−/− mice. Our studies support a pivotal role of Mkp-1 as a negative regulator of mucosal immune responses and highlight its protective function against inflammatory bowel disease.
Keywords: inflammation, prolapse, inflammatory bowel disease
inflammatory bowel disease (IBD) is characterized by chronic intestinal inflammation (35). The two major types of IBD are ulcerative colitis (UC), and Crohn's disease (CD). These two diseases differ in their location, pattern of distribution, depth of involvement within the intestinal mucosa, and histological lesions. Although the etiology of IBD is still incompletely understood, studies have suggested that IBD appears to result from a dysregulated immune response to luminal antigens (35, 40, 56). Genome-wide association studies have defined over 70 distinct susceptibility loci for CD (4, 20), including NOD2, an intracellular sensor for muramyl dipeptide, a component of all bacteria (31, 32, 37, 49). Although NOD2 mutations confer risk, they are neither required nor sufficient for developing CD. Remarkable progress has also been made to identify susceptibility loci for UC (35, 40). Two recent genome-wide association studies have identified additional UC risk loci, increasing the number of UC susceptibility loci to nearly 50 (1, 43). Highlighting the commonality between UC and CD, at least 28 risk loci were shared by both diseases (1). Since many genes associated with increased IBD risk encode proteins regulating the immune response, it has been hypothesized that IBD is caused by inappropriate activation of mucosal immunity toward dietary factors and/or intestinal microflora in individuals with a genetic predisposition (35, 40, 50, 56). In addition to the intestinal tract, IBD also presents extraintestinal manifestations affecting the joints, skin, eyes, and oral cavity (2). IBD is also associated with an increased risk of colon cancer (48). It has been speculated that extraintestinal manifestations are due to the same immunological phenomena underlying the intestinal pathophysiology (2). Current treatment options for IBD include established anti-inflammatory and immunosuppressive therapies (5, 16, 28, 41, 47, 55, 60).
IL-10 is one of the most potent anti-inflammatory cytokines and plays an important role in modulating the immune response (66). Numerous reports have demonstrated that the primary biological function of IL-10 is restraining the inflammatory response. IL-10 is expressed in a variety of cell types including T cells, monocytes/macrophages, and dendritic cells, as well as intestinal epithelial cells (15, 46). IL-10 blocks the secretion of a large number of proinflammatory cytokines. Moreover, IL-10 regulates the differentiation and proliferation of several types of immune cells such as T cells, B cells, natural killer cells, and mast cells (3). The Il-10 knockout mouse is a well-established murine model of IBD (6, 7, 17, 18). When housed in a conventional environment, Il-10 knockout mice spontaneously develop enterocolitis between 7 and 11 wk of age (6, 18). Colitis in Il-10 knockout mice is associated with anemia, weight loss, and increased mortality (17, 18). This wasting syndrome has been correlated with increased TNF-α levels in the spleen and serum of Il-10 knockout mice (17). However, when these mice were housed in a specific pathogen-free (SPF) environment, clinical signs and histological lesions were less severe. It has been demonstrated that IL-10 deficiency is associated with intestinal inflammation and an exaggerated Th-1 and Th-17 response to normal enteric flora characterized by overproduction of IL-12 (17, 64). Initially, it was reported that anti-IL-12 and anti-IFN-γ monoclonal antibodies prevented colitis in Il-10 knockout mice, suggesting that enterocolitis in this model is predominantly a Th-1-mediated disease (17, 58). However, subsequent studies indicate that IFN-γ-producing CD4+ T cells (Th-1 cells) mediate colitis in Il-10−/− mice and IFN-γ itself contributes to the induction phase of the disease in pups but is not necessarily required for sustaining the chronic phase of colitis in adult mice (6, 18, 53). More recently, it has been shown that IL-23, which shares a common p40 subunit with IL-12, is essential in the Il-10−/− mouse model of colitis and promotes chronic intestinal inflammation via IL-17 and IL-6, likely through promoting Th-17 cell differentiation and expansion (68).
Mkp-1 was the first MAPK-selective protein phosphatase discovered that dephosphorylates both phosphotyrosine and phosphothreonine residues on MAPKs (8, 14, 21, 22, 38, 51, 59). Since Mkp-1 deactivates MAPKs and is strongly induced by the mitogenic stimuli and stress that activate MAPKs, Mkp-1 is considered an important feedback control mechanism regulating the MAPKs (38, 59). Several studies conducted using Mkp-1 knockout cells demonstrated that p38 and JNK, but not ERK, are the preferred substrates of Mkp-1 (13, 27, 70). However, these studies do not exclude the possibility that Mkp-1 can also inactivate ERK in response to certain stimuli, particularly when Mkp-1 is expressed in high levels. We have previously shown that Mkp-1 knockout mice are more sensitive to LPS-induced endotoxic shock (70). Macrophages and dendritic cells derived from Mkp-1-deficient mice exhibit a skewed cytokine production profile: Mkp-1−/− cells produce more TNF-α, IL-6, and IL-10 but less IL-12 and IFN-γ than do Mkp-1+/+ cells (26). Hammer et al. have found that IL-10 augments the expression of Mkp-1, an endogenous negative regulator of p38 and JNK (70), thus establishing Mkp-1 as an effector of IL-10 in the anti-inflammatory function.
In this report, we examined the role of Mkp-1 in the context of an Il-10 knockout mouse model of IBD. We found that mice lacking both Mkp-1 and Il-10 (double knockout) are highly susceptible to the development of IBD characterized by inflammation and hyperplasia of the intestinal tract, particularly the colon and rectum, as well as conjunctivitis and blepharitis. We have also shown that immune responses of double knockout mice are skewed toward exaggerated Th-1 and Th-17 responses with excessive production of various proinflammatory cytokines within the large intestines, splenocytes, and lymph node cells. These data provide important insights regarding the interaction between IL-10 and Mkp-1 in this model of colitis and suggest that Mkp-1 serves as an important regulator in the intestinal immune response to luminal antigens. Our findings indicate a novel protective role for Mkp-1 against the development of IBD and may have important clinical implications in the field of IBD.
MATERIALS AND METHODS
Animals.
Cryopreserved embryos of Mkp-1 knockout mice (Mkp-1+/− and Mkp-1−/−) were provided by Bristol-Myers Squibb Pharmaceutical Research Institute (19). These mice were backcrossed to 129 mice for at least 10 generations in the Institute of Toxicology and Genetics. Il-10−/− mice on a 129 genetic background [129(B6)-Il10tm1Cgn/J] housed under SPF conditions were purchased from the Jackson Laboratory (Bar Harbor, ME). Mkp-1−/− mice on the 129 genetic background were crossed with Il-10−/− mice to yield wild-type (Mkp-1+/+/Il-10+/+), Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− (double knockout) mice. All animals were hosted in the Association for Assessment and Accreditation of Laboratory Animal Care-certified SPF facility of the Research Institute at Nationwide Children's Hospital. Animals received care in accordance with National Institutes of Health guidelines. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital.
Genotyping.
Mice were genotyped by PCR using genomic DNA isolated from tails as templates. Wild-type and mutant Mkp-1 alleles were detected in separate reactions. Two primers (5′-ATG GTG ATG GAG GTG GGC ATC CTG-3′ and 5′-CTG GTA GTG ACC CTC AAA GTG G-3′) were used to detect the wild-type allele of Mkp-1. The knockout mutant allele of Mkp-1 was detected by using primers 5′-CCA GGT ACT GTG TCG GTG GTG C-3′ and 5′-AGG TGA GAT GAC AGG AGA TC-3′. The wild-type and knockout mutant alleles of Il-10 were detected in a single reaction using three primers (5′-GTG GGT GCA GTT ATT GTC TTC CCG-3′, 5′-GCC TTC AGT ATA AAA GGG GGA CC-3′, and 5′-CCT GCG TGC AAT CCA TCT TG-3′), according to the recommendation of the Jackson Laboratory.
Reagents.
LPS (Escherichia coli 055:B5) was purchased from Calbiochem (La Jolla, CA). IL-4, IL-5, IL-6, IL-10, IL-12 p70, IL-13, and IFN-γ ELISA kits were purchased from BD Biosciences (San Jose, CA). TNF-α and IL-17A ELISA kits were purchased from eBioscience (San Diego, CA).
Histological scoring of inflammation and IBD in colon sections.
The colon was removed at necropsy from Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. The tissue was flushed with cold 1× PBS and dissected into ascending colon, transverse colon, descending colon, and rectum. These tissue specimens were fixed in 10% formalin overnight and then placed in 1× PBS for 1 day. Eyes and eyelids were also removed at necropsy. These specimens were fixed in 10% formalin for 4 days and then placed in 1× PBS for 1 day. Both intestinal and eye samples were then embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin (H&E) for histological evaluation.
To calculate the inflammation score, H&E-stained sections were blindly scored based on four criteria: inflammation severity, inflammation extent, crypt damage, and percentage of tissue involvement (Table 1). The total inflammation score was calculated by adding the inflammation scores from the ascending, transverse, and descending colons and rectum. The maximum possible inflammation score per animal is 56. The IBD scoring system was based on three parameters: mucosal hyperplasia, goblet cell depletion, and infiltration of lymphoid aggregates (Table 2). Similarly, the scores of all four segments were added together to calculate the total IBD score. The maximum IBD score per animal is 36.
Table 1.
Histological colitis scoring
| Feature Scored | Score | Description |
|---|---|---|
| Inflammation severity | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe | |
| Inflammation extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Crypts lost; surface epithelium present | |
| 4 | Crypts and surface epithelium lost | |
| Percentage of involvement | 0 | 0% |
| 1 | 1–25% | |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 75–100% |
Table 2.
Histological IBD scoring
| Feature Scored | Score | Description |
|---|---|---|
| Mucosal hyperplasia | 0 | Normal |
| 1 | Mild (epithelium twice normal thickness) | |
| 2 | Moderate (epithelium 2–3 times normal thickness) | |
| 3 | Severe (epithelium 4 or more times normal thickness) | |
| Goblet cell depletion | 0 | None (0% goblet cell loss) |
| 1 | Mild (1–35% goblet cell loss) | |
| 2 | Moderate (36–70% goblet cell loss) | |
| 3 | Severe (71–100% goblet cell loss) | |
| Infiltration of lymphoid aggregates | 0 | Normal |
| 1 | Mild (a few focal infiltrates) | |
| 2 | Moderate (Focal to multifocal infiltrates) | |
| 3 | Severe (Multifocal to diffuse infiltrates) |
Isolation and characterization of lymphocytes from mesenteric lymph nodes and colon.
Mesenteric lymph nodes were isolated and dissociated into single-cell suspensions. Intraepithelial lymphocytes in the colon tissues were isolated essentially as previously described (65). Briefly, colons were washed with PBS to remove the luminal contents, opened longitudinally, and cut into 0.5-cm lengths. The segments were incubated in Ca2+- and Mg2+-free HBSS containing 5 mM EDTA and 1 mM DTT for 30 min at 37°C with gentle shaking. Dissociated crypts were allowed to sediment for 15 min and intraepithelial lymphocytes were further purified by Percoll gradient centrifugation. After centrifugation, these cells were washed once with complete medium and finally cultured in RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT).
For intracellular cytokine staining, lymph node lymphocytes and intraepithelial lymphocytes were stimulated for 4 h with Leukocyte Activation Cocktail (BD Pharmingen), which contains PMA, a calcium ionophore (ionomycin), and the protein transport inhibitor BD GolgiPlug (brefeldin A), according to manufacturer's recommendations. Cells were stained for surface antibodies against CD3 and CD4, then fixed and permeabilized by using the Fix/Perm kit (BD Biosciences), and finally stained with IL-17A and IFN-γ antibodies. All antibodies were from eBioscience. Data were acquired on LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star).
ELISA.
Splenocytes and mesenteric lymph node cells were plated into six-well plates at a density of 2×106 cells per well and then stimulated with 100 ng/ml LPS for 24 h. IL-6, IL-12 (p70), IFN-γ, and TNF-α concentrations in the media were assayed by ELISA as previously described (57, 70). The intestine was harvested from 8-wk-old mice and divided into five parts: duodenum, jejunum, ileum, proximal colon, and distal colon. These tissues were homogenized in lysis buffer (10 mM HEPES, pH 7.4, 50 mM β-glycerophosphate, 1% Triton X-100, 10% glycerol, 2 mM EGTA, 1 mM dithiothreitol, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 2 μM leupeptin, 2 μM aprotinin, and 1 mM PMSF). After centrifugation at 16,100 g for 10 min, the supernatants were collected, and protein concentrations in the supernatants were measured via Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). IFN-γ, TNF-α, IL-6, IL-12 (p70), IL-17A, and IL-23 levels in the tissue homogenates were assayed by ELISA and normalized to the corresponding protein concentrations. ELISA for cytokine levels in the cell culture medium was carried out as previously described (12, 57, 70).
Northern blotting and real-time RT-PCR.
Splenocytes and mesenteric lymph node cells isolated from mice were treated with LPS for 24 h and total RNA was collected by use of TRIzol (Invitrogen). Northern blot analysis was carried out by using a mouse Il-12 p35 cDNA probe essentially as described previously (12). The membrane was stripped and reprobed with a Gapdh cDNA to normalize for RNA loading. To perform real-time RT-PCR, the first-strand cDNA of each sample was synthesized by use of a reverse transcription kit (Invitrogen). Quantitative real-time PCR was performed by using an ABI Prism 7900-HT sequence system (Applied Biosystems, Foster City, CA) with the QuantiTect SYBR Green PCR kit (Qiagen, Germantown, MD) in accordance with the manufacturer's instructions. The following primers were used: Il-12 (p40) forward 5′-TTG CTG GTG TCT CCA CTC AT-3, Il-12 (p40) reverse 5′-GGG AGT CCA GTC CAC CTC TA-3′; hypoxanthine-guanine phosphoribosyltransferase (Hprt) forward 5′-AGC CTA AGA TGA GCG CAA GT-3′, Hprt reverse 5′-TTA CTA GGC AGA TGG CCA CA-3′. The Hprt gene was amplified and served as a control. The PCR reactions were performed in triplicate by use of 1 μl of first-strand cDNA product, gene-specific primer pairs, and SYBR Green PCR Master Mix (Invitrogen). The experiments were repeated twice. After SYBR Green PCR amplification, data acquisition and subsequent data analyses were performed using the GeneAmp 5700 sequence detection system (version 1.3). The threshold value of the ΔRn was set at 0.5. The PCR cycle where ΔRn crosses 0.5 is called the threshold cycle (Ct). The expression was normalized to Hprt mRNA levels. The relative induction is calculated by the formula of 2−ΔΔCt.
Western blot analysis.
Western blot analysis was carried out as previously described (11, 57, 69, 70). Rabbit polyclonal antibodies against phospho-p38, phospho-JNK, and phospho-ERK were purchased from Cell Signaling (Danvers, MA). To normalize for protein loading, blots were stripped and probed with a β-actin monoclonal antibody purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunohistochemistry.
Paraffin-embedded tissue sections of descending colons and rectum of Il-10 knockout and double knockout mice were deparaffinized in xylene and rehydrated in graded ethanol. Antigen retrieval was performed by using a 0.01 M citrate buffer at pH 6.0 in a steamer for 30 min. The slides were then processed by using a mouse-to-mouse HRP AEC staining kit from ScyTek (Logan, UT) according to the manufacturer's recommendations. Specifically, sections were incubated overnight with antibodies against phospho-histone H3 (1:200 dilution; Upstate Biotechnology, Waltham, MA), Ki-67 (1:500, BD BioSciences, San Diego, CA), CD3 (1:2,000; BioLegend, San Diego, CA), B220 (1:1,000; BioLegend), and α-occludin (1:250; Santa Cruz Biotechnology). After the reactions with the peroxidase-labeled secondary antibodies, color was developed with 3-amino-9-ethylcarbazole (AEC), and the sections were counterstained with hematoxylin. A positive reaction was indicated by a red color. Sections were scored as previously described (42). Briefly, 20 intact crypts in both the descending colon and rectum were identified for each animal (3 animals per group) and the total number of cells with positive nuclear staining was counted.
Terminal deoxynucleotidyl transferase dUTP-mediated nick end labeling (TUNEL) staining was performed following the manufacturer's instructions (In Situ Cell Death Detection Kit, Roche, Indianapolis, IN) and counterstained with hematoxylin.
Crypt length.
Average crypt length of three animals per group was calculated for Il-10 knockout and double knockout mice as follows: 20 intact crypts were identified from both the colon and rectum, and the length of each crypt was measured from the crypt base basement membrane to the tip of the luminal epithelium by use of Image-Pro Plus (Media Cybernetics, Bethesda, MD).
Statistics.
The results from the experiments assessing the differences in cytokine production and scores between genotypes production were analyzed by Student's t-test. Statistical analysis was carried out by use of the SPSS statistical software program (SPSS, Chicago, IL). Differences were considered significant when P < 0.05.
RESULTS
Mkp-1 deletion accelerates the development of IBD in Il-10 knockout mice.
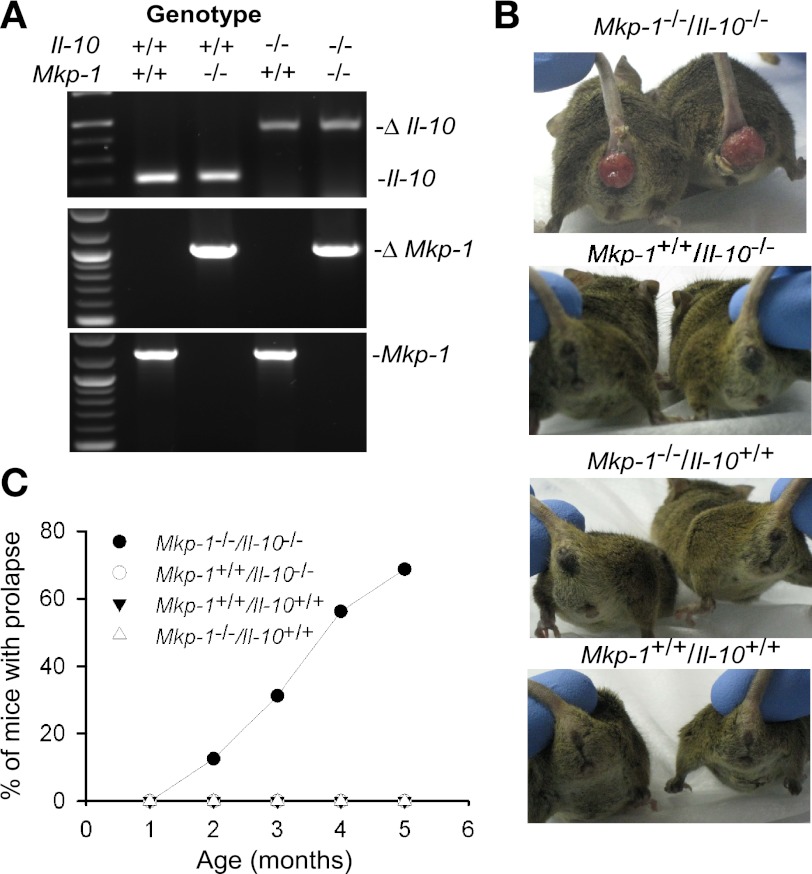
To understand the role of Mkp-1 in the immune system and the interaction between IL-10 and Mkp-1, we crossed Mkp-1−/− mice on a 129 background with Il-10−/− mice that are also on a 129 background to produce Mkp-1+/−/Il-10+/− progenies. These progenies were allowed to interbreed to produce Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice (Fig. 1A). Like Mkp-1−/− mice on mixed background (19), Mkp-1−/− mice on a 129 background were healthy and fertile, and no abnormal phenotype was observed. In our SPF facility, the Il-10 knockout mice on the 129 genetic background did not develop rectal prolapse in the first 5 mo of life. In contrast, the mice deficient in both Mkp-1 and Il-10 developed severe prolapse 2–3 mo after birth (Fig. 1, B and C). These mice exhibited poor growth and high mortality. In fact, the prolapse was so severe that these mice often had to be euthanized shortly after the onset of the disease.
Fig. 1.
Mice deficient in both Mkp-1 and Il-10 develop a high incidence of rectal prolapse. Mice were housed in a specific pathogen-free (SPF) environment and scored for the development of rectal prolapse over 5 mo. A: genotyping of Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice by PCR. B: representative images of rectal prolapse of Mkp-1−/−/Il-10−/− mice. C: time course of rectal prolapse in various genotype groups (n = 16 for each genotype).
It has been demonstrated that Il-10 knockout mice suffer from anemia and weight loss (17). We examined the effect of Mkp-1 deficiency on the growth of mice that already lack Il-10. We also monitored the development of colitis signs in mice lacking Il-10 and Mkp-1 genes. Starting at 8 wk of age, Mkp-1+/+/Il-10−/−, Mkp-1+/−/Il-10−/−, and Mkp-1−/−/Il-10−/− mice were weighed weekly and monitored for the development of clinical signs of colitis until they reached 24 wk of age. The clinical scoring criteria were defined based on six parameters: loss of more than 5% of body weight, development of rectal prolapse, presence of perianal mucus, rectal bleeding, stool consistency, and death. Mice were given a score of either 0 (parameter absent) or 1 (parameter present) for each of those parameters, and the maximum possible score per mouse = 6. The disease in certain animals was so severe that they had to be euthanized. Double knockout mice (Mkp-1−/−/Il-10−/−) had higher clinical scores compared with both Mkp-1+/+/Il-10+/+ and Mkp-1+/+/Il-10−/− mice (Fig. 2). Surprisingly, Mkp-1+/−/Il-10−/− mice also exhibited higher levels of wasting and developed more severe colitis than Mkp-1+/+/Il-10−/− mice. These results suggest that the loss of one copy of Mkp-1 in Il-10−/− mice is sufficient to accelerate the development of colitis in an SPF environment.
Fig. 2.
Knockout of Mkp-1 not only accelerates the induction of colitis but also exacerbates the severity of colitis in Il-10 knockout mice. Mkp-1+/+/Il-10+/+, Mkp-1+/+/Il-10−/−, Mkp-1+/−/Il-10−/−, and Mkp-1−/−/Il-10−/− mice housed in a SPF environment were monitored weekly to score the severity of their colitis. The clinical scoring criteria included 6 parameters: loss of more than 5% of body weight, rectal prolapse, perianal mucus, rectal bleeding, wet stool consistency, and death. Mice were given a score of either 0 (parameter absent) or 1 (parameter present) for each parameter (maximum possible score per mouse = 6). Scores in the graph were averaged from individual animals in each group (n = 20–24 per group) and presented as means ± SE. *P < 0.05, compared with Mkp-1+/+/Il-10−/− mice; ‡P < 0.05, compared with Mkp-1+/+/Il-10+/+ mice (Student's t-test). Note the more severe disease in Mkp-1+/−/Il-10−/− mice relative to Mkp-1+/+/Il-10−/− mice.
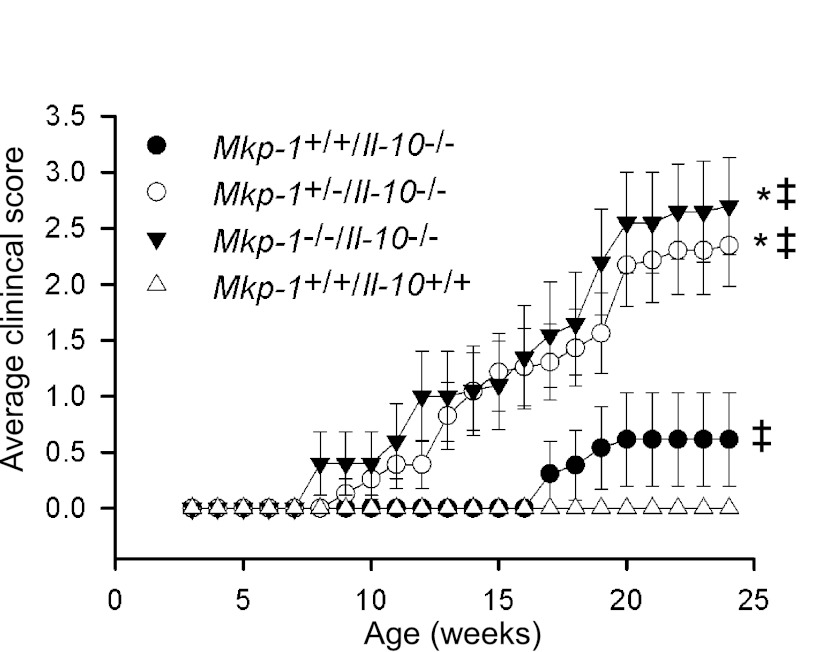
Colons from Mkp-1/Il-10 double knockout mice display more inflammation and mucosal hyperplasia than those from mice only lacking IL-10.
To understand the severe colitis phenotype in the double knockout mice, the colons of Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice were sectioned into four parts and stained with H&E. Histologically, colons from both wild-type and Mkp-1 knockout mice are morphologically normal, showing no signs of mucosal inflammation, hyperplasia, or crypt damage (data not shown). Colons of Il-10 knockout mice showed mild signs of multifocal inflammation with larger intervening regions of histologically normal colon (Fig. 3A). The inflammatory infiltrates were generally limited to the mucosa and submucosa and did not extend into the muscle layers, serosa, or gut-associated lymphoid tissue. In contrast, colons and rectums of double knockout mice exhibited diffuse transmural inflammation as well as mucosal hyperplasia (Fig. 3, A and B). Double knockout mice (Mkp-1−/−/Il-10−/−) had more severe goblet cell depletion and infiltration of lymphoid aggregates than Il-10 knockout mice. Colon sections of double knockout mice often appeared whitish, a characteristic of suppurative colitis (Fig. 3B). Inflammatory cells extended into both the lamina propria and submucosa. Immunohistochemistry staining of Mkp-1−/−/Il-10−/− colon tissues with CD3 and B220 antibodies revealed infiltration of both B and T lymphocytes into the lamina propria, with the majority being CD3+ T cells (Fig. 3C). Both T and B lymphocytes in the lamina propria of Mkp-1−/−/Il-10−/− mice were substantially more abundant than those in the lamina propria of Mkp-1+/+/Il-10−/− mice, whereas very few T and B lymphocytes were seen in the tissues of wild-type and Mkp-1−/−/Il-10+/+ mice (data not shown). Inflammation also affected a larger portion (∼50% of the total lumen surface area) of the colon in double knockout mice than in Il-10−/− mice (∼25% lumen surface area). Furthermore, inflammatory lesions tended to be continuous and widespread in the double knockout mice, whereas lesions in the Il-10−/− mice were mostly isolated and smaller.
Fig. 3.
Mkp-1 deficiency exacerbates the epithelial proliferation and inflammation of the large intestine of Il-10 knockout mice. Mice were housed in a SPF environment and euthanized at 8 wk of age. The colon was excised and fixed for histology and immunohistochemistry analyses. The colon of wild-type (Mkp-1+/+/Il-10+/+) and Mkp-1 knockout (Mkp-1−/−/Il-10+/+) mice appeared normal (data not shown). A: histology of hematoxylin and eosin (H&E)-stained colon sections. Note the marked mucosal epithelial hyperplasia in the double knockout (Mkp-1−/−/Il-10−/−) section, but not in the Mkp-1+/+/Il-10−/− section. B: mucosal leukocytes infiltration with crypt abscess in the Mkp-1+/+/Il-10−/− section. Image on the right is a high-magnification image of the crypt abscess. C: immunohistochemical detection of T lymphocytes (anti-CD3, top) and B lymphocytes (anti-B220, bottom) in the colon section of the Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice. D: immunohistochemical detection of the mitotic marker phospho-histone H3 in the rectal mucosa of Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice. E: detection of apoptosis in the colon section of the Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice by TUNEL assay. F: immunohistochemical detection of α-occludin in colon, with enhanced positive (brown) signal in the Mkp-1+/+/Il-10−/− section than in the Mkp-1−/−/Il-10−/− section. After immunohistochemical staining the sections were counterstained with hematoxylin. Images shown are representative of at least 3 different sections.
Within colonic and rectal mucosa, the double knockout mice (Mkp-1−/−/Il-10−/−) also had increased goblet cell depletion and more marked mucosal hyperplasia compared with Il-10 knockout mice (Fig. 3A). Consistent with the severe rectal prolapse, severe mucosal hyperplasia was observed in the double knockout mice. The crypts in the rectums of double knockout mice were significantly longer than in Mkp-1+/+/Il-10−/− mice (503.6 ± 78.7 vs. 160.1 ± 24.9 μm at 8 wk; P < 0.05). To assess cell proliferation in the rectal tissues, the rectum sections were stained with an antibody against phospho-histone H3, a mitotic marker (24, 29). Although the colonic crypts in Il-10 knockout mice displayed relatively few phospho-histone H3-positive cells, substantially more phospho-histone H3-positive cells were seen in the colon sections of double knockout mice (Fig. 3D). Although most phospho-histone H3-positive cells are localized in the base of the crypts, mitotic cells were also detected in the upper portion of the crypt epithelia, consistent with epithelial hyperplasia. To address the question of whether decreased apoptosis in the colon crypts contributes to the hyperplastic phenotype, we performed TUNEL assays to evaluate apoptosis in colon crypts. Compared with Il-10−/− mice, Mkp-1−/−/Il-10−/− mice had at least comparable, if not more, apoptotic cells in their colonic crypts (Fig. 3E). Additionally, although the tight junction protein α-occludin was abundantly expressed on the surface epithelium in the Il-10−/− mice, as indicated by the strong immunohistochemical reactivity, α-occludin protein level was very low in the colons of double knockout mice (Fig. 3F), suggesting disruption of the tight junction-mediated epithelial barrier function in the colon (61).
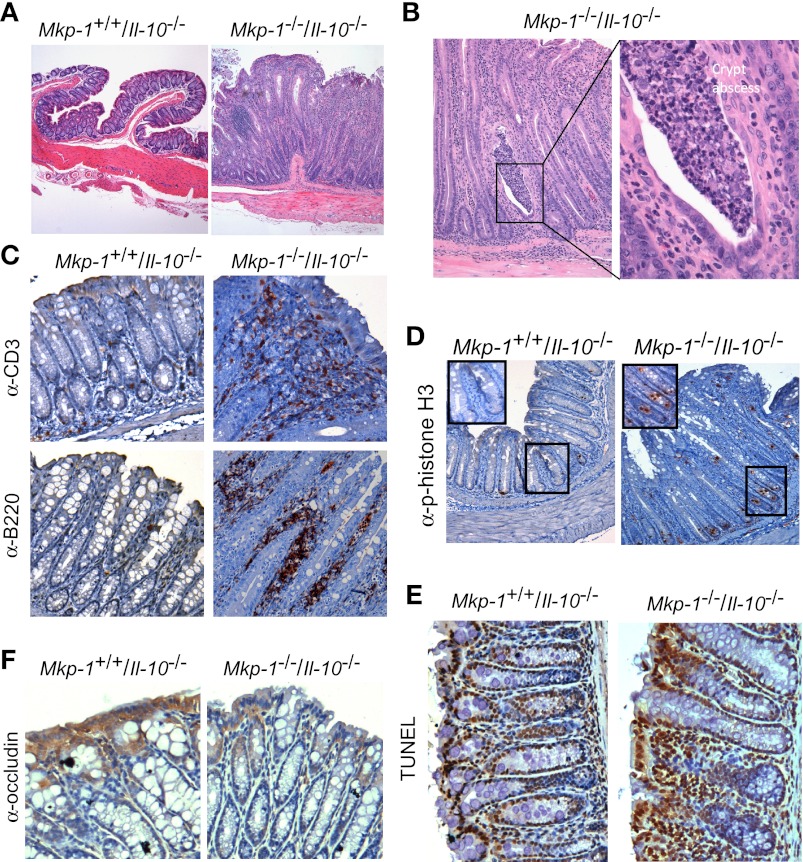
To quantify the degree of inflammation and the severity of IBD in this model, the H&E specimens from the colon of 8-wk-old Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, and Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice were blindly scored by the inflammation scoring criteria in Table 1, and the scoring criteria for IBD severity are shown in Table 2. Double knockout mice had significantly higher inflammation and IBD scores than Il-10 knockout mice (Fig. 4, A and B). Double knockout mice (Mkp-1−/−/Il-10−/−) had more severe mucosal hyperplasia, goblet cell depletion, and infiltration of lymphoid aggregates than Il-10 knockout mice. Moreover, the inflammation severity and extent, as well as presence of crypt abscesses and percent involvement, were higher in double knockout mice than in Il-10 knockout mice.
Fig. 4.
Double knockout mice exhibit higher histological inflammation and inflammatory bowel disease (IBD) scores than Il-10 knockout mice. The colon was removed at necropsy from 8-wk-old Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. The tissue was flushed with cold PBS and dissected into ascending colon, transverse colon, descending colon, and rectum. The colon of Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+ mice were normal. A: inflammation scores of the colon of Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. To calculate the inflammation score, H&E-stained sections were blindly scored based on the criteria listed in Table 1. The total inflammation score was calculated by adding the inflammation scores from the ascending, transverse, and descending colons and rectum (maximum possible inflammation score per animal is 56). B: IBD scores of the colon of Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. The IBD scoring system was based on the parameters outlined in Table 2. Similarly, the scores of all 4 segments were added together to calculate the total IBD score (maximum IBD score per animal is 36). Values shown are means ± SE of at least 3 animals. *P < 0.05, compared with Mkp-1+/+/Il-10−/− mice (Student's t-test).
Mkp-1−/−/Il-10−/− mice develop conjunctivitis and blepharitis.
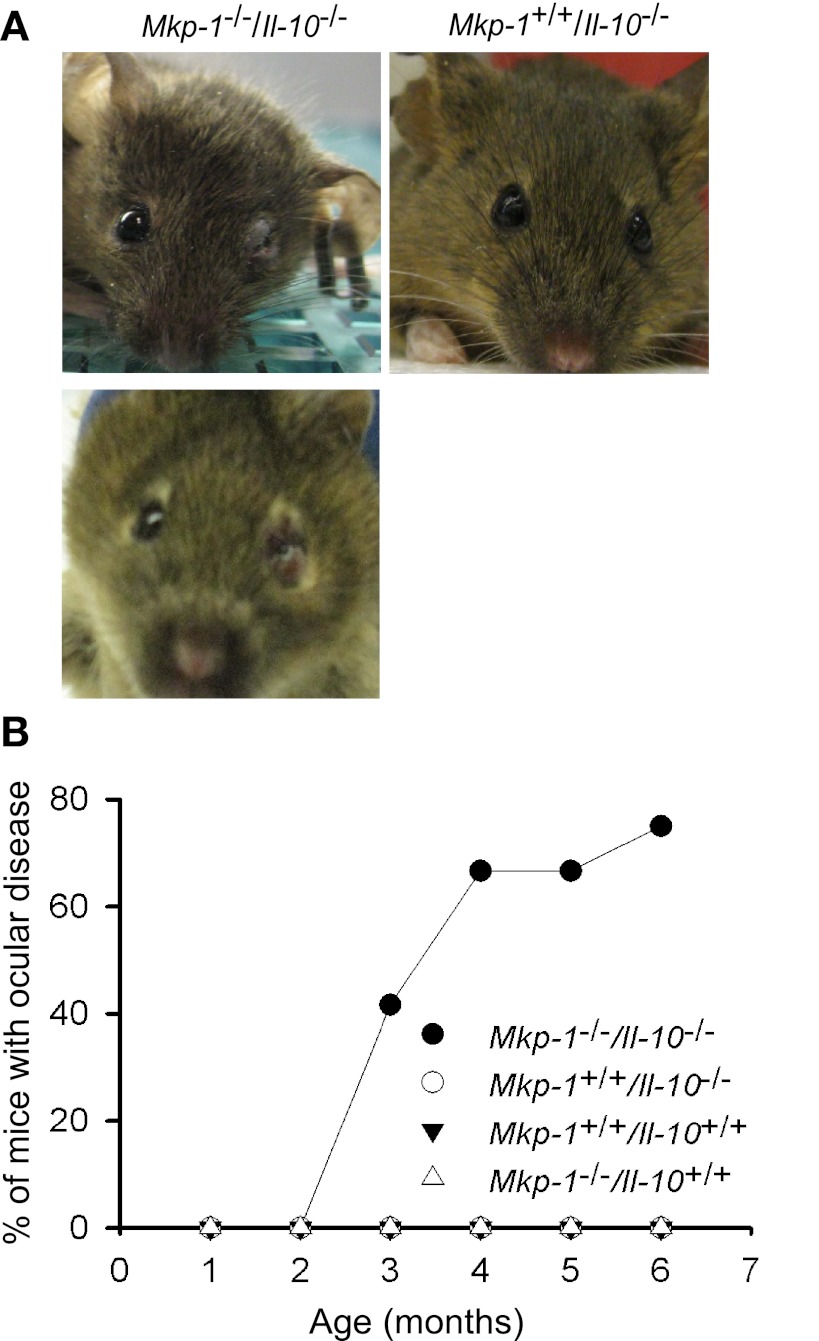
In addition to the more severe colitis phenotype, double knockout mice (Mkp-1−/−/Il-10−/−) also exhibited a high prevalence of unilateral and bilateral eyelid edema (Fig. 5A), indicated by swelling of the upper and lower eyelids. Swelling was moderate to severe and routinely hindered visualization of the globe, although no ocular discharge was observed. The periocular abnormalities were first noted at 2 mo of age, and by 6 mo of age, ∼80% of the mice were affected (Fig. 5B). There appeared a strong association between the rectal prolapse and ocular abnormalities. For example at age 3.5 mo, 3 of 11 double knockout mice (27%) developed both rectal prolapse and ocular disease, 2 mice (18%) developed only prolapse, whereas 9% developed only ocular disease, suggesting a potential mechanistic association between prolapse and periocular lesions.
Fig. 5.
Mkp-1/Il-10 double knockout mice develop periocular lesions. Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice were housed in a SPF environment and scored for grossly visible eye abnormality over 6 mo. A: representative images of periocular swelling in Mkp-1/Il-10 double knockout mice. B: time course of the development of periocular lesions. Graphical representation showing the percentage of mice that develop ocular disease (n = 12 for each genotype).
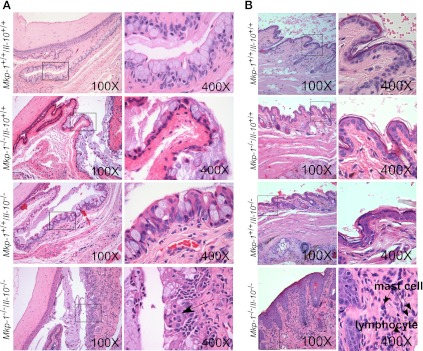
To understand the ocular disease phenotype observed in the double knockout mice, eyes and the periocular tissues, including eyelids and conjunctiva, were harvested from all four mouse genotypes for routine histological analysis of H&E-stained sections. There were no histological lesions within the eyes of the wild-type, single, or double knockout mice (data not shown). When periocular structures were examined, the palpebral conjunctiva of Mkp-1+/+/Il-10+/+ mice was normal, and the palpebral conjunctiva of Mkp-1−/−/Il-10+/+ and Mkp-1+/+/Il-10−/− showed a slight greater number of goblet cells and mild accumulation of mucus within the conjunctival space (Fig. 6A). However, the palpebral conjunctival mucosa of double knockout mice was markedly thickened with goblet cell hyperplasia and occasional clusters of degenerate and nondegenerate neutrophils (intraepithelial abscesses). The moderate conjunctival goblet cell hyperplasia in the double knockout mice was often accompanied by marked accumulation of mucus in the conjunctival space. The conjunctival stroma was also infiltrated with large numbers of neutrophils, with fewer lymphocytes and plasma cells, and this cellular infiltration also extended into the conjunctival mucosa and accumulated mucus (Fig. 6A).
Fig. 6.
Double knockout mice exhibit conjunctival and eyelid lesions. Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice were housed in a SPF environment and were euthanized at 3 mo of age. Eyes and periocular tissues were excised, routinely fixed, and embedded, and sections were stained with H&E. A: conjunctival lesions in double knockout mice. Mkp-1+/+/Il-10+/+ mice lacked conjunctival lesions (top row). Mkp-1−/−/Il-10+/+ and Mkp-1+/+/Il-10−/− mice showed moderately increased goblet cell numbers and mild increased mucus accumulation in the conjunctival space (2nd and 3rd rows). The conjunctival mucosa of Mkp-1−/−/Il-10−/− mice was markedly thickened with moderate goblet cell hyperplasia and moderate to marked mucus accumulation in the conjunctival space (bottom row). Additionally, the conjunctival stroma was expanded by mild edema and infiltrated by neutrophils, with fewer lymphocytes and plasma cells. B: eyelid lesions in Mkp-1/Il-10 double knockout mice. The haired skin of double knockout mouse eyelids mice was markedly thickened and infiltrated by lymphocytes and plasma cells, with scattered clusters of mast cells. Wild-type, Mkp-1−/−/Il-10+/+, or Mkp-1+/+/Il-10−/− mouse eyelids lacked lesions. Column on the right contains high-resolution images of the boxed areas in left column.
In wild-type, Mkp-1−/−/Il-10+/+, and Mkp-1+/+/Il-10−/− mice, the eyelids were histologically normal, with an epithelium composed of a single layer of keratinized squamous epithelium with regularly spaced adnexal structures (hair shafts and glands) and only few scattered resident inflammatory cells (Fig. 6B). In contrast, the eyelid epithelium of the double knockout mice was considerably thickened by inflammatory infiltrates. Lymphocytes and plasma cells extended throughout the dermis, along with occasional foci of mast cells (Fig. 6B).
Knockout of Mkp-1 further exacerbates the production of Th-1 and Th-17 cytokines in Il-10 knockout mice.
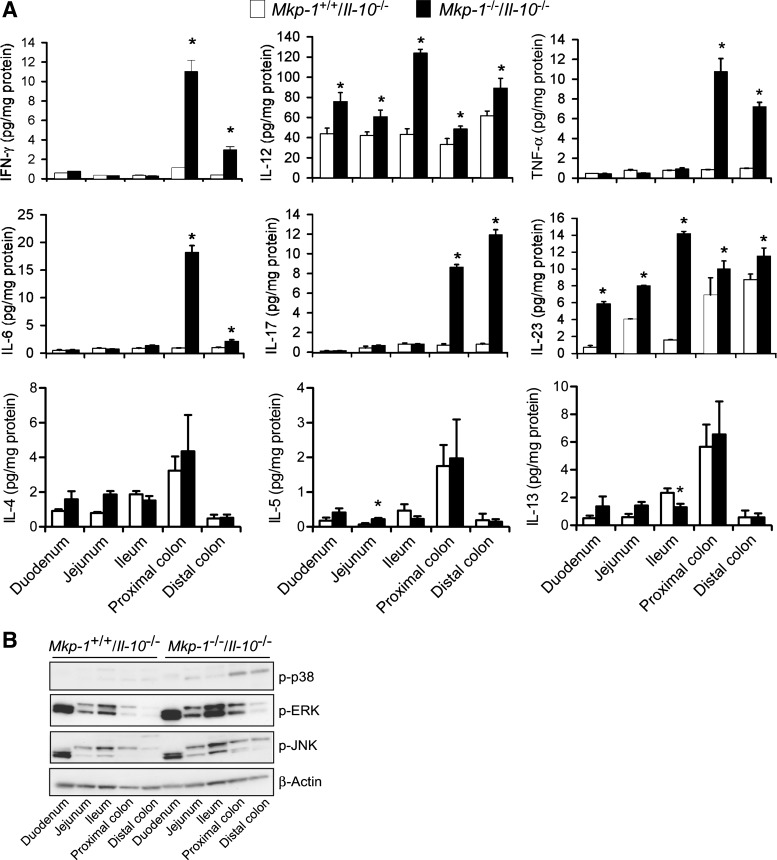
To understand the mechanisms underlying the accelerated colitis development in the double knockout mice, we assessed tissue cytokine levels in the different regions of the intestinal tract. Intestines were harvested from 8-wk-old Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice, divided into five sections (duodenum, jejunum, ileum, proximal colon, and distal colon), and homogenized to extract proteins. Endogenous cytokine levels in the different intestinal sections were measured by ELISA (Fig. 7A). The small intestine of Il-10 knockout mice produced low levels of IFN-γ, TNF-α, IL-6, IL-17, and IL-23, although a significant amount of IL-12 was detected in the small intestine. This is not surprising, since colitis in Il-10−/− mice primarily affects the colon and rectum (17, 18). Interestingly, more IL-12 and IL-23 were detected in the small intestine of the Mkp-1−/−/Il-10−/− mice than in those of the Mkp-1+/+/Il-10−/− mice. Remarkably, all six proinflammatory cytokines of the Th-1 and Th-17 classes, including IFN-γ, TNF-α, IL-6, IL-17, IL-12, and IL-23, were significantly more abundant in both the proximal and distal colons in the Mkp-1−/−/Il-10−/− mice than in Mkp-1+/+/Il-10−/− mice. In contrast, Th-2 cytokines, including IL-4, IL-5, and IL-13, were comparable in the colon tissues between the two genotypes. Similar to what were seen for the Th-1 and Th-17 cytokines, the levels of these Th-2 cytokines in the small intestinal tissue were also very low. These findings suggest that double knockout mice exhibit polarized Th1 and Th17 responses.
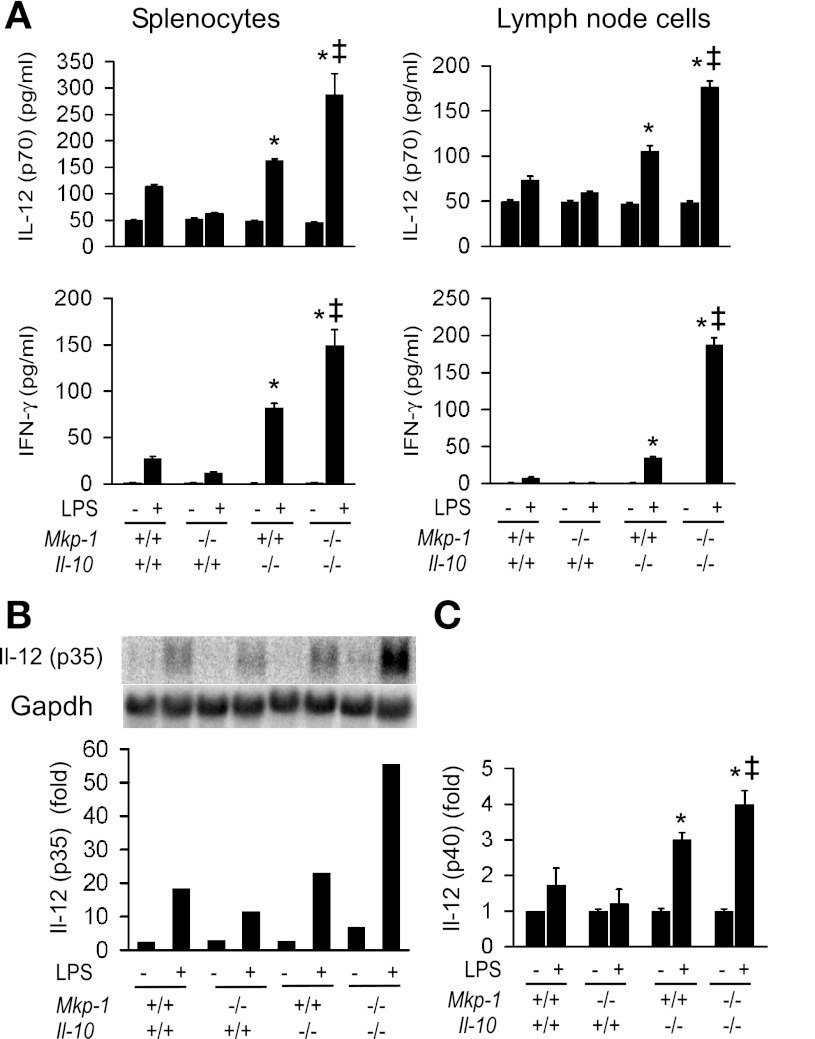
Fig. 7.
Colons from Mkp-1/Il-10 double knockout mice contain higher levels of proinflammatory cytokines. The intestine of both double knockout and Il-10 knockout mice were divided into duodenum, jejunum, and ileum, as well as proximal and distal colons. Tissues were homogenized to extract soluble proteins. A: levels of cytokines in distinct intestinal regions. IFN-γ, IL-4, IL-5, IL-6, IL-12 (p70), IL-13, IL-17, IL-23 (p19/p40), and TNF-α in the tissue extracts were assessed by ELISA. Values were normalized to total protein content in the tissue homogenates and presented as means ± SE from at least 3 different animals. *P < 0.05, compared with level in the Mkp-1+/+/Il-10−/− tissue (Student's t-test). B: activities of distinct MAPKs. The active forms of MAPKs in the tissue homogenates were detected by Western blot analyses, using antibodies against phospho-MAPKs. Comparable protein loading was verified by Western blot analysis using an antibody against β-actin (bottom). Presented are the representative results of at least 3 experiments.
To investigate the effects of Mkp-1 deletion on MAPK activities in the intestinal tissues, active forms of the MAPKs were assessed by Western blot analyses using the phospho-specific antibodies (Fig. 7B). There was no appreciable difference in the activity of any MAPK in the duodenum between the two groups. However, the activities of all three MAPKs (p38, ERK, and JNK) were markedly higher in jejunum, ileum, proximal and distal colons of the Mkp-1−/−/Il-10−/− mice than in the Mkp-1+/+/Il-10−/− mice.
Knockout of Mkp-1 on an Il-10-null background further exaggerates the leukocyte inflammatory response.
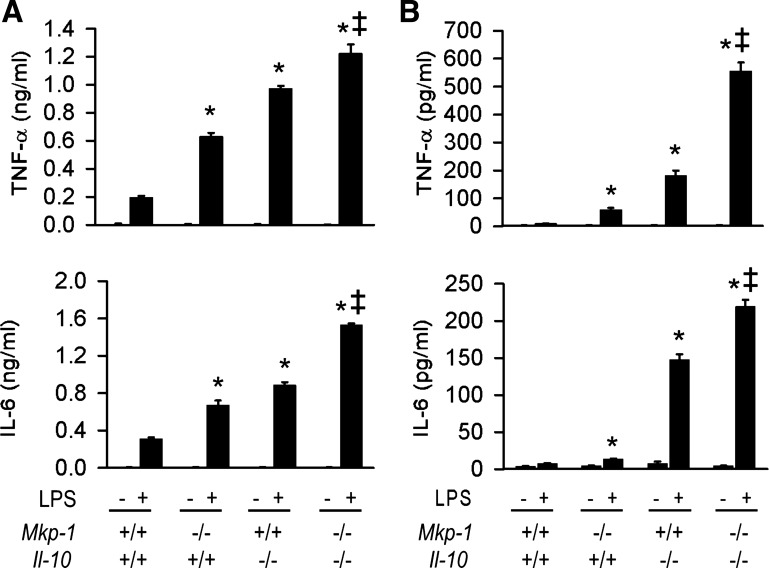
Since it has been shown that lack of Il-10 in mice skews the immune response toward one that is predominantly Th-1 (17), we examined whether lack of Mkp-1 would cause a further shift toward a hyper-Th1 response in the Il-10-deficient mice. Splenocytes and mesenteric lymph node cells were isolated from Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. Cells were treated with LPS for 24 h and the media were collected at the end of the treatment period. Levels of secreted TNF-α and IL-6 were assayed by ELISA (Fig. 8). Untreated control splenocyte produced low levels of TNF-α and IL-6 (Fig. 8A). LPS stimulation of splenocytes enhanced the production of these cytokines. Although both splenocytes from Mkp-1−/−/Il-10+/+ and Mkp-1+/+/Il-10−/− mice produced more TNF-α and IL-6 than their Mkp-1+/+/Il-10+/+ counterparts, Mkp-1−/−/Il-10−/− lymphocytes produced the most TNF-α and IL-6. Similar phenomena were observed in the lymph node cells (Fig. 8B), indicating that Mkp-1 deletion further exaggerated inflammatory cytokine production in the Il-10-deficient cells.
Fig. 8.
Effects of Mkp-1 and Il-10 knockout on the production of TNF-α and IL-6 in innate immune cells during the response to LPS. Splenocytes and mesenteric lymph node cells were isolated from Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. Cells were treated with LPS (100 ng/ml) for 24 h. Concentrations of TNF-α and IL-6 in the media were assayed by ELISA. A: cytokine production by splenocytes. B: cytokine production by mesenteric lymph node cells. Data are presented as the means ± SE of at least 3 independent experiments. *P < 0.05, compared with LPS-stimulated cytokine production in wild-type cells. ‡P < 0.05, compared with LPS-stimulated cytokine production in Mkp-1+/+/Il-10+/+ cells (Student's t-test).
To elucidate the effect of Mkp-1 deletion on the Th-1 cytokine production, we assessed the production of Il-12 and IFN-γ, two cytokines associated with the Th-1 response (Fig. 9A). As expected, knockout of Il-10 enhanced the production of IL-12 (p70) and IFN-γ in both splenocytes and lymph node cells. Knockout of Mkp-1 on an Il-10-null background further augmented the production of both IFN-γ and IL-12. Similar phenomena were observed in both splenocytes and lymph node cells. Northern blot analysis indicated that Il-12 p35 was markedly induced by LPS. Moreover, LPS-induced Il-12 p35 mRNA was substantially elevated in splenocytes lacking both Mkp-1 and Il-10 relative to wild-type splenocytes or splenocytes lacking either Mkp-1 or Il-10 (Fig. 9B, left). Likewise, real-time RT-PCR indicated that the level of Il-12 p40 mRNA after LPS stimulation was also higher in splenocytes lacking both Mkp-1 and Il-10 relative to the other groups (Fig. 9C).
Fig. 9.
Effects of Mkp-1 and Il-10 knockout on Th-1 cytokine production in innate immune cells during the response to LPS. Splenocytes and lymph node cells were isolated from Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. Cells were treated with LPS (100 ng/ml) for 24 h. Total RNA was harvested to examine the expression of IL-12 subunits. A: IL-12 (p70) and IFN-γ production in response to LPS by splenocytes and lymph node cells isolated from Mkp-1+/+/Il-10+/+, Mkp-1−/−/Il-10+/+, Mkp-1+/+/Il-10−/−, and Mkp-1−/−/Il-10−/− mice. Concentrations of IL-12 (p70) and IFN-γ in the media were assayed by ELISA. B: Il-12 p35 mRNA expression in unstimulated and LPS-stimulated splenocytes. Il-12 p35 mRNA expression was detected by Northern blot analysis, using a murine Il-12 p35 cDNA. The blot was stripped and reprobed with a cDNA of murine Gapdh to normalize RNA loading. The intensity of the Il-12 p35 signal was quantified by use of the ImageQuant system, normalized to Gapdh signal, and presented in the graph. Data shown are results from a representative experiment. The level of Il-12 p35 mRNA in unstimulated Mkp-1+/+/Il-10+/+ splenocytes was set as 1. C: Il-12 p40 mRNA expression in unstimulated and LPS-stimulated splenocytes. Il-12 p40 expression was assessed by real-time RT-PCR. The level of Il-12 p40 mRNA in unstimulated Mkp-1+/+/Il-10+/+ splenocytes was set as 1. Data were presented as means ± SE of at least 3 independent experiments. *P < 0.05, compared with LPS-stimulated level in Mkp-1+/+/Il-10+/+ splenocytes; ‡P < 0.05, compared with LPS-stimulated level in Mkp-1+/+/Il-10−/− splenocytes (Student's t-test).
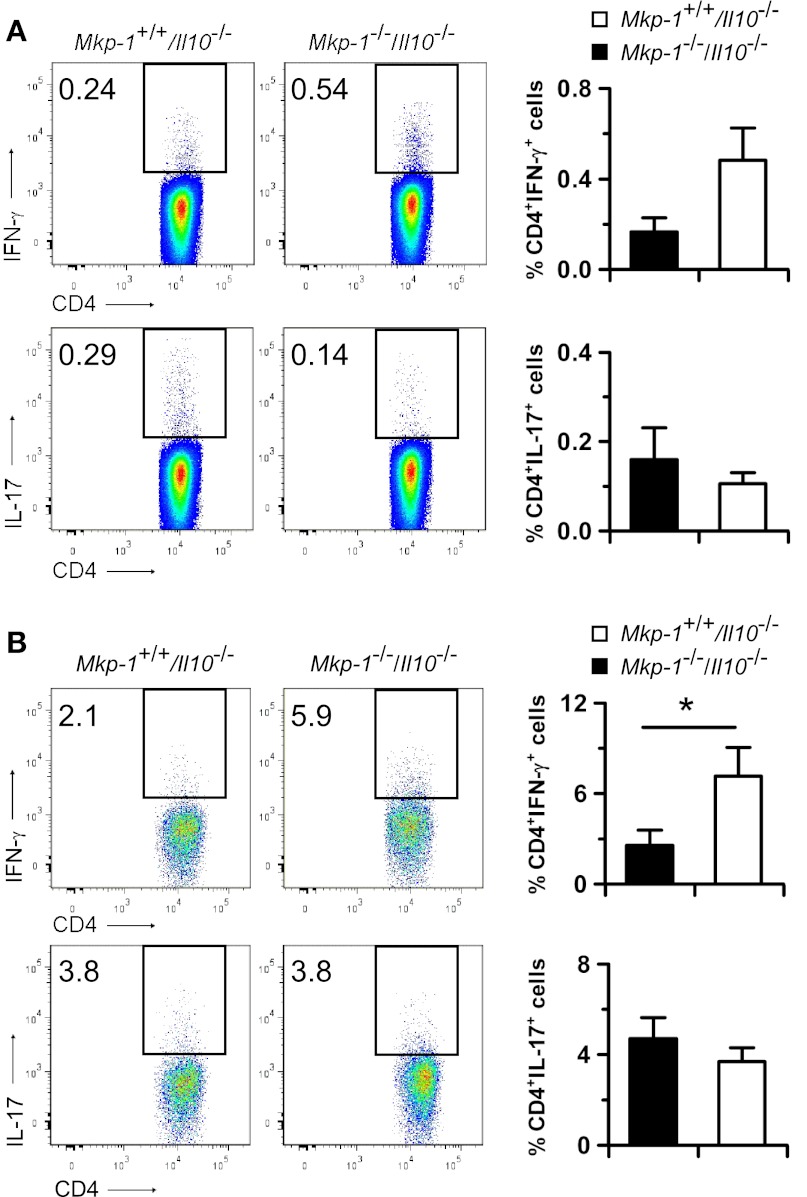
To characterize the T lymphocytes in mesenteric lymph nodes and colonic epithelia, lymphocytes were activated by using PMA and a calcium ionophore in the presence of GolgiPlug to block cytokine secretion. The cells were then stained with antibodies against CD4, IFN-γ, and IL-17 to assess Th-1 (CD4+IFN-γ+) and Th-17 (CD4+IL-17+) cells. At the age of 12 wk, the percentages of both Th-1 and Th-17 cells in the mesenteric lymph nodes were comparable between Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice (Fig. 10A). Consistent with the observation that substantially more T lymphocytes infiltrated into the colons of Mkp-1−/−/Il-10−/− mice than into those of Mkp-1+/+/Il-10−/− mice (Fig. 3C), greater numbers of intraepithelial lymphocytes were also isolated from the Mkp-1−/−/Il-10−/− colons (data not shown). We found that in Mkp-1−/−/Il-10−/− mice Th-1 cells constituted a significantly higher portion among the colonic intraepithelial CD4+ T cells than in Mkp-1+/+/Il-10−/− mice, supporting the idea that Th-1 cells play an important role in the induction of colitis in this model system. However, the percentages of colonic intraepithelial Th-17 cells in Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice were comparable. Very similar profiles in mesenteric lymph node and colonic intraepithelial T cells were found for both Mkp-1+/+/Il-10−/− and Mkp-1−/−/Il-10−/− mice at age 35 wk (data not shown).
Fig. 10.
Characterization of mesenteric lymph node and intraepithelial T cells. Mice were housed in SPF environment and euthanized at 12 wk of age. Lymphocytes were isolated from mesenteric lymph nodes and epithelium through Percoll gradient centrifugation. Lymphocytes were activated with PMA and a calcium ionophore for 4 h in the presence of BD GolgiPlug. Cells were then fixed, stained with antibodies against CD3, CD4, IFN-γ, and IL-17, and analyzed by flow cytometry. A: quantification of Th-1 and Th-17 cells in mesenteric lymph nodes. B: quantification of Th-1 and Th-17 cells in intraepithelial lymphocytes. Representative flow cytometry plots are shown on the left with the cells of interest defined in the rectangles. The percentage of Th-1 and Th-17 cells are presented in the graph on the right as means ± SE of 3 to 4 animals. *P < 0.05, compared with Mkp-1+/+/Il-10−/− samples (Student's t-test).
DISCUSSION
Mkp-1 is a critical negative regulator of the inflammatory response. In response to microbial components, Mkp-1 is strongly induced and acts as a feedback control mechanism to inactivate p38 and JNK. By inactivating p38 and JNK in a delayed fashion, Mkp-1 restrains the inflammatory response and limits the production of inflammatory cytokines. Previously, we have reported that Mkp-1-deficient mice exhibit a heightened inflammatory response in models of endotoxemia and bacterial sepsis (23, 62, 70). In this report, we demonstrate that mice deficient in both Il-10 and Mkp-1 are prone to the development of severe colitis, a chronic inflammatory disease. Compared with previous models of IBD using single knockout Il-10-deficient mice, our double knockouts developed a more pronounced disease phenotype characterized by rectal prolapse, rectal bleeding, and weight loss (Figs. 1–4). The gross observations were corroborated by histological examination via an objective, blinded scoring system (Fig. 4). We also present evidence of a new inflammatory disorder of the eyes not previously reported in Il-10 knockout mice: blepharitis and conjunctivitis both occurred frequently in double knockout mice (Figs. 5 and 6). Taken together, our results support the conclusion that IL-10 and Mkp-1 interact to modulate immune homeostasis in the colon as well as the eye. Our studies raise the possibility that dysregulated Mkp-1 activity contributes to the development of IBD in humans and that Mkp-1 may serve as a basis for novel diagnostics or therapeutics for IBD patients.
Eye inflammation in Il-10/Mkp-1 double knockout mice.
An unexpected finding of the present study is the discovery of inflammatory changes in the eyelids and conjunctivae of double knockout animals that do not occur in either the Il-10 single knockout mice or the Mkp-1 single knockout mice. Forty percent of our double knockout mice demonstrated periocular inflammation by 3 mo of age and the prevalence increased to 75% by 6 mo of age (Fig. 5). Extraintestinal manifestations are a common aspect of IBD in humans and contribute significantly to discomfort and morbidity. Among the myriad extraintestinal symptoms suffered by IBD patients, inflammation of the eyes is relatively common (44). Of note, humans with IBD typically develop anterior uveitis, iritis, scleritis, and episcleritis (33). In contrast, our double knockout mice demonstrated blepharitis and conjunctivitis. At this time, it is unknown whether differential distribution of innate immune cells or MKP-1 expression in humans accounts for the disparity. However, as with colitis, it is possible that our double knockout mice may have value in future exploration of Mkp-1-directed therapeutics for extraintestinal ocular disease in IBD patients.
Mkp-1 in the Il-10 knockout mouse model of IBD.
The Il-10 knockout mouse is an established model of human chronic colitis. Il-10 knockout mice reliably develop chronic colitis as a function of age (6, 17, 18, 53). Previous investigators have established the role of a number of proinflammatory cytokines in the development of colitis in Il-10−/− mice, as the result of absent IL-10-mediated anti-inflammatory effects. Elevated levels of IL-6, IL-12, IL-17, IL-23 and IFN-γ have been documented (6, 45, 68), as has amelioration of colitis in animals treated with neutralizing antibodies directed against these cytokines (6, 18, 52, 53, 68). Given the role of Mkp-1 in the IL-10 signaling pathway and the interaction between Mkp-1 and IL-10, we sought to determine the relative importance of Mkp-1 in the development of chronic colitis by establishing Il-10/Mkp-1 double knockout mice. Mkp-1 is an established critical regulator of systemic inflammation in a variety of experimental systems and models of human disease (39). Mkp-1-deficient mice experience increased inflammation, organ failure, and mortality following challenge with various inflammatory stimuli including LPS, peptidoglycan, and live E. coli (23, 62, 70). Previous work has shown heightened production of many proinflammatory cytokines in Mkp-1−/− animals following exposure to LPS including TNF-α, IL-1β, IL-6, and MCP-1 (13, 27, 54, 70). These cytokine production patterns are associated with increased mortality in these animals in models of systemic infection or inflammation. Interestingly, Mkp-1 knockout mice also produce dramatically higher levels of anti-inflammatory IL-10 than do wild-type mice after LPS challenge (13, 23, 27, 62, 70). The observed phenotype of increased inflammation and mortality despite the high levels of IL-10, in conjunction with the finding that IL-10 enhances Mkp-1 expression (26), led us to postulate that Mkp-1 deficiency superimposed on Il-10 knockout mice would further exacerbate the colitis phenotype of Il-10-deficient mice. The worsened colitis we observed in the double knockout relative to the Il-10 single knockout mice provide strong support for a complex bilateral interaction between IL-10 and Mkp-1 in the control of intestinal inflammation. Our results suggest that in the absence of both Il-10 and Mkp-1, two critical negative regulators of inflammatory responses, minor inflammatory stimuli, such as those that routinely occur in the colonic mucosa of wild-type mice, may result in an exaggerated cytokine production by leukocytes, leading to chronic colonic inflammation. Additionally, Mkp-1 knockout mice exhibit compromised bacterial clearance activities (23, 25, 36). The defect in bacterial containment or clearance may also contribute to the persistent inflammation seen in the colon and rectum of Mkp-1/Il-10 double knockout mice.
Mechanistically, colitis in the Il-10 knockout mice is caused by exacerbation of the Th-1 and Th-17 responses (52, 68). It has been shown that a critical function of IL-10 is to restrain an overzealous Th-17 immune response, by activating the regulatory T cells (9, 10). IL-10 also strongly inhibits Th-1 responses, both by blocking IL-12 production and by attenuating the effects of IFN-γ (34, 67). We have previously shown that IFN-γ inhibits Mkp-1 induction in macrophages during the response to LPS, and knockout of Mkp-1 alleviates the necessity of IFN-γ priming for inducible nitric oxide synthase induction in resident macrophages (63). In this sense, an important function of IFN-γ is to suppress Mkp-1 expression, thus enhancing inflammation. On the other hand, IL-10 enhances the expression of Mkp-1 (26) and thereby controls the expression of many cytokine genes. In the absence of IL-10, Mkp-1 can still function to restrict the inflammatory responses, albeit not as potently as in the presence of IL-10. Since Mkp-1 is functionally positioned at the crossroads between IL-10 and IFN-γ, the absence of Mkp-1 compromises anti-inflammatory mechanisms and simultaneously enhances IFN-γ effects, shifting the intestinal tissues to a more proinflammatory milieu. The higher IFN-γ levels in the colons of Mkp-1/Il-10 double knockout mice compared with Il-10 knockout mice is a conceivable mechanism driving accelerated colitis induction in the double knockout mice (Figs. 1 and 2).
Previously, it has been shown that IL-10 signaling in regulatory T cells is crucial for suppressing Th-17 cells and for maintaining intestinal homeostasis (10). As an important downstream effector molecule induced by IL-10, Mkp-1 could play an important role in this process. The absence of Mkp-1 can compromise the function of regulatory T cells. Additionally, overexpression of IL-6 and IL-23 as a result of Mkp-1 knockout further favors the expansion of the procolitogenic Th-17 cells in the large intestine of Mkp-1/Il-10 double knockout mice, consistent with the elevated IL-17 levels in the colon tissues we observed (Fig. 7). Recently, Huang et al. (30) have shown that dendritic cell-expressed Mkp-1 programs reciprocal Th1 and Th17 cell differentiation by modulating IL-12-STAT4 and IL-6-STAT3 axes and by regulating dendritic cell-mediated T cell cytokine receptor expression. We found that the innate immune effector cells from both spleens and mesenteric lymph nodes in the double knockout mice produced significantly more IL-12, IL-6, and IFN-γ, cytokines favoring Th-1 and Th-17 responses (Fig. 8). Indeed, more lymphocytes, predominantly T cells, were found in the lamina propria of Mkp-1/Il-10 double knockout mice than in the Il-10 knockout mice. Characterization of the intraepithelial T cells, but not mesenteric lymph node T cells, revealed a greater proportion of Th-1 cells in the double knockout mice (Fig. 10B). The enhanced Th-1 response provides a plausible explanation for the acceleration of colitis in the double knockout mice, since Th-1 cells and IFN-γ are involved in the induction phase of colitis (6, 18, 53). Interestingly, we did not find a greater proportion of Th-17 cells among the CD4+ intraepithelial T lymphocytes in double knockout mice compared with those in Il-10 knockout mice (Fig. 10B). However, because total T lymphocytes were more abundant in the colons of double knockout mice than in the Il-10 knockout mice (Fig. 3C), the similar proportion of Th-17 cells will translate to substantially more total Th-17 cells in the colon of the double knockout mice, explaining the greater IL-17 levels in the colonic tissue of double knockout mice (Fig. 7A). The markedly enhanced Th-1 response and the dramatically greater lamina propria lymphocyte population are also consistent with the greater levels of proinflammatory cytokines in the colon tissues (Fig. 7).
In conclusion, by establishing the Il-10/Mkp-1 double knockout mouse, we have shown that Mkp-1 serves an important role in the restraint of inflammation and the development of colitis in an animal model of IBD. We have correlated grossly observable inflammation of the intestinal tract with histological and biochemical evidence of hyperinflammation in the double knockouts that exceeds the inflammatory burden of Il-10 single knockouts. We have also found exaggerated Th-1 and Th-17 responses in the colons of Mkp-1/Il-10 double knockout animals. Our data add to the growing body of literature supporting the critical role of Mkp-1 in the control of inflammatory processes and provide novel information for the pathogenesis of IBD.
GRANTS
This work was supported by grant R01AI57798 from National Institute of Allergy and Infectious Diseases, grant R01HL75261 from National Heart, Lung, and Blood Institute, grant R01DK059656 from National Institute of Diabetes and Digestive and Kidney Diseases, and funding from The Research Institute at Nationwide Children's Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R. Matta, J.A. Barnard, J. Yan, and Y. Liu designed the experiments; R. Matta, J.A. Barnard, L.M. Wancket, J. Yan performed the experiments; R. Matta, J.A. Barnard, L.M. Wancket, J. Yan, J. Grieves, W.J. Frazier, and L.D. Nelin analyzed the data; R. Matta, W.J. Frazier, L.D. Nelin, and Y. Liu wrote the manuscript; A.C.B. Cato generated Mkp-1 knockout mice on 129 background.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Nan Zhang and Xianxi Wang for excellent technical assistance.
REFERENCES
- 1. Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, de Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43: 246– 252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis 40, Suppl 2: S253– S259, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev 55: 241– 269, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40: 955– 962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369: 1641– 1657, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98: 1010– 1020, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med 182: 99– 108, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Charles CH, Sun H, Lau LF, Tonks NK. The growth factor-inducible immediate-early gene 3CH134 encodes a protein-tyrosine-phosphatase. Proc Natl Acad Sci USA 90: 5292– 5296, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986– 991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34: 566– 578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen P, Hutter D, Liu P, Liu Y. A mammalian expression system for rapid production and purification of active MAP kinase phosphatases. Protein Expr Purif 24: 481– 488, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol 169: 6408– 6416, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 103: 2274– 2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem 271: 6497– 6501, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci USA 96: 13938– 13943, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2011. November 23 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17. Davidson NJ, Fort MM, Muller W, Leach MW, Rennick DM. Chronic colitis in IL-10−/− mice: insufficient counter regulation of a Th1 response. Int Rev Immunol 19: 91– 121, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 184: 241– 251, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13: 925– 931, 1996 [PubMed] [Google Scholar]
- 20. Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, de Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42: 1118– 1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem 272: 16917– 16923, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA 95: 3014– 3019, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frazier WJ, Wang X, Wancket LM, Li XA, Meng X, Nelin LD, Cato AC, Liu Y. Increased inflammation, impaired bacterial clearance, and metabolic disruption after gram-negative sepsis in Mkp-1-deficient mice. J Immunol 183: 7411– 7419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 274: 25543– 25549, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Hammer M, Echtenachter B, Weighardt H, Jozefowski K, Rose-John S, Mannel DN, Holzmann B, Lang R. Increased inflammation and lethality of Dusp1−/− mice in polymicrobial peritonitis models. Immunology 131: 395– 404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammer M, Mages J, Dietrich H, Schmitz F, Striebel F, Murray PJ, Wagner H, Lang R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur J Immunol 35: 2991– 3001, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med 203: 15– 20, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology 130: 323– 333, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Hendzel MJ, Wei Y, Mancini MA, Van HA, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106: 348– 360, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Huang G, Wang Y, Shi LZ, Kanneganti TD, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity 35: 45– 58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411: 599– 603, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Inohara N, Ogura Y, Nunez G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol 5: 76– 80, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Isaacs KL. Extra-intestinal manifestations. In: Advanced Therapy of Inflammatory Bowel Disease, edited by Bayless TM, Hanauer SB. Hamilton: Decker, 2001, p. 267– 274 [Google Scholar]
- 34. Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 93: 1456– 1463, 1999 [PubMed] [Google Scholar]
- 35. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307– 317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, Sers C, Lang R, Hammerstein P, Lucius R, Hartmann S. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog 7: e1001248, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125: 47– 57, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem 270: 8377– 8380, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases—regulating the immune response. Nat Rev Immunol 7: 202– 212, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298– 306, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Yang Z, Neurath MF, Salfeld J, Veldman GM, Schwertschlag U, Strober W. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med 351: 2069– 2079, 2004 [DOI] [PubMed] [Google Scholar]
- 42. McDorman KS, Chandra S, Hooth MJ, Hester SD, Schoonhoven R, Wolf DC. Induction of transitional cell hyperplasia in the urinary bladder and aberrant crypt foci in the colon of rats treated with individual and a mixture of drinking water disinfection by-products. Toxicol Pathol 31: 235– 242, 2003 [DOI] [PubMed] [Google Scholar]
- 43. McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagace C, Li C, Green T, Stevens CR, Beauchamp C, Fleshner PR, Carlson M, D'Amato M, Halfvarson J, Hibberd ML, Lordal M, Padyukov L, Andriulli A, Colombo E, Latiano A, Palmieri O, Bernard EJ, Deslandres C, Hommes DW, de Jong DJ, Stokkers PC, Weersma RK, Sharma Y, Silverberg MS, Cho JH, Wu J, Roeder K, Brant SR, Schumm LP, Duerr RH, Dubinsky MC, Glazer NL, Haritunians T, Ippoliti A, Melmed GY, Siscovick DS, Vasiliauskas EA, Targan SR, Annese V, Wijmenga C, Pettersson S, Rotter JI, Xavier RJ, Daly MJ, Rioux JD, Seielstad M. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 42: 332– 337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mintz R, Feller ER, Bahr RL, Shah SA. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis 10: 135– 139, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Montufar-Solis D, Schaefer J, Hicks MJ, Klein JR. Massive but selective cytokine dysregulation in the colon of IL-10−/− mice revealed by multiplex analysis. Int Immunol 20: 141– 154, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683– 765, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S. Guidelines for the management of inflammatory bowel disease in adults. Gut 60: 571– 607, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther 18, Suppl 2: 1– 5, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603– 606, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Podolsky DK. Inflammatory bowel disease. N Engl J Med 347: 417– 429, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270: 7420– 7426, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 278: G829– G833, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Rennick DM, Fort MM, Davidson NJ. Studies with IL-10−/− mice: an overview. J Leukoc Biol 61: 389– 396, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 176: 1899– 1907, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology 135: 1442– 1447, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577– 594, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J Biol Chem 279: 54023– 54031, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 122: 94– 105, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487– 493, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 337: 1029– 1035, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol 169: 1901– 1909, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, Liu Y. Knockout of Mkp-1 enhances the host inflammatory responses to Gram-positive bacteria. J Immunol 178: 5312– 5320, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Wang X, Zhao Q, Matta R, Meng X, Liu X, Liu CG, Nelin LD, Liu Y. Inducible nitric-oxide synthase expression is regulated by mitogen-activated protein kinase phosphatase-1. J Biol Chem 284: 27123– 27134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Watanabe N, Ikuta K, Okazaki K, Nakase H, Tabata Y, Matsuura M, Tamaki H, Kawanami C, Honjo T, Chiba T. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci 48: 408– 414, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2: 2307– 2311, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation—a continuing puzzle. Immunology 113: 281– 292, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yao Y, Li W, Kaplan MH, Chang CH. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med 201: 1899– 1903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 116: 1310– 1316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhao Q, Shepherd EG, Manson ME, Nelin LD, Sorokin A, Liu Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem 280: 8101– 8108, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203: 131– 140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]