Abstract
The transmembrane glycoprotein CD98 is known to be involved in intestinal inflammation. In the present study, we found that CD98 overexpression in intestinal epithelial cells does not normally affect the expression of colonic (epithelial and immune cell) microRNAs (miRNAs), small noncoding RNAs that posttranscriptionally regulate a wide variety of biological processes. However, upon dextran sulfate sodium (DSS) treatment, the expression of several colonic miRNAs, but not miRNAs from other tissues such as liver and spleen, were differentially regulated in mice overexpressing CD98 in epithelial cells compared with wild-type (WT) animals. For example, the level of colonic miRNA 132 was not affected by DSS treatment in WT animals but was upregulated in mice overexpressing CD98 in intestinal epithelial cells. Other colonic miRNAs, including colonic miRNA 23a and 23b, were downregulated in WT animals after DSS treatment but not in colonic epithelial cell CD98-overexpressing mice. Interestingly, the expression of potential miRNA target genes affected intestinal epithelial cells that overexpress CD98 and cell types that did not overexpress CD98 but were in close proximity to CD98-overexpressing intestinal epithelial cells. Taken together, these observations show that the combination of an inflammatory context and intestinal epithelial cell expression of CD98 affects the regulation of miRNA expression in colonic epithelial and immune cells. This is new evidence that protein expression modulates miRNA expression and suggests the existence of regulatory crosstalk between proteins and miRNAs in diseases such as colitis.
Keywords: inflammation, epithelial cells
cd98 is a type ii transmembrane protein encoded by SLC3A2. The protein complex is a heterodimer consisting of a glycosylated heavy chain covalently linked to one of the several nonglycosylated light chains to form an amino acid transporter (12, 45). CD98 is found in all cell types, with the exception of platelets, and has the highest expression in the gastrointestinal (GI) tract and the tubules of the kidney (34, 35, 45). CD98 interacts directly with β1- and β3-integrin, and this interaction is important for integrin signaling, which controls a number of biological processes such as cell proliferation, survival, and epithelial cell adhesion/polarity (8, 14, 32, 33).
CD98 expression increases during intestinal inflammation both in vivo and in vitro (27, 34, 44). Also CD98 overexpression in intestinal epithelial cells (IECs) exacerbated the inflammatory response in dextran sulfate sodium (DSS)-induced colitis including increased expression of proinflammatory cytokines (33). These results suggest that CD98 might be regulating the expression of certain modulators, which in turn regulate the expression of pro- and/or anti-inflammatory proteins in the GI tract.
MicroRNAs (miRNAs) are short (18–24 nucleotide), noncoding RNA that downregulate gene expression by binding to the 3′ untranslated end of mRNA (4, 11). This interaction between miRNA and mRNA is not perfect, so one miRNA can bind to numerous mRNAs and one gene can be regulated by many miRNAs (29, 30). miRNAs carry out regulatory roles in a number of biological events such as cell proliferation, apoptosis, and differentiation (3, 10, 41). Given the continuous and rapid renewal of the intestinal epithelium, miRNA-mediated effects on proliferation, apoptosis, and differentiation may be particularly important in this tissue (16).
In the present study, we explored whether intestinal epithelial CD98 expression controlled the expression of colonic miRNAs in either a normal or an inflammatory context. We examined colonic miRNA profiles in the intestinal epithelium of mice overexpressing CD98, with or without DSS-induced colitis.
MATERIALS AND METHODS
Experimental animals.
Six- to seven-week-old FVB male mice were used in all experiments. FVB transgenic (Tg) mice overexpressing CD98 specifically in the IECs were generated as previously described (33). All mice were group housed in standard cages under a controlled temperature and photoperiod (12-h:12-h light/dark cycle). All procedures using mice were in accordance with the approval of Emory University Institutional Animal Care.
DSS-induced colitis.
Colitis was induced in wild-type (WT) and Tg littermates by replacing regular drinking water with 3% (wt/vol) DSS (MP Biomedicals). Age- and sex-matched mice receiving regular drinking water served as controls. DSS-treated mice were weighed daily and killed after 7 days of treatment.
RNA extraction and microRNA microarray.
Total RNA was extracted from colonic tissue of mice by RNeasy Mini kit (Qiagen) according to the manufacturer's instruction. Small RNA including miRNA was extracted by RNeasy Mini kit following the instructions given in the Qiagen supplementary protocol for purification of miRNA from animal cells. Yield and quality of RNA was verified by Beckman Spectrophotometer. The RNA samples were pooled from two littermates per group. A size fractionation step with YM-100 Microcon filter was done, which isolates nucleotides of ∼200 bp or less (LC Sciences). MicroRNA microarrays was performed by using the μParaflo microfluidic chip technology (LC Sciences). Probes for the arrays were developed using version 16 of the miRBase sequence database (www.mirbase.org). Data analysis for the arrays was performed using t-test, ANOVA, and/or clustering analysis.
Real-time RT-PCR.
cDNA was generated from the total RNAs isolated above using the NCode miRNA first-strand cDNA synthesis kit (Invitrogen) or Maxima first-strand cDNA synthesis kit (Fermentas) according to the manufacturer's instruction. Expression of mature miRNAs or total RNA were quantified by real-time RT-PCR using Maxima SYBR Green/ROX qPCR Master Mix (Fermentas). The universal reverse primer provided in the NCode miRNA first-strand cDNA synthesis kit and the specific miRNA forward primers were used. Small RNA 234 and 36B4 were used as housekeeping genes. Fold induction was calculated using the Ct method as follows: ΔΔCt=(CtTarget − Cthousekeeping)group 1 − (CtTarget − Cthousekeeping)group 2, and the final data were derived from 2−ΔΔCT. Sequences of all the primers used for real-time RT-PCR are given in Table 1.
Table 1.
Primers used in this study
| Primer | Sequence | Description |
|---|---|---|
| snoRNA234 | GCGCGGAACTGAATCTAAGTGATTTAACAA | small RNA 234 forward primer |
| mmT-miR-132F | TAACAGTCTACAGCCATGGTCG | miRNA 132 forward primer |
| mmT-miR-1937cF | ATCCCGGAAGAGCCCCCA | miRNA 1937c forward primer |
| mmT-miR-150F | TCTCCCAACCCTTGTACCAGTG | miRNA 150 forward primer |
| mmT-miR-1894-3pF | GCAAGGGAGAGGGTGAAGGGAG | miRNA 1894-3p forward primer |
| mmT-miR-375F | TTTGTTCGTTCGGCTCGCGTGA | miRNA 375 forward primer |
| mmT-miR-200cF | TAATACTGCCGGGTAATGATGGA | miRNA 200c forward primer |
| mmT-miR-2137F | GCCGGCGGGAGCCCCAGGGAG | miRNA 2137 forward primer |
| mmT-miR-200bF | TAATACTGCCTGGTAATGATGA | miRNA 200b forward primer |
| mmT-miR-762F | GGGGCTGGGGCCGGGACAGAGC | miRNA 762 forward primer |
| mmT-miR-429F | TAATACTGTCTGGTAATGCCGT | miRNA 429 forward primer |
| mmT-miR-23aF | ATCACATTGCCAGGGATTTCC | miRNA 23a forward primer |
| mmT-miR-23bF | ATCACATTGCCAGGGATTACC | miRNA 23b forward primer |
| mmT-miR-92aF | TATTGCACTTGTCCCGGCCTG | miRNA 92a forward primer |
| CD163-F | GGACAGATCTGGGGTGAAGA | CD163 RT-PCR forward primer |
| CD163-R | ATCCCTGCTGTGGGTACAAG | CD163 RT-PCR reverse primer |
| Tjap1-F | CCAGGGACTGGAAGAAATCA | Tjap1 RT-PCR forward primer |
| Tjap1-R | CCTCCAGGCACTCCATAAAA | Tjap1 RT-PCR reverse primer |
| 36B4-F | TCCAGGCTTTGGGCATCA | 36B4 RT-PCR forward primer |
| 36B4-R | CTTTATCAGCTGCACATCACTCAGA | 36B4 RT-PCR reverse primer |
miRNA, microRNA; Tjap1, tight junction-associated protein 1.
MiRNA target prediction.
Potential target genes of miRNAs were determined by three different miRNA target prediction algorithms: PicTar (http://pictar.mdc-berlin.de) (26), MicroCosm (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/) (17), and TargetScan (http://www.targetscan.org/) (18). Matchminer program (http://discover.nci.nih.gov/matchminer/index.jsp) (7) was then used to determine genes that were identified by at least two algorithms.
The enriched functional groups from the list of genes among the miRNA targets was determined by using the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 software (http://david.abcc.ncifcrf.gov/home.jsp) (21).
Protein extraction and Western blot analysis.
Approximately 5–10-mm pieces of colon tissue from control and DSS-treated WT and Tg mice overexpressing CD98 were homogenized in radioimmune precipitation assay buffer (150 mM NaCl, 0.5% sodium deoxycholate, 50 mM Tris·HCl, pH 8, 0.1% SDS, 0.1% Nonidet P-40) supplemented with protease inhibitors (Roche Diagnostics) on ice. The homogenates were centrifuged at 12,000 revolution/min for 10 min at 4°C. Total cell lysates were resolved on 4–15% gradient polyacrylamide gels (Bio-Rad) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were then probed with relevant primary antibodies: CD163 (sc-33560; Santa Cruz Biotechnologies), tight junction-associated protein 1 (Tjap1) (ab80444; Abcam), and β-actin (3700; Cell Signaling Technology). After washes, membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (GE Healthcare), and blots were detected using the Enhanced Chemiluminescence Detection kit (GE Healthcare Amersham).
Statistical analysis.
Values were expressed as means ± SE. Statistical analysis was performed using unpaired two-tailed t-test by GraphPad Prism 5 software. P < 0.05 was considered statistically significant.
RESULTS
Intestinal epithelial CD98 overexpression aggravates DSS-induced colitis.
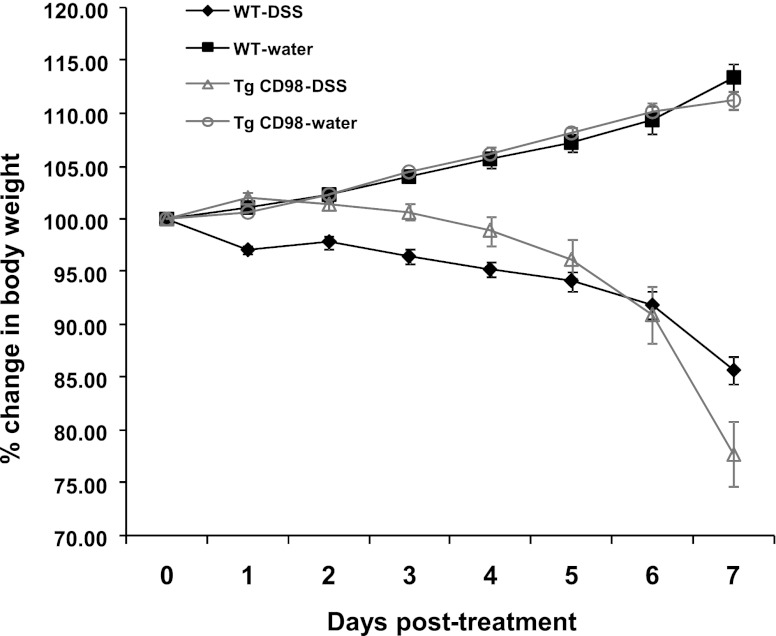
We previously generated Tg mice specifically overexpressing CD98 in IECs including colonocytes (33). In such animals, the CD98 transgene is under the control of the villin promoter. As previously reported, after 7 days of DSS treatment, CD98-Tg mice undergoing DSS-induced colitis lost a greater percentage of body weight (22.22 ± 3.1%) than did WT animals (14.28 ± 1.31%) after 7 days of DSS treatment. Note that, when weight loss in CD98 mice approached 20% body weight, they were euthanized. Control littermates drinking DSS-free water showed normal increases in body weight over the same period (Fig. 1).
Fig. 1.
Intestinal epithelial CD98 overexpression results in extensive body weight loss during colitis. Dextran sodium sulfate (DSS)-induced colitis results in higher percentage of body weight loss in intestinal epithelial cell (IEC)-specific CD98 overexpressing transgenic (Tg) mice compared with the wild-type (WT). WT and Tg littermates were administered regular drinking water (control) and 3.0% DSS for 7 days; n = 6/group.
Intestinal epithelial CD98 expression modulates the expression of miRNAs during colitis.
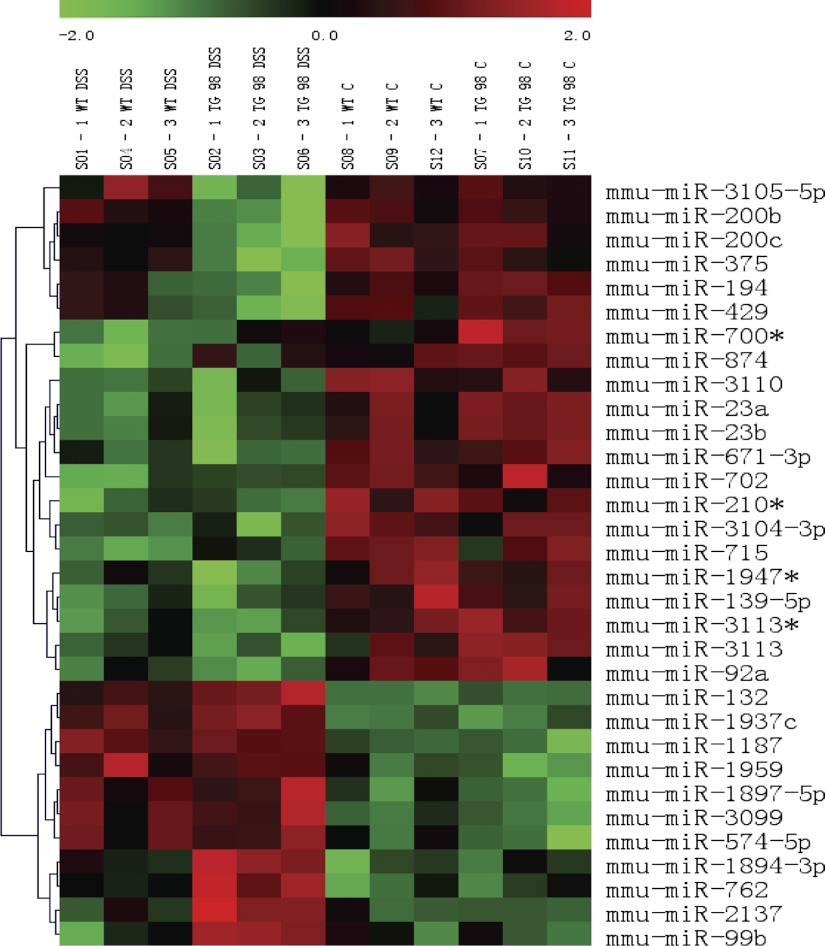
Earlier work showed that CD98 Tg mice were more susceptible to DSS-induced colitis than WT animals, but the exact role played by CD98 remains unclear. miRNAs have been shown to play important roles in biological processes, such as cell proliferation (6) and immunity (39), and are also involved in IEC differentiation (10). We obtained miRNA expression profiles from the colons of WT and CD98 Tg mice with DSS-induced colitis and control littermates. To this end, WT and CD98 Tg mice exposed to DSS for 7 days were killed, along with controls (no DSS treatment), and RNA was extracted from all colons. miRNA microarray analysis was performed using μParaflo technology. Standard arrays allowing evaluation of mature miRNA levels were employed. The levels of 1,040 mouse miRNAs were assayed. We identified miRNA transcripts differentially expressed in WT and Tg DSS-treated and control groups with P values of <0.01, <0.05, and <0.1. A total of 32 miRNA transcripts had a P value of <0.01 (Fig. 2 and Table 2), but 19 were of low signal intensity (<500) and were thus discarded; the remaining 13 miRNAs were included in further analysis.
Fig. 2.
MicroRNA (miRNA) microarray clustering graph of colonic miRNA in WT and Tg CD98 mice colon. A total of 32 miRNA transcripts had differential expression in the colon WT and CD98 Tg mice administered with 3% DSS and normal water (control) for 7 days. miRNA transcripts having P value <0.01 are shown above.
Table 2.
Colonic miRNA transcripts differentially expressed in colon of WT and Tg mice treated with DSS or control in miRNA microarray
| No. | Reporter Name | P value | WT DSS Mean | Tg 98 DSS Mean | WT C Mean | Tg 98 C Mean |
|---|---|---|---|---|---|---|
| 1 | mmu-miR-132 | 5.43E-06 | 528 | 1,001 | 174 | 200 |
| 2 | mmu-miR-1937c | 6.54E-05 | 1,724 | 3,022 | 142 | 133 |
| 3 | mmu-miR-375 | 3.68E-04 | 3,072 | 1,259 | 4,138 | 3,334 |
| 4 | mmu-miR-200c | 8.13E-04 | 5,753 | 2,879 | 7,943 | 7,739 |
| 5 | mmu-miR-2137 | 8.15E-04 | 337 | 1,221 | 297 | 239 |
| 6 | mmu-miR-1894-3p | 1.24E-03 | 397 | 1,555 | 215 | 284 |
| 7 | mmu-miR-200b | 1.28E-03 | 5,852 | 1,677 | 6,309 | 6,029 |
| 8 | mmu-miR-762 | 1.93E-03 | 4,597 | 10,782 | 3,251 | 3,688 |
| 9 | mmu-miR-429 | 3.01E-03 | 446 | 129 | 617 | 849 |
| 10 | mmu-miR-194 | 3.12E-03 | 814 | 284 | 1,041 | 1,554 |
| 11 | mmu-miR-23b | 4.06E-03 | 4,372 | 3,997 | 7,638 | 9,701 |
| 12 | mmu-miR-23a | 5.86E-03 | 3,385 | 3,297 | 6,384 | 8,434 |
| 13 | mmu-miR-92a | 6.65E-03 | 797 | 608 | 1,361 | 1,587 |
| Following transcripts are statistically significant but have low signals | ||||||
| 14 | mmu-miR-574-5p | 7.52E-03 | 473 | 496 | 287 | 186 |
| 15 | mmu-miR-139-5p | 8.26E-03 | 368 | 346 | 823 | 740 |
| 16 | mmu-miR-99b | 9.19E-03 | 492 | 930 | 534 | 496 |
| 17 | mmu-miR-1187 | 1.66E-04 | 244 | 246 | 98 | 79 |
| 18 | mmu-miR-671-3p | 7.96E-04 | 20 | 16 | 33 | 34 |
| 19 | mmu-miR-700 | 8.43E-04 | 21 | 28 | 29 | 43 |
| 20 | mmu-miR-3113 | 1.01E-03 | 23 | 18 | 31 | 43 |
| 21 | mmu-miR-715 | 1.84E-03 | 40 | 52 | 84 | 72 |
| 22 | mmu-miR-3113 | 1.95E-03 | 22 | 19 | 33 | 39 |
| 23 | mmu-miR-874 | 2.08E-03 | 15 | 25 | 29 | 36 |
| 24 | mmu-miR-210 | 2.61E-03 | 35 | 35 | 52 | 47 |
| 25 | mmu-miR-3099 | 2.82E-03 | 76 | 88 | 33 | 27 |
| 26 | mmu-miR-3105-5p | 3.91E-03 | 26 | 12 | 23 | 24 |
| 27 | mmu-miR-3104-3p | 4.18E-03 | 44 | 44 | 83 | 77 |
| 28 | mmu-miR-1959 | 4.52E-03 | 21 | 19 | 9 | 6 |
| 29 | mmu-miR-1897-5p | 6.21E-03 | 92 | 105 | 57 | 46 |
| 30 | mmu-miR-702 | 6.30E-03 | 33 | 39 | 64 | 63 |
| 31 | mmu-miR-1947 | 7.67E-03 | 21 | 16 | 32 | 29 |
| 32 | mmu-miR-3110 | 7.88E-03 | 27 | 27 | 47 | 43 |
P < 0.01.
WT, wild-type; Tg, transgenic; DSS, dextran sodium sulfate; C, control.
We also compared transcripts in WT and Tg mice treated with DSS, WT and Tg controls (no DSS treatment), WT control and WT DSS-treated, and Tg controls and Tg DSS-treated animals. Transcripts differing in levels significant at P < 0.01 and of signal strengths >500 were selected for further analysis (Table 3). Notably, when WT and Tg control data were compared, no miRNA transcript fulfilled any criterion mentioned above, indicating that CD98 overexpression under basal conditions does not influence differential miRNA expression. In contrast, one miRNA transcript was differentially expressed when WT DSS-treatment data were compared with those from Tg DSS-exposed animals (Table 3), and three such transcripts were differentially expressed in Tg controls compared with Tg DSS-treated mice (Table 3). All miRNAs listed in Table 3 also featured in the list of 13 miRNAs that were differentially expressed in WT and Tg DSS-treated and control animals (Table 2), with the exception of miRNA 150 (Table 3).
Table 3.
Colonic miRNA transcripts differentially expressed in WT DSS-treated group, Tg control, and Tg CD98 DSS-treated group
| No. | Reporter name | P value | Mean | Tg 98 DSS Mean |
|---|---|---|---|---|
| 1 | mmu-miR-1894-3p | 9.12E-03 | 397 | 1,555 |
| 1 | mmu-miR-1937c | 3.94E-03 | 133 | 3,022 |
| 2 | mmu-miR-150 | 7.64E-03 | 1,017 | 255 |
| 3 | mmu-miR-1894-3p | 9.84E-03 | 284 | 1,555 |
| Following transcripts are statistically significant but have low signals | ||||
| 4 | mmu-miR-106b | 8.12E-03 | 33 | 21 |
| 5 | mmu-miR-3113 | 8.86E-03 | 39 | 19 |
Colonic miRNA transcript differentially expressed in WT DSS-treated group vs. Tg CD98 DSS-treated group. Colonic miRNA transcripts that were differentially expressed in Tg control and Tg DSS-treated group. The first row is for WT DSS Mean, and all subsequent rows are for Tg 98 C Mean, thus the repetition of number 1.
P < 0.01.
Quantification of the levels of selected miRNAs using real-time RT-PCR.
After analysis of miRNA microarray data, 14 miRNAs were selected for quantification of expression levels using real-time RT-PCR. miRNA 2137 and miRNA 1894–3p did not yield peaks upon melting curve analysis (employing real-time RT-PCR) using appropriate probes. The mature miRNA1937c is in fact a tRNA fragment and is no longer considered to be a miRNA (www.mirbase.org). The expression levels of the remaining 11 colonic miRNAs, obtained by real-time RT-PCR, were in agreement with the miRNA microarray data (Figs. 3, 4, and 5). In addition, we demonstrated that miRNAs 762, 132, and 92a are also expressed in other organs, such as spleen and liver (Fig. 6). However, these miRNAs are not regulated by DSS treatment in WT or Tg mice overexpressing CD98 (Fig. 6). These observations support the idea that the observed regulation of miRNAs in the colon during DSS treatment is tissue specific (colon) and indicate that colonic CD98 expression is responsible for the differential regulation of colonic miRNAs between WT and CD98 Tg mice.
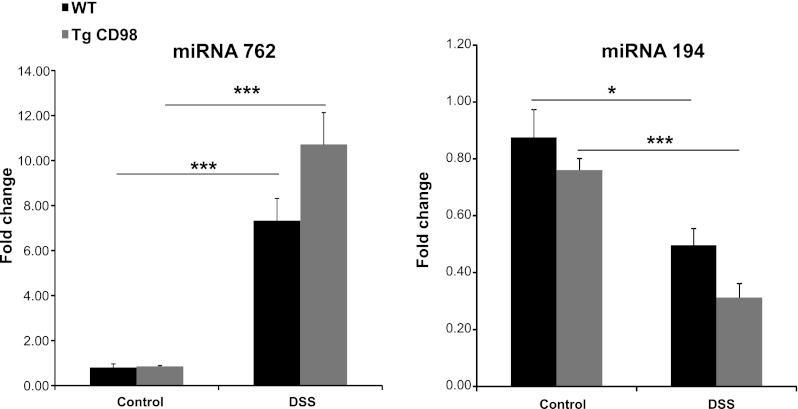
Fig. 3.
Colonic miRNAs expression are modulated in DSS-treated WT and Tg mice. Colonic miRNA 762 expression increases, whereas the expression of colonic miRNA 194 expression decreases in WT and Tg mice colon on DSS treatment as shown by real-time RT-PCR. Values represent means ± SE of n = 6/group. *P < 0.05 and ***P < 0.001.
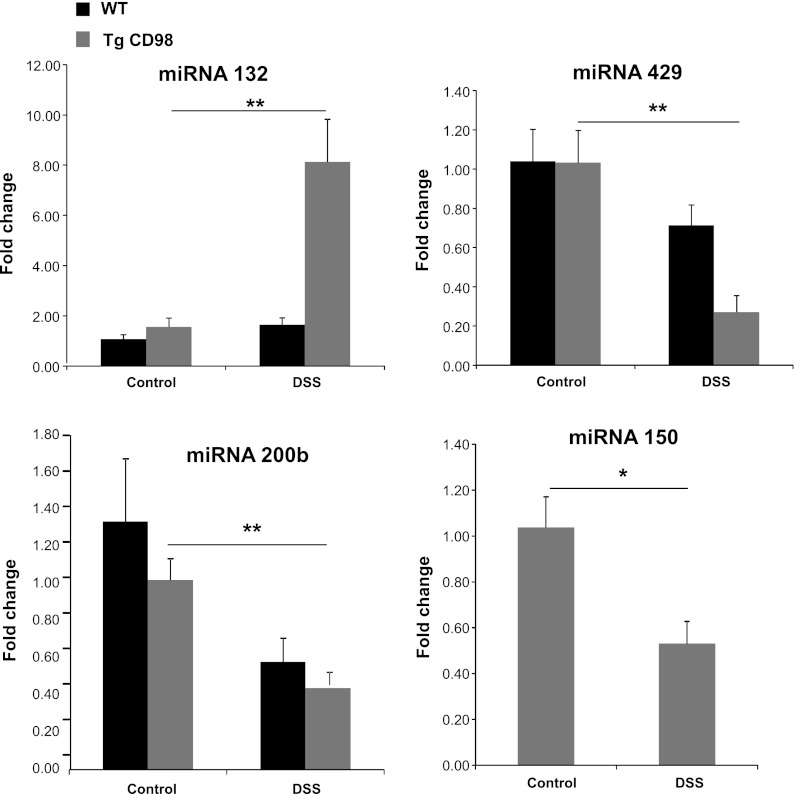
Fig. 4.
Colonic miRNA expressions are modulated in DSS-treated Tg mice. Colonic miRNA 132 expression increases, whereas the expression of colonic miRNA 429, 200b, and 150 decreases in Tg mice colon on DSS treatment compared with Tg control as shown by real-time RT-PCR. Because, in miRNA, microarray transcript of miRNA 150 was differentially regulated in Tg mice and not in WT mice (Table 3), real-time RT-PCR was done to confirm miRNA 150 expression in Tg mice only. Values represent means ± SE of n = 6/group. *P < 0.05 and **P < 0.005.
Fig. 5.
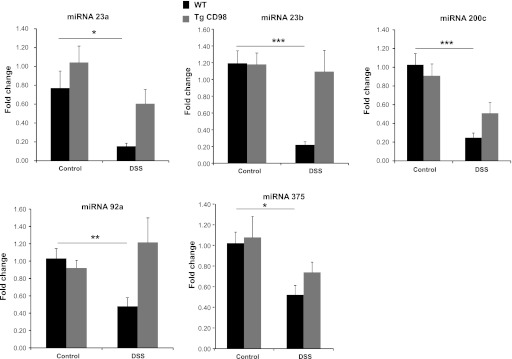
Colonic miRNA expressions are downregulated in DSS-treated WT mice. Expression of colonic miRNA 23a, 23b, 200c, 92a, and 375 were downregulated in DSS-treated WT compared with WT control mice, whereas their expression in Tg mice is nonsignificant under the same conditions as shown by real-time RT-PCR. Values represent means ± SE of n = 6/group. *P < 0.05, **P < 0.005, and ***P < 0.001.
Fig. 6.
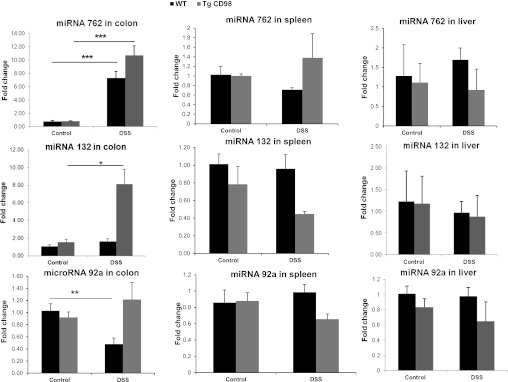
Modulation of miRNA expression is colon specific upon DSS treatment. Expression of miRNA 762, 132, and 92a in the colon changes significantly in DSS-treated WT, Tg, or both animals compared with control, but their expression in spleen and liver remains unchanged or nonsignificant. Values represent means ± SE of n = 6/group. *P < 0.05, **P < 0.005, and ***P < 0.001.
Colonic miRNAs 762 and 194 are differentially expressed in WT and Tg mice upon DSS treatment.
As shown in Fig. 3, expression of colonic miRNA 762 was upregulated, whereas colonic miRNA 194 was downregulated in both WT and Tg mice when colitis was induced by DSS treatment. Forty potential target genes of colonic miRNA 194 (Supplemental Sheet 1; supplemental material is available for this article online at the American Journal of Physiology Gastrointestinal and Liver Physiology website) were identified using the bioinformatics approach described in materials and methods. No potential target gene for colonic miRNA 762 was found in the Pictar database, but 35 genes were listed as its potential targets in Targetscan and Microcosm databases (Supplemental Sheet 1).
Functional groups enriched among the potential target genes of colonic miRNA 762 and 194 (a total of 75 genes, Supplemental Sheet 1) were identified using DAVID software. Application of the Functional Annotation Clustering tool revealed a total of eight annotation clusters; 28 genes were not included in any cluster. Among the eight clusters, the most enriched functional gene groups (Enrichment Scores >1.00, the higher the enrichment score, the more enriched that particular group) were involved in lipid metabolism, transcriptional regulation, and encoding of transcriptional regulators with zinc finger motifs (Table 4).
Table 4.
Enriched functional group among the gene targets of colonic miRNA 762 and 194 as predicted by DAVID software
| Annotation Cluster | Enrichment Score | Enriched Functional Group Gene Annotation / Function | Total Gene Count |
|---|---|---|---|
| 1 | 1.21 | Domain: PH | 5 |
| Lipid degradation | 3 | ||
| Hydrolase | 6 | ||
| 2 | 1.06 | Transcription regulation | 12 |
| Transcription | 12 | ||
| Activator | 5 | ||
| Nucleus | 20 | ||
| DNA binding | 9 | ||
| 3 | 1.01 | Zinc finger region: PDH type 1&2 | 6 |
| Chromatin regulator | 4 | ||
| Zinc finger | 12 | ||
| Metal binding | 11 |
A total of 75 genes were the potential target of colonic miRNA 762 and 194. Genes with enrichment score >1.00 shown above. (The higher the enrichment score, the more enriched that particular group).
Colonic miRNAs 132, 429, 200b, and 150 are differentially expressed in Tg mice upon DSS treatment.
Figure 4 shows that the expression of colonic miRNA 132 was upregulated and that of colonic miRNA 429, 200b, and 150 were downregulated in Tg mice but not in WT animals during DSS treatment. Because in miRNA microarray transcripts of miRNA 150 were differentially regulated in Tg mice and not in WT mice (Table 3), real-time RT-PCR was done to confirm miRNA 150 expression in Tg mice only. This suggests that CD98 might directly or indirectly modulate expression and/or stability of these four miRNAs in the colon when colitis is present. Bioinformatics revealed that 238 genes were potential targets of these four miRNAs (Supplemental Sheet 1). Use of the Functional Annotation Clustering tool revealed a total of 15 clusters; 90 genes were not clustered. The most enriched functional groups (Enrichment Scores >1.00) encoded transcriptional regulators, cell cycle proteins, proteins involved in isopeptide binding, and low-density lipoprotein (LDL) receptor domain- and epidermal growth factor (EGF) domain-containing proteins (Table 5).
Table 5.
Enriched functional group among the gene targets of colonic miRNAs 132, 429, 200b, and 150 as predicted by DAVID software
| Annotation Cluster | Enrichment Score | Enriched Functional Group Gene Annotation/Function | Total Gene Count |
|---|---|---|---|
| 1 | 3.36 | Nucleus | 68 |
| Repressor | 15 | ||
| Transcription regulation | 34 | ||
| Transcription | 34 | ||
| DNA binding | 27 | ||
| 2 | 2.28 | Mitosis | 24 |
| Cell cycle | 5 | ||
| Nucleus | 20 | ||
| 3 | 2.01 | Ubl conjugation | 15 |
| Cross-link: Glycyl lysine isopeptide | 6 | ||
| Isopeptide bond | 7 | ||
| 4 | 1.74 | LDL-receptor domain class A 1–8 | 24 |
| LDL-receptor repeat class B 1–6 | 18 | ||
| Endocytosis signal | 3 | ||
| Endocytosis | 4 | ||
| EGF-like domain 1–3 | 17 | ||
| 5 | 1.43 | Lipid moiety binding region: GPI-anchor amidated serine | 5 |
| GPI-anchor | 5 | ||
| Lipoprotein | 12 | ||
| Propeptide | 6 | ||
| 6 | 1.14 | EGF-like domain 1,3–8 | 29 |
A total of 238 genes were the potential target of these four miRNAs. Gene groups with enrichment score >1.00 shown above (The higher the enrichment score, the more enriched that particular group).
LDL, low-density lipoprotein; EGF, epidermal growth factor; GPI, glycophosphatidylinositol; Ubl, ubiquitin.
Colonic miRNAs 23a, 23b, 200c, 92a, and 375 are differentially expressed in WT mice upon DSS treatment.
In WT mice treated with DSS, expression of colonic miRNA 23a, 23b, 200c, 92a, and 375 were downregulated compared with WT control mice, but the expression levels were similar in control and DSS-treated Tg mice (Fig. 5). This indicates that intestinal epithelial CD98 positively regulates expression of these five colonic miRNAs in the inflamed colon. A total of 477 genes are potential targets of these five colonic miRNAs (Supplemental Sheet 1). Functional Annotation Clustering identified a total of 25 clusters; 130 genes were not part of any cluster. The most enriched functional groups (Enrichment Scores >1.00) encoded transcriptional regulators, protein kinases, EGF domain- and LDL receptor domain-containing proteins, actin- and myosin-binding proteins, and calcium-binding proteins (Table 6).
Table 6.
Enriched functional group among the gene targets of colonic miRNAs 23a, 23b, 200c, 92a, and 375
| Annotation Cluster | Enrichment Score | Enriched Functional Group Gene Annotation/Function | Total Gene Count |
|---|---|---|---|
| 1 | 9.17 | Transcription regulation | 71 |
| Nucleus | 128 | ||
| DNA-binding | 64 | ||
| Transcription | 71 | ||
| Activator | 31 | ||
| 2 | 1.86 | Transferase | 43 |
| Serine/threonine protein kinase | 17 | ||
| Nucleotide binding | 48 | ||
| ATP-binding | 90 | ||
| Protein kinase | 41 | ||
| Proton acceptor binding site | 18 | ||
| 3 | 1.61 | Endocytosis | 8 |
| EGF-like domain 1,3 and 6 | 29 | ||
| LDL-receptor domain class A 1–3 | 9 | ||
| LDL-receptor repeat class B 1–6 | 18 | ||
| EGF-like domain 2; calcium binding | 4 | ||
| 4 | 1.44 | EGF-like domain 7–9; calcium binding | 9 |
| EGF-like domain 6 | 5 | ||
| 5 | 1.32 | Myosin | 5 |
| Actin-binding region | 14 | ||
| Myosin head-like domain | 4 | ||
| Calmodulin-binding | 6 | ||
| Motor protein | 5 | ||
| IQ domain | 3 | ||
| 6 | 1.27 | Golgi apparatus | 19 |
| Lumenal topological domain | 15 | ||
| Signal-anchor | 13 | ||
| 7 | 1.25 | Calcium binding region 1–2 | 14 |
| EF hand domain 1–2 | 15 | ||
| 8 | 1.22 | EGF-like domain 1–6 | 30 |
| EGF-like domain 4 and 5; Calcium binding | 6 | ||
| Extracellular matrix | 6 |
A total of 477 genes were the potential target of these 5 miRNAs. Genes with enrichment score >1.00 shown above (The higher the enrichment score, the more enriched that particular group).
Overexpression of CD98 in intestinal epithelial cells modulates colonic miRNAs that target genes in epithelial and nonepithelial cells.
In our experiments, we observed that CD98 expression in IEC specifically modulates the expression of colonic miRNAs. The observed differences in colonic miRNA expression likely reflect changes in both epithelial and nonepithelial (i.e., immune) cells. For this we looked at the mRNA and protein expression of an epithelial protein Tjap1, a potential gene target of miRNA 132 (Supplemental Sheet 1), and a nonepithelial protein CD163, a potential gene target of miRNA 23a and 23b (Supplemental Sheet 1). Tjap1, also referred to as Pilt, is localized toward the cytoplasmic face of tight junctions and in Golgi complex (22). CD163 is specifically expressed in monocytes/macrophages (1) and has been implicated in Crohn's disease (31) and DSS-induced colitis (13). As shown in Fig. 7A, mRNA and protein levels of Tjap1 (molecular weight 61 kDa) were lower in Tg mice treated with DSS compared with Tg control and DSS-treated WT and control mice (Fig. 7A). Tjap1 mRNA and protein levels are in agreement with colonic miRNA 132 expression levels (Fig. 4). mRNA and protein levels of CD163 (molecular weight 130 kDa) were low in WT and Tg mice without DSS treatment. Moreover, the expression of CD163 mRNA and protein was higher in WT mice treated with DSS than in DSS-treated CD98 transgenic mice (Fig. 7B). These results are consistent with the observed colonic expression of miRNA 23a and 23b in WT and Tg mice with or without DSS treatment (Fig. 5). Collectively, these results suggest that overexpression of CD98 in IECs modulates colonic miRNAs that target genes in epithelial and nonepithelial cells.
Fig. 7.
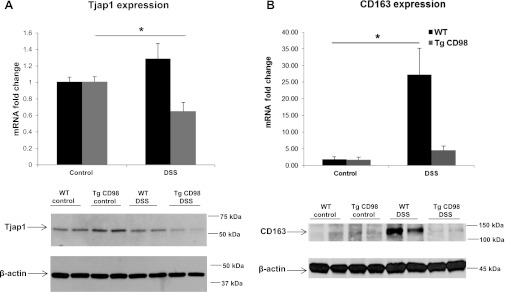
A: tight junction-associated protein 1 (Tjap1) is regulated by colonic miRNA 132 in DSS-treated Tg mice. mRNA and protein expression of Tjap1 (molecular weight 61 kDa), a potential target of colonic miRNA 132, is decreased in DSS-treated Tg mice colon compared with Tg control mice, whereas its expression remains unchanged or nonsignificant in WT DSS-treated and WT control mice. B: CD163 is regulated by colonic miRNA 23a and 23b in DSS-treated WT mice. mRNA and protein expression of CD163 (molecular weight 130 kDa), a potential target of colonic miRNA 23a and 23b, is increased in colonic tissue of DSS-treated WT mice compared with WT control mice, whereas its expression remains low in DSS-treated and control Tg mice. Values represent means ± SE of n = 6/group. *P < 0.05.
DISCUSSION
Intestinal epithelial CD98 expression plays a crucial role in controlling homeostatic and innate immune responses in the gut (33). Such expression is increased during intestinal inflammation, such as occurs in patients with inflammatory bowel disease (IBD) (33). To date, regulation of CD98 expression has been investigated at both the transcriptional and posttranscriptional levels (34, 44). For example, IFN-γ-mediated transcriptional upregulation of intestinal epithelial CD98 has been described. More recently, it has been shown that miRNA 7 modulates epithelial CD98 expression during IEC differentiation (34). The latter study found that miRNAs are involved in posttranscriptional regulation of epithelial CD98 expression. In the present work, we investigated the effect of epithelial CD98 expression on regulation of colonic miRNA expression in the inflamed and noninflamed colon.
We found that CD98 overexpression in IECs did not affect miRNA expression in colonic cells at a basal level. In contrast, we previously demonstrated that intestinal epithelial overexpression of CD98 affected the expression levels of more than 300 genes involved in various biological processes including apoptosis, the defense response, cell proliferation, and cell migration (33). Together, these data suggest that overexpression of CD98 specifically regulates mRNA expression but has no effect on colonic miRNA expression. However, upon exposure to DSS, the expression of some colonic miRNAs but not miRNAs from other tissues such as liver or spleen is differentially regulated in mice overexpressing epithelial CD98. For example, expression of colonic miRNA 132 in WT mice is not affected upon exposure to DSS, but colonic miRNA 132 is upregulated in animals overexpressing intestinal epithelial CD98. Interestingly, miRNA 132 is one of the most highly inducible transcripts known (40). Perhaps inflammation renders CD98 effective to upregulate colonic miRNA 132 levels. Other colonic miRNAs, including miRNA 23b and 92a, are downregulated in WT mice after DSS treatment but not in animals overexpressing epithelial CD98. This shows that the combination of an inflammatory context and expression of intestinal epithelial CD98 affects regulation of colonic miRNA synthesis or stability. In other words, CD98 overexpression in IECs specifically modulates colonic miRNA expression during intestinal colitis.
It has been recently reported that specific human colonic miRNAs are differentially expressed in IBD (9, 36, 42). However, the mechanism underlying this deregulation has not been studied. Consistent with the results of the present study, where we show that expression of miRNA 375 is downregulated in WT mice treated with DSS compared with control, a previous report has shown human miRNA 375 being downregulated in patients with active ulcerative colitis compared with normal individuals (43). However, because gene targets of a given specific miRNA are not necessarily similar among different species, it would be difficult to compare the changes in miRNA expression observed in our mouse experimental model with those obtained in studies on humans. However, our experimental model could serve as a helpful tool for examining how expression of specific proteins regulates miRNAs under intestinal inflammation.
It is well known that a single miRNA can bind to hundreds of mRNA sequences. Thus a change in the expression level of a single miRNA will affect the expression levels of numerous proteins and thus modulate multiple biological functions. For example, epithelial CD98 overexpression induces upregulation of colonic miRNA 132 during colitis. Thus potentially all colonic miRNA 132-targeted gene products will be expressed at lower levels when epithelial CD98 is overexpressed. Our data suggest that genes involved in lipid metabolism and transcriptional regulation, and genes encoding transcriptional regulators with zinc finger motifs, will be downregulated under such conditions.
We found that, in DSS-induced colitis, intestinal epithelial cell CD98 expression regulates colonic miRNAs and their targeted genes, specifically the epithelial Tjap1 and nonepithelial monocyte/macrophage-specific protein, CD163. Studies have shown that, during intestinal inflammation, tight junction proteins play a role in colonic barrier dysfunction (38, 2, 20), and, because Tjap1 is colocalized at the tight junctions (22), it might also be involved in this process. CD163 has been implicated to play a crucial role in controlling inflammation by induction of anti-inflammatory pathways (5, 25, 37, 46). Lower levels of Tjap1 and CD163 in DSS-treated Tg mice colon compared with DSS-treated WT might be one of the reasons for a severe inflammatory response in Tg mice during colitis compared with WT mice. These findings suggest that the changes in colonic miRNA also occur in intestinal macrophages as shown by CD163 expression, in addition to epithelial cells. Moreover, our data support the idea that these changes are initiated by intestinal epithelial CD98 expression. We speculate that, under conditions of intestinal inflammation, upregulated intestinal epithelial cell CD98 transduces signals either directly or indirectly through cell/cell interactions and consequently affects miRNA expression in other cells that are in contact with intestinal epithelial cells. It has recently been demonstrated that a tumor-suppressive miRNA is secreted and transported between cells and exerts a gene-silencing effect in recipient cells (15, 23, 24). Given this important discovery, we cannot rule out the possibility that intestinal epithelial cells that overexpress CD98 secrete colonic miRNAs that are taken up by other cell types, such as intestinal macrophages, resulting in regulation of recipient cell target genes.
We found that, during colitis, expression of epithelial CD98 (via an effect on miRNA synthesis/stability) is linked to the expression of transcription factors, cell cycle proteins, proteins involved in isopeptide binding, and LDL receptor domain- and EGF domain-containing proteins. This is the first report to show that protein expression modulates colonic miRNA synthesis/stability and suggests the presence of regulatory crosstalk between protein and miRNA in diseases such as colitis. Importantly, in our model, upregulation of intestinal epithelial CD98 in an inflamed cellular environment mimics the overexpression of mucosal CD98 in patients with IBD (19, 28). Under such conditions, alteration of colonic miRNA expression levels may play an important role in disease.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and by National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-071594 (to D. Merlin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.A.C., S.A., S.V.S., and D.M. conception and design of research; M.A.C., S.A., and Y.Y. performed experiments; M.A.C., S.A., S.A.I., B.X., E.V., Y.Y., H.L., and D.M. analyzed data; M.A.C., S.A., S.A.I., B.X., E.V., Y.Y., H.L., and D.M. interpreted results of experiments; M.A.C. and S.A.I. prepared figures; M.A.C. and D.M. drafted manuscript; M.A.C., S.A.I., B.X., E.V., H.L., and D.M. edited and revised manuscript; M.A.C., S.A., S.A.I., B.X., Y.Y., H.L., and D.M. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Akila P, Prashant V, Suma MN, Prashant SN, Chaitra TR. CD163 and its expanding functional repertoire. Clin Chim Acta 413: 669– 674, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Amasheh S, Fromm M, Gunzel D. Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201: 133– 140, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Ambros V. The functions of animal microRNAs. Nature 431: 350– 355, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281– 297, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and anti-inflammatory stimuli. J Leukoc Biol 67: 97– 103, 2000. [PubMed] [Google Scholar]
- 6. Bueno MJ, Perez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle 7: 3143– 3148, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Bussey KJ, Kane D, Sunshine M, Narasimhan S, Nishizuka S, Reinhold WC, Zeeberg B, Ajay W, Weinstein JN. MatchMiner: a tool for batch navigation among gene and gene product identifiers. Genome Biol 4: R27, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantor JM, Ginsberg MH, Rose DM. Integrin-associated proteins as potential therapeutic targets. Immunol Rev 223: 236– 251, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Dalal SR, Kwon JH. The role of microRNA in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 6: 714– 722, 2010. [PMC free article] [PubMed] [Google Scholar]
- 10. Dalmasso G, Nguyen HT, Yan Y, Laroui H, Srinivasan S, Sitaraman SV, Merlin D. MicroRNAs determine human intestinal epithelial cell fate. Differentiation 80: 147– 154, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis BN, Hata A. Regulation of microRNA biogenesis: a miRiad of mechanisms. Cell Commun Signal 7: 18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deves R, Boyd CA. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol 173: 165– 177, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Fang K, Bruce M, Pattillo CB, Zhang S, Stone R, 2nd, Clifford J, Kevil CG. Temporal genomewide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiol Genomics 43: 43– 56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390: 81– 85, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Furuta K, Lichtenberger R, Helariutta Y. The role of mobile small RNA species during root growth and development. Curr Opin Cell Biol 24: 211– 216, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Gordon JI. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol 108: 1187– 1194, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154– D158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91– 105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, Ginsberg M, Sethi T. CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J Biol Chem 279: 54731– 54741, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol 590: 1035– 1044, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44– 57, 2009. [DOI] [PubMed] [Google Scholar]
- 22. Kawabe H, Nakanishi H, Asada M, Fukuhara A, Morimoto K, Takeuchi M, Takai Y. Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. J Biol Chem 276: 48350– 48355, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem 287: 1397– 1405, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285: 17442– 17452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kowal K, Silver R, Slawinska E, Bielecki M, Chyczewski L, Kowal-Bielecka O. CD163 and its role in inflammation. Folia Histochem Cytobiol 49: 365– 374, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet 37: 495– 500, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Kucharzik T, Lugering A, Yan Y, Driss A, Charrier L, Sitaraman S, Merlin D. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab Invest 85: 932– 941, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Lasky LA. How integrins are activated. Nature 390: 15; 17, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15– 20, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 115: 787– 798, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Liu S, Russo PA, Baldassano RN, Sullivan KE. CD68 expression is markedly different in Crohn's disease and the colitis associated with chronic granulomatous disease. Inflamm Bowel Dis 15: 1213– 1217, 2009. [DOI] [PubMed] [Google Scholar]
- 32. Merlin D, Sitaraman S, Liu X, Eastburn K, Sun J, Kucharzik T, Lewis B, Madara JL. CD98-mediated links between amino acid transport and beta 1 integrin distribution in polarized columnar epithelia. J Biol Chem 276: 39282– 39289, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen HT, Dalmasso G, Torkvist L, Halfvarson J, Yan Y, Laroui H, Shmerling D, Tallone T, D'Amato M, Sitaraman SV, Merlin D. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest 121: 1733– 1747, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen HT, Dalmasso G, Yan Y, Laroui H, Dahan S, Mayer L, Sitaraman SV, Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem 285: 1479– 1489, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen HT, Dalmasso G, Yan Y, Obertone TS, Sitaraman SV, Merlin D. Ecto-phosphorylation of CD98 regulates cell-cell interactions. PLoS One 3: e3895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis 18: 187– 193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schaer DJ, Boretti FS, Hongegger A, Poehler D, Linnscheid P, Staege H, Muller C, Schoedon G, Schaffner A. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics 53: 170– 177, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci 1165: 294– 300, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity 26: 133– 137, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 102: 16426– 16431, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228– 234, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis 16: 1729– 1738, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135: 1624– 1635 e1624, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Yan Y, Dalmasso G, Sitaraman S, Merlin D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-γ. Am J Physiol Gastrointest Liver Physiol 292: G535– G545, 2007. [DOI] [PubMed] [Google Scholar]
- 45. Yan Y, Vasudevan S, Nguyen HT, Merlin D. Intestinal epithelial CD98: an oligomeric and multifunctional protein. Biochim Biophys Acta 1780: 1087– 1092, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the downregulatory phase of the inflammatory process. Exp Cell Biol 55: 295– 304, 1987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.