Abstract
ANG II is a potent renal vasoconstrictor and profibrotic factor and its activity is enhanced by oxidative stress. We sought to determine whether renal oxidative stress was persistent following recovery from acute kidney injury (AKI) induced by ischemia-reperfusion (I/R) injury in rats and whether this resulted in increased ANG II sensitivity. Rats were allowed to recover from bilateral renal I/R injury for 5 wk and renal blood flow responses were measured. Post-AKI rats showed significantly enhanced renal vasoconstrictor responses to ANG II relative to sham-operated controls and treatment of AKI rats with apocynin (15 mM, in the drinking water) normalized these responses. Recovery from AKI for 5 wk resulted in sustained oxidant stress as indicated by increased dihydroethidium incorporation in renal tissue slices and was normalized in apocynin-treated rats. Surprisingly, the renal mRNA expression for common NADPH oxidase subunits was not altered in kidneys following recovery from AKI; however, mRNA screening using PCR arrays suggested that post-AKI rats had decreased renal Gpx3 mRNA and an increased expression other prooxidant genes such as lactoperoxidase, myeloperoxidase, and dual oxidase-1. When rats were infused for 7 days with ANG II (100 ng·kg−1·min−1), renal fibrosis was not apparent in sham-operated control rats, but it was enhanced in post-AKI rats. The profibrotic response was significantly attenuated in rats treated with apocynin. These data suggest that there is sustained renal oxidant stress following recovery from AKI that alters both renal hemodynamic and fibrotic responses to ANG II, and may contribute to the transition to chronic kidney disease following AKI.
Keywords: fibrosis, reactive oxygen species
acute kidney injury (AKI) is becoming recognized as a significant factor predisposing chronic kidney disease (CKD) (14, 18) but the pathophysiological processes linking AKI and CKD are not well-defined. To investigate the connection between AKI and CKD, several studies have utilized a model of AKI secondary to ischemia-reperfusion (I/R) injury. In rats, total renal blood flow (RBF) is reduced between 50 and 75% within 1 day of reperfusion, which coincides with a dramatic rise in serum creatinine (1, 2, 13). Subsequent to this, recovery ensues and both RBF and creatinine are typically restored to sham-operated control levels within 1–2 wk (2). To regenerate the damaged tubular epithelia, there is a concomitant increase in cell proliferation to replace lost cells. Subsequent migration and redifferentiation rebuild the tubule in a process that is typically complete within 2–4 wk (25, 32, 36).
Previous studies demonstrated that I/R-induced AKI in rats may lead to CKD, which is manifested as proteinuria and interstitial fibrosis that develops slowly following the initial recovery response (3, 5, 6, 12, 26). Several studies also showed a secondary decline in glomerular filtration rate (GFR) and the development of hypertension following recovery from AKI (21, 22, 30) Therefore, postischemic kidneys, even following successful recovery from AKI, may be considered predisposed to CKD.
While regeneration restores GFR and tubular structure without overt disease, the post-AKI kidney is not normal (2, 5, 6, 36). Subtle alterations in renal structure and function may persist representing a potential basis for transition to CKD. For example, permanent peritubular capillary rarefaction following recovery from AKI is evident before renal fibrosis, but may exacerbate hypoxia and contribute to the subsequent development of CKD (5). In addition, there is a persistent alteration in the expression of vasoactive and inflammation-related genes following recovery from AKI (7). Finally, physiological mechanisms controlling renal vascular tone may be altered. For example, postischemic kidneys show impaired pressure-natriuresis and medullary blood flow (MBF) responses to increased renal perfusion pressure (RPP) (8, 28), a feature that may predispose salt-sensitive hypertension in post-AKI rats (34).
Interestingly, altered vascular responses have also been observed in remote (i.e., nonrenal) sites following recovery from AKI. At 5 wk post-AKI, rats demonstrated enhanced systemic pressor responses to ANG II infusions (4). Such responses could also be studied in skeletal muscle arteries either using the in situ cremaster preparation or in isolated gracilis arteries in vitro (4). Recent data suggest that increased ANG II responses result from persistent and generalized oxidative stress that amplifies ANG II activity (29).
Increased oxidant stress is a well-known feature of renal I/R injury. I/R rapidly promotes the generation of superoxide and other reactive oxygen species (ROS) products such as hydrogen peroxide and hydroxyl radical (24). Studies using anti-oxidant treatments suggest that the early rise in ROS contributes to tissue damage and the loss of function in cisplatin-, mercury-, glycerol-, or I/R-induced models of AKI (24). However, there is little known regarding the resolution of oxidant stress following recovery from AKI. Based on the view that AKI predisposes CKD, persistent ROS activity following recovery may represent a potential mediator of progressive CKD.
ANG II is well-known as both a renal vasoconstrictor and as a promoter of renal fibrosis. Because of the link between ROS and increased ANG II responsiveness, we hypothesized that persistent ROS activity following recovery from AKI would alter renal hemodynamic and fibrotic responses to ANG II, features with potential relevance to progression of CKD. Therefore, we utilized a model of recovery from AKI, which is predisposed to develop CKD, and evaluated both acute and chronic ROS-dependent ANG II responses.
METHODS
Animals.
Male Sprague-Dawley rats (initial weight ∼250 g) were utilized in all studies. Care of the rats before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols had received prior approval by the Institutional Animal Care and Use Committees at Indiana University.
Study protocols.
Study I was designed to determine the effect of recovery from I/R injury on the persistent oxidative stress and ROS-induced changes in renal hemodynamic responses to ANG II. The time line and study groups are outlined in Fig. 1. Rats were acclimated to a standard lab diet (AIN 76A, Dyets) containing 0.4% NaCl before surgery. Rats were subjected to bilateral I/R injury or sham surgery and allowed to recover for ∼5 wk (specifically ranging between 33 and 37 days post-I/R); this time point is typically associated with a resolution of serum creatinine and a reestablishment of renal tubular morphology. Some post-I/R rats were treated with apocynin (15 mM) in the drinking water for the final week. Animals were then prepared for studies to examine acute hemodynamic responses to ANG II.
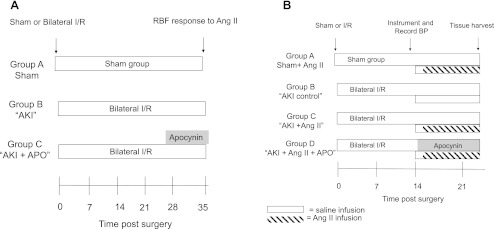
Fig. 1.
Experimental schema to determine the potential role of persistent reactive oxygen species (ROS) activity on alterations of renal function following acute kidney injury (AKI). A: summarizes the design of study I to determine the role of ischemia-reperfusion (I/R) injury and recovery on ANG II-induced alterations in renal hemodynamic responses. Rats are maintained on standard 0.4% NaCl diet during 5 wk of recovery from bilateral I/R or sham surgery. Some rats were treated with apocynin-supplemented drinking water (15 mM) during the final week of recovery. At 5 wk, rats were evaluated for renal hemodynamic responses to ANG II. B: summarizes the time line for study II to determine the role of ROS on blood pressure (BP) and renal fibrotic response to chronic ANG II infusion. At 2 wk following recovery from sham or I/R surgery, animals were chronically instrumented with telemetric BP devices and chronic venous infusions lines. After 2 days of baseline measurements under saline infusion, the increase in blood pressure and fibrosis was determined in response to a 7-day infusion of ANG II. RBF, renal blood flow; APO, apocynin.
Study II was designed to determine the effect of ROS on the development of ANG II-induced renal fibrosis and sustained elevations in blood pressure following recovery from AKI. At day 14 of recovery, rats were chronically instrumented with indwelling venous catheters for infusion and telemetric femoral artery catheters for blood pressure measurements. Rats received saline for 2 days and then either saline or ANG II (100 ng·kg−1·min−1, in saline) for 7 days in a delivery volume of 4 ml/day. In contrast to study I, the chronic infusion of ANG II was initiated at day 16. The initiation of ANG II at this time point was designed to evaluate the effects of ANG II during the final stages of physiological remodeling (25, 32, 36), but following the reestablishment of serum creatinine.
Surgeries.
AKI was induced by bilateral I/R injury to the kidneys by clamping the renal pedicle for 40 min using a surgical approach that has been described previously under anesthesia-induced ketamine (100 mg/kg) and pentobarbital sodium (25–50 mg/kg) (29). To evaluate the degree of injury, tail blood samples (0.2 ml) were collected into heparinized tubes and plasma was obtained for measurement of creatinine (Beckman Creatinine Analyzer II).
Evaluation of renal hemodynamic response to ANG II.
In study I, after 5 wk of recovery from I/R injury or sham, rats were anesthetized and prepared for RBF analysis as described previously (28, 34). Animals were instrumented with an inflatable cuff around the aorta, which served to control RPP. Catheters were placed in both the carotid and femoral artery to measure RPP. The initial mean arterial pressure (MAP) of all rats used in this study was 104 ± 2 mmHg and this was not different between the groups. Anesthetized rats with mean blood pressure <90 mmHg were euthanized and not included in the analysis. Each of the two femoral veins was cannulated for intravenous infusions of either saline or ANG II (Sigma) containing solution. Animals were volume expanded with an intravenous infusion of 2% bovine serum albumin in 0.9% NaCl at a rate of 2 ml·h−1·100 g body wt−1. The perfusion rates through each of the two lines were controlled to allow ANG II infusion to be altered in one line, while the rate of saline was simultaneously reduced; this allowed the net fluid delivery rate to be held constant.
Total RBF was measured using an ultrasonic Doppler flowmeter (model T206, Transonic Systems, Ithaca, NY), while outer MBF was measured by implanting optical probes for laser Doppler flowmetry (Transonic) to a depth of ∼4.0 mm beneath the surface, as described previously (28, 34). Following a 30-min equilibration period, ANG II was delivered at increasing doses for 15 min each, during which time RPP was maintained at baseline levels by adjusting the pressure in the aortic cuff. RBF data were collected and summarized from the final 5 min of stable measurements at each dose.
Measurement of ROS in the kidney.
The evaluation of renal superoxide utilized a modification of the procedure described by Gueler et al. (16) for the oxidation of dihydroethidium (DHE). Kidneys were removed from deeply anesthetized rats and one kidney was rapidly immersed in ice-cold HEPES-Tyrode buffer (132 mM NaCl, 4 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 10 mM HEPES, and 5 mM glucose, pH 7.4) that had been bubbled with 100% O2. Renal tissue slices were prepared using a hand microtome at a thickness of ∼200 μm and stored in cold buffer until use (typically <15 min). The slices were then placed in oxygenated buffer supplemented with 5 μM DHE (Invitrogen) at 37°C for 15 min and the reaction was stopped by immersion of the sections in ice-cold methanol. After 2 days, the slices were counterstained with DAPI and mounted on glass slides with 0.1% Mowiol. Images were obtained using a Zeiss LSM NLO confocal microscope equipped with AR and HeNe lasers and a ×40 water immersion lens. DHE signal was obtained by excitation at 545 nm and detection at 565–615.
For quantification of DHE, a minimum of eight images was obtained from the renal outer medulla using the standard ×1 zoom setting corresponding to an area of 0.053 mm2. Analysis was carried out using Image J software following image thresholding with identical parameters across all images and positive signal was expressed as percent surface area. As a control to validate this method, tempol (100 μM, Sigma) or PEGylated superoxide dismutase (400 U/ml, Sigma) was included in the incubation buffer. This treatment reduced the fluorescent signal by ∼75 or ∼90%, respectively, in post-AKI tissues.
Chronic infusion of ANG II and measurement of blood pressure.
For study II, rats were subjected to a second survival surgery at 14 days to implant the telemetric blood pressure transducers and chronic venous lines as described previously (28, 34). The exteriorized venous infusion line was suspended above the cage using a swivel mount (Harvard Apparatus) to allow free movement of the animal as described (28, 34). The line was connected to a syringe pump for chronic infusion of saline or ANG II. Blood pressure was measured as described previously (8, 27, 34) using DSI Dataquest ART acquisition software.
Renal histology and immunohistochemistry.
Formalin-fixed tissues were embedded in paraffin and stained using Masson's Trichrome stain for assessment of interstitial fibrosis. Tissues were visualized for light microscopy using a Nikon Optiphot-2 upright microscope equipped with a Spot digital camera and image acquisition software (version 3.4.5; Diagnostic Instruments). For quantitative analysis, five random images through renal cortex (∼0.24 mm2) were used to obtain interstitial fibrosis scores. Arbitrary points were overlaid on images using Image J software. For fibrosis scores, a point is counted if it overlays a trichrome-positive interstitial region on the image. In the cortex, the area occupied by the glomeruli was excluded from analysis. Data are expressed as percent points intersecting a fibrotic area. Immunohistochemistry was carried out for S100A4 as previously described (35).
mRNA analysis.
Total RNA samples were obtained from whole kidneys using TRIzol (Invitrogen). Quantitative real-time PCR was conducted using the rat “oxidative stress and antioxidant defense” RT2 Profiler PCR array (rat, no. PARN-065) and the RT2 real-time SyBR Green/Rox PCR kit (all purchased from SA Biosciences, Frederick, MD). Real-time PCR was performed on ABI Prism 7900 HT (Applied Biosystems), according to the manufacturer's instructions (29). Individual Q-PCR primer sets were obtained from SA Biosciences to validate array results and to measure other genes of interest not contained within the array.
Statistical analysis.
All data are expressed as means ± SE. Differences in means were established by Student's t-test or one-way ANOVA with Student-Newman-Keuls post hoc test. For quantitative PCR (Q-PCR), the ΔΔCt method was used with the aid of a Microsoft excel spreadsheet containing algorithms provided by the manufacturer. Fold-changes were then calculated and expressed as log-normalized ratios of values from postischemic/sham-operated tissues. Gene expression changes were considered significant if log-normalized ratio was outside the 95% confidence interval of the mean of all log ratio within the comparison (29). Follow-up analysis of selected genes was carried out by comparing values expressed as 2^Ct and Student's t-test used compare differences between groups.
RESULTS
In study I, serum creatinine values rose in AKI rats to 2.8 ± 0.3 mg/dl within 1 day of I/R. These values returned to levels observed in sham-operated control rats (0.4 ± 0.1 mg/dl) within 5 wk of recovery. Rats treated with apocynin in their drinking water from week 4 to 5 had similar levels of creatinine as those without treatment. Total RBF was evaluated in rats following 5 wk of recovery from AKI. There were no differences in total RBF at baseline in sham-operated control rats vs. post-AKI rats when normalized per kidney (sham 8.9 ± 1.0 ml/min; AKI 9.2 ± 1.0 ml/min) or when normalized per 100 g body wt (sham 2.6 ± 0.3 ml/min; AKI 2.3 ± 0.3 ml/min). However, when normalized per gram of kidney weight, RBF was significantly lower in post-AKI rats relative to sham (sham 7.2 ± 0.8 ml/min; AKI 5.5 ± 0.6 ml/min, P < 0.05 by Student's t-test). The relative lower values in post-AKI rats when expressed in this fashion are due to the ∼30% increase in renal mass. This hypertrophy commonly observed following recovery from AKI may be due to increased interstitial cells, tubular hypertrophy, and/or edema (35, 36). Regardless, the reduced RBF when normalized in this fashion is consistent with our previous report (5).
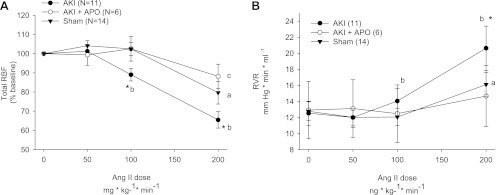
We evaluated the RBF responses to acute ANG II infusion under conditions in which RPP was maintained at baseline levels. ANG II significantly decreased RBF and significantly increased renal vascular resistance (RVR) in sham-operated control rats. By comparison, post-AKI rats manifested a significantly greater response in RBF and RVR compared with shams. Post-AKI rats also showed significant responses at lower doses of ANG II than shams (Fig. 2, A and B). Previous studies showed that apocynin treatment of post-AKI rats abrogates the enhanced peripheral vasoconstriction in response to ANG II (29). In the current study, 1 wk of apocynin abrogated the enhanced RBF response to ANG II (Fig. 2, A and B). In contrast, renal MBF responses measured by laser Doppler were not significantly affected by ANG II infusion and were not significantly influenced by AKI or apocynin (not shown).
Fig. 2.
Changes in total RBF (A) and renal vascular resistance (RVR; B) are shown for anesthetized rats following 5 wk of recovery from either sham operation or bilateral renal I/R. Some animals received APO in the drinking water for 1 wk before evaluation of renal hemodynamic response. N for each group is shown in parentheses. A and B: a, b, and c indicate a change in value relative to baseline within each group, P < 0.05; *P < 0.05 in post-AKI group vs. sham-operated and APO-treated groups, using ANOVA and Student-Newman-Keuls post hoc test.
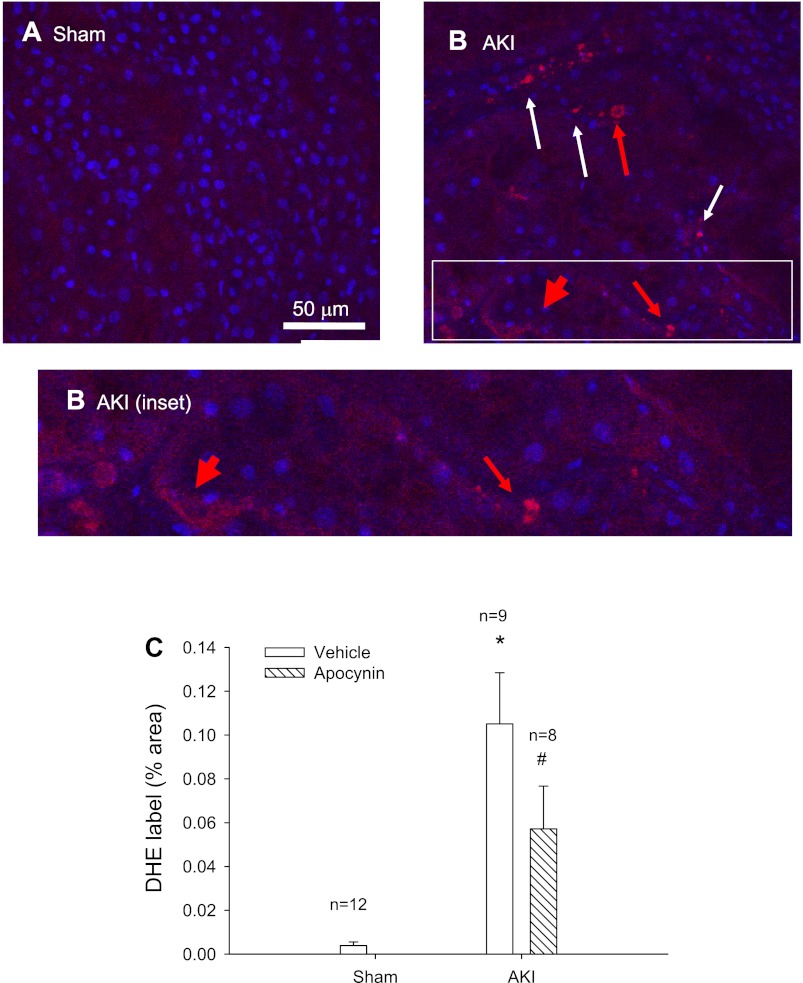
Renal oxidant stress was evaluated by measuring DHE uptake in freshly isolated kidney slices. Under these conditions, no DHE fluorescence was observed in slices obtained from sham-operated control rats (Fig. 3A) while it was consistently detected in several structures throughout kidneys of post-AKI rats. The DHE fluorescence was apparent in punctuate structures with a nuclear morphology (Fig. 3B), which was more apparent in the tubulointerstitial area (white arrows). Much of the fluorescent signal could be localized to nuclei (red arrow). In addition, a more diffuse DHE fluorescence was apparent in the tubular epithelium, which was not clearly localized to the nucleus (thick red arrow). Kidneys following 5 wk of recovery from AKI had significantly higher DHE fluorescence than shams (Fig. 3C), while DHE incorporation was significantly reduced following treatment with apocynin (Fig. 3C).
Fig. 3.
Hydroethidium incorporation into rat kidney tissue slices following recovery from I/R-induced AKI. Shown are representative confocal images through renal outer medulla of a sham-operated rat (A) and a rat at 5 wk of recovery from AKI (B). Hydroethidum fluorescence is shown in red while DAPI counterstaining is shown in blue. Hydroethidium signal was rarely observed in sham-operated samples, while multiple structures evidenced incorporation following recovery from AKI, primarily in unidentified interstitial cells (thin white arrow). Fluorescent signal was also apparent in some nuclear structures (thin red arrow) and diffusely (thick red arrow) in many tubular structures. Magnification is shown in A. C: quantitative analysis of hydroethidium incorporation in rat kidney slices comparing dihydroethidium (DHE) signal derived from tissues of rats following sham surgery, or post-AKI rats treated with or without APO in the drinking water. N refers to the number of different animals in each comparison and values for each comparison derived from analysis of 8 images per animal/treatment. *P < 0.05 post-AKI vs. sham. #P < 0.05 treated AKI groups vs. control AKI group, by Student's t-test.
To investigate further whether recovery from AKI promotes sustained oxidant stress, additional samples were used to measure kidney malondialdehyde (MDA) content. MDA levels were elevated in post-AKI kidneys relative to shams after 5 wk of recovery (0.34 ± 0.1 vs. 0.63 ± 0.06 nmol/mg protein; n = 4 per group, P < 0.05 by Student's t-test).
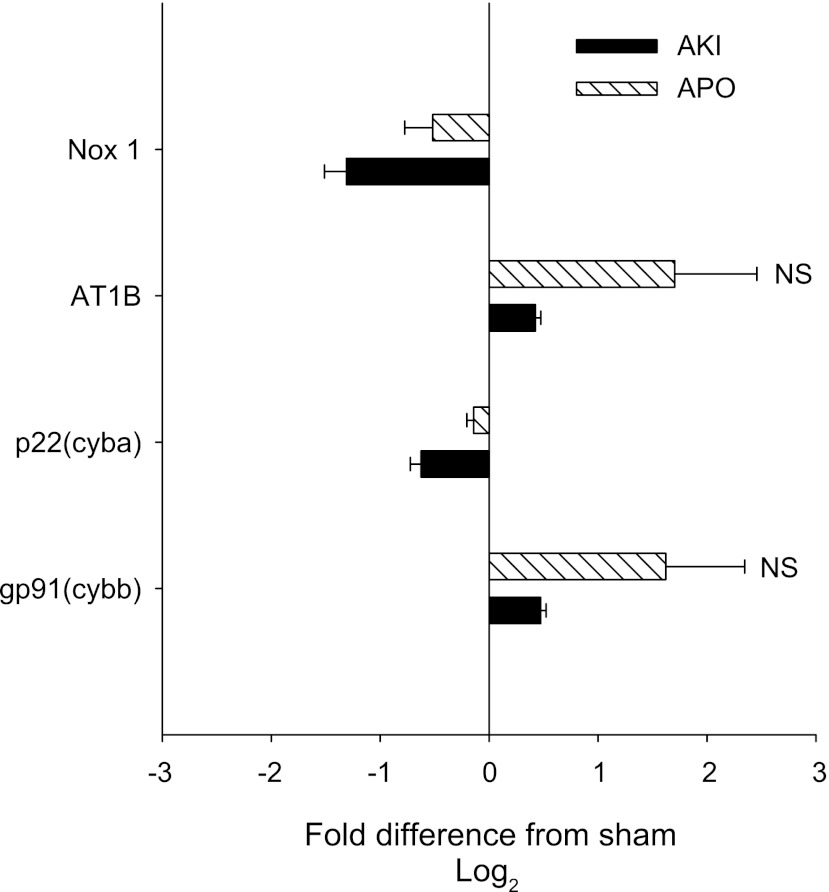
We next sought to determine whether 5 wk of recovery from AKI was associated with altered expression of genes with functions related to oxidant stress. The effects of AKI on the expression of NADPH oxidase subunits and ANG II receptor mRNA are shown in Fig. 4. Surprisingly, AKI did not significantly alter the renal expression of Nox1, p22 (cyba), gp91, and AT1B mRNA relative to sham-operated controls. Moreover, apocynin did not significantly alter the expression of these mRNAs. Further studies utilized a Q-PCR pathway array to evaluate the expression of multiple oxidant-related genes. In addition to the NADPH oxidase genes measured in Fig. 4, the Q-PCR array contained primers for Nox-4, which was also not affected by AKI (Fig. 5).
Fig. 4.
Quantitative (Q)-RT-PCR analysis of NADPH oxidase-associated genes in kidney following recovery from AKI. Data are derived from total RNA from whole kidney of sham-operated, AKI-, and AKI-APO-treated rats. Values for 2^ΔCt were generated by normalizing to RPLP1 gene expression. The data are expressed as Log(2) ratios relative to the sham-operated control, such that lower expression in the AKI relative to sham is indicated by a negative value, while a higher expression is indicated by a positive value. No values were significantly different by Student's t-test. Note the Nox4 gene expression is shown in Fig. 5.
Fig. 5.
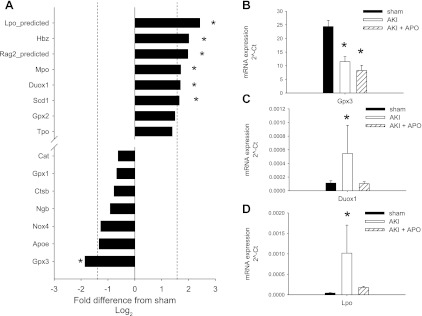
Q-RT-PCR analysis of oxidant-associated genes in kidney following recovery from AKI. Total RNA derived from whole kidney of sham-operated rats and rats following recovery from I/R using “oxidant stress and anti-oxidant” pathway arrays in kidneys of sham and post-AKI rats (see methods). A: data are expressed as Log(2)-normalized ratios relative to the expression in sham; therefore, greater expression in AKI vs. sham corresponds to a positive value and a lower expression in AKI is represented by a negative value. Shown are data from 14 of the 84 genes evaluated (the 7 most upregulated and 7 downregulated). A 95% confidence interval of Log(2) ratios was determined to be ±1.6 and was used to define significance (dotted line). B, C, and D: data from individual Q-PCR verification of array results for the GPX3, dual-oxidase 1 (Duox1), and lactoperoxidase (LPO) genes, respectively, in a wider number of samples (n = 6 per group) and in APO-treated rats. Data are expressed as means ± SE of 2^ΔCt values normalized to the RPLP1 genes. *P < 0.05 relative to sham control by Student's t-test.
However, up to six mRNAs were identified as significantly enhanced and one mRNA was significantly repressed in post-AKI kidneys relative shams (Fig. 5A). Some of these genes were subjected to further validation across a larger number of tissue samples. Follow-up Q-PCR analysis of the most abundantly expressed gene on the array, Gpx3, verified that it was significantly downregulated in post-AKI kidneys (Fig. 5B). Another gene, dual-oxidase 1 (Duox-1), was chosen for further validation because it has homology with NADPH oxidase (19); its expression was also enhanced in post-AKI kidneys relative to sham (Fig. 5C). Finally, we also verified the prominent induction of lactoperoxidase (LPO) mRNA in kidney following AKI (Fig. 5D). Both Duox-1 and LPO mRNA expression were significantly inhibited by treatment with apocynin.
Previous studies showed that AKI enhances the acute pressor response to ANG II (4, 29), but the effects of chronic ANG II stimulation on blood pressure are not known. Moreover, while tubular regeneration is complete within 2–5 wk without prominent renal fibrosis (6), progressive CKD can be hastened using interventions such as elevating sodium intake or reduced renal mass (5, 6, 34, 36). Therefore, study II was conducted to determine whether ROS activity enhanced the ability of ANG II to promote renal fibrosis and/or hypertension following recovery from AKI.
Baseline MAPs ranged between 105 and 107 mmHg before ANG II infusion, and these values were not different among experimental groups. Chronic infusion of ANG II (100 ng·kg−1·min−1) elevated blood pressure significantly in all groups. In response to ANG II, there was no difference in MAP of AKI rats (164 ± 7 mmHg) relative to sham rats (154 ± 4 mmHg). However, when post-AKI rats were treated with apocynin, the MAP response to ANG II was significantly lower (141 ± 5 mmHg, P < 0.05; a single outlying data point satisfying Grubb's criteria was removed for this analysis).
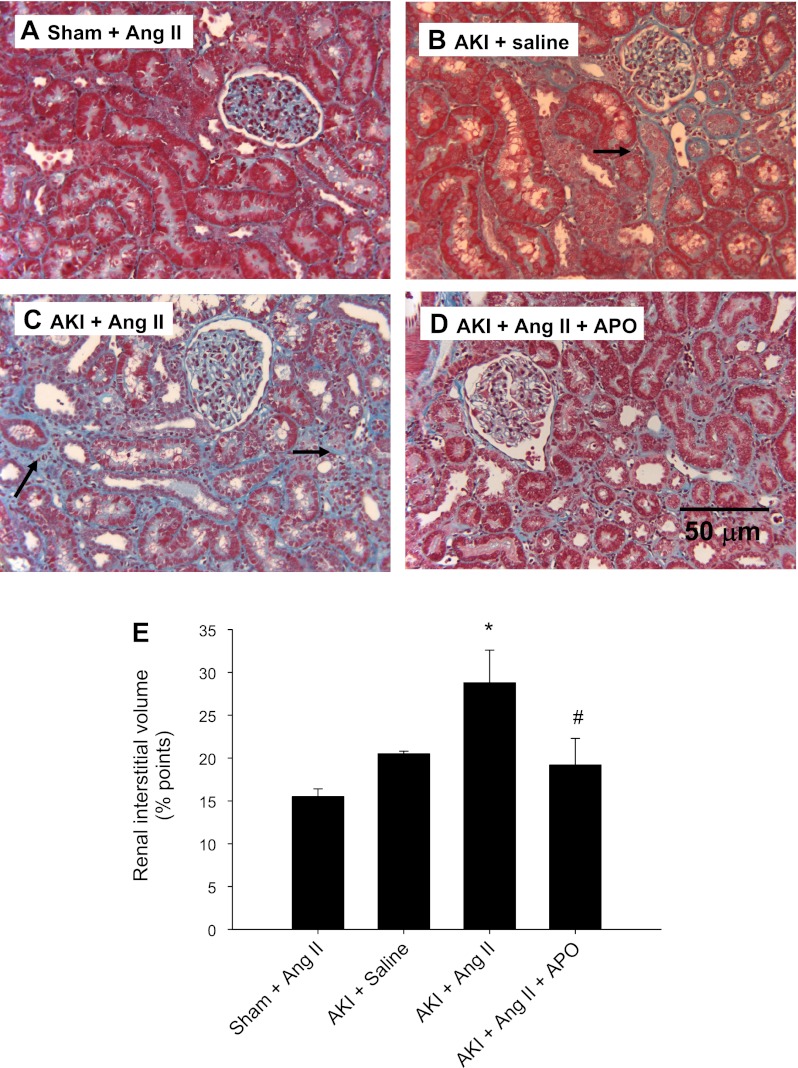
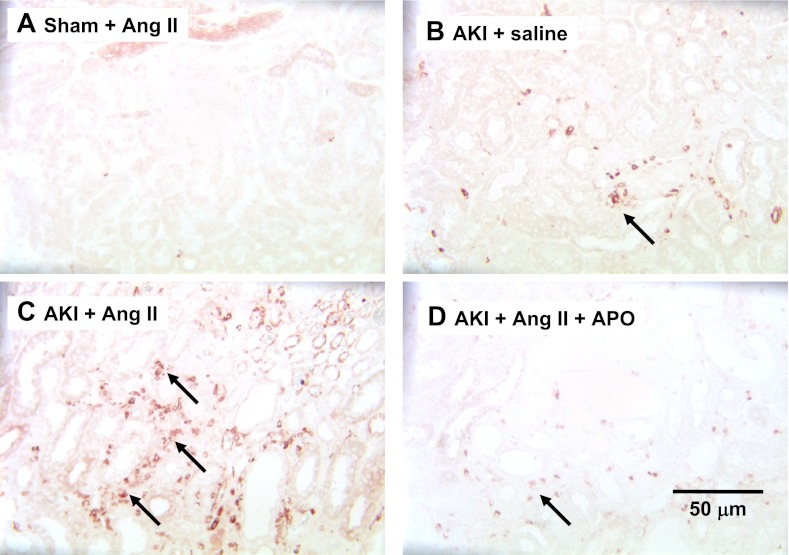
The effects of AKI, ANG II, and apocynin on renal structure are shown in Fig. 6. ANG II infusion into sham rats did not result in evidence of interstitial fibrosis (Fig. 6). Relative to these rats, AKI resulted in a small increase in tubulointerstitial space that was significantly enhanced by ANG II infusion (Fig. 6B vs. 6C). Apocynin treatment significantly reduced the fibrotic effect of ANG II in post-AKI rats (Fig. 6, D and E). In addition, we examined the deposition of renal fibroblasts by S100A4 immunohistochemistry. S100A4-positive cells were prominent in post-AKI rat kidneys following ANG II infusion relative to post-AKI rats infused with saline alone, while the S100A4 staining was reduced in rats treated with apocyinin (Fig. 7).
Fig. 6.
Effect of chronic ANG II infusion on renal morphology and interstitial fibrosis in sham-operated and postischemic rats. Representative Masson's trichrome-stained sections through rat renal cortex are shown from a sham-operated rat (A), post-AKI rat supplemented with saline alone (B), a post-AKI rat supplemented with ANG II (C), and a post-AKI rat with ANG II and APO (D). Trichrome-positive interstitial areas were apparent following AKI but more prominent following ANG II infusion (black arrows), and less apparent in APO-treated rats. Magnification is shown in D. E: quantitative analysis of interstitial volume scores based on trichrome-stained sections derived from counting the number of points in arbitrary grid that overlay the interstitial space. Data are means ± SE. * And #P < 0.05 in ANG II-AKI group relative to sham and AKI-ANG II and APO-treated groups, respectively.
Fig. 7.
Effect of chronic ANG II infusion on S100A4-containing cells in kidney following AKI. Representative immunohistochemical images through rat renal outer medulla are shown from a sham-operated rat infused with ANG II (A), post-AKI rat supplemented with saline alone (B), a post-AKI rat supplemented with ANG II (C), and a post-AKI rat with ANG II and APO (D). Black arrows indicate positively stained interstitial cells most prevalent in ANG II infusion into AKI rats. Magnification shown in D.
DISCUSSION
The mechanisms linking AKI and progressive CKD have remained elusive. Hypoxia, which may be promoted by capillary rarefaction post-AKI (5), is a predominant feature leading to interstitial fibrosis by activating the synthesis of a number of profibrotic genes and the production of extracellular matrix (11). ANG II plays a critical role in blood pressure and fluid homeostasis, but it also plays an active role in tissue remodeling. ANG II has direct profibrotic activity by increasing the expression of extracellular matrix genes and by enhancing the activity of a number of interstitial cell types such as macrophages, myofibroblasts, fibrocytes, and lymphocytes (21). Hemodynamic alterations are also likely to participate in renal fibrosis induced by ANG II either by exposing the kidney to elevated perfusion pressure (23) or by enhancing hypoxia secondary to vasoconstriction (21).
Many studies over the last decade described the synergistic relationship between ROS and ANG II signaling (15, 30, 31). Recently, it has been shown that recovery from AKI in rats induces a prolonged state of systemic vascular hyperreactivity to ANG II for at least 5 wk post-I/R (4, 29). However, post-AKI rats do not have elevated renin or circulating ANG II relative to shams (4, 29). The increased ANG II responsiveness can be observed in isolated skeletal muscle blood vessels and was shown to be a function of oxidant stress since the responses were abrogated in vitro by either tempol or apocynin (29).
The current study extends these observations and verifies that the renal vasculature shares a similar enhanced sensitivity to ANG II following recovery from AKI. Renal vasoconstriction to ANG II was evaluated by measuring RBF under conditions of constant RPP. A motivation for this approach is that ANG II evokes a stronger systemic pressor response in post-AKI rats vs. sham rats (29), providing a potentially stronger myogenic stimulus. When controlling for this, the current results suggest that the increased vasoconstriction is based on enhanced ANG II signaling within the kidney. The renal vasoconstriction may be related to increased oxidant stress since these responses were normalized by treatment with apocynin.
It is tempting to speculate that the altered hemodynamic responses to ANG II may predispose renal disease or hypertension following AKI. Enhanced vasoconstriction particularly in the renal medulla may exacerbate hypoxia in this region. However, we were not able to demonstrate any definitive MBF response to ANG II following AKI.
Previous studies demonstrated the importance of ROS on the manifestation of AKI (24), but few studies investigated prolonged oxidant stress following recovery from AKI. Recently, Kim et al. (20) reported that markers of oxidant stress (i.e., lipid peroxidation and tissue hydrogen peroxide levels) were elevated within 1 day of I/R in the mouse kidney. The induction of these markers was not transient and they remained sustained at peak levels for at least 2 wk as repair and regeneration ensued. Furthermore, the administration of a superoxide dismutase mimetic ameliorated the degree of renal scaring observed following recovery, suggesting that oxidant stress participates in the AKI to CKD transition.
Our results are consistent with and advance the results from Kim et al., by demonstrating persistent oxidant stress 5 wk following recovery from I/R-induced AKI in rats. We recently reported that post-AKI rats had elevated circulating MDA levels following 5 wk of recovery from AKI (29). In addition, kidney tissue MDA content was increased by about twofold, but provided no information on the cellular sources of oxidant stress. Therefore, we investigated sites of DHE incorporation in freshly isolated kidney slices and demonstrated that the most prominent uptake occurred in the interstitial area, with typical strong nuclear signal in this region. The specific cell type/types are not yet known, but the localization is consistent either with vascular cells, or in one of the many types of interstitial cells deposited following AKI. In tubular epithelial cells, nuclear staining was less prominent than in interstitial cells, but there was widespread diffuse labeling in many tubular structures. Whether such staining correlates with superoxide activity is unclear. However, no similar signal was ever observed in kidneys from sham rats and it was abolished by anti-oxidant treatment in vitro (not shown). These results suggest that both interstitial and tubular compartments contribute to oxidant stress post-AKI and may contribute to altered vascular responses.
The cellular and molecular mechanisms mediating increased ROS post-AKI are not completely identified. Given the sensitivity of RBF responses to the putative NADPH oxidase inhibitor apocynin, it was surprising that NADPH oxidase-related mRNAs were not chronically influenced by AKI. Although potentially a source of controversy, these seemingly incongruent results are consistent with our prior study on gracilis artery following AKI. In that study, gracilis arteries showed no evidence of increased NADPH oxidase expression, but apopcynin treatment in rats blocked DHE incorporation and reduced ANG II responses in vitro (29).
Several potential interpretations exist for the lack of increased NADPH oxidase and the effect of apocynin: 1) apocynin is acting with general antioxidant properties rather than as an NADPH oxidase inhibitor (17), 2) NADPH-dependent oxidant signal derives from a small proportion of the kidney cells, too small to detect from whole kidney extracts. In support of this possibility, De Miguel et al. (10) measured increased NADPH oxidase subunits in T cells isolated from Dahl salt-sensitive (S) rats placed on high-salt diet, but the increase could not be measured using whole kidney extracts. In recent studies, we demonstrated persistent deposition of activated T-lymphocytes at 5 wk following recovery from AKI (8).
Finally, a third possibility regarding the effect of apocynin on AKI-induced ROS relates to changes in the activity of other factors that influence superoxide metabolism. A decrease in anti-oxidant defenses might result in increased ROS generation without altering NADPH activity. For these reasons, it is noteworthy that our microarray approach indicated the sustained reduction in the anti-oxidant Gpx3 following I/R. Similarly, Kim et al. (20) previously demonstrated a persistent reduction in superoxide dismutase and catalase activity post-AKI. These observations suggest that increased oxidant stress following regeneration may result from a failure to reestablish anti-oxidant defenses.
In addition, our microarray approach identified several other prooxidant genes, potentially influencing disease progression, such as myeloperoxidase (22). We also identified the increased expression of Duox-1 in the postischemic kidney. To the best of our knowledge, this represents the first report of this gene being expressed in a model of kidney injury or disease. Importantly, Duox-1 shares homology with NADPH oxidase (19), so it is possible that Duox-1 may mediate some of the effects described here. However, such speculation should be tempered due to a limited understanding of its role in vivo.
Our prior studies focused on ROS-dependent modulation of acute ANG II responses, but they provided no information regarding the potential role of ROS on ANG II-induced renal fibrosis or the maintenance of elevated blood pressure. Therefore, we carried out studies on post-AKI rats subjected to chronic infusions of ANG II.
In contrast to our acute studies (4, 29), chronic ANG II infusion over 7 days did not result in a sustained increases in blood pressure following AKI. Importantly, ANG II significantly enhanced postischemic fibrosis, and this response was blocked by apocynin treatment. Studies by Mori and Cowley (23) demonstrated the combined effect of direct vs. pressure-mediated activity on renal fibrosis. Whether the increased fibrosis observed in our study is due directly to ANG II-mediated fibrotic activity or additional effects of added perfusion pressure could not be determined in the current experiment. However, these studies do support a role for persistent oxidant stress in this process.
There is a developing view that numerous alterations in renal structure or function following injury predispose CKD. Sustained hypoxia, impaired tubular regeneration, altered renal vascular responses, and activation of immune cells have all been suggested to mediate the AKI to CKD transition (2, 8, 9, 36). The results of the current study support the view that unresolved oxidant stress following recovery from AKI has the potential to alter renal function and represents an additional pathway that may contribute to the progression of CKD.
With regard to potential clinical relevance, recent studies suggest that too few post-AKI patients receive significant follow-up care despite the prevalence of long-term residual effects of AKI (33) . Such studies highlight the need that additional criteria are needed to identify patients at risk for developing CKD following AKI. The assessment of oxidant stress represents a relatively simple target to evaluate during follow-up, which may identify patients with susceptibility for chronic complications. As there is an increasing interest in evaluating long-term outcomes of AKI, future studies should incorporate an assessment of oxidative stress as a potential predictive marker for development of CKD.
GRANTS
This work was supported by National Institutes of Health Grant DK063114 to D. Basile. A. G. Beal and D. Schleuter were supported by the Indiana University Purdue University-Indianapolis Undergraduate Life Health Science Internship program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.B. conception and design of research; D.P.B., E.C.L., A.G.B., D.S., and J.L.F. analyzed data; D.P.B. interpreted results of experiments; D.P.B. and J.L.F. prepared figures; D.P.B. drafted manuscript; D.P.B. edited and revised manuscript; D.P.B. approved final version of manuscript; E.C.L., A.G.B., D.S., and J.L.F. performed experiments.
REFERENCES
- 1. Arendshorst WJ, Finn WF, Gottschalk CW. Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res 37: 558–568, 1975 [DOI] [PubMed] [Google Scholar]
- 2. Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–13, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to ANG II following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 289: R1770–R1776, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia following ischemia/reperfusion injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Basile DP, Fredrich K, Alausa MT, Vio C, Liang M, Greene AL, Cowley AW., Jr Identification of persistently altered gene expression in kidney following functional recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 288: R953–R963, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am J Physiol Renal Physiol 302: F625–F635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int 67: 1002–1009, 2005 [DOI] [PubMed] [Google Scholar]
- 10. De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int 57: 2375–2385, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Gellai M, Jugus M, Fletcher T, DeWolf R, Nambi P. Reversal of postischemic acute renal failure with a selective endothlin-A receptor antagonist. J Clin Invest 93: 900–906, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldstein S, Devarajan P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol 26: 509–522, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Greiendling K, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–504, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Gueler F, Rong S, Mengel M, Park JK, Kiyan J, Kirsch T, Dumler I, Haller H, Shushakova N. Renal urokinase-type plasminogen activator (uPA) receptor but not uPA deficiency strongly attenuates ischemia reperfusion injury and acute kidney allograft rejection. J Immunol 181: 1179–1189, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but is an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawahara T, Quinn M, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7: 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 297: F461–F470, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int 64: 1956–1967, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Nath K, Norby S. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304: 905–912, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Pagtalunan M, Olson J, Tilney N, Meyer T. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol 10: 366–373, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Pechman K, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium sensitive hypertension following recovery from acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phillips SA, Pechman KR, Leonard EC, Friedrich JL, Bian JT, Beal AG, Basile DP. Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol 298: R1682–R1691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajagopalan PR, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seshiah P, Weber DS, Rocic P, Valppu L, Taniyama Y, Greiendling K. Angiotensin II stimulation of NADPH oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Tubular dilatation in the repair process of ischaemic tubular necrosis. Virchows Arch 425: 281–290, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Siew ED, Peterson JF, Eden SK, Hung AM, Speroff T, Ikizler TA, Matheny ME. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 23: 305–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Spurgeon KS, Donohoe DL, Basile DP. Transforming growth factor-β in acute renal failure: receptor expression, influence in cell proliferation, cellularity and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]