Abstract
Spinal cord transection (SCT) leads to an increase in spontaneous contractile activity in the isolated bladder that is reminiscent of an overactive bladder syndrome in patients with similar damage to the central nervous system. An increase in interstitial cell number in the suburothelial space between the urothelium and detrusor smooth muscle layer occurs in SCT bladders, and these cells elicit excitatory responses to purines and pyrimidines such as ATP, ADP, and UTP. We have investigated the hypothesis that these agents underlie the increase in spontaneous activity. Rats underwent lower thoracic spinal cord transection, and their bladder sheets or strips, with intact mucosa except where specified, were used for experiments. Isometric tension was recorded and propagating Ca2+ and membrane potential (Em) waves were recorded by fluorescence imaging using photodiode arrays. SCT bladders were associated with regular spontaneous contractions (2.9 ± 0.4/min); ADP, UTP, and UDP augmented the amplitude but not their frequency. With strips from such bladders, a P2Y6-selective agonist (PSB0474) exerted similar effects. Fluorescence imaging of bladder sheets showed that ADP or UTP increased the conduction velocity of Ca2+/Em waves that were confined to regions of the bladder wall with an intact mucosa. When transverse bladder sections were used, Ca2+/Em waves originated in the suburothelial space and propagated to the detrusor and urothelium. Analysis of wave propagation showed that the suburothelial space exhibited properties of an electrical syncitium. These experiments are consistent with the hypothesis that P2Y-receptor agonists increase spontaneous contractile activity by augmenting functional activity of the cellular syncitium in the suburothelial space.
Keywords: spinal cord injury, spontaneous contraction, Ca2+ waves, ADP
the overactive bladder in patients is associated with spontaneous transient increases in pressure that occur particularly during filling and, if sufficiently severe, contribute to lower urinary tracts symptoms of frequency, urgency, and incontinence (1). The origins of this common pathology are unclear but can be mimicked in animals in which the bladder outflow is artificially obstructed or the spinal cord is transected (SCT; T8–T9) (12). Whole rat bladders from an SCT model demonstrate large, regular contractions (∼2/min) that coincide with waves of raised intracellular Ca2+ and depolarization (Em) propagating across the surface but originating from one or very few foci. This is in contrast to normal animals where more frequent (5–10/min), low-amplitude contractions are associated with multiple Ca2+/Em foci (12, 14). The detrusor layer of the bladder wall contributes the bulk of tissue and hence most of the Ca2+/Em signals, but an intact mucosa (urothelium and suburothelium) is required to generate large propagating waves, as well as to enhance spontaneous contractile activity (13, 25).
A cellular target for an understanding of such activity is a population of suburothelial interstitial cells (ICs) (24). In response to physiological stimuli such as mechanical stretch, the urothelium releases transmitters such as ATP and acetylcholine (9, 29), and it is proposed that ICs are intermediates in the pathways upon which these transmitters act. The overactive bladder is associated with an increase in IC number in animal and human tissue (12, 21); agents that target c-kit receptors on ICs, such as Glivec, reduce spontaneous activity (3), and ICs generate large depolarizing responses to transmitters, such as ATP (28). Excitatory responses from suburothelial ICs to ATP are via P2Y agonists; ADP and UTP generate similar responses to ATP itself (28), and the most readily labeled purinoceptor is the P2Y6 subtype (26). P2Y agonists induce relaxation of the detrusor muscle itself (16). Thus one paradigm for studying the influence of the mucosa and ICs on spontaneous activity is to characterize the action of ADP and other P2Y agonists, such as UDP and UTP, on the contractile function and intercellular signaling of bladder wall tissue with an intact mucosa from SCT animals.
METHODS
Animal preparations.
Bladders were isolated from adult Sprague-Dawley rats that had SCT at level T8–T9 as described previously (12). Briefly, rats were operated under anesthesia with an isoflurane/O2 mixture (2%-98%), and after a laminectomy the dura and spinal cord were cut. Gelfoam was packed between the cut ends, the muscle and skin were sutured, and the animals were allowed to recover with prophylactic antibiotics (100 mg ampicillin or 2 mg·kg−1·day−1 gentamycin for 2 wk). For these first 2 wk following the operation, bladders were emptied two to three times per day by manual abdominal compression, until the animals' own micturition reflexes recovered. Sham-operated animals received the same operative preparation but without the laminectomy and did not have postoperative abdominal compression. Animals were used between 4 and 6 wk after the operation. All procedures conformed to institutional guidelines and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee or followed European Community Council Directive 86/609/EEC and the UK Animal Act (1986) as appropriate.
Optical imaging and force measurements.
SCT rats that were to be used for combined optical imaging and force measurements were euthanized by CO2 inhalation and exsanguination. The bladders were removed following a laparotomy and placed immediately in Tyrode's solution (below). Bladders were loaded with the Ca2+- and Em-sensitive fluorochromes Rhod-2A and di-4-ANEPPS, respectively, and two types of preparation were prepared. A sheet preparation was generated by cutting the bladder longitudinally, from base to dome, along the ventral surface containing the mucosa or with the mucosa removed from half the area by blunt dissection. The preparation was placed in a recording chamber, impaled at one end with pins, and at the other end tied to an isometric force transducer. The preparation was superfused at 1–2 ml/min with Tyrode's solution at 37°C. Intracellular Ca2+ and Em were measured by an optical imaging system that used 16 × 16 photodiode arrays to record 256 separate images that mapped the preparation surface at 500 Hz. Isochronal maps were generated to record the progression of Ca2+/Em waves, centered on transients with the shortest delays as the initiating points. The second preparation imaged transverse sections of the bladder wall. The bladder wall was sliced with a sharp razor, and the exposed edge was placed uppermost in a superfusion chamber to permit optical imaging of the edge. The methods have been described in detail previously (13, 14).
Isolated detrusor strips (5 mm length, <1 mm diameter) from SCT rat bladders were also used. The bladder was opened as a sheet, and the mucosa was removed or left intact before strips were cut from the dorsal wall of the dome. Preparations were tied between a fixed hook and an isometric force transducer and superfused at 37°C with Tyrode's solution, as above. Spontaneous contractions were recorded continuously. At the end of each experiment, the wet weight of the preparation was recorded to normalize tension measurements.
Solutions.
The Ca2+-free solution contained (mM) 114 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11.7 glucose, pH 7.4 gassed with 5% CO2-95% O2. Tyrode's solution contained (mM): NaCl 118, KCl 4.0, MgCl2 1.0, NaH2PO4 0.4, NaHCO3 24, CaCl2 2.5, glucose 6.1, Na pyruvate 5.0, pH 7.4 gassed with 5%CO2-95%O2. Intervention agents were added from aqueous 10 mM stock solutions to achieve the desired concentration in Tyrode's solution.
Immunofluorescence and confocal imaging.
Bladders from adult male Sprague-Dawley rats (350 g) were quickly removed, washed with PBS, and the mid-cross sections were embedded in OCT and then snap frozen in liquid nitrogen. Cryosections (10 μm) were then placed on poly-l-lysine-coated slides and stored at −20°C until use. Cryosections were fixed with 4% paraformaldehyde for 4 min at room temperature (RT), washed with PBS, and then incubated with a blocking buffer (PBS, 1% BSA, 0.1% Triton-X) for 1 h at RT followed by overnight incubation at 4°C in the primary antibody (rabbit anti-P2Y6, 1:200 Sigma-Aldrich). Sections were then washed with PBS and incubated for 1 h at RT with the secondary antibody (goat anti-rabbit IgG Alexa Fluor 594, 1:200, Millipore). To identify cellular nuclei, the fluorescent nucleic acid binding dye TO-PRO-3 Iodide (1:300, Invitrogen) was added with the secondary antibody. After a final step of washing, slides were air dried and mounted with Vectashield (Vector Laboratories). Slides were viewed at ×60 magnification (oil-immersion lens) using a Zeiss inverted laser-scanning confocal fluorescent Axiovert 135 microscope. Images were acquired using Zen 9000 software and analyzed with Zeiss axovision and Image-J software.
Data presentation, analysis, and calculations.
Data are shown as means ± SD; n is the number of preparations. Differences between data sets were tested by Student's paired or unpaired t-tests; the null hypothesis was rejected at P < 0.05. Spontaneous activity, frequency, and amplitude in detrusor strip preparations were counted using a custom-designed macro after digitization of the primary data.
Intracellular resistivity, Ri, of the suburothelial cellular network was calculated from the one-dimensional cable equation for a uniformly conducting electrical response (7)
| 1 |
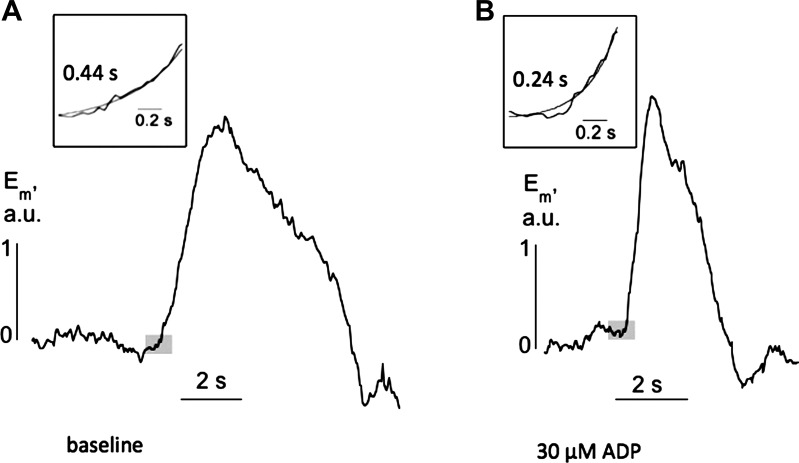
where a is the cell radius, Cm is the specific membrane capacitance (1 μF/cm2), τm is the membrane time constant of the cell propagating the signal, and τ* is the time constant of the exponential base of the propagating signal (see Fig. 7).
Fig. 7.
Individual propagating membrane potential transients from a transverse section of a SCT rat: A: in baseline conditions. B: in the presence of 30 μM ADP. Insets: basal regions of the transients used to calculate the variable τ*. Shaded boxes show the regions from which the insets were analyzed.
RESULTS
Spontaneous contractile activity in SCT rat preparations: effect of purines.
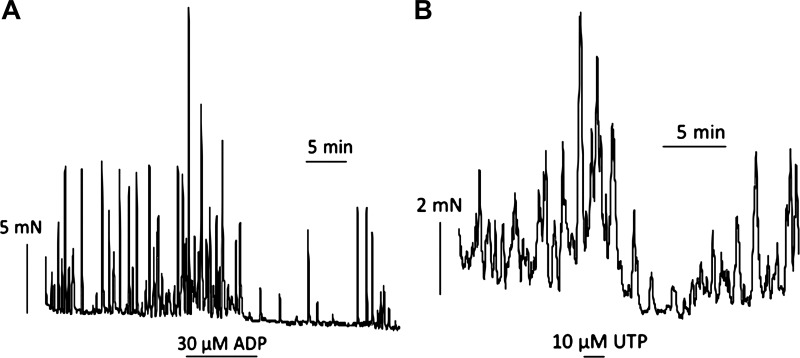
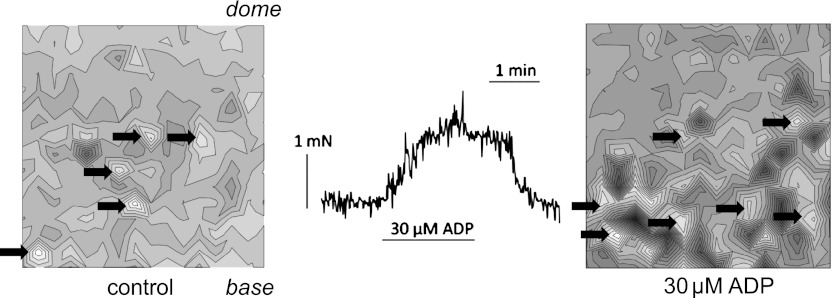
All bladder preparations from SCT rats exhibited spontaneous contractions; the mean tension was 21.5 ± 5.7 mN/g, at a frequency of 2.9 ± 0.4/min (SE, n = 8). Figure 1A shows the effect of 30 μM ADP on spontaneous activity with several consistent observations: 1) a large initial contraction; 2) an increase in contraction magnitude during the exposure; and 3) on removal of ADP, a transient reduction of spontaneous activity before a return to preintervention activity. Table 1 shows the amplitude and frequency of contractions before (baseline conditions) and during ADP, as well as the coefficient of variation (mean/SD) for the two variables. ADP significantly increased the contraction amplitude but had no effect on frequency. Furthermore, the coefficient of variation of these variables was unaffected by ADP, which indicates that the coordination of the pathways responsible for generating the contraction within the tissue mass was not altered by ADP. Figure 1B shows the response to UTP (10 μM), and Table 1 shows that similar conclusions were derived compared with ADP. Contraction amplitude was increased, but frequency, or the variability of these characteristics, was unaltered. Figure 1 shows that ADP and UTP also generated a significant rise in baseline tension (8.7 ± 6.3 mN/g, n = 9), and thus a final contractile characteristic was the tension integral, measured for 10 min before and during the intervention from a baseline of the preintervention tension. ADP and UTP both significantly increased the tension integral (Table 1). Two experiments each with ADP and UDP also showed increased amplitude and tension integral, but no effect on frequency of spontaneous contractions.
Fig. 1.
Spontaneous contractile activity from spinal cord transected (SCT) rat bladder sheets from an intact mucosa. A: exposure to 30 μM ADP. B: exposure to 10 μM UTP for durations as indicated by the lengths of the horizontal bars.
Table 1.
Effect of 30 μM ADP or UTP on spontaneous contractions in mucosa-intact bladder preparations from SCT rats
| Amplitude, mN/g | Amplitude, Coefficient of Variation | Frequency, no./min | Frequency, Coefficient of Variation | Integral, mN·min−1·g−1 | |
|---|---|---|---|---|---|
| Baseline | 30.6 ± 21.1 | 0.35 ± 0.16 | 2.8 ± 1.0 | 0.51 ± 0.21 | 14.5 ± 3.9 |
| ADP (n = 7) | 42.9 ± 18.7* | 0.57 ± 0.20 | 3.1 ± 1.2 | 0.39 ± 0.14 | 23.1 ± 4.0* |
| Baseline | 23.8 ± 19.0 | 0.58 ± 0.34 | 2.6 ± 1.2 | 0.53 ± 0.20 | 11.9 ± 3.4 |
| UTP (n = 8) | 50.1 ± 32.5* | 0.62 ± 0.52 | 2.7 ± 0.7 | 0.41 ± 0.18 | 34.6 ± 24.7* |
Values are means ± SD. SCT, spinal cord transected. The amplitude and frequency of the contractions as well as their coefficients of variation (= SD/mean) are quoted. The integrals of these contractions above the baseline are also given.
P < 0.05.
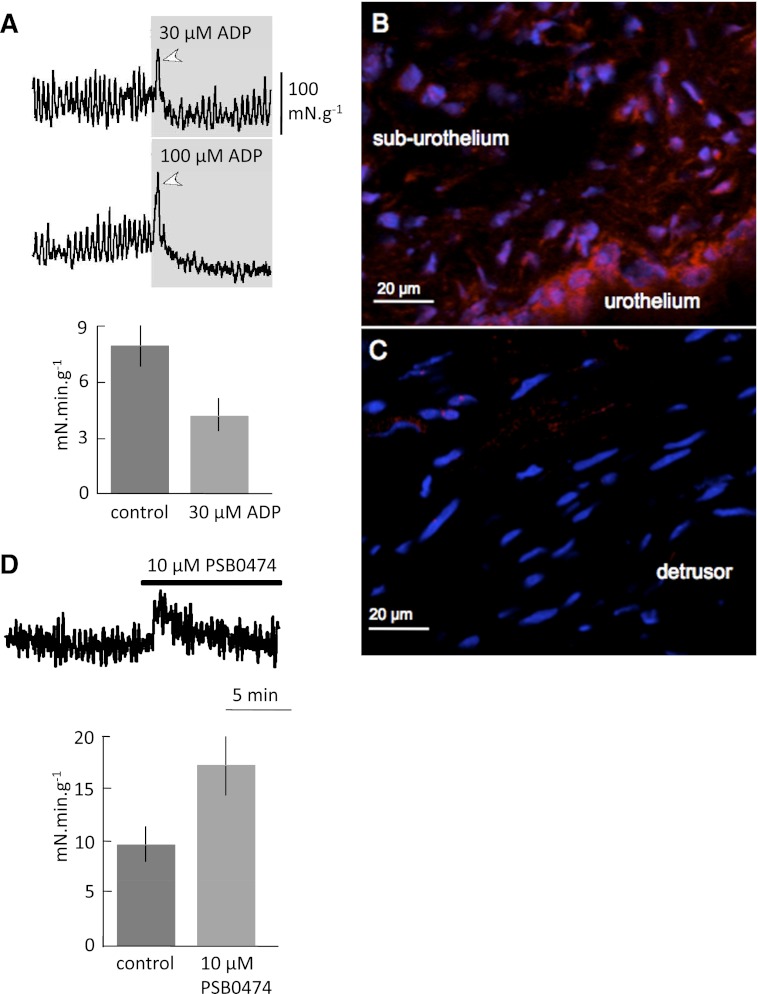
Experiments using ADP with small strips of detrusor from SCT animals from which the mucosa had been removed are shown in Fig. 2A. With 30 or 100 μM ADP, there was a large contraction on initial exposure (arrowhead) but then a reduction of baseline tension and the amplitude of spontaneous contractions. The tension integral was also reduced, illustrated for 30 μM ADP. There was no significant effect on the frequency of spontaneous contractions generated by these strips (2.5 ± 0.3 vs. 1.9 ± 0.2/min; baseline vs. ADP, n = 3).
Fig. 2.
A: Spontaneous contractile activity from detrusor strips, with mucosa removed, of SCT rat bladders. Top traces show response to 30 and 100 μM ADP added for the duration shown in the grey boxes. The bar chart shows the tension integral in baseline conditions and in the presence of 30 μM ADP. B: P2Y6 receptor labeling (red) in the rat mucosa; nuclei are labeled with TO-PRO-3 (blue). C: P2Y6 and TO-PRO-3 labeling in the detrusor layer. D: detrusor strips with intact mucosa. Top trace shows response to 10 μM PSB0474, added for the duration of the thick bar above the trace. The bar chart shows the tension integral in baseline conditions and in the presence of 10 μM PSB0474.
Suburothelial ICs in guinea pigs were readily labeled by P2Y6 immunofluorescent markers (26). We sought to verify that similar labeling was present in the rat bladder wall. Figure 2B shows that labeling was observed in the suburothelial space as well as the urothelium. In the suburothelium, P2Y6 labeling was observed near TO-PRO-3-labeled nuclei and thus was considered to have a cellular distribution; more intense labeling was also detected in the urothelium, similar to that in guinea pig bladders (26). In the detrusor layer, labeling was very sparse (Fig. 2C); only a few punctate labels were observed. Similar observations were made in sections from three different bladders. Therefore, the effects of a P2Y6-selective agonist (PSB0474) on basal tone and spontaneous contractions were measured in detrusor strips from SCT rats with an intact mucosa. PSB0474 (10 μM) significantly increased basal tension, as well as the amplitude of spontaneous contractions (Fig. 2D) but had no significant effect on their frequency (2.9 ± 0.4 vs. 2.8 ± 0.3/min; baseline vs. PSB0474, n = 3). The tension integral was also increased significantly by PSB0474 (Fig. 2D). These effects of PSB0474 were similar to those of ADP (tension integral increased from 7.8 ± 13 to 10.9 ± 1.4, n = 6) and to ADP or UTP on the bladder sheets with an intact mucosa (Fig. 1), and unlike the effect of ADP on mucosa-denuded muscle strips. Note that removal of the mucosa reduced the baseline amplitude of the spontaneous contractions (1.4 ± 0.05 vs. 0.50 ± 0.09 mN/mg; n = 6; P < 0.001), and increased frequency (2.2 ± 0.6 vs. 3.5 ± 0.2; n = 6; P < 0.05).
Action of ADP and UTP on Ca2+/Em waves in bladder sheets.
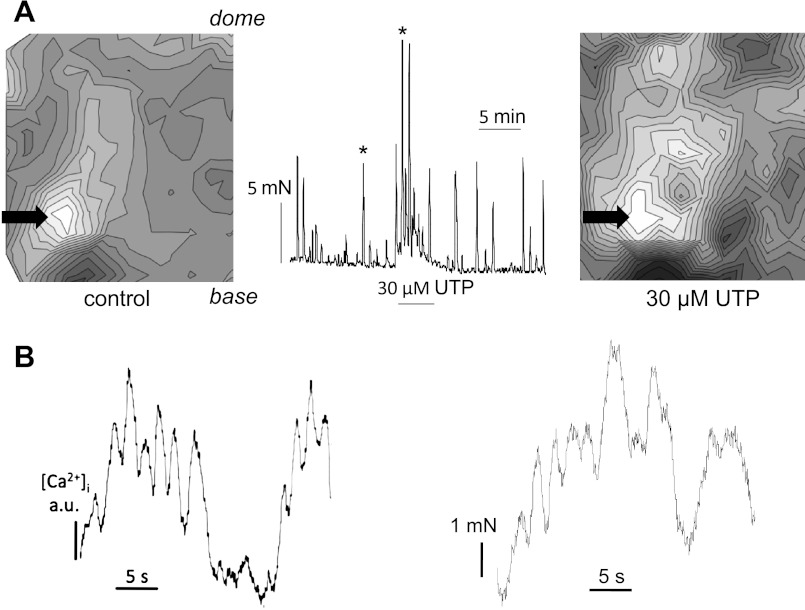
Spontaneous contractions in bladders from SCT rats are associated with propagated Ca2+ and Em waves originating from one or very few focal sites (12, 14). These experiments aimed to determine whether ADP and UTP 1) enhanced these waves and 2) required an intact mucosa. Figure 3A shows Ca2+ waves from a SCT rat bladder sheet with an intact mucosa. The left-hand map was obtained in baseline conditions; the lightest areas correspond to where Ca2+ transients occurred with the shortest delay and darker areas to successively longer delays. A single focal area was observed near the base of the bladder (arrow). In four preparations, Ca2+ waves propagated in the base-dome axis at 4.8 ± 1.7 mm/s in baseline conditions; the corresponding velocity for Em waves was 5.6 ± 1.6 mm/s. Conduction in the lateral plane was generally slower, as seen by the closer isochrones, with velocities of 0.8 ± 0.3 and 1.3 ± 0.9 mm/s for Ca2+ and Em waves, respectively. The right-hand map shows a corresponding Ca2+ wave map in the presence of 30 μM UTP. The tension trace between the maps corresponds to the experiment from which they were obtained; the two arrows correspond to contractions in baseline conditions (Fig. 3A, left) and in the presence of 30 μM UTP (Fig. 3A, right). UTP increased basal contractile tone and the amplitude of spontaneous contractions; the Ca2+ wave map was taken when enhancement of contractile activity was maximum and at steady state. The focal initiation site in the Ca2+ wave map was enhanced, particularly in the base-dome axis to a wider area of the preparation, although it did not alter the origin. In this example, conduction velocity was increased from 4.5 to 6.5 mm/s. The acceleration of conduction velocity may be expected to coordinate a larger area of the bladder surface and thus increase the magnitude of the subsequent contraction.
Fig. 3.
Ca2+ wave maps from bladder sheets with an intact mucosa (isochronal interval 100 ms). The base and dome regions of the sheets are indicated in the left-hand maps. A: SCT rat bladder. Maps show sheets in the baseline condition (left) and in the presence of 30 μM UTP (right). The tension trace corresponding to the experiment from which the maps were constructed is shown. Arrows mark the spontaneous contractions corresponding to the 2 maps. UTP was added for the duration of the bar below the trace. The arrows indicate the origins of the propagating wave. B: correspondence between the time course of a Ca2+ transient from a single pixel and contractile activity in a bladder preparation during exposure to 30 μM ADP.
Figure 3B shows that Ca2+ transients were indeed associated with contractile activity. The example, obtained in the presence of 30 μM ADP, shows Ca2+ transients from one pixel in an optical imaged array near to the focal point of Ca2+ wave propagation and contractile activity of the preparation. The similarity of the traces suggests contraction is associated with these Ca2+ waves.
Spontaneous Ca2+ waves and contractile activity in normal rat preparations.
Figure 4 shows an example of Ca2+ waves and spontaneous contractions in a bladder sheet from an unoperated animal with an intact mucosa, demonstrating multiple foci (arrows). Addition of ADP did not coalesce the small, individual foci but preserved the pattern; the same observation was made in three preparations. The corresponding tension trace shows that ADP generated a rise in basal tension overlain throughout by small spontaneous contractions. ADP (30 μM) had no significant effect on the frequency (8.0 ± 1.8 vs. 9.0 ± 1.2/min; n = 3, P > 0.05) or amplitude (1.08 ± 0.20 vs. 1.07 ± 0.06 mN/g; n = 3, P > 0.05) of spontaneous contractions; however, basal tone increased significantly (4.27 ± 1.91 mN/g; n = 3). Data were also obtained from small mucosa-attached detrusor strips of six sham-operated animals. ADP (30 μM) again had no effect on the frequency (9.4 ± 0.5 vs. 8.7 ± 0.4/min, n = 9, P > 0.05) or amplitude (0.98 ± 0.16 vs. 0.76 ± 0.24 mN/mg; n = 6, P > 0.05) of spontaneous contractions, but did increase basal tension by a small amount 0.17 ± 0.01 mN/mg, n = 6, i.e., 17.3 ± 2.4% of the baseline spontaneous contraction amplitude.
Fig. 4.
Ca2+ wave maps for a bladder sheet from an unoperated animal. Maps show the sheet in baseline solution (left) and in the presence of 30 μM ADP (right) and the corresponding tension trace. ADP was added for the duration of the bar below the trace. Arrows mark focal origins of activity.
Role of the mucosa in Ca2+/Em waves.
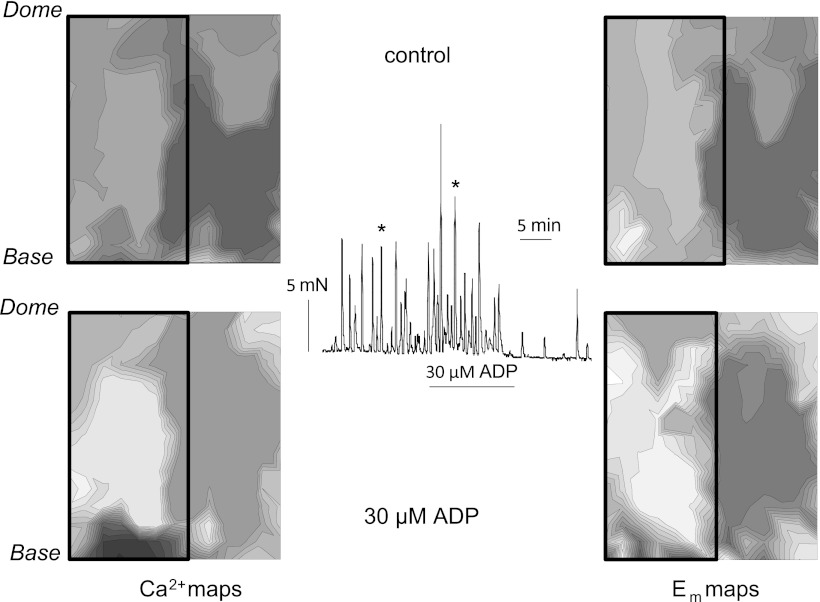
Previous experiments have shown that an intact mucosa enhances spontaneous detrusor contractile activity (25). Its importance in the generation of Ca2+/Em waves, and any role of ADP, was also investigated. Figure 5 shows an experiment using SCT rat bladders from which half the mucosa had been removed; the intact portion is denoted by the boxes on the left side of each of the Ca2+ and Em maps. In baseline conditions (top maps) prominent Ca2+ and Em foci were generated on the half with an intact mucosa, although there was a smaller, secondary wave in the denuded half of the preparation in both maps. ADP increased the distance between isochrones in both the Ca2+ and Em maps (bottom maps), although the position of the focal points did not greatly alter. This indicates that in the presence of ADP, conduction of Ca2+ and Em waves was accelerated in the region with an intact mucosa, although the spread to the denuded portion remained slow. In this example, the Ca2+ wave conduction velocity was enhanced from 4.8 to 8.6 mm/s and Em wave conduction velocity from 5.6 to 9.3 mm/s in the mucosa-intact region.
Fig. 5.
Ca2+ (left) and membrane potential (Em; right) maps from a SCT rat bladder sheet. The mucosa was removed from the right-hand side of the sheet; the box marks the area of intact mucosa. Maps were obtained before (top) and during (bottom) the application of 30 μM ADP. Arrows on the tension trace mark the spontaneous contractions corresponding to the maps. ADP was added for the duration of the bar below the trace.
Action of ADP and UTP on Ca2+/Em waves in bladder transverse sections.
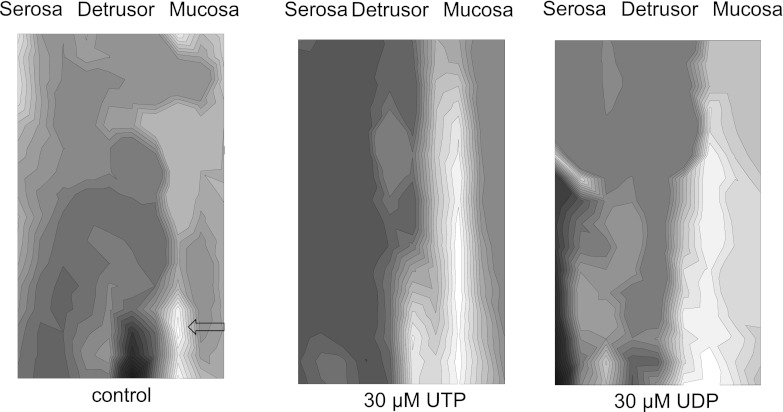
When bladder sections were imaged, Ca2+ and Em waves originated from the mucosal surface and propagated eventually to the detrusor layer. Figure 6 shows Ca2+ isochronal maps in baseline conditions (left) and in the presence of 30 μM UTP (middle) or UDP (right). In this example, under baseline conditions two small areas of focal activity (arrows) were observed on the mucosa/detrusor boundary. Propagation of signals was quicker in the longitudinal direction (top to bottom), along the suburothelial layer and slower in the transverse direction, especially in the direction toward the detrusor, as seen by the higher density of isochrones. It was clear that the wave origins are submucosal (i.e., not at the extreme right of the maps). This observation was preserved when activity was enhanced by UTP or UDP and is consistent with the hypothesis that spontaneous activity arises within the suburothelial region of the bladder wall. Similar observations were made in the presence of ADP (not shown). Under baseline conditions, longitudinal and transverse conduction velocities (CVL and CVT) were 0.5 ± 0.2 and 0.09 ± 0.02 mm/s, respectively (n = 4). CVL was increased significantly (P < 0.05, n = 4) to 2.3 ± 1.1, 1.2 ± 0.5 and 1.9 ± 0.5 mm/s in the presence of ADP, UDP, and UTP, respectively. Similarly, CVT was increased significantly to 0.5 ± 0.1, 0.5 ± 0.2 and 0.5 ± 0.2 mm/s in the presence of ADP, UDP, and UTP.
Fig. 6.
Ca2+ maps from a transverse section of a SCT rat bladder in the baseline condition (left) and during the subsequent application of 30 μM UTP and 30 μM UDP. The positions of 2 loci for Ca2+ waves are indicated by the black arrow on the baseline map. The UTP map was obtained immediately after the baseline map. The UDP map was obtained after 15-min washout of UTP.
Electrical characteristics of the suburothelial space.
In many preparations, Em waves spread along the suburothelial space from a focal point (Fig. 6) at a velocity much greater (>10-fold) than in the transverse plane. Under such circumstances, signals were considered to be one-dimensional within a functional syncitium, and this permitted estimation of the resistance, Ri, of the cellular network (ICs): Eq. 1. These cells have fine projections from a central body with a radius, a, of ∼0.25 μm (27) and a value for τm of 70 ms (28). Figure 7 shows typical individual Em transients from the area of suburothelial propagation under baseline conditions (Fig. 7A) and in the presence of 30 μM ADP (Fig. 7B). The value of τ* (Eq. 1) was estimated from the basal region of the transient (shaded boxes, Fig. 7) and had a value of 0.45 ± 0.16 s under baseline conditions (n = 11, 4 preparations) and decreased to 0.27 ± 0.07 s (n = 12, 4 preparations) in the presence of ADP or UTP (data combined). Values for a longitudinal conduction velocity of 0.27 ± 0.097 cm and 0.37 ± 0.099 cm/s (n = 4) in the absence and presence of ADP/UTP allowed calculation of Ri and yielded values of 1,095 ± 357 and 903 ± 121 Ω·cm, respectively, and were not significantly different. Thus Ri was similar in both conditions, and the increase in conduction velocity was due to a decrease in the variable τ* (see below).
DISCUSSION
Spontaneous activity in spinal cord injury.
SCT at the T8–T9 level generates an overactive bladder phenotype of large, fairly regular bladder contractions. The objective of this study was to gain insight into the cellular and tissue pathways contributing to this phenotype. This overactive behavior was mirrored in isolated bladder preparations by increased spontaneous contractile activity, suggesting that at least part of the increased response is myogenic (6, 12). With bladders from unoperated animals, spontaneous activity consisted of smaller and more frequent contractions and was mirrored by optical imaging as multiple focal points of activity. Although increased spontaneous activity in bladders from SCT rats may be due in part to altered properties of detrusor muscle, this and other studies (13) suggest that an intact mucosa is important for full expression of increased spontaneous activity. Moreover, optical mapping of transverse sections of the bladder wall showed here that Ca2+ and Em activity originated in the mucosa and propagated to the detrusor. The concordance between contractile activity and optical signals of propagating Ca2+ and Em waves suggests a causal relationship, and we propose that such activity originates in the suburothelial space. It is not possible to determine whether mucosal-detrusor interaction is due to a diffusible agent released from the mucosa or whether it requires a physical connection. However, there was a considerable delay of signal at the mucosa-detrusor border, which may be interpreted as a requirement for a diffusible factor at least at this site. In addition, these experiments also showed that activity originated not at the apical edge of the mucosa but rather in the suburothelial layer and spread both toward the urothelium as well as to the detrusor. In principle, Ca2+ an Em signals could spread across the urothelium, as this region labels strongly for the gap junction protein Cx26 (10); however, these experiments lacked the spatial resolution to confirm this fact.
Action of purines and pyrimidines on spontaneous activity.
The contractile responses in SCT preparations were significantly augmented by exogenous purines (ADP and ATP) and pyrimidines (UTP and UDP). They were mirrored by an increase in Ca2+ and Em signaling in bladder sheets with an intact mucosa and in the mucosal layer of transverse sections, all suggesting an involvement of P2Y receptors (19). These agents all ultimately produce relaxation of isolated detrusor smooth muscle (2, 5, 16), and preservation of the relaxatory response to ADP in detrusor from SCT animals was confirmed in these experiments. Thus it is unlikely that the increased contractile and Ca2+/Em activity is due to a direct action on the detrusor layer. No single P2Y receptor is likely to be responsible for the augmentation of activity considering that a range of purine and pyrimidines produced similar changes to spontaneous activity. P2Y1 receptors are activated most effectively by ADP, whereas UTP is a potent activator of P2Y2 and P2Y4 receptors (19). The P2Y6 receptor is most potently activated by UDP, and the role of this receptor was corroborated by an increase in spontaneous activity by the selective agonist PSB0474. P2Y1, P2Y2, and P2Y4 receptors have all been localized to the urothelium in the cat bladder (4). Furthermore, strong labeling for P2Y6 receptors was found in suburothelial ICs in the guinea pig bladder, with weaker labeling of P2Y2 and P2Y4 receptors but none for P2Y1 (26).
The ability of P2Y agonists to augment spontaneous activity, especially in the overactive bladder, could arise from ATP that is released from the urothelium/suburothelium during stresses such as bladder wall stretch and raised transmural pressure. ATP is readily broken down to ADP and other metabolites by endonucleotidases present in the bladder wall (22) that could act on ICs to generate excitatory responses. Moreover, there is evidence that such a system is upregulated in the overactive bladder to produce the larger and better coordinated spontaneous contractions, including an increase in IC number (25) and augmented ATP release (15, 17). We propose that this P2Y-mediated pathway represents a target for the reduction of overactive bladder spontaneous contractions.
Syncitial properties of the suburothelial cellular network.
Immunohistochemical studies have demonstrated that a major site for the gap junction protein Cx43 is suburothelial ICs, suggesting that they are capable of transmitting intercellular signals (24). These experiments provide direct evidence for signal propagation along the suburothelial layer, more quickly than transversely to the detrusor or urothelium. In a subset of experiments, longitudinal transmission was more than 10-fold greater than in transverse dimensions, and thus it was possible to treat this layer as a one-dimensional multicellular syncitium with only ∼10% error (20). Using a solution of the cable equation for signals of constant propagation velocity (methods), a value for intracellular resistivity of ∼1,000 Ω·cm was calculated both in baseline conditions and with P2Y agonists. This value is comparable to 600–800 Ω·cm in smooth muscle (18, 23) but greater than that in better-coupled syncitia, such as ventricular myocardium (226 Ω·cm) (8). The important conclusion is that the suburothelial space has the required electrical properties to permit relatively rapid propagation of signals over distances of many cell lengths and thus permit coordination of significant areas of the bladder wall.
Increased conduction velocity with P2Y agonists was not due to a reduction in Ri but rather to a decrease in the value of τ*. The parameter τ* represents the initial depolarization rate of a propagating wave and is determined by the strength of local circuits from adjacent regions undergoing electrical activity; the magnitude of local circuits in turn depends largely on the density of inward currents generating the electrical response (11). Because P2Y agonists increase inward currents in ICs (28), this will accelerate the propagation rate of electrical signals within the suburothelium to enhance the coordination of signals. This analysis gives further weight to the hypothesis that ICs in the suburothelial space act as a functional syncitium whose coordinating role can be augmented by locally produced exogenous agents.
Conclusion.
Bladders from SCT rats generate large spontaneous contractions and propagating waves of intracellular Ca2+ and membrane depolarization, which require an overlay of mucosa on the detrusor smooth muscle. These activities are augmented by P2Y receptor agonists such as ADP, UTP, and UDP. The Ca2+ and Em waves originate in the suburothelium of the mucosa and spread to the detrusor, a phenomenon accelerated by P2Y receptor agonists. The mucosa may be modeled as a functional synctium to account for wave propagation. This study shows the crucial importance of the mucosa in determining the contractile properties of bladders with an overactive phenotype and the influence of purinergic modulators that are generated by the urothelium.
GRANTS
This work was funded by grants to C. H. Fry, R. I. Jabr (EU FP7 INComb, Pfizer), J. S. Young (AgeUK), and A. J. Kanai (NIH/NIDDK DK 057284).
DISCLOSURES
C. H. Fry is a member of an advisory board to Eli Lilly and is carrying out a joint project with Y. Takeda.
AUTHOR CONTRIBUTIONS
Author contributions: C.H.F., J.S.Y., R.I.J., C.M., Y.I., and A.J.K. provided conception and design of research; C.H.F., J.S.Y., R.I.J., C.M., and Y.I. performed experiments; C.H.F., J.S.Y., and R.I.J. analyzed data; C.H.F., J.S.Y., R.I.J., C.M., Y.I., and A.J.K. interpreted results of experiments; C.H.F. and R.I.J. prepared figures; C.H.F. drafted manuscript; C.H.F., J.S.Y., R.I.J., C.M., Y.I., and A.J.K. edited and revised manuscript; C.H.F., J.S.Y., R.I.J., C.M., and A.J.K. approved final version of manuscript.
REFERENCES
- 1. Abrams P. Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology 62: 28–37: 2003 [DOI] [PubMed] [Google Scholar]
- 2. Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107: 603–613, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Biers SM, Reynard JM, Doore T, Brading AF. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int 97: 612–616, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287: F1084–F1091, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L. The biphasic response of rat vesical smooth muscle to ATP. Br J Pharmacol 114: 1557–1562, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brading AF. A myogenic basis for the overactive bladder. Urology 50, Suppl 6A: 57–67, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Chapman RA, Fry CH. An analysis of the cable properties of frog ventricular myocardium. J Physiol 283: 263–282, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooklin M, Wallis WR, Sheridan DJ, Fry CH. Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ Res 80: 765–771, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haefliger JA, Tissières P, Tawadros T, Formenton A, Bény JL, Nicod P, Frey P, Meda P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res 274: 216–225, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Hunter PJ, McNaughton PA, Noble D. Analytical models of propagation in excitable cells. Prog Biophys Mol Biol 30: 99–144, 1975 [DOI] [PubMed] [Google Scholar]
- 12. Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293: F1018–F1025, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Renal Physiol 295: F454–F461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry CH. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar V, Chapple CR, Rosario D, Tophill PR, Chess-Williams R. In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur Urol 57: 1087–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 16. McMurray G, Dass N, Brading AF. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br J Pharmacol 123: 1579–1586, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munoz A, Smith CP, Boone TB, Somogyi GT. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem Int 58: 295–300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohba M, Sakamoto Y, Tokuno H, Tomita T. Impedance components in longitudinal direction in the guinea-pig taenia coli. J Physiol 256: 527–540, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 20. Rihana S, Lefrançois E, Marque C. A two dimension model of the uterine electrical wave propagation. Conf Proc IEEE Eng Med Biol Soc: 1109–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Roosen A, Datta SN, Chowdhury RA, Patel PM, Kalsi V, Elneil S, Dasgupta P, Kessler TM, Khan S, Panicker J, Fry CH, Brandner S, Fowler CJ, Apostolidis A. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur Urol 55: 1440–1448, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Stella J, Bavaresco L, Braganhol E, Rockenbach L, Farias PF, Wink MR, Azambuja AA, Barrios CH, Morrone FB, Oliveira Battastini AM. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol 28: 260–267, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Sui GP, Coppen SR, Dupont E, Rothery S, Gillespie J, Newgreen D, Severs NJ, Fry CH. Impedance measurements and connexin expression in human detrusor muscle from stable and unstable bladders. BJU Int 92: 297–305, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Sui GP, Wu C, Roosen A, Ikeda Y, Kanai AJ, Fry CH. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol 295: F688–F697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int 97: 1327–1331, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 2321–243, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S. Non-neuronal cholinergic system in human bladder urothelium. Urology 67: 425–430, 2006 [DOI] [PubMed] [Google Scholar]