Abstract
The adult kidney contains a population of low-cycling cells that resides in the papilla. These cells retain for long periods S-phase markers given as a short pulse early in life; i.e., they are label-retaining cells (LRC). In previous studies in adult rat and mice, we found that shortly after acute kidney injury many of the quiescent papillary LRC started proliferating (Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. J Am Soc Nephrol 20: 2315–2327, 2009; Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. J Clin Invest 114: 795–804, 2004) and, with cell-tracking experiments, we found upward migration of some papillary cells including LRC (Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. J Am Soc Nephrol 20: 2315–2327, 2009). To identify molecular cues involved in the activation (i.e., proliferation and/or migration) of the papillary LRC that follows injury, we isolated these cells from the H2B-GFP mice and found that they migrated and proliferated in response to the cytokine stromal cell-derived factor-1 (SDF-1). Moreover, in a papillary organ culture assay, the cell growth out of the upper papilla was dependent on the interaction of SDF-1 with its receptor Cxcr4. Interestingly, location of these two proteins in the kidney revealed a complementary location, with SDF-1 being preferentially expressed in the medulla and Cxcr4 more abundant in the papilla. Blockade of Cxcr4 in vivo prevented mobilization of papillary LRC after transient kidney ischemic injury and worsened its functional consequences. The data indicate that the SDF-1/Cxcr4 axis is a critical regulator of papillary LRC activation following transient kidney injury and during organ repair.
Keywords: cytokines, precursor cell, adult stem cell, acute kidney injury
the kidney papilla contains a population of cells with several characteristics of organ-specific stem cells (46, 47). The cells are easily detectable because, like other adult stem cells (8, 12, 14, 24, 31, 62), they are low cycling and retain S-phase labels when given as a short pulse followed by a long time of chase. To mark the cells, the label is administered during a period of intense growth (e.g., embryonic life or shortly after birth) so that all cells incorporate the label but during the following period of chase it is diluted and lost in the progeny of dividing cells and selectively retained by low/noncycling cells. We have detected such cells in the kidney papilla of both rats and mice pulse-chased with bromodeoxyuridine (BrdU) (47) and in genetically modified mice that conditionally express a fusion protein of histone 2B-green fluorescent protein (H2B-GFP) under the control of doxycycline (46). In the normal adult kidney, most of these papillary label-retaining cells (LRC) are, unsurprisingly, in growth arrest, but we found in the upper papilla a small population of them that were cycling (46), indicating that they generate new cells and explaining that the number of papillary LRC slowly decreases with age. In marked contrast to the normally low cycling frequency of the papillary LRC, many of these cells proliferated shortly after acute kidney injury, likely generating many new cells as the S-phase label disappeared into this progeny (46, 47). In the aggregate, these results suggested that the papillary LRC are precursor/stem cells of the adult kidney and are involved in normal organ homeostasis and repair from injury.
The kidney has a great capacity to regenerate from transient injury causing severe apoptosis and necrosis, and there is widespread cell proliferation during repair. Recent genetic labeling experiments indicate that most if not all of the new epithelial cells generated following transient kidney injury derive from terminally differentiated epithelia (21). However, many organs with multiple cell types contain several precursor/stem cells (2, 41, 48, 68), and in the kidney a precursor cell that generates a specialized glomerular epithelial cell called a podocyte has recently been described (4, 52). Moreover, it has been found in several organs that the type of stem/precursor cells activated during normal homeostasis differs from that activated after injury (15, 32, 57) and that even might vary depending on the type of injury (15).
A striking characteristic of some adult, organ-specific stem cells (or their immediate progeny) is their ability to migrate (19, 35, 66, 70) and to proliferate while migrating (42, 53, 61, 70). In many organs, adult stem homing and migration are critical for replacement of old cells (9, 12, 43, 62) and regeneration of damaged tissues (3, 7, 18, 22, 23, 32, 38, 51, 53, 66, 70). Indeed, using vital dyes in cell-tracking experiments we found that, both under normal conditions and after transient ischemic injury, there was an upward migration of cells in the kidney papilla and that some of these cells were LRC (46). To identify molecular cues inducing proliferation and migration (i.e., activation) of the quiescent papillary LRC, we have used a variety of in vitro and in vivo approaches and found that the chemokine stromal cell-derived factor-1 (SDF-1) and its receptor Cxcr4 are critical regulators of papillary LRC activation following transient ischemic injury. Interestingly, we found that SDF-1 is likely to exert this effect because its expression in the kidney is highly restricted to the medulla.
MATERIALS AND METHODS
Reagents.
Collagens I and IV were from BD Biosciences (Bedford, MA). bFGF, GDNF, SDF-1, and PDGF were all from R&D Systems (Minneapolis, MN). BrdU, doxycyclin, the Rho-kinase inhibitor Y-27632, AMD3100, dexamethasone, l-thyroxinem, and insulin-transferrin-sodium selenite media supplement were from Sigma (St. Louis, MO). 4,6,-Diamidino -2-phenylindole (DAP)I was from Invitrogen (Carlsbad, CA). Rhodamine-coupled Dolicos biflorus agglutinin was from Vector Laboratories (Burlingame, CA). Following are antibodies and sources: mouse monoclonal anti-BrdU was from Roche (Indianapolis, IN); rabbit polyclonal anti-Ki67 was from Novocastra (Newcastle Upon Tyne, UK) and Abcam (Cambridge, MA); for nestin, rabbit polyclonal was from Abcam; for aquaporin-2 (AQP2), rabbit polyclonal was from Sigma; for V-ATPase B1/2, rabbit polyclonal was from Santa Cruz Biotechnology; for Cxcr4, rabbit polyclonals ab7199 and ab2074 (both were from Abcam); for SDF-1 IHC, rabbit polyclonal ab25117 was from Abcam; for SDF-1 neutralization, mouse monoclonal was from R&D Systems; and mouse monoclonal anti-GAPDH was from Chemicon/Millipore (Bedford, MA). Irrelevant rabbit and mouse IgG and secondary antibodies were from Jackson Laboratories (West Grove, PA).
Cell chemotaxis assay.
To screen chemotactic/growth factors for their ability to induce migration of papillary cells, kidney papillae from mice transgenic for SV40-T antigen linked to the MHC-1 promoter (Immortomouse, Charles River) were dissected from mice homozygous for the transgene. After mincing and digestion of the papillae, cells were isolated and cultured as previously done (46, 47). Transwell chambers with filters of 12-mm diameter and 12-μm pore size (Costar) were coated on their upper surface with either 5 μg/ml of collagen I or 2.5 μg/ml collagen IV for 30 min and rinsed with PBS. Cells (1 × 105/cm2) were seeded on the filters, and the filters were placed in tissue culture wells with culture media containing different ligands below the filters. After 4 days in culture at 37°C, cells on the upper part of the filter were removed with a cotton swab, and cells in the contralateral side were fixed with methanol and stained with DAPI (Molecular Probes). Cells were quantified by visually (×200 magnification) counting the number of cells within six random separate fields for each filter. For each data point, three independent filters were counted and results averaged.
Culture of cells and papillae.
Unless specified, cell cultures were carried out in the following media: DMED containing 2 mM glutamine, 1× nonessential amino acids, 1× nucleosides, 1× mercaptoethanol, 15% EmbryoMax fetal calf serum (all form Chemicon), and penicillin/streptomycin (Invitrogen). Unless specified, papillae were cultured in serum-free media, containing DMEM/Ham's F12 (Invitrogen) with 2 mM glutamine and antibiotics plus 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, 20 ng/ml dexamethasone, 20 ng/ml l-thyroxine, 50 ng/ml bFGF, and 20 ng/ml EGF.
Generation of papillary LRC in rats and mice.
Sprague-Dawley rats and H2B-GFP mice (46) were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee approved the protocol. Rats with BrdU-retaining cells in the papillae were obtained as previously described (47). To obtain papillary LRC in mice, we used transgenic mice that express hybrid histone 2B-GFP molecules (H2B-GFP) under the control of tetracycline-regulatory element (46, 62). As described (46), pregnant females were given drinking water containing 2 mg/dl of doxycyclin starting after plug detection and until day of delivery. Following withdrawal of doxycyclin, pups expressing H2B-GFP were allowed to grow until 3 mo of age.
Kidney papilla three-dimensional organ culture assay.
Kidney papillae from 3-mo-old Sprague-Dawley rats were isolated with sterile technique on HBSS. For the three-dimensional (3D) gel assay, a mixture of collagen IV, 10× DMED, and 0.34 N NaOH at the ratios of 1:10, 1:2.5, and 1:5, respectively, were added to a solution of collagen I. Of this collagen mixture, 0.3 ml were then added to wells of at 12-well tissue culture plate and incubated at 37°C for 30 min to allow cross-linking of the collagen gel. Next, an isolated papilla plus 0.6 ml of the collagen mixture were then added to each well and the plate was again incubated at 37°C for 30 min to allow gel formation. Finally, appropriate amounts of tissue culture media were added, and the plates were incubated in tissue-culture conditions. Every day for the next 5 days, under a dissecting microscope, the papillae were photographed and the outgrowth at each side of the upper part of the papilla was quantified by grid analysis.
Kidney papilla organ culture assay.
For the organ culture outside the collage gel, isolated papillae from rats pulse-chased with BrdU (47) or from the H2B-GFP mouse (46) were placed on 0.4-μm pore size Transwell filters (Costar) and surrounded by ∼100 μl of cell culture growth media as done with embryonic kidneys (45). The filters with the papillae were then placed inside tissue culture wells containing growth media to the level of the filters and incubated in standard tissue culture conditions. For the experiments in which the media was supplemented with SDF-1, the two papillae from the same mouse were simultaneously incubated in different filters, and the media of one of the two papillae was supplemented by 200 ng/ml of SDF-1.
Isolation and culture of papillary LRC from H2B-GFP mice.
Adult H2B-GFP mice, pulse-chased as described, were euthanized and their kidney papillae isolated. After incubation with antibodies to mark bone marrow-derived and endothelial cells, GFP+ cells were isolated by FACS as described (46) and plated in tissue culture conditions. Several attempts to grow the cells were unsuccessful as the cells died in the first few days after isolation. As described for human embryonic stem cells (65), to inhibit apoptosis the Rho-kinase inhibitor Y-27632 (10 μM) was added to the culture of the isolated cells, which then successfully expanded. Y-27632 was kept in the culture media for the first two passages, after which it was removed from the media without changing cell growth.
Flow cytometry.
Papillae from three H2B-GFP mice pulse-chased with doxycycline were isolated, and single-cell suspensions were obtained as described (46, 47). Staining for cell surface antigens was preceded by Fc blocking (TruStain FcX, anti-mouse CD16/32; BioLegend, San Diego, CA) for 15 min, after which cells were incubated with PE anti-mouse CD3 antibody (clone 17A2), APC anti-mouse CD19 antibody (clone 6D5), PE/Cy7 anti-mouse CD11c antibody (clone N418), and PerCP/Cy5.5 anti-mouse CD68 antibody (clone FA-11), all from BioLegend. Cells were acquired with a LSR II flow cytometer (BD Biosciences) and analyzed with FACS DiVa software.
Papillary LRC mobilization assay.
GFP+ cells from kidney papillae were isolated and cultured as described above, and after attachment to the plates the culture media was removed and substituted for DMEM media containing glutamine and penicillin/streptomycin plus 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, and 20 ng/ml dexamethasone (all from Sigma), which was changed every 3 days. Six to nine days later, the cells had aggregated as spheres, at which time fresh media was added in the presence or absence of SDF-1 (200 ng/ml) and, again, changed every third day.
Papillary LRC proliferation assay.
Cells (1 × 104) were seeded in wells of a 96 well tissue culture plate and cultured with the media detailed above for cell culture except that concentration of fetal calf sera was decreased to 0.5%. Three days later, the media was changed and in selected wells bFGF (50 ng/ml) or SDF-1 (200 ng/ml) was added to the media, which was changed again 3 days later. Cell growth was assayed by using a CellTiter 96 AQueous Solution Cell Proliferation Assay (Promega) as detailed by the manufacturer. For each data point, absorbance (490 nm) was read in four wells with Spectra MaxPLus (Molecular Devices).
Immunohistochemistry.
For Cxcr4 and SDF-1 detection in kidney parenchyma, adult mice and rat kidneys were fixed in 10% formalin and mounted in paraffin blocks. After sectioning, slides were processed for immunohistochemistry, incubated with primary antibodies (or equal amounts of irrelevant IgG), and developed as detailed in an ImmPRESS reagent Kit (Vector Laboratories, Burlingame, CA). In the sections incubated with specific antibodies, a signal was only considered positive when it developed while the section incubated with irrelevant IgG remained negative.
Effect of transient renal ischemia and ADM3100 administration on LRC.
Sprague-Dawley rats (∼10 mo old), pulse-chased with BrdU as described (46) were anesthetized with isofluorane and had an Alzet osmotic minipump (Cupertino, CA) subcutaneously implanted. The pump contained either saline or a solution of AMD3100 (20 mg/ml) delivered at 4.2 mg·kg−1·day−1. Twenty-four hours after pump placement, to induce transient renal ischemic injury, the left renal artery was clamped for 45 min as described (46, 47). Three weeks later, rats were euthanized and both of their kidneys were harvested. Frozen kidney sections were obtained as described (47) and after immunostaining with an antibody to BrdU plus DAPI, the number of BrdU+ cells was quantified for each papilla. To obtain this number, in three separate 5-μm sections of each papilla block, six random fields were selected with the DAPI signal, and the relative number of BrdU-positive cells vs. the total number of DAPI-positive cells was determined.
Effect of transient bilateral renal ischemia during ADM3100 administration.
Two-month-old male Sprague-Dawley rats were anesthetized with ketamine/xylazine as described (47) and had an Alzet osmotic minipump subcutaneously implanted. The pump contained either saline or a solution of AMD3100 (22 mg/ml) delivered at 21 mg·kg−1·day−1. Twenty-four hours after pump placement, to induce transient renal ischemic injury, via posterior subcostal incisions both renal arteries were accessed and clamped for 45 min. The animals were euthanized 8 days later, and their kidneys were harvested; after fixation with 4% PFA, frozen kidney sections were obtained as described. For plasma creatinine determinations, in addition to samples obtained the day of renal artery clamping and the day of death, ∼150 μl of blood were obtained from the tail vein 1 and 2 days after transient ischemic injury. Plasma creatinine was determined by the enzymatic method (26) using the creatinine assay kit (Abcam) with the samples filtered through a 10-kDa MW cut-off filter before assay and using the fluorescence assay procedure.
Kidney sections from each animal were used to quantify cell proliferation and apoptosis. For proliferation, there independent 5-μm sections were immunolabeled with an antibody to Ki67 (46, 47) and the number of positive cells in three random fields of the different areas of the kidney in each slide was quantified under a fluorescence microscope. For apoptosis, three kidney sections from each kidney were assayed by the terminal transferase-dUTP-nick-end labeling (TUNEL) assay (47) with the In Situ Cell Death Detection Kit, TMR red (Roche), and the number of positive cells was quantified as described for Ki67.
Immunoblotting.
For Cxcr4 and SDF-1 abundance in different regions of the kidney, kidneys from 2- to 3-mo-old Sprague-Dawley rats were harvested in ice-cold HBSS. The kidneys were halved along the coronal plane so that the papilla remained divided. One-half of a papilla was next isolated, and the kidney parenchyma that had been adjacent to it and that is under the cortex was identified as the medulla and dissected. Cortical tissue was obtained by sectioning ∼1 mm of tissue from the outside edge of the kidney. Tissues were then placed in homogenization buffer (250 mM sucrose; 10 mM imidazole, and 1 mM EDTA, pH 8.0) containing Protease Inhibitor Cocktail Tablets (Roche) and minced into small fragments. Next, samples were homogenized with a Dounce or Micropestle (Eppendorf) homogenizer and centrifuged at 5,000 rpm for 5 min. After protein determination, samples were processed for SDS-PAGE and immunoblotting as described. Quantification of the data was done with ImageJ software.
Statistical analysis.
Data are means ± SE and were analyzed by ANOVA with correction for multiple comparisons with either Bonferroni correction or, when nonparametric data (e.g., ratios), Dunn's test. Where appropriate, Student's t-test was used.
RESULTS
Cxcr4/SDF-1 axis regulates papillary LRC in vitro.
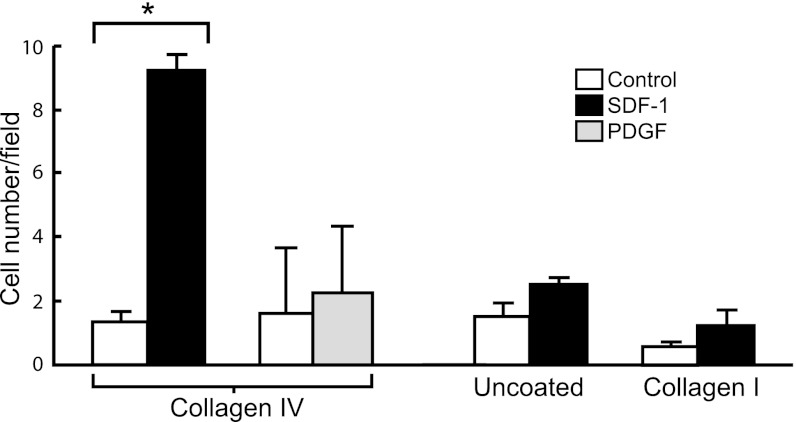
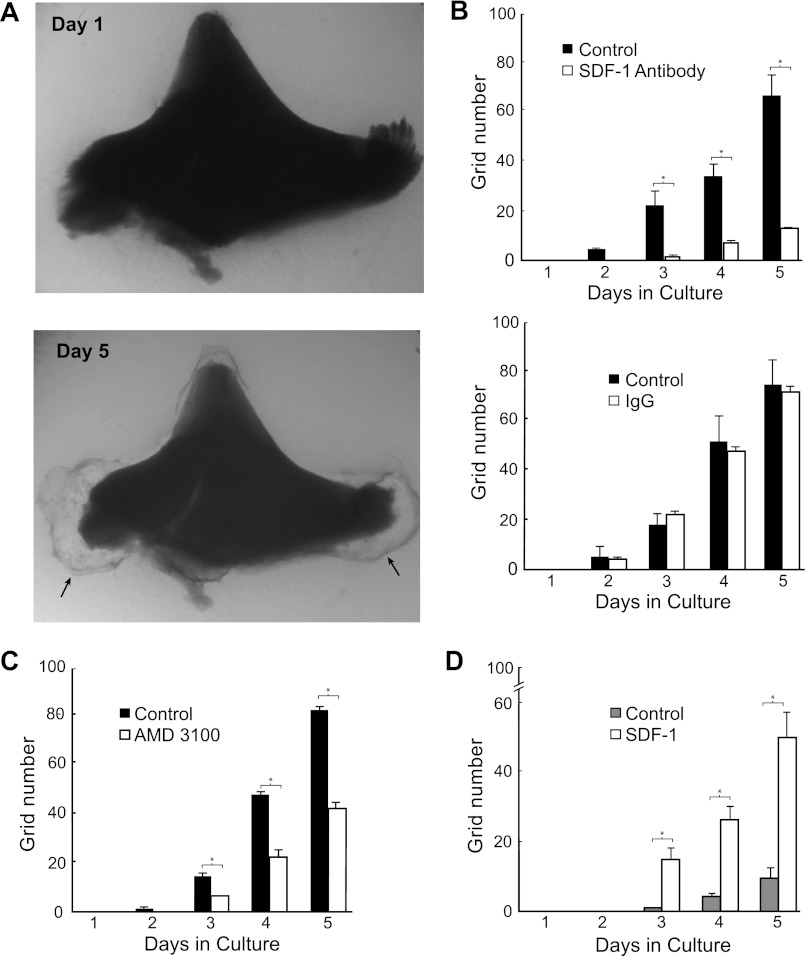
To search for molecular cues regulating papillary LRC mobilization, we first screened several chemotactic/growth factors known to be present in the kidney and examined their ability to induce migration of cultured mouse papillary cells across filters. As shown in Fig. 1, papillary cells readily migrated across collagen IV-coated filters in response to a gradient of SDF-1 but failed to respond to PDGF. To replicate some of the features of cell migration in vivo and examine whether SDF-1 also induced mobilization of papillary cells residing in the papilla, we used a papillary organ culture assay. As shown in Fig. 2A, outgrowths from cultured papillae imbedded into 3D gels of collagens I and IV could easily be detected, and, interestingly, most of the outgrowth occurred from the sides of the upper papilla (see arrows), a region that we previously identified as harboring chains of proliferating papillary LRC (46). Quantification by grid assay of the rate in which this outgrowth appeared (Fig. 2B) demonstrated that a substantial component of it was mediated by SDF-1 as it was significantly inhibited by either an SDF-1 blocking antibody (Fig. 2B, top) while it was unaffected by the same concentration of irrelevant rabbit IgG (Fig. 2B, bottom). In addition, the Cxcr4 receptor blocker AMD3100 also inhibited cellular outgrowth (Fig. 2C). Moreover, when papillae were cultured in the absence of growth factors cell outgrowth was decreased (see control bars in Fig. 2D), but it was then markedly stimulated by addition of SDF-1.
Fig. 1.
Rat papillary cell migration across filters. Cells seeded on collagen IV-coated filters migrated across the filters in the presence of a gradient of stromal cell-derived factor-1 (SDF-1) but failed to migrate to a gradient of PDGF. SDF-1-induced migration required the presence of collagen IV since SDF-1 was ineffective in uncoated filters or filters coated with collagen I. *P < 0.01; n = 4–6 individual experiments for each ligand.
Fig. 2.
SDF-1/Cxcr4 axis induces cell growth in rat papilla organ culture. A: example of isolated papilla cultured inside 3D gels made of collagens I and IV. Note cellular outgrowth, particularly in the lateral regions of the top papilla (arrows). B: surface area occupied by the cellular outgrowth from the top papilla was inhibited by a neutralizing antibody to SDF-1 (top, n = 4; P < 0.001) but it was not affected by the same concentration of irrelevant rabbit IgG (Bottom, n = 4). C: Cxcr4 blocker AMD3100 also inhibited cellular outgrowth (n = 6; P < 0.01). D: in the absence of bFGF and EGF, papillary outgrowth was reduced (compare with control values in B and C), but it was enhanced by the addition of SDF-1 (n = 6; P < 0.001). *P < 0.05.
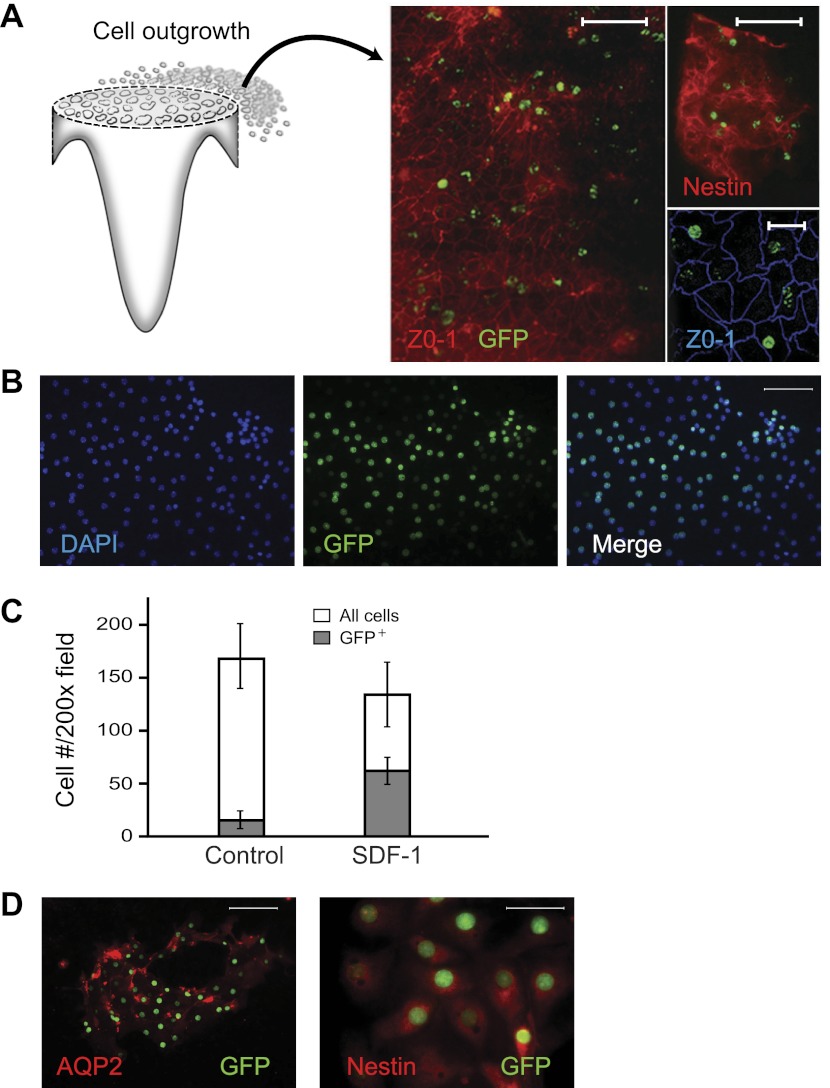
We next examined whether papillary LRC were capable of exiting the papilla in organ culture. Because the papillary cell outgrowth imbedded in the 3D gel proved impossible to isolate for analysis, papillae from rat and mice containing LRC were cultured in organ culture on filters as is done with embryonic kidneys (45). After 8 days in culture, in the experiments with papillae from rats pulse-chased with BrdU, many cells had exited the papilla (Fig. 3A) and attached to the filters, and a substantial number of these cells were BrdU labeled; in three experiments, these cells accounted for 27 ± 5% of the total number of cells. Similarly, in papillary cultures from H2B-GFP mice, many of the cells attached were GFP positive (Fig. 3B). Interestingly, under control culture conditions, 10 ± 3% of the cells on the filters were GFP positive, and this fraction increased to 56 ± 12% of the total number of cells in the presence of SDF-1 in the culture media (Fig. 3C; n = 7; P < 0.001). To characterize the identity of the LRC that exited the papilla, cells on the filters were probed with antibodies to AQP2, nestin, and the V-ATPase B1/2. Occasionally, groups of cells that were GFP positive were also positive for AQP2 (Fig. 4D), suggesting that they were principal cells of the collecting duct; cells that could be identified as intercalated cells (i.e., positive for the V-ATPase B1/2) were not detected. However, most GFP-positive cells were nestin positive, suggesting that might be of interstitial origin (46).
Fig. 3.
In vitro migration of papillary label-retaining cells (LRC) from rat and mice. A: rat kidney papillae from animals given bromodeoxyuridine (BrdU) as pups and chased for ∼ 9 mo were surgically isolated and cultured. After 8 days of in vitro culture, kidney papillae were removed from the filters and the cells growing on the filters (see cartoon) were stained, showing numerous BrdU-positive cells; the stained cells are from 3 different experiments, labeled with an anti-BrdU antibody (green), an antibody to either zonula occludens (ZO)-1 (left; scale bar = 50 μm), or to nestin (scale bars: top = 50 μm; bottom = 20 μm). B: kidney papillae from H2B-green fluorescent protein (GFP) mice with LRC were similarly cultured, and many of the cells in the filters were LRC (i.e., GFP+, scale bar = 50 μm). C: ff the cells in the filters, the fraction of LRC was significantly greater in the filters of the papillae that were cultured with SDF-1 (n = 7; P < 0.001). D: both types of LRC were detected on the filters, LRC from the collecting duct, i.e., aquaporin-2 (AQP2; scale bar = 50 μm), and likely from the interstitium (nestin; scale bar = 25 μm), with the latter being the most abundant.
Fig. 4.
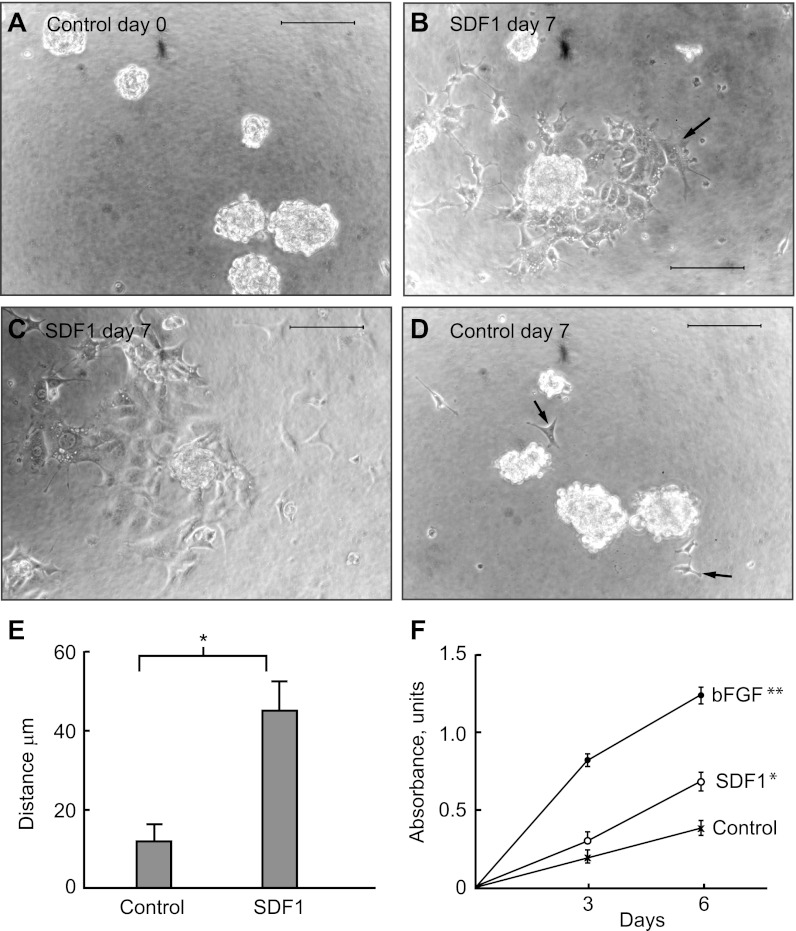
Papillary LRC from H2B-GFP mice migrate and proliferate in response to SDF-1. A: LRC isolated from H2B-GFP mice and grown in serum-free media formed spheres. B: 7 days after the addition of SDF-1, many cells moved a considerable distance from the spheres (arrow), which, in turn, diminished in size or disappeared altogether (C). D: in contrast, in the absence of SDF-1 (control), very few cells (arrows) separated from the spheres and stayed close to them. Bars = 100 μm. E: distance at which cells located from the edge of the spheres in the absence (control) or presence of SDF-1 in the media after 7 days (*P < 0.01; 3 spheres were analyzed in each individual experiment with n = 7 for control and n = 8 for SDF-1 cultures). F: when grown in culture media containing 0.5% FCS, cell number increased markedly in response to bFGF and modestly but significantly in response to SDF-1 (*P < 0.01; **P < 0.001).
To directly determine whether SDF-1 regulates papillary LRC, we isolate live papillary LRC (GFP+) from the H2B-GFP mouse (46) by FACS and grew them in vitro. These cells showed most of the characteristics of the cells that we previously isolated from papillae of BrdU-labeled rats (47), prominent among them being formation of spheres (Fig. 4A). To test whether SDF-1 increased mobility of the papillary LRC, cultures of the cells that had formed spheres were exposed to SDF-1 and followed for the appearance of cells outside the spheres. As shown in Fig. 4B, 7 days after SDF-1 addition multiple cells had exited the spheres and located at a considerable distance from them while the spheres decreased in size, or disappeared altogether (Fig. 4C). In contrast, in the absence of SDF-1, cells rarely exited the spheres and these remained intact (Fig. 4D). Quantification of the distance from the edge of the spheres attained by the new cells, both under control conditions and in the presence of SDF-1, is shown in Fig. 4E and indicates that this cytokine induced mobilization of cultured papillary LRC.
Next, to examine whether SDF-1 also regulated proliferation of papillary LRC in vitro, cells were starved in tissue culture media containing 0.5% of fetal calf serum for 3 days, after which cultures were treated with bFGF or SDF-1. Figure 4F shows that bFGF markedly increased the number of cells over the next 6 days and that, in addition, SDF-1 induced a modest but statistically significant increase in the cell number. In the aggregate, these results indicate that at least in vitro, papillary LRC migrate out of the papilla and respond to SDF-1 with increased mobility and proliferation.
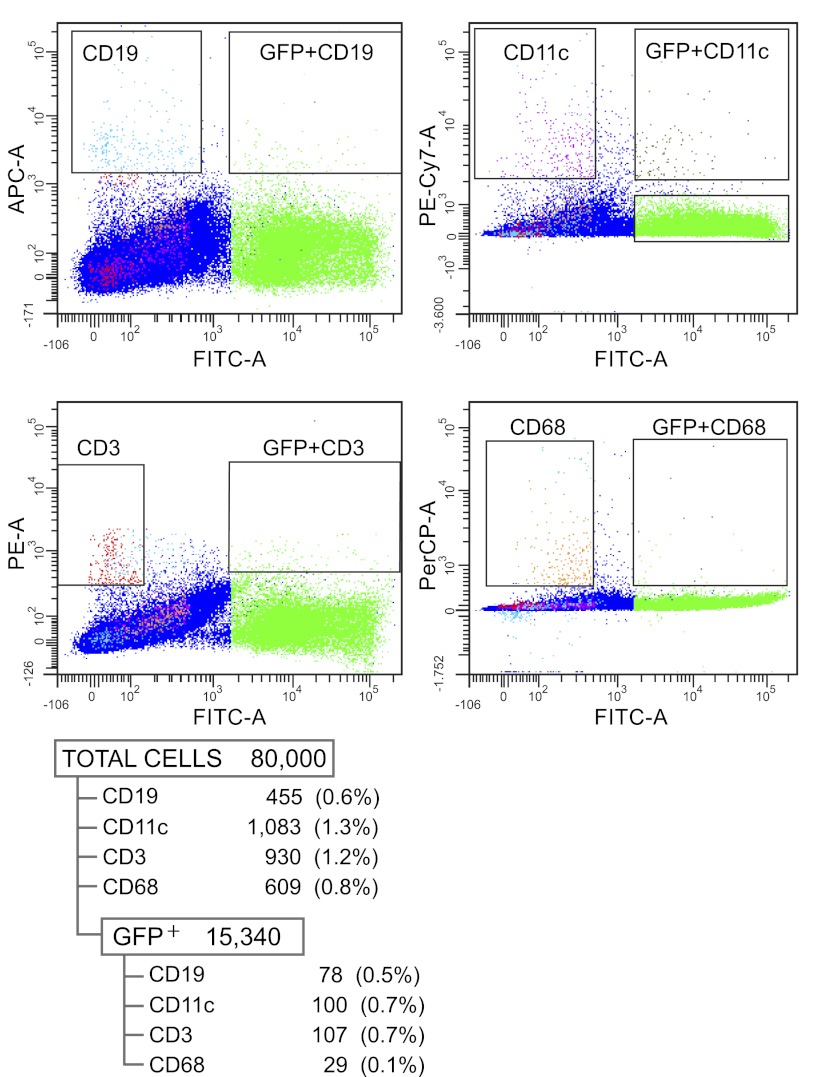
Cxcr4 was first isolated from T lymphocytes, and it is broadly expressed in immune cells, where, among many actions, it controls their migration (25, 29, 37, 64). In addition to blood cells, the kidney contains resident macrophages and dendritic cells, and our finding that LRC isolated from the kidney's papilla migrate and proliferate in response to SDF-1 raises the question of whether they could be immune cells. To examine this, we obtained a papillary cell suspension from kidneys of H2B-GFP mice and examined it for the presence of immune markers. Cells were incubated with labeled antibodies identifying B cells, dendritic cells, T cells, and macrophages. As shown in Fig. 5, of the papillary LRC (GFP+), 0.5% were CD19+ (B cells), 0.7% were CD11c+ (dendritic cells), 0.7% were CD3+ (T cells), and 0.1% were CD68+ (macrophages). The respective fractions in the total population of the papillary cells analyzed were 0.6% for CD19+, 1.3% for CD11c+, 1.2% for CD3+, and 0.8% for CD68+. Hence the numbers of the immune cells as a fraction of the GFP+ were low and, more importantly, there was no enrichment of the immune cell population in the GFP+ cell fraction (i.e., papillary LRC).
Fig. 5.
Analysis of papillary LRC for immune cell markers. Papillary cells from H2B-GFP mice pulse-chased to adulthood were analyzed for expression of GFP (i.e., LRC) vs. CD19 (for B lymphocytes), CD11c (for dendritic cells), CD3 (for T lymphocytes), and CD68 (for macrophages). The table displays quantitative data showing the frequency of immune cells in the total cell papillary population and in the papillary LRC (GFP+) only. In this experiment, cells were obtained from the 6 kidney papillae from 3 mice. The experiment was done twice, and similar results were obtained on both occasions.
Cxcr4 and SDF-1 are preferentially expressed in different regions of the adult kidney.
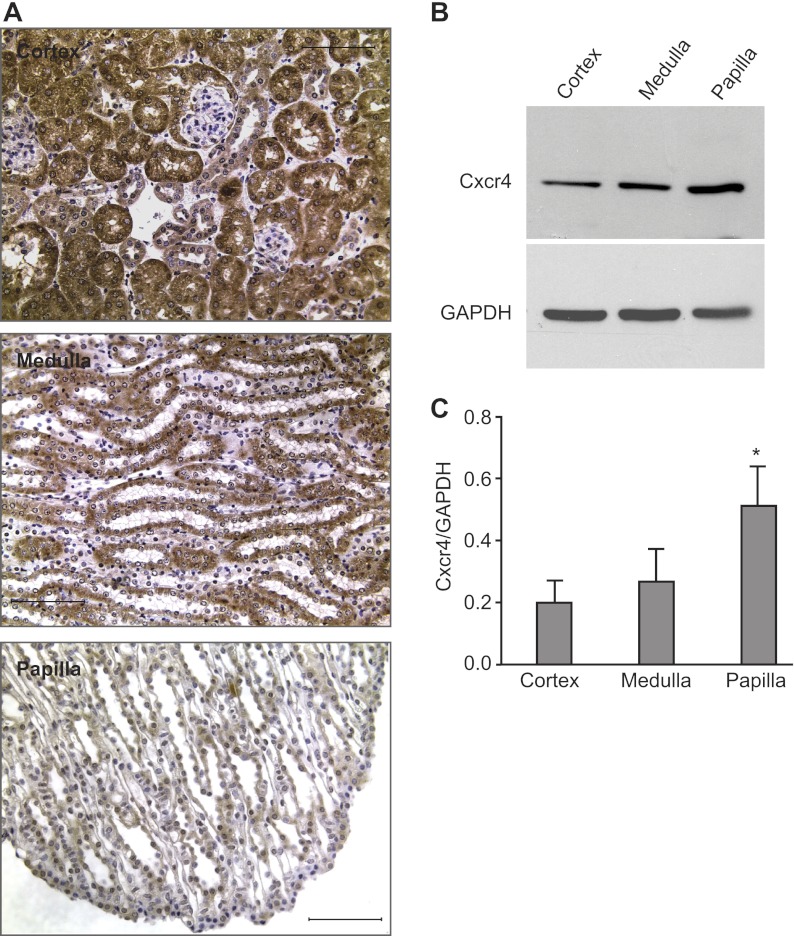
Chemokines regulate cell migration by developing concentration gradients (36, 40), and the SDF-1/Cxcr4 axis frequently controls migration of progenitor cells by expressing these two proteins in a complementary manner (33, 34, 40, 71). To determine where SDF-1 and Cxcr4 are expressed in the adult kidney, we examined their location by immunohistochemistry of rat and mouse kidney sections and immunoblotting of homogenates from different regions of the rat kidney, which due to its larger size allows more precise isolation of different regions. Results in mouse and rat kidneys were identical. Figure 6A shows that Cxcr4 was found in all areas of the kidney, with the glomerular tuft as the sole structure prominently not expressing the protein. While immunohistochemistry showed a Cxcr4 signal was present through the kidney parenchyma (Fig. 6A) and with a strong signal in the cortex and medulla, immunoblots of the homogenates from different areas revealed that the ∼45-kDa band of the glycosylated monomer form of the receptor was significantly more abundant in the papilla than in others parts of the kidney (Fig. 6, B and C).
Fig. 6.
Regional location of Cxcr4 in the adult kidney. A: immunohistochemistry for Cxcr4 in the cortex (top), medulla (middle), and papilla (bottom). As shown, Cxcr4 was widely distributed in the kidney parenchyma. Kidney sections incubated with identical amounts of irrelevant rabbit IgG and simultaneously processed with those with the Cxcr4 antibody gave no signal (not shown). Bars = 50 μm. B: representative immunoblot for Cxcr4 and GAPDH in different regions of the kidney. C: relative abundance of Cxcr4 vs. GAPDH in different regions of the kidney showed that the papilla had a significantly greater amount of the ∼ 45-kDa monomeric form of Cxcr4 than both the cortex and the medulla (*P < 0.05). Data derive from samples of 8 individual experiments, where protein homogenates from the 3 different regions of the kidneys were simultaneously analyzed. Each lane for Cxcr4 contained 24 μg of protein homogenate. Because of the large signal obtained with the antibody to GAPDH, for proper quantification of this protein's abundance in the sample, only one-third (i.e., 8 μg) of the protein homogenates were loaded on the gels.
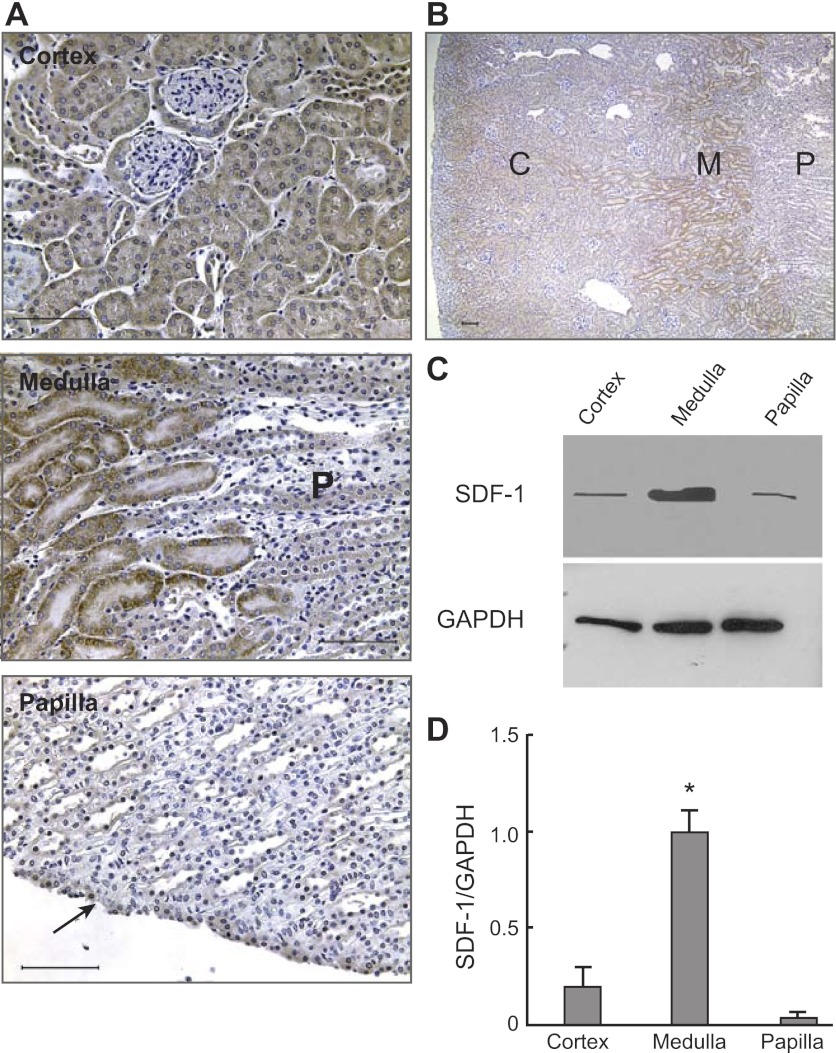
Immunohistochemistry of kidney sections for SDF-1 showed that this cytokine was readily detectable in the cortex, except in glomeruli that were negative (Fig. 7A). There was a particularly robust medullary SDF-1 signal (Fig. 7A) that, interestingly, abruptly disappeared at the medulla-papillary transition as the papilla had little if any signal for SDF-1 (except in the cells of the uroepithelium; Fig. 7A, arrow). The sharp decrease in the SDF-1-immunoreactive signal between the medulla and papilla is illustrated in the middle panel of Fig. 7A (where P indicates papilla) and in the low-power image in Fig. 7B (where C, M, and P indicate cortex, medulla, and papilla, respectively). These results suggested that compared with the cortex and particularly the papilla, the kidney medulla is a site with abundant SDF-1, and immunoblot analysis of the soluble fraction of protein homogenates from different parts of the kidney confirmed this suspicion. As shown in Fig. 7, C and D, while in homogenates of the kidney medulla there was a strong SDF-1 signal, those of the cortex had a markedly lower amount of cytokine and its signal was barely detectable in the papilla.
Fig. 7.
Regional location of SDF-1 in the adult kidney. A: Immunohistochemistry for SDF-1 in the cortex (top), medulla (middle), and papilla (bottom). As shown, SDF-1 was widely distributed in the cortex and medulla, whereas little signal was detected in the papilla (except in the uroepithelium; arrow). Note in the middle picture that the strong signal for SDF-1 in the medulla (left) sharply disappears in the top papilla, marked P. Bars = 50 μm. B: the greater prominence of the SDF-1 signal in the medulla than in other parts of the kidney is illustrated in this low-power image, which shows, from the left, the cortex (C), medulla (M), and papilla (P) regions of the same kidney. Kidney sections incubated with identical amounts of irrelevant rabbit IgG and simultaneously processed with those with the SDF-antibody gave no signal (not shown). Bar = 200 μm. C: representative immunoblot for SDF-1 and GAPDH in different regions of the kidney. D: relative abundance of SDF-1 vs. GAPDH in different regions of the kidney showed that the medulla had a significantly greater amount of SDF-1 than both the cortex and the papilla (*P < 0.01). Data derive from 4 individual blots, each from an independent rat. In each blot, protein homogenates from the 3 different regions of the kidneys were simultaneously analyzed. Each lane for SDF-1 contained 50 μg of protein homogenate. Because of the large signal obtained with the antibody to GAPDH, for proper quantification of this protein's abundance in the sample, only one-fifth (i.e., 10 μg) of the protein homogenates were loaded on the gels.
These results suggest that the kidney medulla is a site of abundant SDF-1 and that Cxcr4, or at least the monomeric species of this receptor, is more abundant in the papilla than other regions of the kidney. Moreover, the transitional zone from the upper part of the papilla to the medulla, where normally a few papillary LRC proliferate but many cycle shortly after injury (46, 47), is a zone with a sharp gradient of SDF-1.
In vivo blockade of Cxcr4 prevents papillary LRC mobilization after ischemic injury.
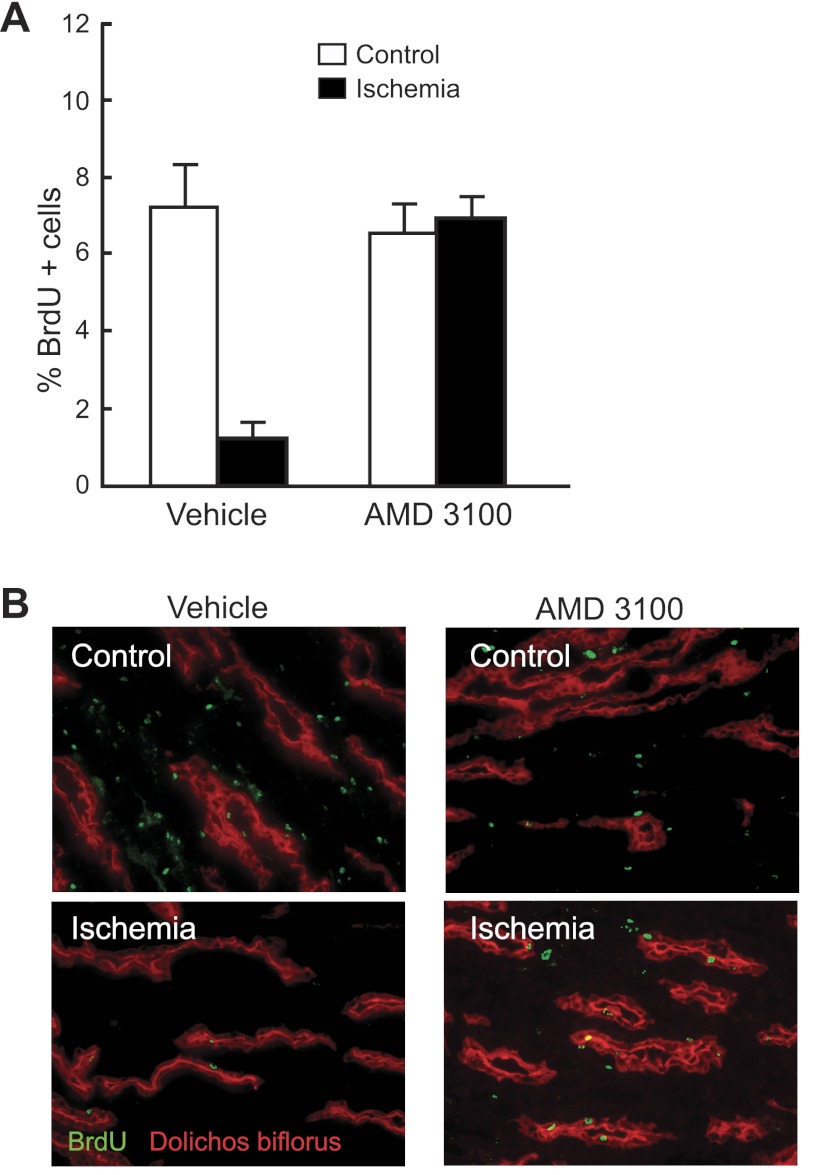
Under normal conditions, very few papillary LRC proliferate and their number only slowly decreases with age (46). However, after transient ischemic kidney injury, many LRC proliferate and after a period of repair, their number markedly decreases (20, 46, 47). Hence, to examine whether the SDF1/Cxcr4 axis regulates papillary LRC activation in vivo, we induced unilateral ischemic acute transient ischemic injury to rats that had been pulsed-chased with BrdU, in both the presence and absence of the Cxcr4 blocker AMD3100 (10). As shown in Fig. 8A, left and in the representative example in Fig. 8B, 3 wk after kidney ischemic injury, there was a significant reduction in the number of papillary LRC in the injured kidney compared with that of the control, noninjured kidney in the animals receiving saline (vehicle). These results confirm our previous findings (46, 47) and likely reflect that BrdU diluted into the progeny of the proliferating papillary LRC. In marked contrast, in the rats that received AMD3100, the average number of papillary LRC in the injured kidney was not different from that in the noninjured contralateral kidney (Fig. 8, A and B), strongly suggesting that binding of SDF-1 to Cxcr4 is required for the activation (i.e., proliferation and/or migration) of the papillary LRC after injury.
Fig. 8.
Mobilization of papillary LRC after transient ischemic injury is prevented by blockade of Cxcr4. A: relative abundance of papillary LRC present in the 2 kidneys (control and ischemia) from rats 3 wk after they underwent transient ischemic injury in the left kidney (black bars). In the group of rats that received only vehicle, the number of LRC in the papilla of the kidney subjected to injury was markedly lower that in the contralateral control kidney (n = 6; P < 0.01). However, in the group of rats that received the Cxcr4 blocker AMD3100, both papillae had a similar abundance of LRC. B: representative sections of papilla from a rat receiving vehicle only; note the absence of BrdU-positive cells (i.e., LRC) in the papilla of the kidney subjected to ischemia. In contrast, the 2 papillae of a rat receiving AMD3100 had similar numbers of BrdU-positive cells. Dolicos biflorus (rhodamine) labels collecting ducts.
Effect of transient kidney injury on Cxcr4 and SDFG-1.
Since Cxcr4 is widely expressed in the kidney, the effect of its blockade on papillary LRC mobilization may be indirect. Precursor germ cells increased Cxcr4 during migration (13), and muscle precursor cells increased Cxcr4 in diseased muscle (11, 49). In addition, tissue hypoxia enhances SDF-1 expression (6), and kidney injury has been shown to increase SDF-1 in the kidney (39, 55). To determine whether papillary Cxcr4 and medullary SDF-1 might increase during papillary LRC activation after transient kidney injury, we induced unilateral ischemia and examined papillary Cxcr4 and medullary SDF-1 by immunoblotting. In experiments with five control rats and five littermates subjected to transient kidney ischemic injury 5 days before analysis, the Cxcr4/GAPDH values averaged 0.8 ± 0.1 for control and 0.9 ± 0.3 for injured kidneys. The respective values for SDF-1/GAPDH averaged 0.9 ± 0.2 vs. 0.7 ± 0.2. None of these differences was statistically significant.
Cxcr4 blockade worsens the kidney's functional response to transient injury.
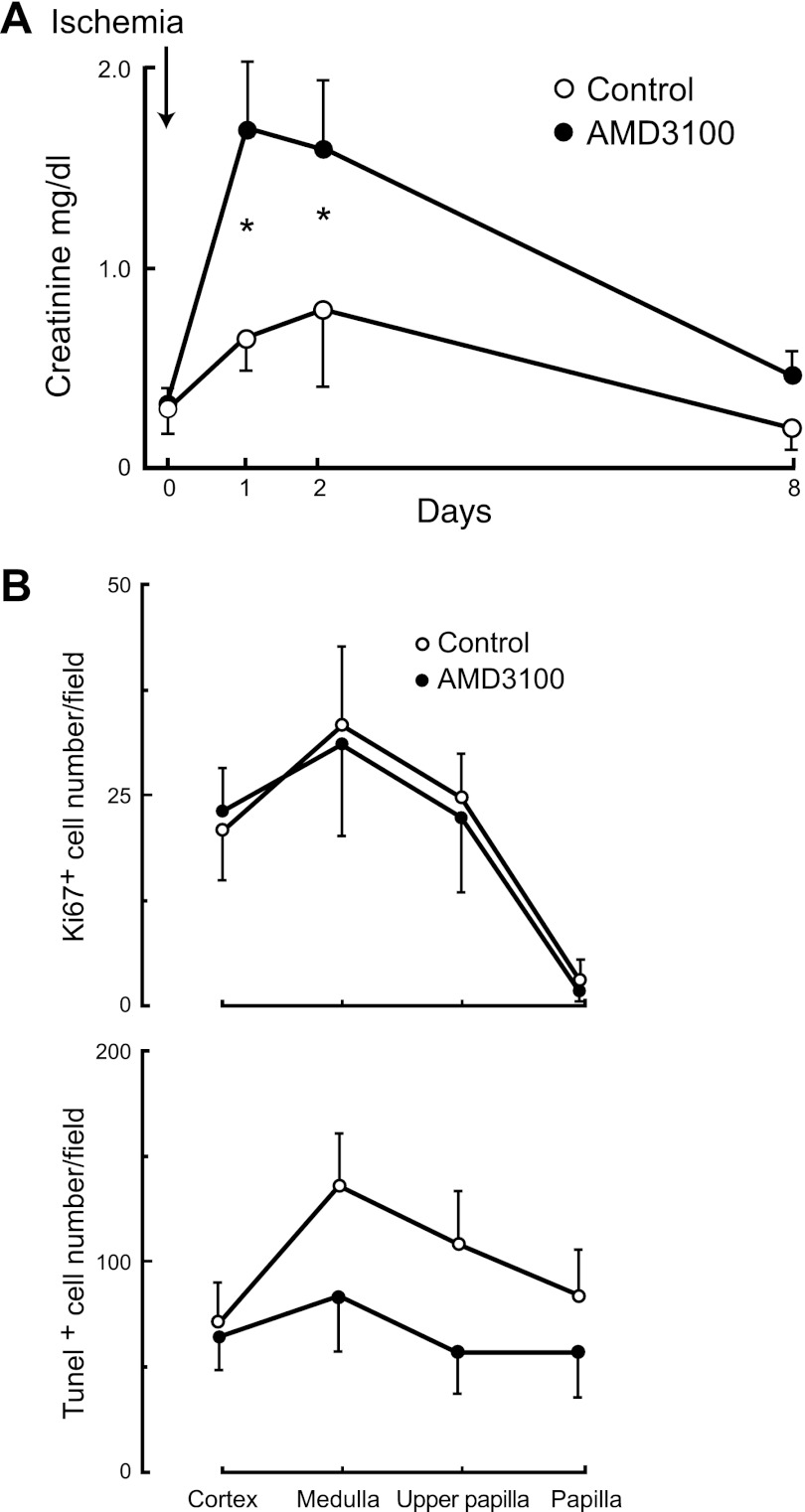
To determine whether blockade of Cxcr4, in addition of preventing mobilization of papillary LRC after injury may also affect the kidney's functional response to it, we induced bilateral transient ischemic injury in rats in the presence and absence of the Cxcr4 blocker ADM3100. As a marker of global kidney function, we measured the concentration of plasma creatinine and, upon harvesting the kidneys, we also determined the apoptotic and proliferative responses to injury. Osmotic minipumps containing saline or saline plus ADM3100 were subcutaneously implanted 1 day before kidney injury and creatinine concentration in plasma was measured on the day of the experiment and several days afterward. Figure 9A, shows that bilateral transient ischemic kidney injury in the animals receiving saline significantly increased plasma creatinine concentration, which returned to the control level by day 8 postinjury. Also shown in the figure is that the rats with blockade of Cxcr4 by AMD3100 had a significantly greater increase in plasma creatinine, indicating a more severe functional response of their kidneys to ischemia.
Fig. 9.
Transient kidney injury during blockade of Cxcr4 worsens kidney dysfunction. A: the rise in creatinine concentration in plasma following bilateral transient kidney ischemic injury was significantly greater in rats receiving the Cxcr4 blocker AMD 3100 (●) than in control rats receiving vehicle only (○); n = 6/group (P < 0.01; *P < 0.05). B, top: cellular proliferation as determined by Ki67-positive cells in kidney sections was not different within the 2 groups of rats; bottom: apoptosis as determined by terminal transferase-dUTP-nick-end labeling (TUNEL) appeared slightly less prominent in the kidney sections of the rats receiving AMD3100 than in the control rats, but the difference was not statistically significant.
As SDF-1 has growth (see Fig. 4F and Refs. 30 and 67) and antiapoptotic actions (30), we examined whether Cxcr4 blockade altered the proliferative and apoptotic responses to transient ischemic kidney injury. As anticipated (Fig. 9B; top), except for the body of the papilla, there were still many cycling cells (Ki67+) in the kidney 8 days after injury, but their number was not significantly different between the control rats and the rats receiving the Cxcr4 blocker. Similarly, as shown in Fig. 9B, bottom, 8 days after ischemic kidney injury there was widespread apoptosis in all areas of the organ in both experimental groups, but the number of TUNEL-positive nuclei was not different in the control rats from those that received AMD3100. In fact, there appear to be fewer apoptotic cells in the latter group, but the difference was not statistically significant. Analysis of sections stained with hematoxylin/eosin revealed no detectable differences between the kidneys of the animals receiving AMD3100 from those receiving vehicle.
DISCUSSION
The signaling molecules that induce papillary LRC to exit their inactive state shortly after transient ischemic injury (46) are unknown, and to find potential candidates we screened several chemotactic and growth factors known to locate in the kidney for their ability to mobilize papillary cells in vitro. The screen led us to focus on SDF-1/Cxcr4, a signaling pathway which frequently displays a complimentary expression motif and which stands unique in its ability to regulate migration and activation of multiple precursor/stem cells particularly during embryogenesis (5, 33, 34, 40, 63, 69, 71) and in adult life (1, 28) during both normal conditions and organ repair from injury (6, 16, 17, 22, 27, 44, 60). Our results suggest that in the kidney SDF-1 and Cxcr4 are regulators of papillary LRC activation.
First, in vitro papillary LRC readily responded to SDF-1 with enhanced proliferation and migration (Figs. 3C and 4). Second, papillary LRC mobilization after transient ischemic injury was prevented by blockade of the SDF-1 receptor Cxcr4 by the bicyclam ADM3100 (Fig. 8), a compound that also worsened the functional consequences of acute transient kidney injury (Fig. 9). Finally, immunoblot analysis of SDF-1 and Cxcr4 in the normal kidney suggests that these two proteins locate in the kidney parenchyma in a complementary manner; SDF-1 was strikingly more prominent in the medulla and, in fact, could barely be detected in the papilla (Fig. 7D) while Cxcr4 was most abundant in the papilla (Fig. 6C). However, for Cxcr4 there was a robust immunohistochemical signal in the cortex and medulla. Since this receptor is known to have multiple structural forms (54) and be actively degraded (50), it is likely that forms of the protein other than the monomeric receptor detected by immunoblot contributed to it.
In the aggregate, our results suggest that after ischemic kidney injury, high SDF-1 concentrations in the medulla regulate papillary LRC proliferation and/or upward migration. However, a definitive test of this hypothesis will require specific deletion of Cxcr4 in papillary LRC. and mouse lines with loxP sites in Cxcr4 (59) provide an initial tool to do this. However, also needed is identification of a gene that is specifically expressed in the LRC or at least in the embryonic cell compartment from which the LRC derive to drive Cre-recombinase and delete Cxcr4 in LRC.
It must also be considered that in addition to the possibility of nonspecific drug effects, the worsened functional response to injury in the animals receiving AMD3100 may well be independent of receptor blockade in the papillary LRC, since Cxcr4 is widely expressed in the kidney (Fig. 6). Other researchers also found that blockade of the Cxcr4/SDF-1 axis worsened the functional consequences of kidney injury (39, 55), and this effect has been attributed to migration and homing of renal precursor cells (39) or bone marrow cells (58) to sites of kidney injury. Also, the SDF-1/Cxcr4 axis is essential for developmental of the renal vasculature (56), and it is involved in recruitment of vascular precursors to ischemic tissues (6), actions that could be important for kidney recovery from injury. Thus to determine whether the worsened renal function during blockade of the SDF-1/Cxcr4 axis and the concomitant inability of the LRC to be activated are causally related will require experiments in animals with specific deletion of Cxcr4 in these cells.
In addition, understanding the function of papillary LRCs will require identification of their cell progeny, a problem best addressed by cell fate genetic studies. In a recent elegant study, Humphreys et al. (21) presented genetic evidence that many if not all new epithelial cells generated after kidney injury derive from fully differentiated epithelial cells, suggesting that papillary LRC do not generate new epithelia cells after injury. Needless to say, there are many other cell types in the kidney, of which papillary LRC could be their precursor. In addition, the function of the cells could be to provide growth/survival factors critical for epithelial maintenance and/or regeneration.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-55388.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.O., A.K., A.E., and Q.A.-A. provided conception and design of research; J.A.O., O.M., F.H.C., C.L., Q.-Y.Z., C.K., M.Z.A., and M.F. performed experiments; J.A.O., O.M., F.H.C., C.L., C.K., and Q.A.-A. analyzed data; J.A.O., Q.-Y.Z., and Q.A.-A. interpreted results of experiments; J.A.O., O.M., C.L., and C.K. prepared figures; J.A.O. and Q.A.-A. drafted manuscript; J.A.O. and Q.A.-A. edited and revised manuscript; J.A.O. and Q.A.-A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kristie Gordon for generous help with the flow cytometry analysis.
REFERENCES
- 1. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 185: 111–120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron 41: 683–686, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4: 49–61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci USA 100: 5319–5323, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122: 289–301, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57: 201–209, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7: 349–359, 2006 [DOI] [PubMed] [Google Scholar]
- 10. De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 77: 1655–1664, 2009 [DOI] [PubMed] [Google Scholar]
- 11. De Paepe B, Schroder JM, Martin JJ, Racz GZ, De Bleecker JL. Localization of the alpha-chemokine SDF-1 and its receptor CXCR4 in idiopathic inflammatory myopathies. Neuromuscul Disord 14: 265–273, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 111: 647–659, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27: 84–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 117: 1249–1259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 174: 231–243, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA 106: 9286–9291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 125: 1151–1163, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Humphreys BD, Czerniak S, Dirocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 101: 18117–18122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96: 25–34, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, Yoshiki R, Tamamura H, Fujii N, Inaba K, Tokura Y. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol 171: 1249–1257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60: 813–823, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 7: 163–173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity 25: 213–224, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C, Le Bousse-Kerdiles MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G0/G1 transition in CD34+ cells: evidence for an autocrine/paracrine mechanism. Blood 99: 1117–1129, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA 97: 13473–13475, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10: 720–726, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Li Q, Shirabe K, Kuwada JY. Chemokine signaling regulates sensory cell migration in zebrafish. Dev Biol 269: 123–136, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Lieberam I, Agalliu D, Nagasawa T, Ericson J, Jessell TM. A Cxcl12-CXCR4 chemokine signaling pathway defines the initial trajectory of mammalian motor axons. Neuron 47: 667–679, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science 271: 978–981, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol 19: 543–548, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10: 463–471, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Masuda T, Isobe Y, Aihara N, Furuyama F, Misumi S, Kim TS, Nishino H, Hida H. Increase in neurogenesis and neuroblast migration after a small intracerebral hemorrhage in rats. Neurosci Lett 425: 114–119, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol 213: 442–456, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA 94: 1908–1913, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 12: 195–206, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Olive M, Mellad JA, Beltran LE, Ma M, Cimato T, Noguchi AC, San H, Childs R, Kovacic JC, Boehm M. p21Cip1 modulates arterial wound repair through the stromal cell-derived factor-1/CXCR4 axis in mice. J Clin Invest 118: 2050–2061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oliver JA, Goldberg MR, Al-Awqati Q. Endothelial cell targeting during renal development: use of monoclonal antibodies. Am J Physiol Renal Physiol 272: F153–F159, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Oliver JA, Klinakis A, Cheema FH, Friedlander J, Sampogna RV, Martens TP, Liu C, Efstratiadis A, Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J Am Soc Nephrol 20: 2315–2327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pisani DF, Clement N, Loubat A, Plaisant M, Sacconi S, Kurzenne JY, Desnuelle C, Dani C, Dechesne CA. Hierarchization of myogenic and adipogenic progenitors within human skeletal muscle. Stem Cells 28: 2182–2194, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 21: 363–371, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 20: 1915–1924, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8: 217–222, 1985 [DOI] [PubMed] [Google Scholar]
- 54. Sloane AJ, Raso V, Dimitrov DS, Xiao X, Deo S, Muljadi N, Restuccia D, Turville S, Kearney C, Broder CC, Zoellner H, Cunningham AL, Bendall L, Lynch GW. Marked structural and functional heterogeneity in CXCR4: separation of HIV-1 and SDF-1alpha responses. Immunol Cell Biol 83: 129–143, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Stokman G, Stroo I, Claessen N, Teske GJ, Florquin S, Leemans JC. SDF-1 provides morphological and functional protection against renal ischaemia/reperfusion injury. Nephrol Dial Transplant 25: 3852–3859, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 20: 1714–1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102: 451–461, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Trampont PC, Tosello-Trampont AC, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol 11: 162–170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res 76: 20–34, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 157: 1257–1265, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science 303: 359–363, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev 19: 2187–2198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang J, Guan E, Roderiquez G, Calvert V, Alvarez R, Norcross MA. Role of tyrosine phosphorylation in ligand-independent sequestration of CXCR4 in human primary monocytes-macrophages. J Biol Chem 276: 49236–49243, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25: 681–686, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Watt DJ, Morgan JE, Clifford MA, Partridge TA. The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol (Berl) 175: 527–536, 1987 [DOI] [PubMed] [Google Scholar]
- 67. Wu Y, Peng H, Cui M, Whitney NP, Huang Y, Zheng JC. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem 109: 1157–1167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yan X, Owens DM. The skin: a home to multiple classes of epithelial progenitor cells. Stem Cell Rev 4: 113–118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yusuf F, Rehimi R, Morosan-Puopolo G, Dai F, Zhang X, Brand-Saberi B. Inhibitors of CXCR4 affect the migration and fate of CXCR4+ progenitors in the developing limb of chick embryos. Dev Dyn 235: 3007–3015, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci 27: 3157–3162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci 5: 719–720, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]