Abstract
Nuclear factor-κB (NF-κB) plays a role in inflammation. However, we recently reported an association between NF-κB and antioxidant enzymes in renal proximal tubules of exercise-trained rats, suggesting its role in antioxidant homeostasis (George L, Lokhandwala MF, Asghar M. Am J Physiol Renal Physiol 297: F1174–F1180, 2009). A direct role of NF-κB in antioxidant homeostasis in renal cells has not been elucidated and warrants investigation. Therefore, we examined whether NF-κB has a direct role in antioxidant homeostasis and redox balance in human kidney-2 cells overexpressing NF-κB-p65 and compared them with the cells overexpressing Nrf-2, a well-known transcription factor involved in antioxidant homeostasis. The ability of NF-κB-p65 to increase antioxidant enzymes, to reduce reactive oxygen species (ROS), and to rescue ROS-induced renal dopamine D1 receptor dysfunction, was studied. The transcription activity of NF-κB-p65 and Nrf-2, measured as luciferase reporter activity, increased in cells overexpressing these nuclear factors. The levels of mRNA and activity of glutathione peroxidase as well as the protein levels of superoxide dismutase-1 and glutamylcystein transferase were increased in cells overexpressing NF-κB-p65 and Nrf-2. Furthermore, the levels of ROS decreased and D1 receptor agonist SKF38393-mediated [35S]GTPγS binding (index of D1 receptor function) increased in the presence of hydrogen peroxide in cells overexpressing NF-κB-p65 and Nrf-2. These results suggest a direct role of NF-κB-p65 in antioxidant homeostasis, contributing to redox balance in renal cells.
Keywords: renal D1 receptor, Nrf-2, oxidative stress, GPCRs
nuclear factor-κb (nf-κb) is an inducible transcription factor which was first discovered to play a role in the regulation of the immune system in response to pathogens (6, 29, 32). Subsequently, it has been implicated in the regulation of other systems affecting transcription of a wide array of target gene families, such as stress response, apoptosis, and receptor genes, and influencing cell survival, differentiation, and proliferation (12, 15, 35). NF-κB has been the focus of research in diseases such as cancer, asthma, and muscular dystrophy (1, 8, 12, 21, 27, 30).
The inflammatory role of NF-κB is well documented. Most of our understanding about NF-κB signaling comes from immune cell research. Many of these studies describe its involvement in the process of inflammation and oxidative stress (19, 25). For example, NF-κB has been linked in the production of surrogate markers of inflammation such as chemokines (CCL5, MCP-1) (36), cytokines (IL-1a, IL-1b, IL-6) (16, 28, 34), and adhesive molecules (VCAM-1, NCAM) (18, 33). Also, it has been suggested to regulate the transcription of the reactive oxygen species (ROS)-producing enzyme NADPH-oxidase and the cellular milieu of oxidative stress (2). For long, it was thought that NF-κB is exclusively involved in the inflammatory and oxidative stress processes. However, our recent study, including the studies from others, implicates its role in the process of antioxidant homeostasis in the kidney and muscle tissues as well as in the blood and neuronal cell lines (13, 14, 23, 31).
A direct role of NF-κB in antioxidant homeostasis, especially in renal cells, has not been established. Therefore, we designed experiments to study the direct role of NF-κB in antioxidant homeostasis and redox balance in human kidney-2 (HK-2) cells. NF-κB-p65 with a nuclear localization segment (NLS) (26) was overexpressed in HK-2 cells, and its ability to increase antioxidant enzyme levels, such as glutathione peroxidase (GPx), superoxide dismutase-1 (SOD-1), and glutamylcystein transferase (GST), was studied. Also, the effect of NF-κB-p65 overexpression on the cellular levels of ROS in the presence of hydrogen peroxide (H2O2) was studied. Since H2O2 mediates dysfunction of the dopamine D1 receptor in renal cells (3), we also studied the ability of NF-κB-p65 to rescue H2O2-mediated dysfunction of the D1 receptor. Furthermore, these effects of NF-κB-p65 were studied in parallel by overexpressing Nrf-2, a well-known transcription factor involved in antioxidant homeostasis (9).
MATERIALS AND METHODS
HK-2 cell cultures.
Human kidney (HK-2) cells of proximal tubular origin were purchased from American Type Culture Collection (ATCC, Manassas, VA). The cells were grown in DMEM/F12 culture media containing 10% fetal bovine serum, 5 ng/ml epidermal growth factor, 50 μg/ml bovine pituitary extract, and a mixture of antibiotics and antimycotics (100 U penicillin, 100 μg streptomycin, and 0.25 μg/ml amphotericin B, Invitrogen, Carlsbad, CA) in a humidified incubator maintained at 37°C under 5% CO2.
Cell transfection with NF-κB-p65 and Nrf-2 expression vectors.
An NF-κB-p65 plasmid in a pShooter vector (pEF/myc/nuc-NF-κB) with a nuclear translocation domain (26) and Nrf-2 plasmid in a pCMV vector were used. pEF/myc/nuc (pShooter) and pCMV/myc/cyto (pCMV) served as the empty vector controls for NF-κB-p65 and Nrf-2, respectively. The cells were grown to 40% confluence and transfected with either an empty vector or NF-κB-p65 and/or Nrf-2 plasmids using a fugene-6 transfection reagent-to-DNA ratio as 3 μl:1 μg as per the manufacturer's protocol (Roche, Indianapolis, IN). The DNA amount in transfection studies was kept at 2 μg by using an appropriate empty vector. All the experiments were conducted 24 h posttransfection.
Transcription activity.
A custom promoter-reporter construct was synthesized (NorClone Biotech Labs, London, ON) where 2,850 bp upstream of the start site in the promoter region of the antioxidant hemeoxygenase-1 (HO-1) enzyme was linked to the firefly luciferase gene in a pGL4.10 vector. The cells were transfected with 1 μg DNA each for the HO-1 promoter-reporter construct and either an empty vector or NF-κB-p65/Nrf-2 plasmids (alone and together). A Renilla luciferase plasmid (0.1 μg pRL-TK) was used as the transfection efficiency control. All the transfections were carried out as above. Luciferase activity as an index of transcriptional activity of NF-κB-p65 and Nrf-2 was conducted using a Dual Luciferase Reporter Assay Kit (Promega, Madison, WI) according to the manufacturer's instructions. Briefly, 24 h after transfection, the cells were washed with PBS and 20 μl of the cell lysate was used to measure firefly luciferase activity using reagent II, followed by determination of Renilla luciferase activity using Stop and Glo reagent. The firefly and Renilla luciferase activities were measured using a Synergy 2 plate reader (Biotek, Winooski, VT), and the results are expressed as the ratio between the activities of firefly and Renilla luciferase.
ROS measurement.
The cellular levels of ROS were determined using H2O2 and the cell-permeable fluorescent probe dichlorofluorescein diacetate (CM-H2DCFDA; Invitrogen) (37, 38). Briefly, cells in 12-well plates were loaded with 5 μM CM-H2DCFDA in PBS for 45 min at room temperature. Thereafter, cells were washed with PBS twice and 2 ml of fresh PBS was added and incubated with vehicle or 5 μM H2O2. The fluorescence intensity as a result of dichlorofluorescein oxidation by H2O2 was recorded over a period of 5 min using the Synergy 2 plate reader (excitation: 492–495 nm/emission: 517–527 nm, Biotek). The cells were detached using trypsin/EDTA (0.5/0.2 g/l), counted, and used to normalize the results.
Immunofluorescence.
The cells were cultured on 1% poly d-lysine-coated glass coverslips and transfected with NF-κB-p65 and Nrf-2 plasmids and their respective empty vectors as described above. Twenty-four hours after transfection, the cells were fixed with 4% ice cold paraformaldehyde in PBS for 10 min and processed for immunofluorescence staining as described earlier (11). Briefly, cells were incubated with blocking buffer (5% normal goat serum and 0.1% Triton X-100 in PBS) followed by separate incubation with rabbit NF-κB-p65 (1:200) and Nrf-2 (1:200) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). After washing with PBS, a cy3-conjugated goat anti-rabbit secondary antibody (1:1,000, Invitrogen) was used to probe the primary antibody. In NF-κB-p65 and Nrf-2 dual-transfected cells, double labeling of NF-κB-p65 and Nrf-2 was conducted using mouse NF-κB-p65 (1:200, Millipore, Billerica, MA) and rabbit Nrf-2 (1:200) primary antibodies and Alexa Fluor 488-conjugated anti-mouse and Alexa Fluor 568-conjugated anti-rabbit secondary antibodies (1:1,000), respectively. Thereafter, the cells were mounted with nuclear dye DAPI containing mount media and scanned under an Olympus 181x series fluorescence microscope using a ×60 oil objective and respective filters. Cells probed with only a secondary antibody resulted in no fluorescence signal and were considered as negative controls (data not shown).
Protein measurement.
Proteins were measured using a BCA protein assay kit (Pierce, Rockford, IL) and BSA as standards.
Western blotting.
The proteins levels of Nrf-2, NF-κB-p65 (Santa Cruz Biotechnology), SOD-1 (Millipore), and GST-M1 (Assay Designs, Ann Arbor, MI) were determined by Western blotting using specific primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology).
[35S]GTPγS binding assay.
[35S]GTPγS binding in response to the D1 receptor agonist SKF38393 was performed as an index of D1 receptor function by a previously described method (13). In brief, 5 μg cell membranes were incubated with vehicle or various concentration of SKF38393 (10 pmol/l to 10 μmol/l) in the presence of [35S]GTPγS (final conc. 0.6 nM) for 1 h at 30°C. Nonspecific [35S]GTPγS binding was determined in the presence of 100 μM GTP. The radioactivity was read on a scintillation counter (Beckman Coulter, Brea, CA).
RT-PCR.
Total cellular RNA was isolated using an RNeasy minikit (Qiagen, Valencia, CA) per the manufacturer's instructions. cDNA was then synthesized from 1 μg RNA using Advantage RT for a PCR kit (Clontech, Mountain View, CA) and used in the PCR to amplify the GPx1 gene using published primers for GPx1 (forward: 5′-GACTACACCCAGATGAACGAGC-3′ and reverse: 5′- CACCAGGAACTTCTCAAAG-3′) (7). The DNA bands were resolved on an 0.8% agarose gel, visualized with ethidium bromide on Image Station, and quantified using software (Alpha Innotech, Cell Biosciences, Santa Clara, CA).
GPx activity.
GPx activity was measured using a commercially available kit (Assay Designs).
Statistical analysis.
Data were analyzed by ANOVA and post hoc Newman-Keuls analysis. P < 0.05 was considered statistically significant.
RESULTS
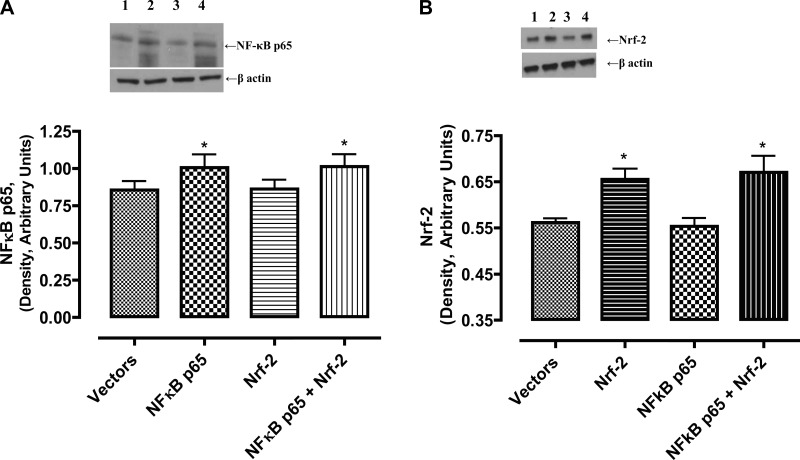
As shown in Fig. 1, NF-κB-p65 overexpression alone or in combination with Nrf-2 increased the levels of NF-κB-p65 proteins (Fig. 1A). Nrf-2 overexpression alone did not affect endogenous levels of NF-κB-p65 proteins (Fig. 1A). Similarly, Nrf-2 overexpression alone or in combination with NF-κB-p65 increased the levels of Nrf-2 proteins (Fig. 1B). NF-κB-p65 overexpression alone did not affect endogenous levels of Nrf-2 proteins (Fig. 1B).
Fig. 1.
NF-κB-p65 and Nrf-2 plasmids increase the levels of their respective proteins in HK-2 cells. Cellular homogenates (20 μg proteins) were used to determine NF-κB-p65 and Nrf-2 by western blotting. A, top: representative blot for NF-κB p65 and protein loading control, β-actin. Lane 1, empty vector; lane 2, NF-κB-p65 plasmid; lane 3, Nrf-2 plasmid; lane 4, NF-κB-p65 and Nrf-2 plasmids. Bottom: densitometric analysis of NF-κB-p65 and β-actin protein bands. Bars are ratios between the densities of NF-κB p65 and β-actin. *Significantly different from empty vector- or Nrf-2 plasmid-transfected cells. B, top: representative blot for Nrf-2 and protein loading control, β-actin. Lane 1, empty vector; lane 2, Nrf-2 plasmid; lane 3, NF-κB-p65 plasmid; lane 4, NF-κB-p65 and Nrf-2 plasmids. Bottom: densitometric analysis of Nrf-2 and β-actin protein bands. Bars are ratios between the densities of Nrf-2 and β-actin. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis. *Significantly different from empty vector- or NF-κB-p65 plasmid-transfected cells (P < 0.05); n = 5 separate experiments.
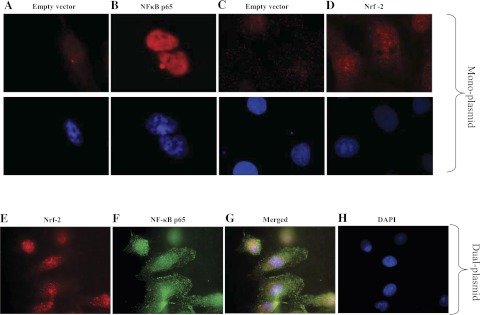
Figure 2 shows the cellular localization of NF-κB-p65 and Nrf-2 upon their overexpressions in HK-2 cells. NF-κB-p65 was localized exclusively in the nuclei (Fig. 2B) whereas Nrf-2 was found to be present throughout the cells (Fig. 2D). Their coexpression mostly localized Nrf-2 in the nuclei (pseudo-red color) (Fig. 2E), whereas, due to NLS, NF-κB-p65 was localized in the nuclei (pseudo-green color) (Fig. 2F). The merging of red (Nrf-2) and green (NF-κB-p65) resulted in yellow staining in the nuclei (Fig. 2G).
Fig. 2.
Cellular localization of NF-κB-p65 and Nrf-2 upon their overexpressions. Cellular localization of NF-κB-p65 and Nrf-2 in monoplasmid (A–D)- and dual-plasmid (E–G)-transfected cells were determined by immunofluorescence (details in materials and methods). Shown are NF-κB-p65 fluorescence staining in empty vector (A, top)- and NF-κB-p65 plasmid (B, bottom)-transfected cells and Nrf-2 fluorescence staining in empty vector (C, top)- and Nrf-2 plasmid (D, top)-transfected cells. A–D, bottom: 4,6-diamidino-2-phenylindole (DAPI)-stained nuclei in corresponding cells. E and F: Nrf-2 (E) and NF-κB-p65 (F) fluorescence staining in cells transfected with dual (Nrf-2+NF-κB-p65) plasmids. G and H: colocalization of Nrf-2- and NF-κB-p65 (H) DAPI-stained nuclei in the same cells where Nrf-2 and NF-κB-p65 staining is localized. The cells in A–H are pseudocolored.
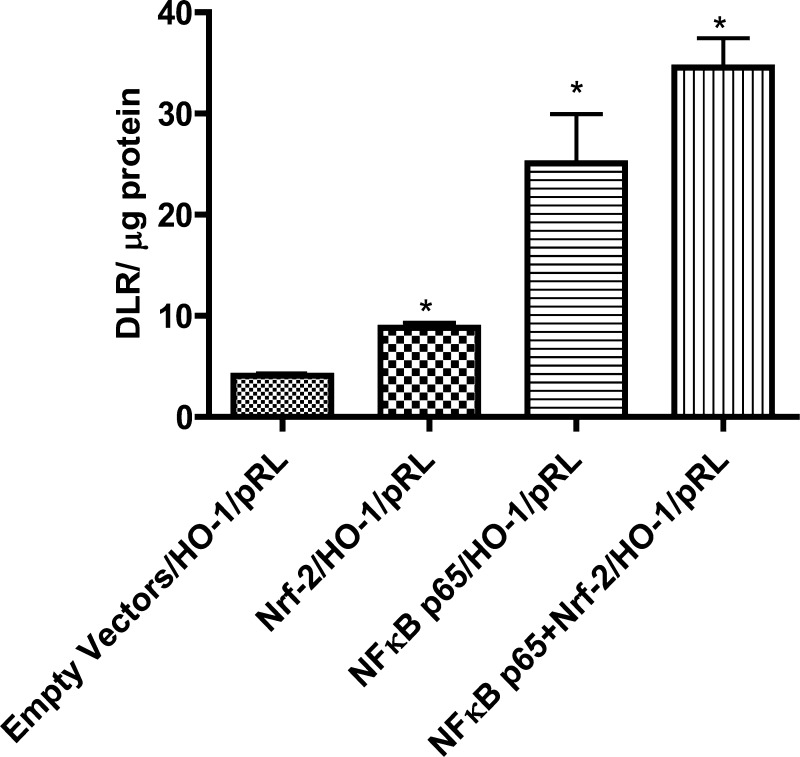
Overexpression of Nrf-2, NF-κB-p65, and their combination increased the luciferase activity by about twofold, fourfold, and sixfold, respectively (Fig. 3).
Fig. 3.
NF-κB-p65 and/or Nrf-2 plasmids increase the transcription of firefly luciferase in HK-2 cells. NF-κB p65 and Nrf-2 transcription activity was determined using hemeoxygenase-1 promoter-firefly luciferase reporter plasmid. Dual luciferase reporter (DLR) is the ratio between the fluorescence intensities of firefly luciferase and Renilla luciferase. Renilla luciferase plasmid was used as a transfection control (details in materials and methods). Results are means ± SE. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis.*Significantly different from empty vectors (P < 0.05); n = 5 separate experiments.
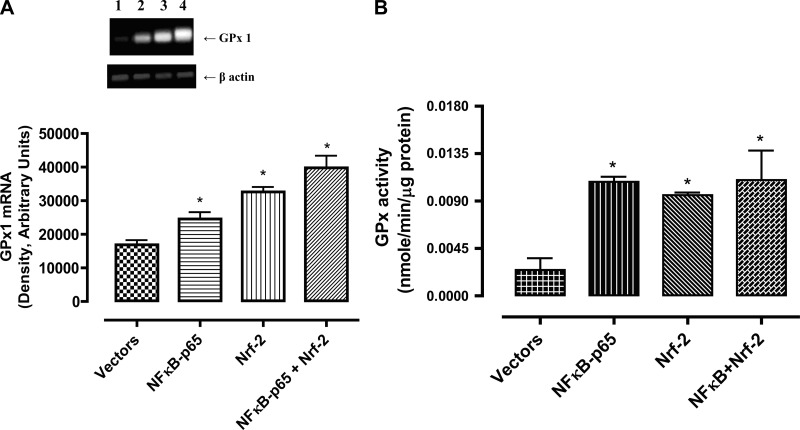
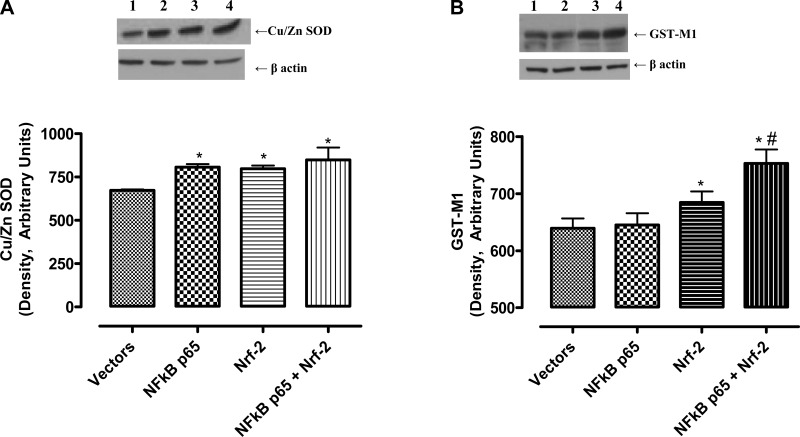
The GPx1 mRNA levels increased with overexpression of NF-κB-p65 and Nrf-2, alone and in combination, in the order of NF-κB-p65 < Nrf-2 < NF-κB-p65+Nrf-2 (Fig. 4A) whereas the activity increased to a similar extent in these cells (Fig. 4B). Furthermore, the levels of SOD-1 protein increased to similar levels in cells overexpressing NF-κB-p65 and Nrf-2 alone or in combination (Fig. 5A). However, the GST-M1 protein levels increased in Nrf-2- but not in NF-κB-p65 overexpressing cells, which further increased in cells overexpressing both NF-κB-p65 and Nrf-2 (Fig. 5B).
Fig. 4.
NF-κB-p65 and/or Nrf-2 overexpression increases the levels of antioxidant enzyme glutathione peroxidase (GPx1) mRNA and activity in HK-2 cells. A: GPx1 and β-actin mRNAs were amplified by RT-PCR using respective primers and resolved on 0.8% agarose gel. Top: representative bands for GPx1 and β-actin. Bottom: densitometric analysis of GPx1 and β-actin bands. The bars are ratios of densities of GPx1 and β-actin bands. Lane 1, empty vector; lane 2, NF-κB-p65; lane 3, Nrf-2; lane 4, NF-κB-p65+Nrf-2 plasmid. B: 20 μg of HK-2 cell homogenates were used to perform GPx1 activity assay. Results are means ± SE. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis.*Significantly different from empty vectors (P < 0.05); n = 5 separate experiments.
Fig. 5.
NF-κB-p65 and/or Nrf-2 overexpression increases the levels of antioxidant enzyme Cu/Zn superoxide dismutase (SOD) and glutathione-S-transferase-M1(GST-M1) in HK-2 cells. A: cellular homogenates (20 μg proteins) were used to determine Cu/Zn SOD and GST-M1 proteins by Western blotting. Top: representative blots for Cu/Zn SOD and loading control, β-actin. Bottom: densitometric analysis of Cu/Zn SOD and β-actin bands. Bars are ratios between the densities of Cu/Zn SOD and β-actin. B, top: representative blots for GST-M1 and loading control, β-actin. Bottom: densitometric analysis of GST-M1 and β-actin bands. Bars are ratios between densities of GST-M1 and β-actin. Lanes are defined as in Fig. 4. Values are means ± SE. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis.*Significantly different from empty vectors (P < 0.05). #Significantly different from NF-κB p65 (P < 0.05); n = 5 separate experiments.
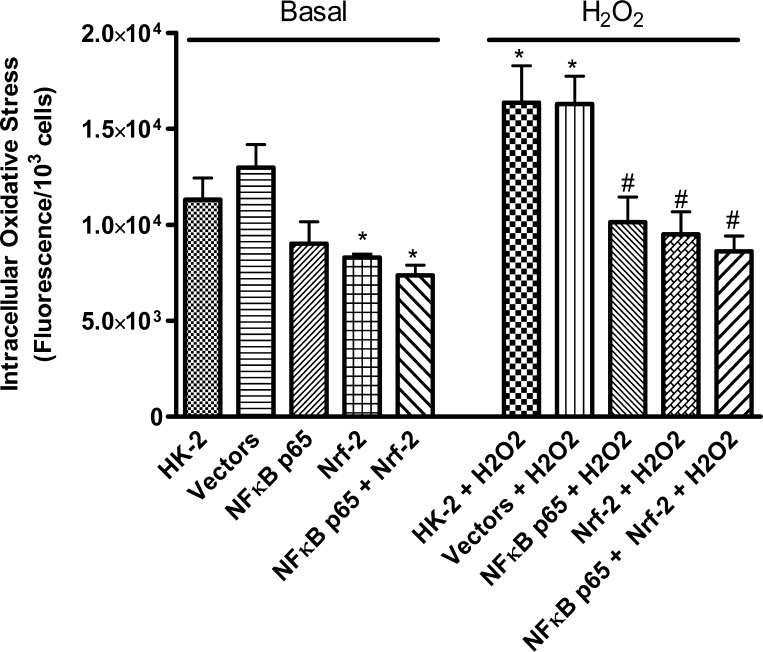
The endogenous levels of CM-H2DCFDA fluorescence intensity, an index of ROS, in empty vector-transfected cells increased, which decreased with NF-κB-p65 and Nrf-2 overexpression (Fig. 6, left). H2O2 treatment increased the levels of fluorescence intensity compared with vehicle treatment (basal) in empty vector-transfected cells, which were attenuated in cells overexpressing NF-κB-p65 and Nrf-2 (Fig. 6, right).
Fig. 6.
NF-κB-p65 and/or Nrf-2 overexpression reduces the intracellular levels of oxidative stress in HK-2 cells. Intracellular levels of oxidative stress was determined by using cell-permeable fluorescence probe CM-H2DCFDA in non- (HK-2), empty vector-, NF-κB-p65-, Nrf-2- and NF-κB-p65+Nrf-2 plasmid-transfected cells. Hydrogen peroxide (H2O2) radical was used to increase intracellular levels of oxidative stress. Values are means ± SE. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis. *Significantly different from basal HK-2- and empty vector-transfected cells (P < 0.05). #Significantly different from H2O2-treated HK-2- and empty vector-transfected cells (P < 0.05); n = 5 separate experiments.
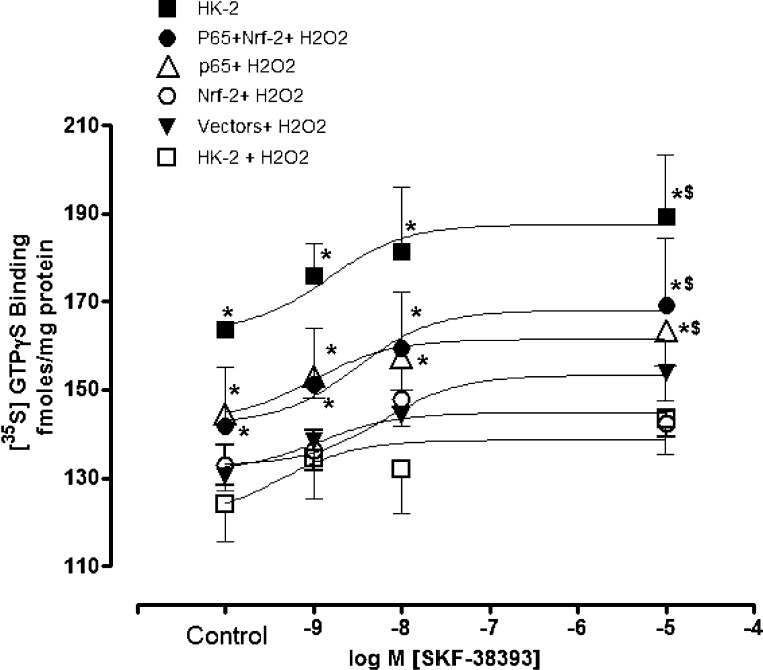
The D1 receptor agonist SKF38393 increased [35S]GTPγS binding in vehicle-treated compared with H2O2 (10 μM/20 min)-treated cells transfected with empty vectors (Fig. 7). SKF38393 increased [35S]GTPγS binding in H2O2-treated cells overexpressing NF-κB-p65 and Nrf-2 (Fig. 7).
Fig. 7.
NF-κB-p65 and/or Nrf-2 overexpression increases dopamine D1 receptor function during oxidative stress in HK-2 cells. D1 receptor function was measured as an index of [35S]GTPγS binding in response to D1 receptor agonist SKF38393. H2O2 radical was used to increase cellular levels of oxidative stress in non- (HK-2), empty vector- , NF-κB-p65-, Nrf-2-, and NF-κB-p65+Nrf-2 plasmid-transfected cells. Values are means ± SE. Data were analyzed by ANOVA and post hoc Newman-Keuls analysis. *Significantly different from corresponding control and SKF38393 in HK-2- and empty vector-transfected cells treated with H2O2 (P < 0.05). $Significantly different from respective control (P < 0.05); n = 5 separate experiments.
DISCUSSION
Our present study clearly demonstrates that NF-κB-p65 in renal cells increases the expression of antioxidant enzymes (GPx1, SOD-1) and enhances the ability of Nrf-2 to increase GST-M1 protein expression. It also reduces the cellular ROS (H2O2) levels and rescues ROS-mediated dysfunction of renal dopamine D1 receptors. These effects of NF-κB-p65 clearly indicate a direct role of NF-κB-p65 in antioxidant and redox homeostasis in renal cells.
The present study actually stemmed from our own findings and the findings from other laboratories demonstrating an association between NF-κB and antioxidant enzymes in the kidney, muscle, and neuronal cells (13, 14, 31). However, these studies could not provide evidence for a causal role of NF-κB in antioxidant enzyme homeostasis. Therefore, we studied the causal role of NF-κB-p65 in antioxidant and redox homeostasis in renal cells by undertaking an approach of overexpressing NF-κB-p65 with a nuclear localization domain (26). This approach was particularly helpful in targeting NF-κB-p65 to the nuclei (Fig. 2), the site of its action. Furthermore, the antioxidant effects of NF-κB-p65 were simultaneously studied and compared by overexpressing the antioxidant Nrf-2 factor (9) as a positive control.
NF-κB is a pleiotropic transcription factor involved in transcription of a wide array of target genes responsible for the immune response, cell survival, stress response, proliferation, and apoptosis (10, 12). Nrf-2 belongs to the Cap-‘n’-collar family of basic region leucine zipper transcription factors (9, 20) and causes gene transcription of detoxifying enzymes and antioxidant enzymes including GPx, SOD, and GCT (9, 20). Both NF-κB-p65 and Nrf-2 plasmids used in our study are not inhibitory to each other (Fig. 1), causing expression of the respective proteins, which seem to interact with each other in the nuclei (yellow staining; Fig. 2G). However, the magnitude of NF-κB-p65 expression is less than that of Nrf-2. The reason for this could be due to the mechanisms by which their homeostasis is maintained in the cells. The half-life of active NF-κB is <30 min whereas the inactive form is relatively stable (17). We have employed an NF-κB-p65 plasmid with a nuclear targeting domain (26) (active NF-κB-p65 form), which caused its protein expression but resulted in lesser cellular accumulation probably due to its shorter half-life.
On the other hand, Nrf-2, under homeostatic conditions, is bound to a protein, keap1, which causes its cytoplasmic sequestration and proteosomal degradation (20, 22). It is likely that Nrf-2 overexpression offsets its Keap 1-mediated degradation, resulting in its greater cellular accumulation.
We further investigated transcription activity of NF-κB-p65 and Nrf-2 by determining hemeoxygenase-1 promoter luciferase reporter activity as well as mRNA levels of an antioxidant enzyme, GPx1. HO-1 promoter has consensus binding sequences for both NF-κB-p65 and Nrf-2. NF-κB-p65 and Nrf-2 alone increased the luciferase activity and GPx1 mRNA levels, which were additive when both the transcription factors were present, suggesting their independent transcription activity for GPx1.
We also measured GPx activity to determine whether the NF-κB-p65- and Nrf-2-induced increase in the GPx1 mRNA levels correlated with enzyme activity. To our surprise, the GPx activity increased to a similar extent in the cells overexpressing NF-κB-p65 and Nrf-2, alone or together, suggesting that enzyme activity, at least with GPx, does not always increase in parallel with the mRNA levels. A recent study conducted in the rat kidney also showed a lack of direct relationship between GPx1 mRNA levels and its activity (24). Low GPx activity was not associated with any change in either GPx1 mRNA or its protein levels (24). The reason for the lack of existence of a direct relationship between GPx1 mRNA and/or protein on its activity may be due to posttranscriptional and posttranslational modifications of its mRNA and protein, respectively. Furthermore, NF-κB-p65 and Nrf-2, alone or together, increased SOD-1 protein to similar levels. Contrary to this, Nrf-2 alone, but not NF-κB-p65 alone, increased the protein levels of another antioxidant enzyme, GST-M1, which further increased when NF-κB-p65 was present. Taken together, these results suggest that NF-κB-p65 and Nrf-2 have differential effects on different antioxidant enzymes and NF-κB-p65 may actually aid Nrf-2 function, or vice versa, for at least GST-M1.
The functional consequences of NF-κB-p65 and Nrf-2 overexpressions were determined by studying their effectiveness in reducing ROS levels and protecting ROS-induced dysfunction of dopamine D1 receptors in the presence of H2O2. ROS cause dysfunction of the dopamine D1 receptor in terms of inability of the receptor agonist SKF38393 to increase [35S]GTPγS binding (3, 11, 13). ROS levels, determined as DCF fluorescence (4), increased with the empty vector, which further increased with H2O2. Both NF-κB-p65 and Nrf-2 overexpressions reduced ROS levels induced by either the empty vector or by H2O2. These results provide evidence that NF-κB-p65 and Nrf-2, alone or together, are effective in maintaining redox balance in renal cells. Another interesting finding of our study is that mere gene transfer (transfection) increases the cellular levels of ROS and both NF-κB-p65 and Nrf-2 could reduce them. This unravels the unique role of NF-κB-p65 and Nrf-2 as antioxidants, which can be undertaken as tools to reduce ROS where ROS might produce confounding effects in the study dealing with gene transfer.
We also tested the role of NF-κB-p65 and Nrf-2 in D1 receptor function in the presence of ROS (H2O2). The renal D1 receptor plays an important role in blood pressure control (5). Dysfunction of the D1 receptor, which is actually caused by ROS, is implicated in the development of essential hypertension and salt-sensitive hypertension (5). We found that a SKF38393-mediated increase in [35S]GTPγS binding (an index of D1 receptor-G protein coupling and function) was attenuated in H2O2-treated cells transfected or not with empty vectors, suggesting ROS induce dysfunction of the D1 receptor. There was a modest increase in [35S]GTPγS binding in H2O2-treated cells overexpressing Nrf-2 alone, which further increased in cells overexpressing NF-κB-p65 alone or with Nrf-2. These results suggest the ability of these factors to rescue D1 receptor function in the presence of ROS. These results further suggest that these molecules have potential as future therapeutic targets to reduce ROS and treat cardiovascular disorders such as hypertension where ROS play a detrimental role.
One intriguing question, however, is that activation of NF-κB on one hand is linked to beneficial effects relating to antioxidant homeostasis (13, 14, 23, 31, present study) while on the other hand it is implicated in inflammatory processes (1, 2, 8). It is likely that the fate of NF-κB action is decided by the instigator proximal to its activation as well as binding between different NF-κB subunits (p50, p52, p65) and may result in different downstream signal for discrete target gene transcription. This is perhaps true given the diverse role played by NF-κB in the immune response, cell survival, stress response, proliferation, and apoptosis (12, 33).
In summary, the results from the present study, to our knowledge for the first time, suggest a direct role of NF-κB-p65 in antioxidant and redox homeostasis in renal cells.
GRANTS
This work was supported by National Institutes of Health/NIA Grants AG039836, AG025056, and AG029904.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.E.G., M.F.L., and M.A. conception and design of research; L.E.G. performed experiments; L.E.G. and M.F.L. analyzed data; L.E.G., M.F.L., and M.A. interpreted results of experiments; L.E.G. prepared figures; L.E.G. drafted manuscript; L.E.G., M.F.L., and M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
NF-κB-p65 and Nrf-2 plasmids were kind gifts from Dr. Mark Nanes (Emory Univ. School of Medicine and the VA Medical Center, Atlanta, GA) and Jefferson Chan (Univ. of California, Irvine), respectively.
REFERENCES
- 1. Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sehnek Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117: 889–901, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281: 5657–5667, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Asghar M, Banday AA, Fardoun Z, Lokhandwala MF. Hydrogen peroxide causes uncoupling of dopamine D1-like receptors from G proteins via a mechanism involving protein kinase C and G-protein-coupled receptor kinase 2. Free Radic Biol Med 40: 13–20, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Asghar M, Lokhandwala MF. Antioxidant supplementation normalizes elevated protein kinase C activity in the proximal tubules of old rats. Exp Biol Med 229: 270–275, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Asghar M, Tayebati SK, Lokhandwala MF, Hussain T. Potential dopamine-1 receptor stimulation in hypertension management. Curr Hypertens Rep 13: 294–302, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Atchison ML, Perry RP. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell 48: 121–128, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Boutet M, Roland L, Thomas N, Bilodeau JF. Specific systemic antioxidant response to preeclampsia in late pregnancy: the study of intracellular glutathione peroxidases in maternal and fetal blood. Am J Obstet Gynecol 200: e531–e537, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Charokopos N, Apostolopoulos N, Kalapodi M, Leotsinidis M, Karamanos N, Mouzaki A. Bronchial asthma, chronic obstructive pulmonary disease and NF-kappaB. Curr Med Chem 16: 867–883, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des 10: 879–891, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Fardoun RZ, Asghar M, Lokhandwala M. Role of nuclear factor kappa B (NF-kappaB) in oxidative stress-induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague-Dawley rats. Free Radic Biol Med 42: 756–764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 16: 1053–1068, 2002 [DOI] [PubMed] [Google Scholar]
- 13. George L, Lokhandwala MF, Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol 297: F1174–F1180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44: 126–131, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol 13: 6231–6240, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hohmann HP, Remy R, Scheidereit C, van Loon AP. Maintenance of NF-kappa B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol 11: 259–266, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem 267: 16323–16329, 1992 [PubMed] [Google Scholar]
- 19. Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 3: 371–382, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36: 1208–1213, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, van der Vliet A. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc 6: 249–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys 433: 342–350, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA 91: 5987–5991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SK, Arunkumar S, Sirajudeen KN, Singh HJ. Glutathione system in young spontaneously hypertensive rats. J Physiol Biochem 66: 321–327, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol 30: 285–296, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Lu X, Farmer P, Rubin J, Nanes MS. Integration of the NfkappaB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem 92: 833–848, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death–a new approach to cancer therapy. J Clin Invest 115: 2625–2632, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mori N, Prager D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood 87: 3410–3417, 2006 [PubMed] [Google Scholar]
- 29. Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326: 711–713, 1987 [DOI] [PubMed] [Google Scholar]
- 30. Panwalkar A, Verstovsek S, Giles F. Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies. Cancer 100: 1578–1589, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci 24: 7324–7334, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47: 921–928, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Simpson CS, Morris BJ. Regulation of neuronal cell adhesion molecule expression by NF-kappa B. J Biol Chem 275: 16879–16884, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, Kim K. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol 51: 71–77, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Sullivan JC, Kalaitzidis D, Gilmore TD, Finnerty JR. ReI homology domain-containing transcription factors in the cnidarian Nematostella vectensis. Dev Genes Evol 217: 63–72, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Wickremasinghe MI, Thomas LH, O'Kane CM, Uddin J, Friedland JS. Transcriptional mechanisms regulating alveolar epithelial cell-specific CCL5 secretion in pulmonary tuberculosis. J Biol Chem 279: 27199–27210, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M. Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int 63: 464–473, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem 276: 25096–25100, 2001 [DOI] [PubMed] [Google Scholar]