Abstract
Objective
To investigate the distribution and the antimicrobial resistance of pathogens in lower respiratory tract infection from 2006 to 2010.
Methods
The sputum specimens from inpatients with lower respiratory tract infection in the First Affiliated Hospital of Nanjing Medical University during the past five years were cultured and identified; the antimicrobial resistance was analyzed by the software WHONET 5.4.
Results
A total of 12,191 isolates were characterized in sputum samples: 73.5% were Gram-negative bacteria, 13.7% were Gram-positive bacteria, and 12.8% were fungi. The isolation rate of Acinetobacter was significantly increasing from 12.8% in 2006 to 26.4% in 2010. The Gram-negative bacterial resistance rate to the second and third generation cephalosporin increased year by year. Decreasing trend, 78.7% in 2006 decreased to 63.5% in 2010 (R2=0.93 and P<0.01), in resistance to clindamycin against Staphylococcus aureus was observed. Worth noting is the drug resistance of Acinetobacter and Klebsiella pneumoniae to carbapenem significantly increased (R2>0.3 and P≤0.05).
Conclusions
The antimicrobial resistance of pathogens in lower respiratory tract infection increased in recent years. The hospitals and government departments should strengthen management of the use of some antibiotics, such as the second/third generation cephalosporin and carbapenem, in order to enhance the effectiveness of medication.
Key Words: Antimicrobial resistance, Acinetobacter, Staphylococcus aureus, lower respiratory tract, carbapenem
Introduction
Lower respiratory tract infections (LRTI) are common bacterial infections among patients in hospital and result in high overall mortality (1,2). It is reported that LRTI account for 3% to 5% of deaths in adults, especially over the age of 60 years, most common pathogens of LRTI are Pseudomonas, Acinetobacter, Klebsiella, Citrobacter, and Escherichia coli (3-5). At present, therapy for community-acquired lower respiratory tract infections (LRTI) is often empirical, and how to choose an effective antimicrobial agent is a new challenge to the clinicians, as the composition and the resistance to antimicrobial agents of infection pathogens was changing frequently. The knowledge of likely prevalent strains along with their antimicrobial resistance pattern will help in better management of patients and framing the antibiotic policy.
The present study, the pathogens profile and their antimicrobial resistance in lower respiratory tract infection from January 2006 to December 2010 in the First Affiliated Hospital of Nanjing Medical University was retrospectively reviewed in order to provide evidence for clinical therapy.
Material and methods
Patients
Patients with lower respiratory tract infections (LRTI) were enrolled from January 2006 to December 2010 in the First Affiliated Hospital of Nanjing Medical University (6). The sputum specimens of only new patients who were enrolled for the first time were included in the study. Single or mixed growth from one patient and consecutive samples from the new patients were included in the study. If the repeat sample was received from the same patient who was already enrolled, it was not included in the study. Acquisition and inoculation of the sputum samples were all accorded to standard operating procedures, following Clinical and Laboratory Standards Institute guidelines (CLSI) (7).
Bacterial identification and antimicrobial susceptibility testing
The bacterial isolates were identified and performed antimicrobial susceptibility testing predominantly through disk susceptibility testing, supplemented by the Vitek 2 system, following Clinical and Laboratory Standards Institute guidelines (CLSI) (7). Antimicrobial agents tracked include: penicillins (penicillin G), cephalosporins (cefazolin, cefuroxime, ceftriaxone, ceftazidime, and cefepime), monobactams (aztreonam), cephamycins (cefoxitin), carbapenems (imipenem and meropenem), compound agents (amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin/tazobactam, cefoperazone/sulbactam), aminoglycosides (amoxicillin), fluoroquinolones (ciprofloxacin and levofloxacin), sulfonamides (cotrimoxazole), macrolides (erythromycin), lincomycins (clindamycin), glycopeptides (vancomycin and teicoplanin). Microbiologic data were extracted from the laboratory information system and converted centrally into a standard format using WHONET 5.4 (WHO, Geneva, Switzerland), with duplicates eliminated according to the guidelines of the CLSI. The following controls strains were included: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923.
Statistical analysis
WHONET 5.4 microbiology laboratory data management software World Health Organization recommended was used for statistical analysis. Changing trends in this use were analyzed by linear regression (R2>0.3 and P≤0.05) (8).
Results
Pathogens distribution
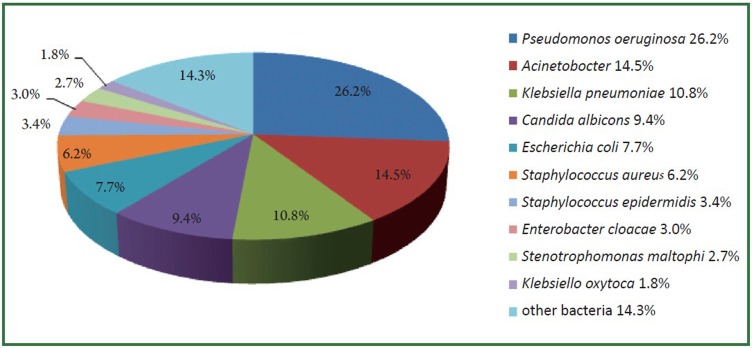
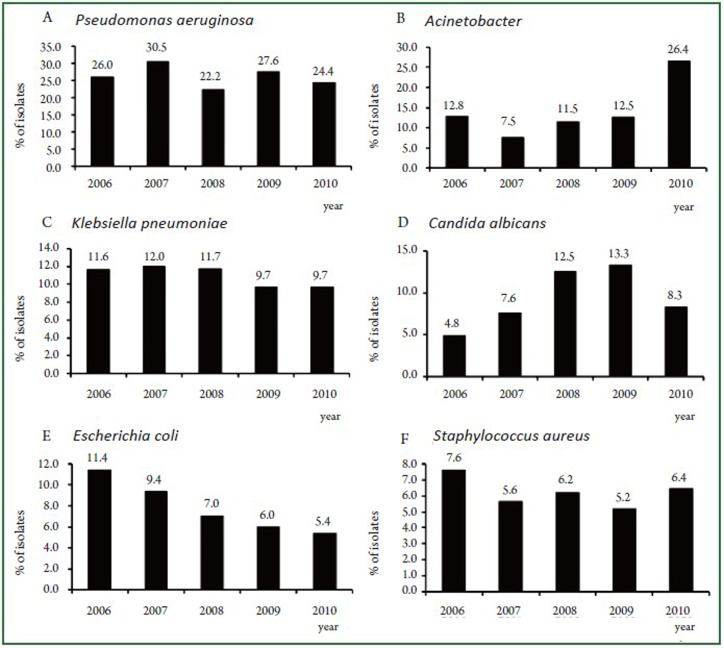
During the study period, a total of 12,191 isolates were characterized in sputum samples from all patients with LRTI. Of these pathogens, 73.5% (8956/12191) were Gram-negative bacteria, 13.7% (1671/12191) were Gram-positive bacteria and 12.8% (1555/12191) were fungi. The top 10 frequently isolated pathogens were Pseudomonas aeruginosa (26.2%), Acinetobacter (14.5%), Klebsiella pneumoniae (10.8%), Candida albicans (9.4%), Escherichia coli (7.7%), Staphylococcus aureus (6.2%), Staphylococcus epidermidis (3.4%), Enterobacter cloacae (3.0%), Stenotrophomonas maltophi (2.7%) and Klebsiella oxytoca (1.8%) (Figure 1). These 10 species accounted for 85.7% of the total number of isolates. Figure 2 (A-F) showed the changing trend of the top 6 pathogens from 2006 to 2010. Pseudomonas aeruginosa accounted for top 1 from 2006 to 2009. The isolation rate of Acinetobacter was significantly increased from 12.8% in 2006 to 26.4% in 2010, more than Pseudomonas aeruginosa (24.4%) in 2010.
Figure 1.
The distribution of the top 10 frequently isolated pathogens, 2006 to 2010.
Figure 2.
Changing trend of top 6 pathogens, 2006 to 2010.
Antimicrobial resistance of Gram-negative bacteria
With regard to to Gram-negative bacteria (GNB), the resistance rate of all GNB to cephalosporins showed the increasing trend although not all showed significantly. Among GNB, the resistance rates were more than 90% in 2010 of Escherichia coli to all cephalosporins, a significant increase in the resistance rate with time was found for cefuroxime (R2=0.86 and P=0.02) and ceftriaxone (R2=0.78 and P=0.04) (Table 1). Klebsiella pneumonia had a more than 80% resistance rate in 2010 to all cephalosporins and significant increasing trends were observed to ceftazidime (R2=0.91 and P=0.01) and ceftriaxone (R2=0.95 and P<0.01) (Table 2). Acinetobacter resistance rate to all cephalosporins were more than 85% in 2010, significant increased against cefepime (R2=0.78 and P=0.05) (Table 3). Significant increasing trends were also observed in Citrobacter to ceftazidime (R2=0.80 and P=0.04) and ceftriaxone (R2=0.82 and P=0.03) and Pseudomonas aeruginosa to ceftazidime (R2=0.81 and P=0.04) (Table 4, 5). The same increasing trend was also found in GNB against aztreonam. Citrobacter, Pseudomonas aeruginosa and Acinetobacter resistance rate were all significant increased to aztreonam (R2>0.3 and P≤0.05) and were all more than 60% in 2010 (Table 3, 4, 5). Worth noting is the drug resistance to carbapenem including imipenem and meropenem has become increasingly serious. Significant increasing trends were found to imipenem (R2=0.78 and P=0.05, R2=0.79 and P=0.04, respectively) and meropenem (R2=0.81 and P=0.04, R2=0.80 and P=0.04, respectively) of Klebsiella pneumoniae and Acinetobacter (Table 2, 3). Most GNB resistance rate increased to compound agents including ampicillin/sulbactam, piperacillin/tazobactam, cefoperazone/sulbactam except Pseudomonas aeruginosa remained stable. Among them, Citrobacter to ampicillin/sulbactam and piperacillin/tazobactam and Acinetobacter to piperacillin/tazobactam and cefoperazone/sulbactam showed significant increasing trends (R2>0.3 and P≤0.05) (Table 3, 4). The only decreasing trend among GNB was observed in resistance to amoxicillin against Escherichia coli (R2=0.88 and P=0.01) while increasing trends against Pseudomonas aeruginosa (R2=0.82 and P=0.03) and Acinetobacter (R2=0.84 and P=0.03) (Table 1, 3, 5).
Table 1. Trends in resistance rate of Escherichia coli to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Cefazolin |
88.5 |
86.4 |
82.3 |
92.6 |
92.2 |
0.25 |
0.39 |
–2.94-5.66 |
Stable |
| Cefuroxime |
84.1 |
89.9 |
89.7 |
95.3 |
94.7 |
0.86 |
0.02 |
0.68-4.63 |
Increasing |
| Ceftazidime |
39.8 |
86.9 |
81.9 |
91.9 |
92.0 |
0.62 |
0.12 |
–4.91-26.79 |
Stable |
| Ceftriaxone |
74.8 |
86.5 |
82.8 |
93.3 |
92.8 |
0.78 |
0.04 |
0.08-8.48 |
Increasing |
| Cefepime |
53.9 |
88.2 |
82.2 |
92.1 |
92.4 |
0.63 |
0.11 |
–3.29-19.47 |
Stable |
| Aztreonam |
52.8 |
88.6 |
82.3 |
92.2 |
91.6 |
0.60 |
0.12 |
–3.96-20.20 |
Stable |
| Imipenem |
1.2 |
4.0 |
3.6 |
2.5 |
3.5 |
0.19 |
0.46 |
–0.87-1.49 |
Stable |
| Meropenem |
2.8 |
4.2 |
7.6 |
3.0 |
3.5 |
<0.01 |
0.98 |
–2.26-2.30 |
Stable |
| Amoxicillin/clavulanate |
46.9 |
32.5 |
36.8 |
37.1 |
50.3 |
0.06 |
0.70 |
–7.34-9.62 |
Stable |
| Ampicillin/sulbactam |
66.7 |
54.5 |
49.5 |
67.6 |
100.0 |
0.41 |
0.24 |
–9.60-25.54 |
Stable |
| Piperacillin/tazobactam |
26.7 |
19.6 |
16.6 |
18.4 |
20.7 |
0.30 |
0.34 |
–5.06-2.42 |
Stable |
| Cefoperazone/sulbactam |
17.6 |
10.4 |
10.9 |
21.5 |
26.4 |
0.44 |
0.23 |
–3.13-8.87 |
Stable |
| Amoxicillin |
29.7 |
25.5 |
24.0 |
20.9 |
10.1 |
0.88 |
0.01 |
–7.37-1.39 |
Decreasing |
| Levofloxacin | 82.0 | 79.7 | 77.8 | 70.7 | 79.5 | 0.26 | 0.38 | –5.70-2.90 | Stable |
Table 2. Trends in resistance rate of Klebsiella pneumoniae to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Cefazolin |
62.3 |
63.5 |
58.8 |
54.5 |
74.1 |
0.10 |
0.60 |
–6.59-9.51 |
Stable |
| Cefuroxime |
54.0 |
69.9 |
63.3 |
61.7 |
74.0 |
0.42 |
0.23 |
–3.64-10.00 |
Stable |
| Ceftazidime |
28.6 |
43.7 |
58.8 |
55.7 |
72.1 |
0.91 |
0.01 |
4.09-15.71 |
Increasing |
| Ceftriaxone |
40.6 |
48.6 |
59.4 |
59.7 |
72.2 |
0.95 |
<0.01 |
4.38-10.48 |
Increasing |
| Cefepime |
30.4 |
63.1 |
60.1 |
56.6 |
72.5 |
0.61 |
0.12 |
–3.70-19.24 |
Stable |
| Aztreonam |
35.9 |
65.2 |
60.1 |
56.7 |
73.4 |
0.56 |
0.14 |
–4.08-17.38 |
Stable |
| Imipenem |
2.3 |
6.1 |
7.5 |
9.5 |
25.2 |
0.78 |
0.05 |
0 .06-9.78 |
Increasing |
| Meropenem |
1.0 |
4.1 |
6.8 |
10.9 |
29.0 |
0.81 |
0.04 |
0 .68-1.88 |
Increasing |
| Amoxicillin/clavulanate |
38.2 |
41.5 |
36.1 |
35.9 |
54.5 |
0.30 |
0.34 |
–4.81-10.21 |
Stable |
| Ampicillin/sulbactam |
49.5 |
53.3 |
43.8 |
50.0 |
66.7 |
0.33 |
0.31 |
–5.03-11.25 |
Stable |
| Piperacillin/tazobactam |
25.0 |
35.1 |
20.2 |
24.3 |
47.0 |
0.24 |
0.41 |
–7.67-14.31 |
Stable |
| Cefoperazone/sulbactam |
15.0 |
10.3 |
10.6 |
20.0 |
38.2 |
0.59 |
0.13 |
–2.95-14.17 |
Stable |
| Amoxicillin |
24.2 |
25.2 |
23.5 |
18.6 |
41.2 |
0.26 |
0.39 |
–5.87-11.35 |
Stable |
| Levofloxacin | 40.6 | 43.2 | 31.3 | 32.6 | 52.6 | 0.06 | 0.69 | –8.41-11.09 | Stable |
Table 3. Trends in resistance rate of Acinetobacter to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime |
65.8 |
69.3 |
61.2 |
83.6 |
93.3 |
0.67 |
0.09 |
–1.99-15.85 |
Stable |
| Ceftriaxone |
71.0 |
93.5 |
87.8 |
96.1 |
98.8 |
0.69 |
0.08 |
–1.35-12.99 |
Stable |
| Cefepime |
51.5 |
56.2 |
51.1 |
76.6 |
88.2 |
0.78 |
0.05 |
0.28-18.48 |
Increasing |
| Aztreonam |
55.2 |
54.3 |
61.3 |
84.3 |
87.4 |
0.86 |
0.02 |
2.42-16.46 |
Increasing |
| Imipenem |
14.4 |
17.0 |
13.9 |
60.2 |
86.3 |
0.79 |
0.04 |
1.20-36.20 |
Increasing |
| Meropenem |
18.9 |
35.5 |
23.4 |
59.4 |
87.1 |
0.80 |
0.04 |
1.24-30.82 |
Increasing |
| Amoxicillin/clavulanate |
71.1 |
75.9 |
61.7 |
84.7 |
94.3 |
0.49 |
0.19 |
–4.91-15.95 |
Stable |
| Piperacillin/tazobactam |
51.0 |
57.4 |
52.8 |
82.2 |
91.3 |
0.81 |
0.04 |
1.16-19.92 |
Increasing |
| Cefoperazone/sulbactam |
8.8 |
13.0 |
10.6 |
43.8 |
55.4 |
0.82 |
0.04 |
1.61-23.19 |
Increasing |
| Amoxicillin |
55.8 |
59.8 |
57.7 |
69.6 |
75.1 |
0.84 |
0.03 |
0 .98-8.70 |
Increasing |
| Ciprofloxacin |
55.6 |
87.5 |
66.7 |
100.0 |
82.1 |
0.35 |
0.29 |
–9.79-22.89 |
Stable |
| Levofloxacin | 61.5 | 57.8 | 58.1 | 75.4 | 80.1 | 0.69 | 0.08 | –1.27-12.23 | Stable |
Table 4. Trends in resistance rate of Citrobacter to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Cefuroxime |
56.7 |
72.4 |
77.2 |
69.0 |
95.0 |
0.69 |
0.08 |
–1.72-16.36 |
Stable |
| Ceftazidime |
28.6 |
40.0 |
56.6 |
47.1 |
80.0 |
0.80 |
0.04 |
0.91-1.07 |
Increasing |
| Ceftriaxone |
37.5 |
71.4 |
73.4 |
75.0 |
95.0 |
0.82 |
0.03 |
1.59-22.12 |
Increasing |
| Cefepime |
20.0 |
43.3 |
42.9 |
47.1 |
33.3 |
0.19 |
0.46 |
–8.38-14.46 |
Stable |
| Aztreonam |
31.4 |
43.3 |
58.5 |
50.0 |
63.2 |
0.78 |
0.05 |
0.14-3.92 |
Increasing |
| Imipenem |
20.0 |
11.1 |
15.8 |
6.9 |
23.8 |
0.00 |
0.93 |
–10.87-11.55 |
Stable |
| Meropenem |
10.0 |
3.4 |
1.8 |
9.1 |
31.6 |
0.42 |
0.24 |
–5.70-15.48 |
Stable |
| Amoxicillin/clavulanate |
77.1 |
85.7 |
85.5 |
78.8 |
90.5 |
0.33 |
0.31 |
–3.25-7.23 |
Stable |
| Ampicillin/sulbactam |
61.9 |
63.0 |
69.4 |
75.0 |
82.0 |
0.96 |
<0.01 |
3.28-7.16 |
Increasing |
| Piperacillin/tazobactam |
15.2 |
14.3 |
25.0 |
41.2 |
52.9 |
0.92 |
0.01 |
4.61-15.85 |
Increasing |
| Cefoperazone/sulbactam |
11.4 |
10.3 |
10.5 |
42.9 |
19.0 |
0.29 |
0.35 |
–8.82-18.38 |
Stable |
| Amoxicillin |
26.5 |
32.1 |
44.6 |
25.8 |
33.3 |
0.02 |
0.81 |
–7.94- 9.40 |
Stable |
| Levofloxacin | 20.6 | 44.8 | 60.6 | 64.3 | 65.4 | 0.83 | 0.03 | 1.75-20.07 | Increasing |
Table 5. Trends in resistance rate of Pseudomonas aeruginosa to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime |
60.8 |
68.3 |
65.4 |
74.4 |
74.9 |
0.81 |
0.04 |
0.43-6.43 |
Increasing |
| Ceftriaxone |
81.1 |
95.0 |
93.0 |
96.8 |
96.2 |
0.61 |
0.12 |
–1.53-7.93 |
Stable |
| Cefepime |
62.3 |
65.7 |
61.2 |
72.1 |
64.7 |
0.17 |
0.49 |
–3.38-5.62 |
Stable |
| Aztreonam |
63.3 |
68.5 |
68.8 |
77.1 |
74.3 |
0.80 |
0.04 |
0.27-5.85 |
Increasing |
| Imipenem |
52.5 |
61.8 |
51.5 |
60.5 |
63.2 |
0.34 |
0.31 |
–3.18-7.20 |
Stable |
| Meropenem |
45.1 |
59.7 |
50.1 |
59.1 |
65.0 |
0.60 |
0.13 |
–2.01-9.85 |
Stable |
| Amoxicillin/clavulanate |
95.8 |
94.5 |
92.3 |
97.1 |
95.5 |
0.03 |
0.78 |
–1.85-2.25 |
Stable |
| Piperacillin/tazobactam |
65.1 |
66.8 |
59.5 |
73.2 |
70.2 |
0.25 |
0.39 |
–3.57-6.89 |
Stable |
| Cefoperazone/sulbactam |
47.2 |
48.7 |
40.4 |
62.8 |
59.4 |
0.44 |
0.22 |
–4.18-11.88 |
Stable |
| Amoxicillin |
57.0 |
56.2 |
58.9 |
63.6 |
63.2 |
0.82 |
0.03 |
0.29-3.66 |
Increasing |
| Ciprofloxacin |
61.3 |
59.2 |
56.7 |
64.7 |
59.7 |
0.02 |
0.84 |
–3.17-3.64 |
Stable |
| Levofloxacin | 68.6 | 67.7 | 66.1 | 67.2 | 63.4 | 0.74 | 0.06 | –2.28-0.09 | Stable |
Antimicrobial resistance of Gram-positive bacteria
With regard to Gram-positive bacteria (GPB), vancomycin was extremely effective against the most common bacteria (Staphylococcus aureus). No vancomycin-resistant isolate of Staphylococcus aureus was found. Few GPB was found resistant to teicoplanin. The GPB resistance rate remained high to penicillins, cephalosporins, piperacillin/tazobactam, levofloxacin and erythromycin and was more than 70% in 2010. The decreasing trend occurred in resistance rate to clindamycin (R2=0.93 and P<0.01) against Staphylococcus aureus (Table 6).
Table 6. Trends in resistance rate of Staphylococcus aureus to antibiotics, 2006 to 2010.
| Antibiotics | 2006 | 2007 | 2008 | 2009 | 2010 | R2 | P | 95% CI | Trend |
|---|---|---|---|---|---|---|---|---|---|
| Penicillin G |
99.4 |
99.2 |
96.8 |
97.7 |
97.6 |
0.52 |
0.17 |
–1.41-0.39 |
Stable |
| Cefazolin |
85.5 |
85.8 |
70.8 |
72.4 |
83.7 |
0.13 |
0.55 |
–9.72-6.32 |
Stable |
| Ceftriaxone |
84.7 |
91.3 |
84.6 |
95.2 |
100.0 |
0.66 |
0.09 |
–1.06-7.96 |
Stable |
| Cefepime |
84.8 |
89.0 |
84.4 |
92.7 |
88.2 |
0.24 |
0.40 |
–2.39-4.49 |
Stable |
| Cefoxitin |
82.3 |
88.1 |
82.9 |
89.3 |
87.7 |
0.35 |
0.30 |
–1.82-4.22 |
Stable |
| Piperacillin/tazobactam |
77.7 |
91.3 |
82.9 |
90.3 |
89.0 |
0.35 |
0.29 |
–3.26-7.58 |
Stable |
| Amoxicillin |
63.1 |
56.8 |
66.7 |
70.5 |
71.0 |
0.63 |
0.11 |
–1.20-7.10 |
Stable |
| Levofloxacin |
84.2 |
77.8 |
90.0 |
86.3 |
78.5 |
0.01 |
0.89 |
–6.30-5.72 |
Stable |
| Cotrimoxazole |
42.1 |
46.0 |
38.4 |
42.3 |
22.9 |
0.54 |
0.16 |
–11.32-2.90 |
Stable |
| Erythromycin |
86.3 |
86.7 |
86.0 |
86.4 |
77.0 |
0.51 |
0.18 |
–5.30-1.52 |
Stable |
| Clindamycin |
78.7 |
76.6 |
68.5 |
64.4 |
63.5 |
0.93 |
0.01 |
–6.36-2.16 |
Decreasing |
| Teicoplanin |
0.0 |
1.7 |
0.0 |
0.8 |
0.0 |
N |
N |
N |
N |
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | N | N | N | N |
N: The data is not suitable for statistical analysis.
Discussion
In this study, Gram-negative bacteria were the predominant pathogens causing LRTI. During the study period of the 5 years, the proportion of Gram-negative bacteria changed little. Candida infections had significantly increased while Gram-positive bacteria decreased. Opportunistic pathogens (such as Candida albicans) ratio gradually increased, which caused by dysbacteria because of long-term use of antibiotics. Pseudomonas aeruginosa remained the most common pathogen (26.5-30.5%) from 2006 to 2009. Wang et al. (8) also reported Pseudomonas aeruginosa was the most common pathogens causing hospital-acquired lower respiratory tract infections in North China and as found in a study in North America (9). The rate of isolation of Acinetobacter increased from 7.5% (2007) to 26.4% (2010) becoming the most common isolate in 2010 in this study. As opportunistic pathogen, Acinetobacter baumannii-infected patients are mainly immunocompromised critically ill patients, the elderly or the patients using immunosuppressive drugs. In this study, Most of Acinetobacter baumannii infection patients came from department of respiratory medicine, neurology, and intensive care units (ICU), because the patients in these sections were all in critical condition, tracheostomy, long hospital stay, lower immune defense, or suffering from a variety of disease, the bacteria was easy to cross-infection in the form of droplets or aerosols. And the severe resistance of Acinetobacter baumannii resulted in the hospital stay of patients who infected with Acinetobacter baumannii prolonged resulting in increased cross-infection, so that the isolation rate increased.
The resistance rate of GNB to most antibiotics showed the increasing trend especially for cephalosporins and carbapenems. Escherichia coli, Klebsiella pneumonia and Acinetobacter had high resistance rates to cephalosporins, which were all more than 70% in 2010. This is probably related to the irrational use of cephalosporins in our hospital in recent years. GNB resistant to cephalosporins had been very serious in China, which is different in Europe and America (10-12). Carbapenems was considered as the most effective antibiotic agent against Gram-negative bacteria in the past. But now, the resistance rates of GNB to carbapenems were increasing gradually. In this study, Klebsiella pneumonia resistance rate significant increased, which was more than 25% in 2010, possibly related to the prevalence of KPC carbapenemase (13). As more and more multi-resistant Pseudomonas aeruginosa, the study found that the efflux pump on the cell membrane of Pseudomonas aeruginosa is one of the main reasons of its multi-drug resistance, the outer membrane protein OprM is the most common among six efflux pumps (14).
Due to the wide application of broad-spectrum antibiotics, the resistance rates of Acinetobacter to most antibiotics have continually increased during recent decades, resistance to carbapenems is most concerning (15). The MYSTIC program of 2007 demonstrated that 74.1% of isolates were susceptible to meropenem and 78.9% were susceptible to imipenem in Europe, compared with much lower susceptibilities of 51.3% and 52.0% in several Asian countries in the SENTRY program of 2006-2007 (16,17). The emergence of carbapenem-resistant Acinetobacter has been described as the sentinel event of antimicrobial resistance. In this study, more than 85% resistance rate was observed in Acinetobacter against carbapenems resulting in the isolates rate of multidrug-resistant Acinetobacter increased year by year. The results were similar with the Chinese Meropenem Surveillance Study (CMSS), which took place from 2003 to 2008, defined a serious carbapenem resistance problem in Acinetobacter. The major mechanism of carbapenem resistance in Acinetobacter is production of OXA β-lactamases, primarily OXA-23, OXA-66 and OXA-58 (13).
No vancomycin-resistant isolate of Staphylococcus aureus was found in this 5-year study. Decreasing trend in resistance to clindamycin against Staphylococcus aureus was observed. This may related to strengthen the clindamycin-induced experimental in our hospital.
Although antimicrobial agents are an important therapeutic weapon in infectious disease (18), the selective pressure, which may lead to antibiotics resistance, was imposed at the same time. In general, new resistances would be detected soon after introduction of new antimicrobial agents. To cope with resistant bacteria, new antibiotics must be developed, however, it became increasingly difficult to develop new ones (19). To prolong the effectiveness of currently available antimicrobial agents, it is essential to know the pathogen distribution and their antibiotic resistance patterns (20). The pathogen profile and their antibiotic resistance patterns was identified by the present study, it would be help the clinicians to facilitate decision-making.
Our investigation showed that Gram-negative bacteria were the predominant pathogens and that antimicrobial resistance is severe in our hospital, which may be related to illegitimate antibiotic use. The treatment of patients with bacterial LRTI is, therefore, becoming more complicated. In particular, the emergence of resistance to commonly prescribed antimicrobial agent by respiratory tract pathogens has compounded the problem. These results highlight the need for systematic interventions to ensure more consistent application of recommended guidelines for antimicrobial use, especially for the second/third generation cephalosporin and carbapenem. The researchers from our hospital found that the total annual consumption of carbapenem has markedly increased. It increased 192 times in 2009 compared with 2001. The consumption of imipenem/cilastatin, meropenem, and total carbapenem is associated with Acinetobacter resistance to piperacillin-tazobactam, ceftazidime, cefepime, amikacin, and levofloxacin (21). The good news is our hospital had formulated the related policy that only the specified senior doctors have the prescription right of above antibiotics.
This 5-year study demonstrated that antimicrobial resistance of pathogens isolated from lower respiratory tracts has become a serious problem with some antibiotics, the hospitals and related government departments should strengthen management of the use of some antibiotics in order to enhance the effectiveness of medication.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 81000754) and a grant from the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114).
Disclosure: The authors declare no conflict of interest.
References
- 1.Tao Y, Yan Z, Sha J, Zhu Z, Lei H. Severe gastroparesis causing postoperative respiratory complications in a heart-lung recipient. J Thorac Dis. 2010;2:121-123 [PMC free article] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903 [DOI] [PubMed] [Google Scholar]
- 3.Kuba PK, Sharma J, Sharma AK. Complication of warfarin therapy presenting as empyema. J Thorac Dis. 2011;3:74-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonlugur U, Bakici MZ, Akkurt I, Efeoglu T. Antibiotic susceptibility patterns among respiratory isolates of Gram-negative bacilli in a Turkish university hospital. BMC Microbiol. 2004;4:32 10.1186/1471-2180-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay C, Bhargava A, Ayyagari A. Role of mechanical ventilation & development of multidrug resistant organisms in hospital acquired pneumonia. Indian J Med Res. 2003;118:229-235 [PubMed] [Google Scholar]
- 6.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138-1180 10.1183/09031936.05.00055705 [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Analysis and presentation of cumulative susceptibility test data; approved guideline, 2nd ed. Document M39-A2, vol. 25, no. 28. Wayne (PA): Clinical and Laboratory Standards Institute;2006. [Google Scholar]
- 8.Wang Y, Zhang R, Li W, Feng Y, Leng T. Serious antimicrobial resistance status of pathogens causing hospital-acquired lower respiratory tract infections in North China. J Int Med Res. 2009;37:899-907 [DOI] [PubMed] [Google Scholar]
- 9.Hoban DJ, Biedenbach DJ, Mutnick AH, Jones RN. Pathogen of occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000). Diagn Microbiol Infect Dis. 2003;45:279-285 10.1016/S0732-8893(02)00540-0 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Yu Y, Xie X, et al. In-vitro antibacterial activities of cefpiramide and other broad-spectrum antibiotics against 440 clinical isolates in China. J Infect Chemother. 2000;6:81-85 10.1007/PL00012156 [DOI] [PubMed] [Google Scholar]
- 11.Glupczynski Y, Delmée M, Goossens H, Struelens M, Belgian Multicenter ICU Study Group Distribution and prevalence of antimicrobial resistance among gram-negative isolates in intensive care units (ICU) in Belgian hospitals between 1996 and 1999. Acta Clin Belg. 2001;56:297-306 [DOI] [PubMed] [Google Scholar]
- 12.Barlow M, Hall BG. Experimental prediction of the natural evolution of antibiotic resistance. Genetics. 2003;163:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao YH, Giske CG, Wei ZQ, Shen P, Heddini A, Li LJ. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist Updat. 2011;14:236-250 [DOI] [PubMed] [Google Scholar]
- 14.Li XZ, Poole K, Nikaido H. Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob Agents Chemother. 2003;47:27-33 10.1128/AAC.47.1.27-33.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39:105-114 10.1016/j.ijantimicag.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Turner PJ. MYSTIC Europe 2007: activity of meropenem and other broad-spectrum agents against nosocomial isolates. Diagn Microbiol Infect Dis. 2009;63:217-222 10.1016/j.diagmicrobio.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN. Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY Surveillance Program. J Antimicrob Chemother. 2009;63:55-59 10.1093/jac/dkn434 [DOI] [PubMed] [Google Scholar]
- 18.Salyers AA, Whitt DD. Revenge of the microbes: how bacterial resistance is undermining the antibiotic miracle. Washington, DC: ASM Press;2005. [Google Scholar]
- 19.Brötz-Oesterhelt H, Sass P. Postgenomic strategies in antibacterial drug discovery. Future Microbiol. 2010;5:1553-1579 10.2217/fmb.10.119 [DOI] [PubMed] [Google Scholar]
- 20.Woodford N, Livermore DM. Infections caused by Gram-positive bacteria: a review of the global challenge. J Infect. 2009;59:S4-S16 10.1016/S0163-4453(09)60003-7 [DOI] [PubMed] [Google Scholar]
- 21.Cao J, Song W, Gu B, et al. Correlation Between Carbapenem Consumption and Antimicrobial Resistance Rates of Acinetobacter baumannii in a University-Affiliated Hospital in China. J Clin Pharmacol. 2012. Epub ahead of print 10.1177/0091270011435988 [DOI] [PubMed] [Google Scholar]