Abstract
This is the case of a 63 year-old male who was diagnosed adenocarcinoma in the left upper lung with ipsilateral malignant pleural effusion. At diagnosis it had already spread to left pulmonary HLN (hilar lymph node) and left supraclavicular lymph node and mediastinal lymph nodes. The patient received combined chemotherapy with bevacizumab and GP (gemcitabine and carboplatin) for 6 courses. Disease progression on chest CT scan was recognized, daily treatment with oral gefitinib (250 mg/day) was commenced. One week later, he was admitted under the impression of gefitinib-related interstitial pneumonitis, gefitinib was discontinued immediately and methylprednisolone with BIPAP assisted ventilation were used. The patient was followed up for 2 months after the start of treatment with corticosteroids and BIPAP assisted ventilation and remained well.
Key Words: Gefitinib, interstitial pneumonitis, non-invasive BIPAP-ventilation
Background
Lung cancer remains the leading cause of malignancy-related mortality worldwide, with over one million cases diagnosed yearly (1). Non-small-cell lung cancer (NSCLC) accounts for >80% of all lung cancers. Because it is typically diagnosed at an advanced stage, chemotherapy (CT) remains the cornerstone of treatment, however, conventional treatment of NSCLC has apparently reached a plateau of effectiveness in improving survival of patients. For better toxicity profile than conventional CT, molecular targeted therapies for cancer become a new therapeutic areas in recent years. Gefitinib (Iressa) is an Epidermal Growth Factor Receptor Type 1/tyrosine kinase (HER1/EGFR) inhibitor and block the signal transduction pathway implicated in the proliferation and survival of cancer cells (2,3). The development of gefitinib in the treatment of advanced non-small-cell-lung cancer (NSCLC) raised a great enthusiasm among physicians. Gefitinib is well tolerated and less toxic compared to conventional cytotoxic drugs, but gefitinib-related interstitial lung disease (ILD) has been reported as a serious adverse effect (4). Here, we reported a case of acute lung injury induced by gefitinib that was detected in the early phase with high-resolution (HR) CT, and successfully treated with corticosteroid and BIPAP assisted ventilation therapy.
Case report
A 63-year-old man presented with cough and dyspnoea on exertion. He had been previously healthy until adenocarcinoma in the left upper lung with ipsilateral malignant pleural effusion and left pulmonary HLN (hilar lymph node) and left supraclavicular lymph node and mediastinal lymph nodes metastasis (T1N3M1a, stage IV) was diagnosed in May 2010. He received combined chemotherapy with bevacizumab (Avastin) 400 mg d0/3 weeks and gemcitabine 1,600 mg d1, 8/3 weeks and carboplatin 500 mg d1/3weeks for 6 courses. At the same time, left-sided intrathoracic instillation of OK-432 was given. Disease progression on chest CT scan was recognized in December 2010. Daily treatment with oral gefitinib (250 mg/day) was commenced. At this time his CT scan showed no lung tissue abnormality in the right upper lobe (Figure 1). The patient then received gefitinib (250 mg/day) on December 20, 2010. However, aggravated dyspnea on exertion with a dry cough developed after 7 days of gefitinib treatment. Hypoxemia and a 75% of oxyhemoglobin saturation at rest were measured by pulse oximetry. The body temperature was 37.0 °C; pulse rate was 100 beats/min and the blood pressure was 135/85 mmHg. Arterial blood gas analysis at rest (FiO2 =0.29) were illustrated in Table 1 (Values 1). Leucocyte cell count was 1.0×104/mm3 with 77% neutrophils. All cultures and stains for infectious etiologies including common bacteria, fungi, pneumocystis, legionella, nocardia, viruses were negative. Chest CT revealed ground glass opacity (GGO) in the right upper lobe (Figure 2), which suggesting an acute interstitial pneumonia. He was admitted under the impression of gefitinib-related interstitial pneumonitis. After admission, gefitinib was discontinued immediately and methylprednisolone 80 mg/day was started. At the same time, BIPAP assisted ventilation was used. The Ventilator parameters were: IPAP 12 cmH2O, EPAP 4 cmH2O, FiO2 75%, R 18 bpm. Dyspnoea and cough were resolved 7 days after commencement of treatment with methylprednisolone and BIPAP assisted ventilation, and a chest CT taken 10 days after the start of treatments demonstrated a scar-like lesion without GGO in the right upper lobe (Figure 3). Arterial blood gas analysis in room air (FiO2 =0.29) were illustrated in Table 1 (Values 2). The daily dosage of prednisolone was decreased by 20 mg per day depending on the patient’s response. The patient was followed up for 2 months after the start of treatment with corticosteroids and remained well.
Figure 1.
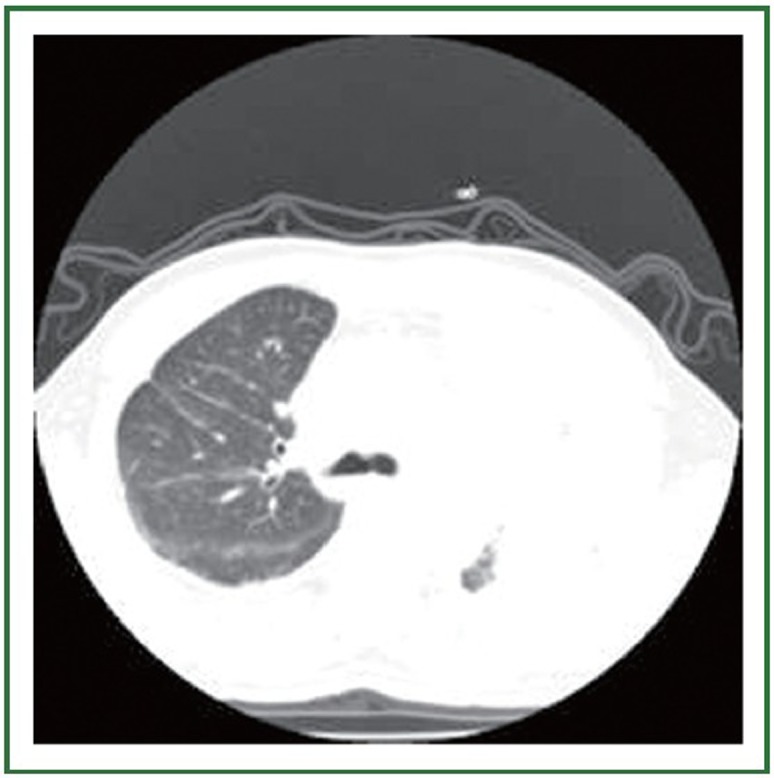
Chest CT scan through the carina level, before gefitinib treatment. No abnormalities were visible in the right upper lobe.
Table 1. Arterial blood gas analysis.
| Values 1 | Values 2 | Normal range | |
|---|---|---|---|
| pH |
7.423 |
7.390 |
7.35-7.45 |
| PaO2 |
37.82 |
138.35 |
83-108 mmHg |
| PaCO2 |
47.29 |
50.98 |
35-48 mmHg |
| ABE | 5.4 | 4.4 | –3.2-3.5 mmol/L |
Figure 2.
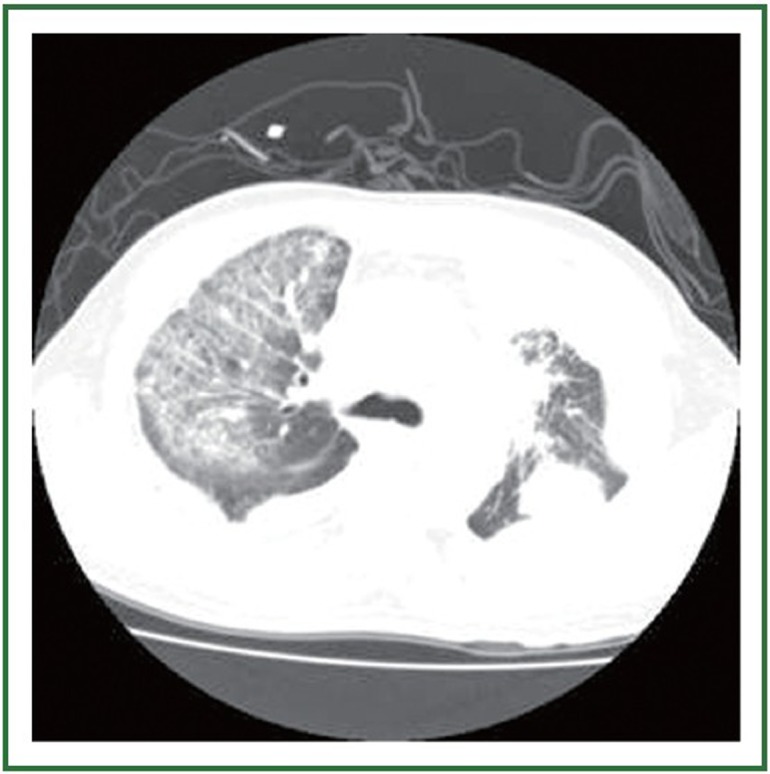
Chest CT scan 1 week later after 1 week of gefitinib therapy, when symptoms developed. CT scan through a similar lung section, suggesting an acute interstitial pneumonia pattern.
Figure 3.
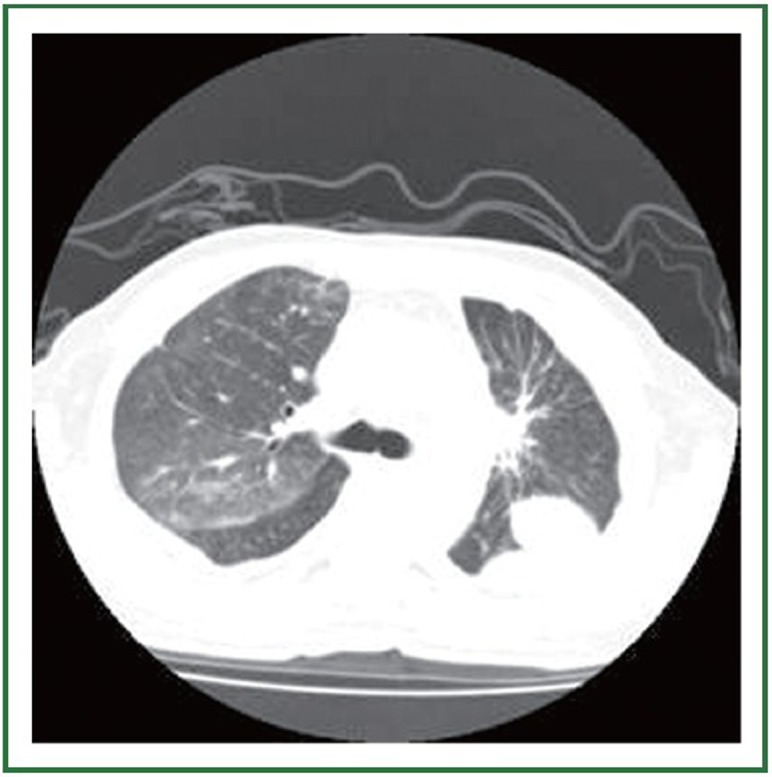
Chest CT scan 10 days after gefitinib had been discontinued and treated with corticosteroid and non-invasive BIPAP-ventilation.
Discussion
Gefitinib is an oral selective inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that may be effective in some patients with advanced non-small-cell lung, ovarian, breast, head and neck, and colon cancers. Members of the EGF family have been implicated in the repair of pulmonary damage (5,6). Therefore, inhibition of EGFR-mediated signalling by gefitinib may impair the repair of bronchioloalveolar epithelium and thereby exacerbate lung injury (7,8), especially in patients with pulmonary comorbidities (9).
Although the incidence of lung injury due to gefitinib is low (10), the number of patients with gefitinib-induced lung injury is likely to increase. Therefore, a strategy for dealing with this is required. There was no randomized controlled trial to guide the management of gefitinib-induced acute interstitial pneumonia. Li-Chiao Kuo et al. (11) used high-dose corticosteroid to treat gefitinib-induced acute interstitial pneumonia,but high-dose corticosteroid is harmful to the body. The case in the present report suggests that lung injury induced by gefitinib could be treated and cured using low-dose corticosteroid and BIPAP assisted ventilation, if detected early. When dyspnoea on exertion developed in this patient 1 week after administration of oral gefitinib, CT was immediately performed and gefitinib discontinued. At that time, this patient was serious, we can’t carry out the transbronchial biopsy. Ten days after discontinuation, HRCT showed progression of lung injury, which was compatible with an AIP pattern. Treatment with corticosteroids and BIPAP assisted ventilation resolved the lung injury, symptoms and the GGO lesions on HRCT, and increased PaO2. Such recovery is possible when lung injury is detected and treated in the early phase of diffuse alveolar damage, before progression to the fibrotic phase of AIP.
Noninvasive ventilation (NIV) has revolutionised the (12) management of patients with acute respiratory failure. It has decreased the need for endotracheal intubation and its attendant complications like nosocomial pneumonia and (13) other intensive care unit-acquired infections. In selected situations like chronic obstructive pulmonary disease and pulmonary oedema, it has also been shown to decrease (14,15) mortality. Positive pressure therapy acts by augmenting (16,17) intrinsic positive end-expiratory pressure (PEEP). Using biphasic positive airway pressure (BiPAP), may improve the respiratory failure in patients with type II blood oxygen level, to improve blood oxygen pressure, oxygen saturation and improved tissue hypoxia.
To effectively follow up patients treated with gefitinib and to prevent deaths from gefitinib-induced lung injury, early recognition and intervention may prevent a fatal outcome, corticosteroids and non-invasive BIPAP-ventilation should be considered if clinically deteriorated especially the patient has severe hypoxia.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96 [DOI] [PubMed] [Google Scholar]
- 2.Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001;13:491-498 [DOI] [PubMed] [Google Scholar]
- 3.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958-2970 [PubMed] [Google Scholar]
- 4.Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361:137-139 [DOI] [PubMed] [Google Scholar]
- 5.Madtes DK, Busby HK, Strandjord TP, Clark JG. Expression of transforming growth factor-alpha and epidermal growth factor receptor is increased following bleomycin-induced lung injury in rats. Am J Respir Cell Mol Biol. 1994;11:540-551 [DOI] [PubMed] [Google Scholar]
- 6.Madtes DK, Rubenfeld G, Klima LD, et al. Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:424-430 [DOI] [PubMed] [Google Scholar]
- 7.Hardie WD, Prows DR, Leikauf GD, Korfhagen TR. Attenuation of acute lung injury in transgenic mice expressing human transforming growth factor-alpha. Am J Physiol. 1999;277:L1045-L1050 [DOI] [PubMed] [Google Scholar]
- 8.Hardie WD, Prows DR, Piljan-Gentle A, et al. Dose-related protection from nickel-induced lung injury in transgenic mice expressing human transforming growth factor-alpha. Am J Respir Cell Mol Biol. 2002;26:430-437 [DOI] [PubMed] [Google Scholar]
- 9.Danson S, Blackhall F, Hulse P, Ranson M. Interstitial lung disease in lung cancer: separating disease progression from treatment effects. Drug Saf. 2005;28:103-113 [DOI] [PubMed] [Google Scholar]
- 10.Armour A. Gefitinib in advanced non-small cell lung cancer:clinical experience in patients of Asian origin. Asia Pac J Clin Oncol. 2007;3:366-378 [Google Scholar]
- 11.Kuo LC, Lin PC, Wang KF, Yuan MK, Chang SC. Successful treatment of gefitinib-induced acute interstitial pneumonitis with high-dose corticosteroid: a case report and literature review. Med Oncol. 2011;28:79-82 [DOI] [PubMed] [Google Scholar]
- 12.Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA. 2002;288:932-935 [DOI] [PubMed] [Google Scholar]
- 13.Girou E, Brun-Buisson C, Taillé S, Lemaire F, Brochard L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290:2985-2991 [DOI] [PubMed] [Google Scholar]
- 14.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;CD004104. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Non-invasive ventilation in acute cardiogenic pulmonary oedema. Postgrad Med J. 2005;81:637-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JA, Kraemer RW, Gjerde GE. Inspiratory work and airway pressure with continuous positive airway pressure delivery systems. Chest. 1985;88:519-526 [DOI] [PubMed] [Google Scholar]
- 17.Lenique F, Habis M, Lofaso F, Dubois-Randé JL, Harf A, Brochard L. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Respir Crit Care Med. 1997;155:500-505 [DOI] [PubMed] [Google Scholar]


