Abstract
Introduction
Lung cancer is the leading cause of cancer-associated death. In many countries, adenocarcinoma is the most common histologic type in lung cancer. Previously, few factors are identified to be prognostic indicators for the patients with small lung adenocarcinoma. Recently, the ground glass opacity (GGO) area found on high-resolution computed tomography (HRCT) scanning was identified as a prognostic indicator in some studies. But no clear consensus has been defined.
Methods
The PubMed/MEDLINE, EMBASE, Cochrane library and SpringerLink electronic databases were searched for articles related to ground glass opacity on computed tomography in patients with small lung adenocarcinoma. Data was extracted and analyzed independently by two investigators. An estimate of the hazard ratio (HR) for comparing high GGO ratio with low GGO ratio was extracted. The respective HRs was combined into a pooled HR, and 95% confidence interval (CI) was calculated for each study. The publication heterogeneity was assessed graphically using performing Beggs’ funnel plot. All the statistical tests used in our meta-analysis were performed with STATA version 11.
Results
Thirteen studies, encompassing 2,027 patients, were included in our meta-analysis. Ten of these studies revealed that the GGO ratio in small lung adenocarcinoma is a good prognostic indicator. Seven studies were combined in a meta-analysis using overall survival (OS) as the end point of interest. The weighted HR of 7 studies was 0.85, with relative 95% CI ranging from 0.78 to 0.93 (P=0.009). For the surgical patient population, the primary endpoint of relapse-free survival (RFS) was superior with high GGO area on computed tomography (The combined HR 0.82, 95% CI 0.74-0.90; P=0.007).
Conclusions
The result of our meta-analysis suggested that the GGO area measured on HRCT had a prognostic value of overall survival and relapse-free survival in small lung adenocarcinoma. The GGO ratio may be an independent prognostic factor for small lung adenocarcinoma.
Key Words: GGO ratio, prognostic factor, small lung adenocarcinoma, meta-analysis
Introduction
Lung cancer is the leading cause of cancer death in the world and non-small cell lung cancer (NSCLC) is the majority of lung cancer (1,2). The 5-year survival rate of NSCLC is only 10-15% (3). Studies have revealed that survival of patients is strongly associated with the stage of lung cancer. Expect stage and performance status, no other prognostic factors have been definitively established for lung cancer. Clinical features including gender, age, weight loss and serum markers have also been studied but are found to be not sufficiently accurate for individual patients. Few indicators are identified to be able to assist in predicting therapy response and outcome (4).
The detection rate of small lung adenocarcinoma has been increased due to the widely use of high-resolution computed tomography (HRCT) and computed tomography (CT) screening for lung cancer. The small nodules have been identified in clinic as less than 3 cm in diameter and with the ground glass opacity (GGO) area. Small lung adenocarcinoma could be classified as either ‘air-containing type’ or ‘solid-density type’ according to tumor shadow disappearance rates (TDR) on mediastinal window images or GGO ratio (5). Air-containing type is defined as having areas where tumor opacity on mediastinal window images (TOM) is half or less than half the size of those noted on lung window images. Solid-density type is defined as having areas where TOM is greater than half the size of those noted on lung window images (6).
Recently, more and more studies indicated that it was important to identify the characteristics of the GGO lesions on small lung adenocarcinoma, to determine the appropriate operative mode and indicate the prognosis for each patient. Some findings indicated that lung adenocarcinoma patients with air-containing types after resection demonstrated a better prognosis (7). However, some authors retrospectively analyzed peripheral lung adenocarcinoma with GGOs and defined the GGO ratio as a limited prognostic indicator (8). A pooled analysis of the current studies might provide a better understanding of the GGO area in small lung adenocarcinoma. Therefore, we carried out a systematic review of published studies and a meta-analysis to combine the results of these studies.
Materials and methods
Data sources and keywords
PubMed/MEDLINE, EMBASE, Cochrane library and SpringerLink electronic databases were searched with the keywords ‘ground glass opacity’, ‘small lung adenocarcinoma prognostic’ and ‘GGO’. Latest studies were updated to May 17th, 2011. Titles and abstracts of research articles were screened for relevance according to predetermined inclusion criteria. References of the included studies were also searched to identify relevant articles that might be missed during the primary search.
Selection criteria
In order to define the eligibility for the meta-analysis, a study had to meet the following inclusion criteria: (I) high-resolution CT (HRCT) or thin-slide CT (TSCT) scanned prior to surgery, (II) limited to small lung adenocarcinoma (less than 3 cm in diameter), (III) patients underwent pulmonary lobectomy or segmental resection, (IV) carried out postoperative histopathological examination, (V) assessed the relationship between the GGO areas measured on HRCT and patients survival at least in univariate analysis. The studies have been published as original articles in English and provided sufficient data including hazard ratio (HR) and 95% confidence interval (CI) or survival curve. Meeting proceedings and abstracts were excluded as it cannot provide enough details to assess patient survival information to perform the meta-analysis.
When authors reported on the same patient population or updated data in several publications, only the recent or complete study was included in the analysis to avoid the overlap of information.
Data extraction and quality assessment
From the finally included studies, two investigators independently extracted needed data, such as the first author, the year of publication, journal name, sex and age of subjects, tumor histology, retrospective or prospective set-up of the study. The HR and associated 95% CI were collected from results of the univariate and multivariate analysis. If the needed data was not all reported in the texts, published survival curves would be used to obtain the unreported HR or 95% CI. The survival rates could also be analyzed from the survival curves; and finally, an adjusted HR and 95% CI were searched.
Meanwhile, we assessed the quality of included studies according to the Steele’s methods (European Lung Cancer Working Party quality scale for biological prognostic factors for lung cancer) (9). The overall score indicated the evaluation of several dimensions of the methodology, grouped into the following main categories: The scientific design, the description of the examinations, the generalizability of the results, the analysis of the study data and the association between GGO ratio and patient survival. Each category had a maximum score of 10 points, in which a value between 0 and 2 was attributed to each item. Hence the overall maximum score was 40 points.
Statistical analysis
We analyzed the survival distributions of high GGO ratio group and low GGO ratio group to reveal the association between GGO ratio and survival by hazard ratio (HR). The standard statistical method is to retrieve the HR estimate and its variance from the reported results, or to calculate them directly using parameters given by the authors for the univariate analysis: the 95% CI for the HR, the log-rank statistic, or its p-value. A P value below 0.05 was considered to be significance.
If the only available data of the survival distributions was in the form of graphical representations, we extracted data from the survival curves at different points to reconstruct the HR estimate and its variance. If survival of three or more groups was reported, some cutoff value was used to combine these groups into GGO ratio less than 50% group and greater than 50% group by reference to the statistical method from Williamson et al. (10). The HR and 95% CI for survival comparison in studies evaluated the prognostic value of GGO ratio on high-resolution CT.
We assessed combined HRs was obtained by the use of fixed-effects models in case of absence of heterogeneity and of random-effects models otherwise and the publication heterogeneity between studies by using chi-squared (χ2) test. Selection bias was assessed graphically using performing Beggs’ funnel plot. Interaction tests were performed using χ2 tests. The χ2 tests and I-squared (variation in ES attributable to heterogeneity; I2) statistic were used to assess heterogeneity (11). An I2 value greater than 50% suggested a significant heterogeneity. All statistical analysis in our meta-analysis was performed using STATA version 11 (StataCorp LP, College Station, Texas 77845, USA).
Results
Eligible studies
Six hundred and eighty-six potentially relevant articles were found by searching PubMed/MEDLINE, EMBASE, Cochrane library and SpringerLink electronic databases with the listed keywords. Studies were included according the inclusion criteria. Results were also determined by another investigator. Total 15 articles published in March 2001 to May 2011 were enrolled in the meta-analysis. The final articles for meta-analysis were also screened to avoid the overlap of patients.
The principal characteristics of the 15 studies for the meta-analysis are described in Table 1. The association between GGO ratio and relapse-free survival or overall survival was reported on each study. Most of the studies identified that high GGO ratio was associated with a good prognosis. Three studies revealed the opposite result that high GGO ratio predicted a poor prognosis.
Table 1. Principal characteristics of 15 studies included in the meta-analysis.
| Study | Publication date | nPts | GGO ratio | Computed tomography | Histology | Prognosis factor for survival |
|---|---|---|---|---|---|---|
| Takatoshi Aoki et al. (12) |
2001 |
127 |
0.5 |
HRCT |
AC |
Favorable |
| Ken Kodama et al. (13) |
2001 |
104 |
0.5 |
HRCT |
AC |
Favorable |
| Tetsuro Kondo et al. (6) |
2002 |
137 |
0.5 |
HRCT |
AC |
Favorable |
| Boming Dong et al. (14) |
2002 |
143 |
0.5 |
HRCT |
AC |
Favorable |
| Shodayu Takashima et al. (15) |
2002 |
64 |
0.57 |
HRCT |
AC |
Favorable |
| Shodayu Takashima et al. (16) |
2003 |
52 |
0.5 |
HRCT |
AC |
Unfavorable |
| Kazuya Takamochi et al. (17) |
2004 |
189 |
0.8 |
HRCT |
AC |
Favorable |
| Haruhiko Nakamura et al. (18) |
2004 |
100 |
0.5 |
HRCT |
AC+SCC |
Favorable |
| Masao Nakata et al. (19) |
2005 |
146 |
0.5 |
HRCT |
AC |
Unfavorable |
| Boming Dong et al. (20) |
2005 |
131 |
0.5 |
HRCT |
AC |
Favorable |
| Kunihiko Shimizu et al. (21) |
2005 |
260 |
0.5 |
HRCT |
AC |
Favorable |
| Toshihiko Hashizume et al. (22) |
2008 |
359 |
0.5 |
HRCT |
AC |
Favorable |
| Kotaro Higashi et al. (23) |
2009 |
87 |
0.5 |
HRCT |
AC |
Favorable |
| Haruhiko Nakayama et al. (24) |
2010 |
201 |
0.5 |
HRCT |
AC |
Unfavorable |
| Haruhiro Saito et al. (25) | 2011 | 134 | 0.5 | TSCT | AC | Favorable |
nPts, number of patients; GGO, ground glass opacity; HRCT, high-resolution computed tomography; TSCT, thin-section computed tomography; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Consideration of overlaps between patient cohorts
Two studies in 15 publications were reported by Dong B et al. (14,20). The patients from the first study were likely to be included in the second one. Another two studies were reported by Takashima S et al. (15,16). Although the overlap was not large, we included the late studies by Dong B et al. and Takashima S et al. to avoid the excursion (16,20). Thirteen studies with total 2,027 patients were finally included in our meta-analysis (6,12,13,16-25).
Quality assessment and evaluable studies
Two independent investigators formally analyzed the quality of studies referring to the Steele’s methods and variables that might reflect the quality of studies were recorded. If a variable in a study was not applicable, this theoretically attributable point would not be taken into account in the total of the concerned category. Higher value indicated a better methodological quality. The final quality scores ranged from 55.0% to 75.5%, with a mean of 68.7%, which indicated that these studies were evaluable and all publications included into a final meta-analysis.
Survival data aggregation
Thirteen studies with a total number of 2,027 patients were identified and included in the different subgroups analysis from our previous search. Seven studies were enrolled in a meta-analysis using overall survival (OS) as the end point of interest. Table 2 shows the 7 studies included in the meta-analysis with the GGO ratio for OS analysis. Relapse-free survival (RFS) data were available in eight studies (Table 3). We combined the individual HR into a pooled HR, and 95% confidence interval (CI) were calculated for every study. Three studies provided survivals of three or more groups, so these groups were divided into GGO ratio less than 50% group and greater than 50% group according to the statistical method from Williamson et al. (10).
Table 2. Meta-analysis of GGO ratio for overall survival analysis on small lung carcinoma.
| Study | Publication date | nPts | HR | 95% CI |
|---|---|---|---|---|
| Takatoshi Aoki et al. (12) |
2001 |
127 |
0.64 |
0.41-1.00 |
| Tetsuro Kondo et al. (6) |
2002 |
52 |
0.72 |
0.51-1.00 |
| Shodayu Takashima et al. (15) |
2003 |
52 |
0.10 |
0.90-1.10 |
| Kazuya Takamochi et al. (17) |
2004 |
189 |
0.72 |
0.52-1.00 |
| Haruhiko Nakamura et al. (18) |
2004 |
100 |
0.64 |
0.41-1.00 |
| Boming Dong et al. (20) |
2005 |
131 |
0.68 |
0.48-0.96 |
| Kunihiko Shimizu et al. (21) | 2005 | 260 | 0.78 | 0.61-1.00 |
nPts, number of patients; HR, hazard ratio; CI, confidence interval.
Table 3. Meta-analysis of GGO ratio for relapse-free survival analysis on small lung carcinoma.
| Study | Publication Date | nPts | HR | 95% CI |
|---|---|---|---|---|
| Ken Kodama et al. (13) |
2001 |
104 |
0.68 |
0.46-1.00 |
| Tetsuro Kondo et al. (6) |
2002 |
137 |
0.58 |
0.33-1.00 |
| Masao Nakata et al. (19) |
2005 |
146 |
1.11 |
0.98-1.30 |
| Kunihiko Shimizu et al. (21) |
2005 |
260 |
0.78 |
0.61-1.00 |
| Toshihiko Hashizume et al. (22) |
2008 |
359 |
0.76 |
0.57-1.00 |
| Kotaro Higashi et al. (23) |
2009 |
87 |
0.55 |
0.31-1.00 |
| Haruhiko Nakayama et al. (24) |
2010 |
201 |
0.76 |
0.58-1.00 |
| Haruhiro Saito et al. (25) | 2011 | 134 | 0.71 | 0.51-1.00 |
nPts, number of patients; HR, hazard ratio; CI, confidence interval.
The final HR revealed a survival benefit of high GGO ratio for small lung adenocarcinoma. The square size is proportional to the number of patients included in the study. The weighted HR of 7 studies at overall survival was 0.85, with a 95% confidence interval ranging from 0.78 to 0.93 (P=0.009) (Figure 1A). The total of patients in 8 studies on relapse-free survival was 1,428. As shown in Figure 1B, relapse-free survival did significantly differ between the two groups (The weighted HR 0.82, 95% CI 0.74-0.90; P=0.007).
Figure 1.
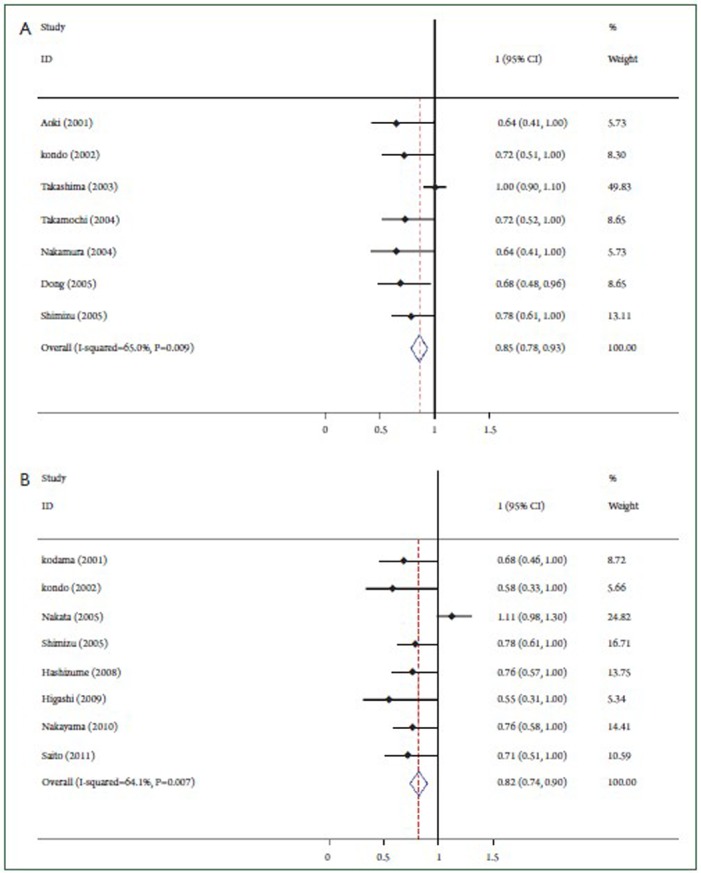
Graphical representation of the prognostic value of GGO ratio on survival in small lung adenocarcinoma. A: Based on overall survival analysis; B: Based on relapse-free survival analysis.
Heterogeneity study
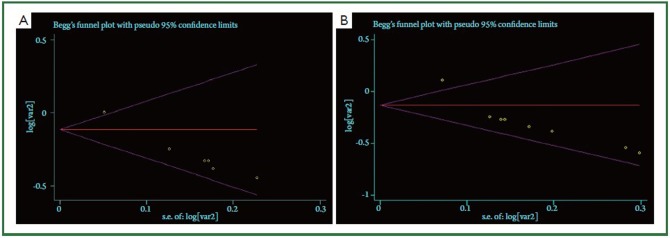
The funnel plot for the 7 reports on overall survival analysis revealed an evidence of publication bias (Figure 2A). Heterogeneity χ2 =17.15 (degrees of freedom, df.=6; P=0.009). I2=65.0%. As showed in Figure 2B, the funnel plot for the studies on relapse-free survival analysis also showed the publication bias. Heterogeneity χ2=19.52 (df.=7; P=0.007). Again high inconsistency was found across studies (I2 statistics =64.1%).
Figure 2.
Funnel plot analysis to detect publication bias. A: Based on overall survival analysis; B: Based on relapse-free survival analysis.
Discussion
We confirmed in our meta-analysis, with 13 original studies and 2,027 patients, that the GGO ratio was a prognostic indicator for small lung adenocarcinoma. After combined the HR from different studies, the final result suggested that high GGO ratio in HRCT predicted a better OS and RFS with a significant statistical value.
Recently, high-resolution computed tomography (HRCT) scan for lung cancer mass has been reported to have an advantage at defining tumors as small as 3 cm in diameter or less. The optimal intervention of these patients can improve the lung cancer survival rate and diminish the mortality.
Previously, pathological type and lymph node status were considered to be the most significant prognostic factors (26), and some prognostic factors have been reported for patients with surgically resected small lung adenocarcinoma. Most adenocarcinomas with a good prognosis showed in the radiography as pure GGO or GGO mixed with a small area of solid attenuation at high-resolution CT (27). Several studies have reported that the ground glass opacity (GGO) at HRCT finally proved to be lung adenocarcinomas in pathology (28). This showed a significant association between the GGO and tumor pathological type. However, the association between GGO ratio in HRCT and survival of small lung carcinoma patients was not identified.
The GGO ratio and TDR possibly reflected grade of tumor malignancy. A tumor with a larger GGO component is likely to be adenocarcinoma in situ or minimally invasive adenocarcinoma, therefore to have low propensity for distant spread. The lung adenocarcinoma smaller than 3 cm with a GGO component of more than 50% at HRCT has a high likelihood of being free of lymph node metastasis or vein involvement. Although lobectomy with mediastinal lymph node dissection is a standard operative procedure for lung cancer, the small adenocarcinoma with more GGO might be managed appropriately by limited resection to decreasing the operative mortality and morbidity as well as improving the performance status of the patients (29).
Our meta-analysis still has several limitations. First, publication bias is a well known problem in meta-analysis. Although we tried to reduce the selection bias to small, this meta-analysis still had a selection bias. This result was expected because the included patients of most studies came from Asia. Therefore, the studies have been published in the English language, which might have induced a verification bias. In our meta-analysis, we included 13 articles in which 12 articles had a GGO ratio of 0.5, only one reported as TDR of 0.8. The proportion of 24 patients with squamous cell cancer histology only reported by Nakamura H et al. was small. Second, since the GGO component has been calculated by using a semi-quantitative method with physician determination, the GGO areas on HRCT or TSCT scanning would be reported with disparity.
Although with these limitations, our meta-analysis still provide a positive and statistically significant result that high GGO ratio in HRCT predicted a better OS and RFS for small lung adenocarcinoma patients.
Conclusions
Our meta-analysis indicated that the GGO ratio had a prognostic value for small lung adenocarcinoma. The GGO ratio on HRCT scan could be an important predictive factor for postsurgical survival in patients with small lung adenocarcinoma. This result revealed the predictive value of GGO ratio for clinic. To further confirm these findings, large, prospective, randomized studies are required.
Acknowledgements
This research was supported by the Natural Science Fund of Jiangsu Province (BK2011658) to Yong Song.
Disclosure: The authors declare no conflict of interest.
References
- 1.Jemal A, Center MM, Desantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300 [DOI] [PubMed] [Google Scholar]
- 3.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment and survivorship. Mayo Clin Proc. 2008;83:584-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 2008;3:457-66. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252-1259 [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Yamada K, Noda K, et al. Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas. Lung Cancer. 2002;36:49-57 [DOI] [PubMed] [Google Scholar]
- 7.Ichiki Y, Hanagiri T, Baba T, et al. Limited pulmonary resection for peripheral small-sized adenocarcinoma of the lung. Int J Surg. 2011;9:155-159 [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Han J, Lee KS, et al. Lung adenocarcinoma as a solitary pulmonary nodule: Prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer. 2009;66:379-385 [DOI] [PubMed] [Google Scholar]
- 9.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705-719 [DOI] [PubMed] [Google Scholar]
- 10.Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337-3351 [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558 [DOI] [PubMed] [Google Scholar]
- 12.Aoki T, Tomoda Y, Watanabe H, et al. Peripheral lung adenocarcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Radiology. 2001;220:803-809 [DOI] [PubMed] [Google Scholar]
- 13.Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. 2001;33:17-25 [DOI] [PubMed] [Google Scholar]
- 14.Dong B, Sato M, Sagawa M, et al. Computed tomographic image comparison between mediastinal and lung windows provides possible prognostic information in patients with small peripheral lung adenocarcinoma. J Thorac Cardiovasc Surg. 2002;124:1014-1020 [DOI] [PubMed] [Google Scholar]
- 15.Takashima S, Maruyama Y, Hasegawa M, et al. Prognostic significance of high-resolution CT findings in small peripheral adenocarcinoma of the lung: a retrospective study on 64 patients. Lung Cancer. 2002;36:289-295 [DOI] [PubMed] [Google Scholar]
- 16.Takashima S, Maruyama Y, Hasegawa M, et al. High-resolution CT features: prognostic significance in peripheral lung adenocarcinoma with bronchioloalveolar carcinoma components. Respiration. 2003;70:36-42 [DOI] [PubMed] [Google Scholar]
- 17.Takamochi K, Yoshida J, Nishimura M, et al. Prognosis and histologic features of small pulmonary adenocarcinoma based on serum carcinoembryonic antigen level and computed tomographic findings. Eur J Cardiothorac Surg. 2004;25:877-883 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H, Saji H, Ogata A, et al. Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer. 2004;44:61-68 [DOI] [PubMed] [Google Scholar]
- 19.Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:1226-1231 [DOI] [PubMed] [Google Scholar]
- 20.Dong B, Sato M, Sakurada A, et al. Computed tomographic images reflect the biologic behavior of small lung adenocarcinoma: they correlate with cell proliferation, microvascularization, cell adhesion, degradation of extracellular matrix, and K-ras mutation. J Thorac Cardiovasc Surg. 2005;130:733-739 [DOI] [PubMed] [Google Scholar]
- 21.Shimizu K, Yamada K, Saito H, et al. Surgically curable peripheral lung carcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Chest. 2005;127:871-878 [DOI] [PubMed] [Google Scholar]
- 22.Hashizume T, Yamada K, Okamoto N, et al. Prognostic significance of thin-section CT scan findings in small-sized lung adenocarcinoma. Chest. 2008;133:441-447 [DOI] [PubMed] [Google Scholar]
- 23.Higashi K, Sakuma T, Ito K, et al. Combined evaluation of preoperative FDG uptake on PET, ground-glass opacity area on CT, and serum CEA level: identification of both low and high risk of recurrence in patients with resected T1 lung adenocarcinoma. Eur J Nucl Med Mol Imaging. 2009;36:373-381 [DOI] [PubMed] [Google Scholar]
- 24.Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer. 2010;116:3170-3177 [DOI] [PubMed] [Google Scholar]
- 25.Saito H, Kameda Y, Masui K, et al. Correlations between thin-section CT findings, histopathological and clinical findings of small pulmonary adenocarcinomas. Lung Cancer. 2011;71:137-143 [DOI] [PubMed] [Google Scholar]
- 26.Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol. 2009;4:951-958 [DOI] [PubMed] [Google Scholar]
- 27.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844-2852 [DOI] [PubMed] [Google Scholar]
- 28.Kondo T, Yamada K, Noda K, et al. Radiologic-prognostic correlation in patients with small pulmonary adenocarcinomas. Lung Cancer. 2002;36:49-57 [DOI] [PubMed] [Google Scholar]
- 29.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926-932, discussion 932-933 [DOI] [PubMed] [Google Scholar]