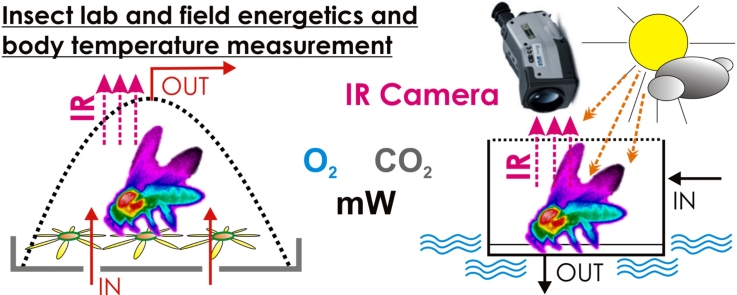
Graphical abstract
.
Highlights
► We demonstrate the benefits of a combined use of infrared thermography with respiratory measurements in insect ecophysiological research. ► Infrared thermography enables repeated investigation of behaviour and thermoregulation without behavioural impairment. ► Comparison with respirometry brings new insights into the mechanisms of energetic optimisation of bee and wasp foraging. ► Combination of methods improves interpretation of respiratory traces in determinations of insect critical thermal limits.
Keywords: Thermography, Respirometry, Energetics, Temperature, Honeybee, Wasp
Abstract
Endothermic insects like honeybees and some wasps have to cope with an enormous heat loss during foraging because of their small body size in comparison to endotherms like mammals and birds. The enormous costs of thermoregulation call for optimisation. Honeybees and wasps differ in their critical thermal maximum, which enables the bees to kill the wasps by heat. We here demonstrate the benefits of a combined use of body temperature measurement with infrared thermography, and respiratory measurements of energy turnover (O2 consumption or CO2 production via flow-through respirometry) to answer questions of insect ecophysiological research, and we describe calibrations to receive accurate results.
To assess the question of what foraging honeybees optimise, their body temperature was compared with their energy turnover. Honeybees foraging from an artificial flower with unlimited sucrose flow increased body surface temperature and energy turnover with profitability of foraging (sucrose content of the food; 0.5 or 1.5 mol/L). Costs of thermoregulation, however, were rather independent of ambient temperature (13–30 °C). External heat gain by solar radiation was used to increase body temperature. This optimised foraging energetics by increasing suction speed.
In determinations of insect respiratory critical thermal limits, the combined use of respiratory measurements and thermography made possible a more conclusive interpretation of respiratory traces.
1. Introduction
Honeybees and vespine wasps are heterothermic insects which change between endothermy (body temperature regulated) and ectothermy (body temperature following changes of ambient temperature closely). During foraging they are mostly endothermic, keeping their body temperature considerably above the ambient temperature [1–5]. Inside the nest some of them are endothermic but the majority of them is ectothermic or only weakly endothermic [6,7]. In the endothermic state they have to cope with an enormous heat loss because of their small body size in comparison to larger endothermic animals like mammals and birds [8,9]. For example, at 20 °C they have to invest more than 650-fold of energy per unit time and mass than a horse to keep the thorax temperature at 38 °C [10]. This is about 120-fold their resting metabolism [10]. In the ectothermic state their energy turnover increases approximately exponentially with ambient temperature [10–13].
For the measurement of body temperature thermocouples have been used by many authors [e.g. 8,9,14–18]. An alternative with special advantages in free ranging insects is the use of infrared thermography [1–5,19–24]. It allows for repeated, simultaneous non-contact measurement of the surface temperature of several body parts, without disturbing the animals’ behaviour or social interactions. By combining thermographic body temperature measurements with the measurement of operative temperature (i.e. the temperature of dead animals), the endothermic part of thermoregulation could be separated from the temperature increase caused by solar radiation in free ranging honeybee and wasp foragers [4]. However, we do not know of thermodynamic models of heat exchange that would allow accurate determination of energy turnover from body temperature measurements. Direct measurement in field experiments is recommended.
The energy turnover for the purpose of thermoregulation has been measured with various methods. Some authors used direct calorimetry [11,25–28]. An alternative is indirect calorimetry. It allows calculation of energy turnover from measurements of O2 consumption or CO2 production. Manometric methods to measure O2 consumption were applied by several authors [12,29–31]. The CO2 production of honeybee foragers was also determined with a titration method by determining the amount of time an air current needed to titrate 112 nequiv. of Ba(OH)2 [32,33]. Flow-through respirometry allowed investigation of flight energetics of honeybees [17,18,34].
During foraging, honeybees and wasps have to balance energetic costs with gain [4,5,35]. As muscular function is temperature dependent [36], a higher body temperature may speed up suction rate considerably [4]. The higher body temperature, on the other hand, increases energetic costs. Bees and wasps foraging in sunshine usually use solar heat gain to increase their body temperature by several degrees [4,5,37]. It is unknown, however, to what extent they use solar radiation to save energy, i.e. to reduce their own endothermic effort. We here demonstrate that a combination of respiratory measurements with infrared thermography provides advanced insights into the mechanisms controlling foraging efficiency in bees and wasps [compare 38,39]. We present experimental setups which even allow the simultaneous investigation of respiration, energy turnover and thermoregulation of foraging honeybees and wasps under field conditions. Special emphasis is given to possible measurement errors and calibration methods needed to get accurate results.
In recent years, in the course of global change, the temperature dependence of energy turnover and the thermal tolerance has become of general importance in insect ecology. A combination of respiration measurements with body temperature measurements with infrared thermography allows for judgement of ectothermy or weak endothermy during determination of resting metabolism [13]. Determination of critical thermal maxima (CTmax) and minima (CTmin), and of lethal temperatures is done by behavioural observation (video) and by a combination of thermolimit respirometry with (automated) activity tracking [31,40]. We here demonstrate that the combination of thermolimit respirometry with infrared thermography allows considerable advances in the interpretation of respiratory traces during such experiments.
2. Experimental
2.1. Setups for combined respiratory and thermographic measurements
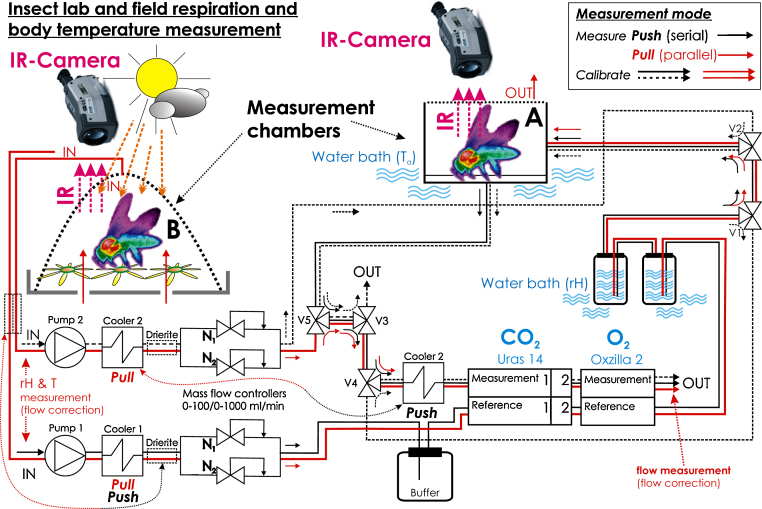
In open-flow investigations of insect respiration different parallel and serial plumbing setups are in use [31]. For general hints on measurement setups see also [41]. Fig. 1 shows a differential measurement setup which allows a fast, (semi-) automated switch between different measurement modes, and simultaneous thermographic measurement of body surface temperature.
Fig. 1.
Experimental setup for simultaneous measurement of energy turnover via respiration (CO2 production, O2 consumption), and body temperature with infrared thermography. The setup allows a fast switch between serial (push) and parallel (pull) plumbing. (A and B) Types and positions of the insect measurement chamber in serial and parallel mode, respectively. In serial mode (A), the Oxzilla 2 was replaced by a Magnos 14 paramagnetic O2 device (ABB). The Magnos 14 allowed exact definition of measurement chamber closing and opening because this produced small shifts in the O2 signal (a somewhat expensive pressure sensor). An flow meter (5860S, Brooks Instrument) could be inserted at the gas outlets (after Oxzilla or URAS) to control the accuracy of the mass flow controllers. Long dotted arrows: positions of drying devices in the two measurement modes (manual switching with quick connectors). URAS 14: ‘1’ and ‘2’ refer to a long (sensitive, 0–250 ppm) and a short (larger measurement range of 0–10,000 ppm) measurement tube in series, respectively. V1–V5: magnetic valves; N1, N2: mass flow controllers with a range of 0–100 and 0–1000 ml/min, respectively (5850S, Brooks Instrument). Ta = ambient air temperature, rH = relative humidity. For further details see text.
2.1.1. Serial mode
Serial plumbing has the advantage that during differential measurements of CO2 production or O2 consumption both the measurement and the reference parts of the sensor devices always are operated at exactly the same flow rate, and only one mass flow controller is needed [31]. In serial mode fresh air was taken from outside the laboratory via a copper tube. A pump fed the air to a peltier-driven cool trap (cooler 1) regulated at a temperature of 10 °C (Fig. 1). This brought the water content of the air to a constant and low level. After the cooler, Drierite columns could be inserted into the gas stream if complete drying was necessary. Afterwards, the air passed a set of mass flow controllers (e.g. Brooks 5850S, Brooks Instrument, Hatfield, USA) with a range of 0–100 and 0–1000 ml/min. The small range mass flow controller was recommended in measurements of tiny individuals like honeybee larvae which may weigh considerably less than a mg in early stages of development [42]. The controller software (Centrol 5; Harnisch, Austria) performed an automated switch to the 0–1000 ml/min mass flow controller if the flow was set to >95 ml/min. In investigations of honeybee and wasp resting metabolism and in thermolimit experiments, a flow of 150 ml/min turned out to provide a good compromise of sensitivity and temporal resolution of CO2 production measurements in a wide range of ambient temperatures (2.5–45 °C). To dampen fluctuations in outside air CO2 content, the air passed through a buffer container after the mass flow controllers (5 L, Fig. 1). A second container (10 L) was inserted in the air path before the pumps if a high baseline stability was required [13,42].
The air then passed the reference tubes of the differential CO2 gas analyser (DIRGA; URAS 14, ABB; operated at an internal temperature of 60 °C). A serial combination of a 0–250 ppm (=long) and a 0–1% (=short) measurement tube always provided a calibrated readout, independent of flow rate setting and intensity of insect respiration. Calibration of the URAS could be automatically performed in regular intervals (e.g. 3 h) both in zero point (against air from outside of the laboratory) and end point (with internal URAS 14 calibration cuvettes), after switching the air flow with magnetic valves to bypass the insect measurement chamber. The resolution of the CO2 measurement equipment was <0.2 ppm. Measurement accuracy was ∼2 ppm. The volumes of CO2 production reported in this paper refer to standard (STPS) conditions (0 °C, 101.32 kPa = 760 Torr).
On demand, an oxygen measurement device (Oxzilla 2, Sable Systems; or Magnos 16, ABB) could be inserted in the gas path after the DIRGA. The Oxzilla 2 can be operated in a differential mode. It then provides a high measurement sensitivity down to the ppm range. However, due to the limitations of the fuel cell sensor technology, one has to be aware of a considerable drift, which requires frequent manual baselining with outside air if the highest accuracy is recommended. The intrinsical sensitivity of the fuel cells to pressure changes, caused for example by the switching of the magnetic valves during automated calibration, did not allow accurate measurement of O2 consumption in the serial plumbing setup shown in Fig. 1. Valve switching has to be smooth (which could be accomplished by stepping motor valves). Therefore, we prefer to use it in parallel mode which is infinitely superior for the very small differences in O2 production the Oxzilla is able to sense (see Section 2.1.2 below). The Magnos 16 is a less sensitive, paramagnetic oxygen measurement device but allows long-duration operation with automated calibration. However, because of its sensitivity to tilting or changes in position it has to remain in a firm position throughout the measurements.
After the CO2 and O2 measurement devices the air passed the insect measurement chamber (‘A’ in Fig. 1). For measurement of resting metabolism [13] and for thermolimit experiments a brass variant of the chamber (inner volume 18 ml) was positioned in a programmable water bath (F33 HT, Julabo) for temperature control. The construction, size and shape of such a chamber, however, had to be adapted to the size of the insect under investigation. In young honeybee larvae only a volume of 2 ml or less (in combination with a low flow rate down to 20 ml/min) guaranteed an acceptable measurement sensitivity [42]. Investigation of large insect pupae require larger volumes [43,44], and much larger ones when investigating chirping energetics of large Mecopoda grasshoppers (e.g. 200 ml) [45].
To avoid desiccation of the insects during longer measurements (e.g. overnight), the relative humidity in the measurement chamber could be adjusted by saturating the air with water vapour by passing it through two flasks with distilled water immersed in a temperature controlled water bath prior to the respirometer chamber (Fig. 1). The temperature of this water bath was adjusted to the dew point temperature that corresponded to the desired relative humidity (rH) at the temperature inside the measurement chamber. The following set of formulas allowed calculation of the dew point temperature (Tdewpoint, °C) to get the desired relative humidity (rHdes, %) at a desired temperature (Tdes, °C)
| (1) |
| (2) |
| (3) |
where VP is vapor pressure (mbar), and SVP is the saturation vapor pressure (mbar).
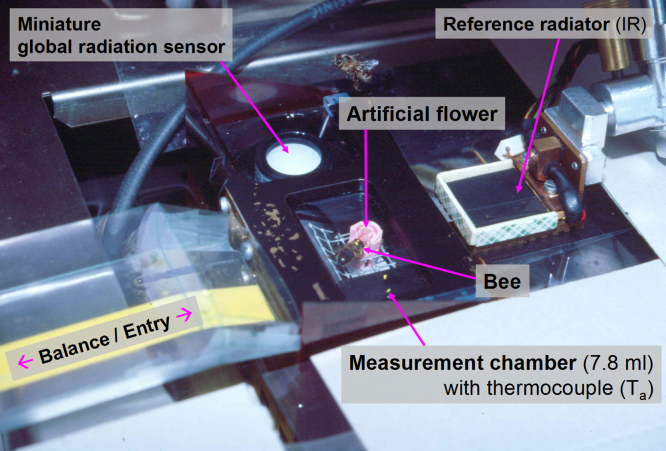
Fig. 2 shows a measurement chamber variant for outdoor measurements (volume variable from 7.8 to 11 ml). This size was optimal for measurement of honeybee and wasp CO2 production during foraging. It was mostly operated at a flow rate of 250 ml/min. Its lid could be opened and closed quickly to give the bees and wasps fast access to an artificial flower inside (Fig. 2, Fig. 6). The flow rate of sucrose solution (‘artificial nectar’), applied via a syringe needle at the artificial flower bottom, could be controlled by a perfusor (Perfusor® compact S; B-Braun, Melsungen). A thermocouple near the foraging insect (∼1 cm) measured the air temperature inside. Solar radiation was measured by a miniature global radiation sensor (FLA613GS/Mini spezial; Ahlborn) in a second chamber nearby milled into the chamber brass block. This sensor was specifically designed for our need of small dimensions. It has a flat sensitivity curve in the range of 380–1100 nm wavelength. Measurement chamber and outside air temperature, and relative humidity and radiation was recorded by ALMEMO® data loggers (2690-8 or 2890-9, with automated sensor detection; Ahlborn). Through the plastic film windows of the chamber (polypropylene or cellophane, see Section 2.3.2 below), solar radiation could reach the bees or wasps and the radiation sensor. As this film was transparent to the infrared, honeybee and wasp body surface temperature could be measured thermographically without disturbance or behavioural impairment of the insects (see Section 2.3 and Fig. 6). The inner surface of the chamber was coated by black lacquer to avoid reflection of solar radiation onto the bee and the radiation sensor. Cooling of this chamber (by immersion in a water bath, Figs. 1 and 2) was indispensable during measurements in sunshine. Otherwise the temperature inside rose quickly above critical levels and the insects no longer entered it. For optimal cooling results, cooling ribs were milled into the chamber base.
Fig. 2.
Outdoor measurement chamber for simultaneous respiratory and thermographic measurements in foraging honeybees in shade and in sunshine (serial mode, A in Fig. 1). For temperature control the chamber was immersed in a water bath. The infrared camera (on top, outside the picture) viewed the bees from the dorsal view. In some experiments the bees were trained to enter and leave the chamber via a balance (left, not shown).
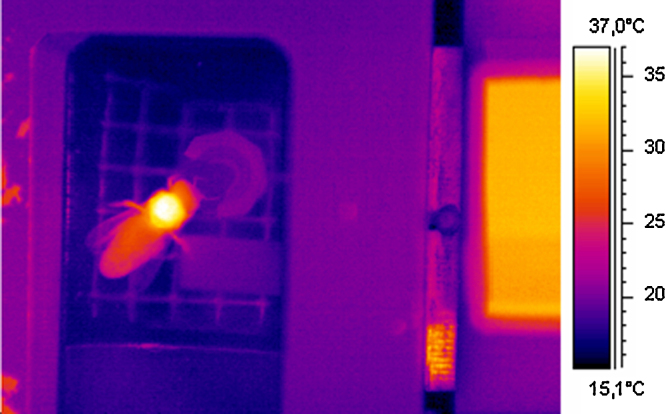
Fig. 6.
Thermogram (SC2000) of a honeybee forager sucking sucrose solution from an artificial flower during simultaneous measurements of body surface temperature and CO2 production (flow-through measurement in serial mode, A in Fig. 1). The thorax was heated by activation of flight muscles, part of the heat has reached the head and the abdomen. Right-hand rectangle: proprietary infrared reference radiator (see Fig. 2), its lower part (dark orange) was covered by the same infrared transmissive plastic film as the measurement chamber lid. Air inlet was from bottom part of the picture (right of the bee), outlet was below the bee.
After the measurement chamber the air passed a second peltier-driven cool trap (cooler 2) before it entered the measurement tubes of the DIRGA (Fig. 1). During system calibration the gas path was automatically switched to cooler 2 and the measurement tubes of the DIRGA. The insects in the measurement chamber were automatically supplied with fresh air via a second set of pump and mass flow controllers. This avoided an unnatural increase of CO2 concentration in the measurement chamber and lengthy flush times after calibration.
As a result of the tube length between the metabolic chamber and the CO2 measuring device the delay between measurement chamber and DIRGA had to be considered, especially if respiratory recordings had to be compared with thermographic measurements. Digital data readout via the RS-232 interfaces of URAS 14 and Oxzilla 2, and control of the DIRGA was done by Centrol 5 software (Harnisch, Austria).
2.1.2. Parallel mode
The setup of Fig. 1 allowed a quick switch from serial to parallel mode. For simultaneous measurement of CO2 production and O2 consumption, e.g. for the measurement of the respiratory quotient (RQ), the parallel mode was preferable because this avoided offsets caused by the pressure impulses of magnetic valve switching. Sensitivity to pressure is an inevitable consequence of the fact that devices like the Oxzilla 2 measure not O2 ‘concentration’ but O2 ‘partial pressure’. In parallel mode the high sensitivity of the Oxzilla could be assessed at utmost accuracy. A brass insect measurement chamber was inserted into one air stream before the pumps. Both measurement and reference gas path received the air from a common inlet which took dry air (via a common large Drierite column) from outside the laboratory. To avoid desiccation the supply with dry air should be avoided if small insects, or their larvae or pupae are to be measured for longer periods of time. Two manual 3-way valves (not shown in Fig. 1) allowed smooth switching of the measurement gas path to bypass the measurement chamber during the regularly necessary manual calibrations of the Oxzilla 2.
For field oxygen consumption measurements with the Oxzilla 2 alone, an independent set of a dual bench/field pump with controllable throughput (PP-2 v2, Sable Systems), a mass flow valve controller (MFC-2, Sable Systems) controlling two mass flow controllers (Side Trak 840-L, Sierra Instruments) was used. The Oxzilla 2 allowed operation at flow rates up to 1 L/min without subsampling. Data readout and digitisation on a laptop computer was accomplished by an Universal Interface (UI-2, Sable Systems) and ExpeData software (Sable Systems). The Drierite columns for air drying (indispensable with the Oxzilla) were inserted in the gas stream before the pumps. This attenuated coupling of pump noise to the measurement chamber at the tube inlet considerably.
A set of two identical measurement chambers was used for these measurements, one for the measuring and another one for the reference air stream (∼50 ml). These consisted of a small plastic film cylinder attached to a glass laboratory funnel. The base of the cylinder was attached to an iron spacer ring to fit the chamber to the underlying artificial flower. The spacer ring provided the iron counterpart of a magnetic catch realised via pieces of hard disc magnets integrated in the base of the artificial flower the bees were sucking from. While the reference chamber remained at a reference flower permanently, the measurement chamber was operated from a distance (∼1.5 m) via a rod to prevent the operators from influencing the measurements with their exhaled air. The artificial flower consisted of an inverted white plastic laboratory cup closer. In its centre a blue plastic cuvette closer provided a source of sucrose solution. Around the sucrose source a ring of holes drilled in the flower base allowed the pumps to suck fresh air from underneath the flower. This way the air stream, regulated at 500 ml/min, washed away the air around the bee sucking at a 1 cm higher position (compare B in Fig. 1). If we had to wait for a forager to visit the artificial flower the measurement chamber was parked at a third, identical flower (with an identical fill height of sucrose). This helped to stabilize the Oxzilla 2 baseline during waiting periods. The Oxzilla was regularly recalibrated against the outside air. Data Evaluation (offset and drift correction, and calculation of integrals for total O2 consumption per stay, etc.) were in these experiments done with ExpeData software (Sable Systems).
2.2. Calibrations for respiratory field measurements
In respiratory investigations of longer duration insect energy turnover can be measured by cutting out a section of a respiratory trace and simple averaging [13,42]. In experiments with unlimited sucrose flow at artificial flowers, however, the duration of stay may be shorter than a minute, especially at high ambient temperatures. Depending on the experimental situation the signal rise and decay (washout) times may exceed the visit duration. To get the mean energy turnover per stay, therefore, a mere cut-out from the CO2 or O2 traces would not give correct results. Therefore, we integrated the total CO2 or O2 signal (including 2 min of washout) and divided the integral by the duration of stay inside the respiratory chamber.
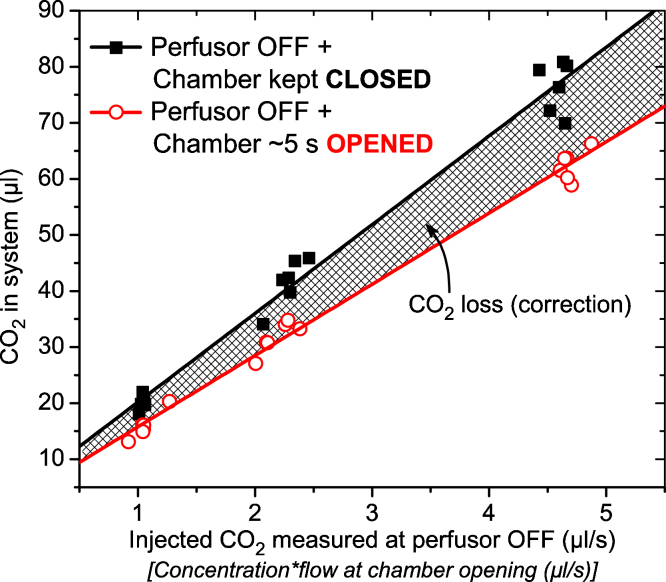
However, chamber opening after the insects’ visit caused a loss of measurement gas. The following calibrations allowed for compensation of this loss (Fig. 3). CO2 was injected into the measurement chamber via a syringe by a perfusor (Perfusor® compact S; B-Braun, Melsungen) to achieve a stable measurement signal. Then (a) the perfusor was turned off and the chamber was kept closed, or (b) the perfusor was turned off and the chamber was opened for ∼5 s (the period of chamber opening when a bee left the chamber). During this period, the chamber was flushed with fresh air because the pump and mass flow controller were still active. This way we got two calibration curves of the amount of CO2 in the system in dependence on the ‘turnover’ (concentration × flow) at the time of perfusor off (Fig. 3). The difference between these two curves represented the CO2 (O2) loss caused by chamber opening. It allowed loss correction in dependence of the respiratory gas concentration at chamber opening (compare CO2 traces in Fig. 7).
Fig. 3.
Results of the calibrations of CO2 loss during field measurements with the measurement chamber shown in Fig. 2. CO2 was injected into the measurement chamber with a perfusor. For details on the measurement procedure see Section 2.2. Correction (loss) = regression[CLOSED condition] − regression[OPENED condition]. CO2 loss (nl) = (VCO2[opening, nl s−1] × 15.826 + 4364.35) − (VCO2[opening, nl s−1] × 12.708 + 3083.59). Field measurements with the Oxzilla 2 (Fig. 5a): O2 loss (nl) = (VO2[opening, nl s−1] × 17.93709 − 7103.78751) − (VO2[opening, nl s−1] × 16.25679 − 8637.51409). Such correction functions have to be determined for every measurement setup sepatately.
Fig. 7.
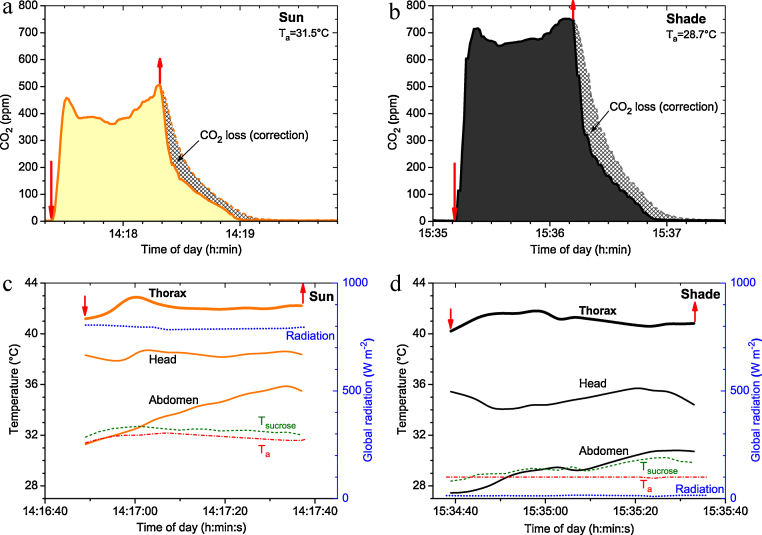
Respiratory traces (a and b) and simultaneously recorded body surface temperature curves (c and d) of a honeybee during foraging of 1.5 M sucrose solution in sunshine and shade, inside the measurement chamber shown in Figs. 2 and 6 (A in Fig. 1). Total CO2 production per stay was determined from the trace integral plus the loss determined from the calibrations as shown in Fig. 3 (hatched areas in Fig. 7). Arrows: entering and leaving of the chamber. Note time delay of ∼37 s of respiratory against thermographic measurements.
2.3. Thermographic and thermocouple calibrations
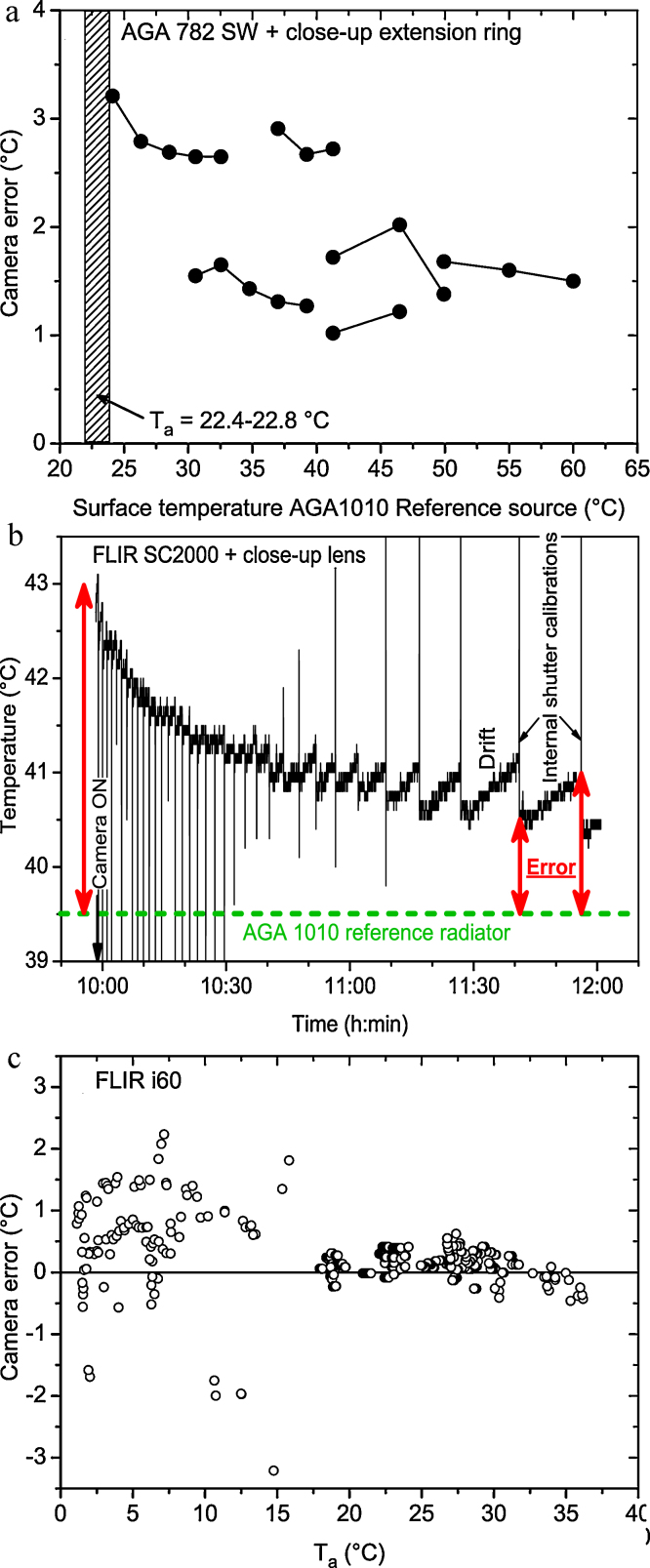
In insect thermoregulation research infrared thermography provides several advantages over conventional thermometry. The insects neither need to be touched, nor impaired in their behaviour or even killed like in measurements with thermocouples. Measurements on the same individual can be repeated many times, and the spatial distribution of the body surface temperature can be evaluated [1]. Meanwhile, the sensitivity of the top bolometer cameras is better than 0.03 K. Measurement accuracy of most cameras, however, is usually only 2 °C or 2% (Fig. 4). In special cases, for example if macro lenses are used, these specifications may not be valid some time after the camera was turned on (Fig. 4b) or at certain ambient conditions (Fig. 4c). Only a few models offer an accuracy down to 1 °C. Therefore, calibration with an external reference radiator is indispensable if an accuracy better than 1 °C has to be guaranteed.
Fig. 4.
Measurement errors of three generations (types) of thermographic cameras (Flir, Inc.). (a) AGA 782SW with 12 mm close-up extension ring (single InSb detector, liquid nitrogen cooled, 3.5–5.6 μm waveband); (b) SC2000 NTS (320 × 240 microbolometer focal plane array, 7–14 μm waveband) with 64/150 close-up lens, error usually (without close-up lens) smaller; (c) i60 handheld camera (180 × 180 microbolometer array, 7–14 μm waveband). Ta = ambient air temperature.
2.3.1. Camera calibration with reference radiator
In our measurements, we used two thermographic systems, a SC2000 NTS or a i60 (FLIR Systems; Danderyd, Sweden). The i60 was calibrated against an AGA1010 reference radiator (FLIR) before and after the bees’ visits at an artificial flower. The AGA1010 had been precision calibrated against a cavity black body radiator of 0.05 °C accuracy [1]. A portable cavity black body radiator can easily be constructed by immersing a cylinder with the inner surface coated with black lacquer (infrared emissivity should be at least 0.95) into a thermal coffee pot filled with warm water. Water temperature can be read from a standard laboratory thermometer or an electronic thermometer. The cavity increases the apparent cavity emissivity to a value >0.995 if the ratio of cylinder depth to radius is at least 10:1 [46]. Reference temperature should be at least 5 °C above ambient temperature for utmost accuracy.
The SC2000 was calibrated against a proprietary, peltier-driven reference radiator (Fig. 2). Its accuracy was ∼0.4 °C (2 SD) for a broad range of ambient temperatures (0–40 °C). The small radiator head (30 mm × 30 mm, 10 mm height) allowed placement close to the insects (Fig. 2). Therefore it was visible in the thermograms even if close-up lenses were used (Fig. 6). This way, every infrared picture could be corrected directly for offset errors.
2.3.2. Attenuation of IR transmissive film
The IR transmissive lid of the measurement chamber were polypropylene or cellophane films used by florists for wrapping of flowers. They provided a good stability and transparency. Polyethylene would have been even more transparent to infrared radiation, but commercially available films were all of rather poor ‘optical’ quality, i.e. their surface was not smooth enough. The attenuation of the infrared radiation by the transmissive film of the measurement chamber lid was compensated for by covering part of the reference source head with a stripe of the same film (Fig. 2). This way errors resulting from ambient reflections via the film surface could be minimised. The attenuation of infrared transmissive films may also, with less precision, be corrected by determination of a special ‘atmospheric transmission’ coefficient. Standard evaluation software will calculate a correction automatically. Because of the slight overpressure inside the measurement chamber the film was slightly curved. Special care had to be taken to avoid different reflection of ambient radiation (room walls, IR camera, operators, etc.) from different parts of the film surface. In outdoor experiments special care had to be taken to avoid reflections of the sky in part of the film. This was done by positioning several layers of corrugated cardboard above the IR camera. This way the lowest cardboard surface resembled ambient air temperature even in sunshine, which is usually used for correction of reflected ambient radiation. In general, however, it has to be noted that thermographic measurement through plastic films should be avoided whenever possible because it inevitably adds additional sources of error.
2.3.3. Honeybee cuticle infrared emissivity
According to the honeybee cuticle infrared emissivity of ∼0.97 [1], ∼3% of solar long-wave infrared radiation is reflected via the cuticle into the infrared camera. In cockchafers (Melolontha melolontha) a similar value of 0.965 was determined in the 3–6 μm wavelength range (see [47]). With standard evaluation software this is compensated for by considering ambient ‘black body’ temperature. During measurements in sunshine, however, there is an additional error. We measured this effect by thermographing insects at different intensities of solar radiation with a long-wave (7–14 μm) infrared camera at a frame rate of 50 Hz (SC2000 NTS). The cuticular surface temperature was measured and then the insects were shaded and their temperature measured again within a few ms. The resulting measurement error turned out to be small in the 7–14 μm waveband. The applied correction factor for reflected solar radiation amounted to 0.2183 °C kW−2 m−2.
2.3.4. Thermocouples
Modern electronic thermometers provide direct readout and storage of thermosensor data (thermistors, Pt100, thermocouples). For measurements in shade all these sensor types provide an accuracy of about 0.1–0.3 °C. We used standard type K (NiCr/NiAl) thermocouples (0.3 mm wire diameter: 5TC-TT-K-30-72, OMEGA) to measure ambient air temperature close (≤1 cm) to the insects. In sunshine, where thermosensors are heated by the radiation, we either shaded the thermosensors (Fig. 2, thermocouple junction above the gas outlet below the artificial flower), or we applied a correction derived from a comparison of two sensors, one shaded and the other one in sunlight. With our thermocouples the correction amounted to 1.4 °C kW−1 m−2.
2.4. Experimental procedures
2.4.1. Oxygen consumption, thermoregulation and profitability of foraging
In a first series of experiments honeybees (Apis mellifera carnica) were trained to gather sucrose solution from an artificial flower in May. A cylindrical measurement chamber, operated from a distance of ∼1.5 m via a rod, was put over the foragers within 2–3 s after arrival. Oxygen consumption was measured with a standalone Oxzilla 2 setup (for details see Section 2.1.2; compare ‘B’ in Fig. 1). When the foragers wanted to leave the flower, the chamber was lifted to let the bees free. As the measurement chamber temperature rose quickly in sunshine, experiments were restricted to shaded conditions in these experiments. Body surface temperature of bees foraging under very similar ambient conditions were measured at an identical feeding station with a Flir i60 handheld IR camera (see Section 2.3). Thermograms were taken after landing and every 10 s until departure.
2.4.2. Energetics, thermoregulation and radiation
In a second series of field experiments, honeybees were trained to forage sucrose solution from inside a brass measurement chamber of 7.9 ml inner volume, immersed in a water bath (Fig. 2). Experiments were conducted in August and September. The water bath allowed control of the air temperature inside the measurement chamber even in bright sunshine. This way, for the first time, a simultaneous comparison of foraging energetics and thermoregulation in sunshine and shade was possible. The respiratory equipment was operated in serial mode (see Section 2.1.1; ‘A’ in Fig. 1). Thermograms were stored digitally with 14 bit resolution at a rate of 3–10 pictures/s on a DOLCH FlexPac computer (Kontron) with ThermaCam Researcher software (Flir).
2.4.3. Thermolimit
The third example of the benefits of the combined use of infrared thermography and flow-through respirometry are experiments to determine the critical thermal respiratory maximum (resp CTmax) of vespine wasps (Vespula vulgaris). The respiratory equipment was operated in serial mode (Section 2.1.1). The wasps were inserted in the measurement chamber at a starting temperature of 25 °C. After 10 min of equilibration the water bath was heated at a rate of 0.25 °C min−1 up to 55 °C. A thermocouple in the measurement chamber centre (see thermograms in Fig. 9) provided readout of actual chamber air temperature on an ALMEMO® 2890-9 data logger (Ahlborn). Behaviour was controlled by infrared thermography. Thermographic scenes were stored at a rate of 10 frames s−1 on a DOLCH FlexPac computer (Kontron). Experiments were conducted in September and October.
Fig. 9.
Thermolimit experiment to determine the upper respiratory critical thermal maximum (resp CTmax) of a wasp (Vespula vulgaris). Shown are temperature ramp (green curve, right axis; thermocouple in measurement chamber), respiratory trace (black curve, left axis), thoracic temperature excess against abdomen (Tth − Tab, pink curve on bottom, right lower axis), and evaluation of respiratory trace variability: absolute difference sum (rADS, light blue) and rADS residuals (dark blue, arbitrary units; see Section 3.3). Thermograms (relative scale of 5 °C) show wasp walking (a), after respiratory failure (resp CTmax) (b), at the maximum of the final thoracic heating bout during the post mortal respiratory peak (c), and dead (d). Thermogram (b) also uncovers the reason for the drop in the Ta curve: the wasp had smeared regurgitated fluid on the thermocouple protection envelope about 3 min before (dark ring in upper right edge).
2.5. Data evaluation
Respiratory data were evaluated in MS Excel (Microsoft Corporation) and Origin 8.1 (OriginLab) or with ExpeData (Sable Systems). Thermographic measurements with the i60 and SC2000 cameras were evaluated with AGEMA-Research or ThermaCam Researcher software (Flir) controlled by a proprietary MS Excel (Microsoft) VBA macro. This macro also extracted the stored environmental data automatically from the logger files at the time of thermographic measurements. Statistics was done with Statgraphics (Statpoint Technologies) and Origin software.
3. Results and discussion
The results presented here are thought as examples that demonstrate the benefits of a combined use of infrared thermography with respiratory measurements to determine insect energy turnover and critical thermal limits (e.g. CTmax).
3.1. Effect of honeybee motivation and ambient temperature on thermoregulation and energetics
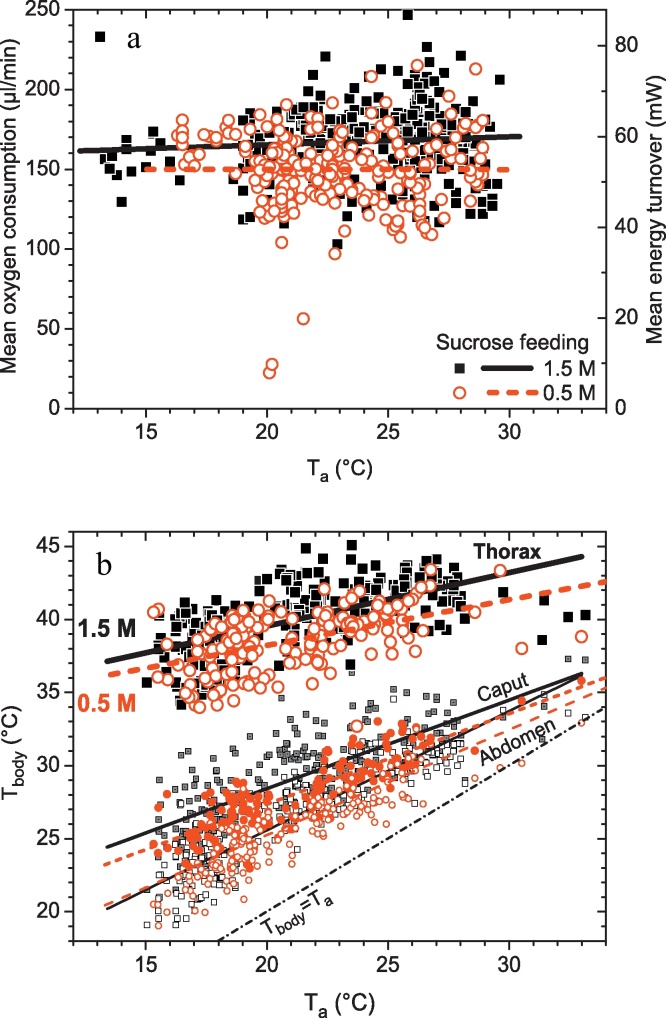
Fig. 5a shows the mean oxygen consumption per stay (measured with the Oxzilla 2) in dependence on ambient air temperature close to the bees (Ta). To our surprise, the oxygen consumption was independent of Ta (ANOVA: F-ratio = 1.06, P = 0.304) but depended highly significantly on food quality (sucrose concentration; F-ratio = 54.74, P < 0.00001; n = 252 and 296 for 0.5 M and 1.5 M concentration, respectively).
Fig. 5.
(a) Oxygen consumption and (b) body surface temperature (means per stay) of honeybees foraging 0.5 M or 1.5 M sucrose solution of unlimited flow from artificial flowers in shade (parallel mode, B in Fig. 1). Oxygen consumption, calculated as the product of flow rate (500 ml/min) and mean O2 concentration per stay (integral of the drift corrected O2 trace plus the calibrated loss during chamber opening, divided by duration of stay; compare Fig. 7), was independend of ambient temperature (Ta) (P > 0.3). Energy turnover calculated as the product of oxygen consumption (L O2 s−1) and the caloric equivalent of sucrose (21.117 kJ L−1 O2). Ta = ambient air temperature close to the bees (≤1 cm). Regression line constants (y = A + B × x) for O2 consumption (a): A = 155.327, B = 0.50201, R2 = 0.00305, P = 0.16877 for 1.5 M feeding; A = 150.365, B = −0.0169, R2 = −0.004, P = 0.97227 for 0.5 M feeding. Constants for body temperature (b) (all regressions: P ≪ 0.00001): thorax: A = 32.677, B = 0.34582, R2 = 0.34166 for 1.5 M feeding; A = 31.973, B = 0.31234, R2 = 0.24446 for 0.5 M feeding; caput: A = 16.2999, B = 0.60934, R2 = 0.59327 for 1.5 M feeding; A = 14.358, B = 0.63958, R2 = 0.69957 for 0.5 M feeding; abdomen: A = 9.5288, B = 0.80189, R2 = 0.78987 for 1.5 M feeding; A = 10.0709, B = 0.73469, R2 = 0.78752 for 0.5 M feeding.
This means that the bees did not invest more energy in thermoregulation to compensate for the expected higher loss of heat at lower ambient temperatures. The thermographic measurements shown in Fig. 5b explain this finding. Thorax surface temperature depended on both Ta and sucrose concentration (ANOVA: F-ratio = 176.16, P < 0.00001 for Ta; F-ratio = 54.74, P < 0.00001 for concentration). With decreasing Ta the bees did not regulate thoracic temperature at a constant level but rather decreased it. This decreased the temperature excess over Ta and this way helped the bees to save energy.
The present findings are in contrast to other measurements where an increase of respiratory turnover with decreasing temperature was determined [12,48]. In both these investigations, however, the bees were in a quite different behavioural state not comparable to the present study. The bees had been confined in a measurement chamber against their ‘will’. Under such conditions they search for an exit from the chamber. To remain ready for flight they keep their body temperature at a rather constant level, independent of Ta [12]. Therefore, they had to invest more energy for thermoregulation at low Ta. In flying honeybees both was reported, the energy turnover increasing [17,34] or remaining nearly constant [18] with decreasing Ta. In all these flight experiments, thorax temperature decreased with decreasing Ta like in the present investigation.
Fig. 5b also shows that our bees managed to thermoregulate to some extent (i.e. increase the thoracic excess over Ta with decreasing Ta) despite their rather constant level of energy turnover. This finding suggests that honeybees on an artificial feeding station not only thermoregulate by regulation of heat production but, to some extent, probably also by regulating heat loss. The most plausible candidates to regulate heat loss at medium to low Ta in non-flying but endothermic bees are regulation of heat transport to the abdomen [8] and respiration (e.g. increase or decrease of ventilation frequency). At high Ta, cooling by fluid droplets regurgitated at their mouthparts is of increasing importance for thermoregulation (see literature in [8]). Thermography allowed simultaneous measurement of all body parts (Fig. 6). Under our experimental conditions the bees regulated the temperature of the head and even of the abdomen to some extent (Fig. 5b). Partial regulation of abdominal temperature was also found in water foragers [4,49] but could not be proved in all measurements of bees foraging from flowers (proofed in [50]; not proofed in [5]).
Fig. 5b also demonstrates that honeybees gathering a 1.5 M sucrose solution (ad libitum) regulated their thorax temperature at a 1–2 °C higher level than bees gathering a 0.5 M sucrose. To achieve this, they had to increase oxygen consumption by about 10–20 μl min−1 (Fig. 5a). This equals an increase in energy turnover of 4.7–7 mW (8.9–13.3%). Our results confirm thermographic measurements of the effect of profitability of foraging on honeybee thermoregulation by Schmaranzer and Stabentheiner [1,2], measurements with thermocouples by Dyer and Seeley [51], and measurements of energy turnover by Balderrama and co-workers [29,30,32,33]
3.2. Simultaneous assessment of energetics and thermoregulation in field measurements
Fig. 6 shows a thermogram of a honeybee sucking from an artificial flower inside the respiratory measurement chamber, viewed through the infrared transmissive plastic film lid. The thorax temperature is increased due to activation of the flight muscles. Haemolymph circulation has transported part of the heat to the head and the abdomen. The abdomen remained always cooler than the head.
An unsolved question in honeybee (and wasp) energetics is the use of solar radiation. Is it used to save energy or to increase body temperature, or both? Fig. 7 shows simultaneous, continuous sample registrations of body surface temperature and CO2 production of foragers sucking 1.5 M sucrose at the artificial flower in sunshine or in the shade at a high ambient temperature (28.7–31.5 °C). The CO2 graphs (Fig. 7a and b) show the effect of the corrections applied to compensate for CO2 loss during chamber opening after the bees’ visits.
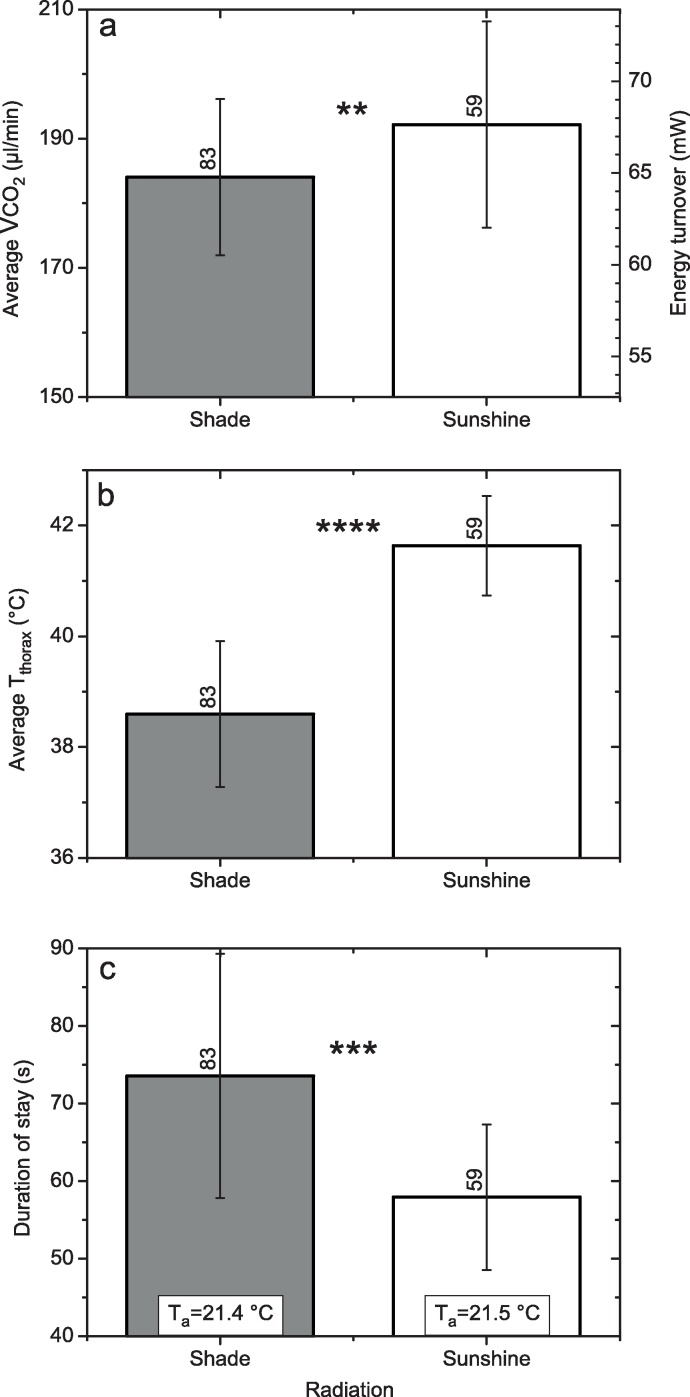
Thorax surface temperature was regulated constantly at a high level (40–43 °C) in both conditions (Fig. 7c and d). Head temperature was kept rather constant not only in the shade but also in sunshine. Since the bees had flown to the feeding station at lower ambient temperatures, their abdominal temperature increased after they entered the rather warm measurement chamber (Fig. 7c and d). This increase was faster in sunshine. As was expected from literature [4,5,8,15,37], the thorax temperature was slightly higher in sunshine. However, CO2 production, and thus energy turnover, was considerably lower in sunshine (Fig. 7a and b). Fig. 8 shows the mean values of a complete experiment at a lower Ta. While the thorax surface temperature was again higher in sunshine (Fig. 8b), CO2 production was also higher (Fig. 8a). This contradicts our expectation that the bees would use the external heat gained from the sun to reduce their own (internal) energetic effort. Instead, they used the sun to increase their thorax temperature by nearly 3 °C. Fig. 8c shows the benefit of this energetic investment. The higher body temperature allowed the bees to increase sucking speed considerably, i.e. to reduce the duration of stay. In water foragers similar results were obtained [4]. This has a considerable effect on foraging efficiency, because the bees can make more foraging trips per day. The comparison of Fig. 8 and Fig. 7 and our own unpublished results, however, suggest that energetic strategies of foraging honeybees are probably more complex. Therefore, more experiments are needed to elucidate the energetic adaptation strategies of foraging honeybees to varying environmental conditions.
Fig. 8.
Average values (±SD, with N) of means per stay of (a) CO2 production rate (VCO2) or energy turnover, (b) thorax surface temperature (Tthorax), and (c) duration of stay at an artificial flower (see Fig. 6) of honeybees foraging 1.5 M sucrose solution (unlimited flow) from an artificial flower (serial mode, A in Fig. 1). CO2 production rate calculated as the product of flow rate (250 ml/min) and mean CO2 concentration per stay (integral of the drift corrected CO2 trace plus the calibrated loss during chamber opening, divided by duration of stay; Fig. 7). Calculation of energy turnover (product of CO2 production and the caloric equivalent of sucrose, 21.117 kJ L−1 O2) done assuming a respiratory quotient (RQ) of 1 (CO2 production equals O2 consumption). **P < 0.01, ***P < 0.0001, ****P < 0.00001 (t test). Global radiation: 27 ± 12.8 W m−2 in shade, and 687 ± 74.5 W m−2 in sunshine. Ta = ambient air temperature in measurement chamber.
3.3. Thermography in thermolimit respirometry
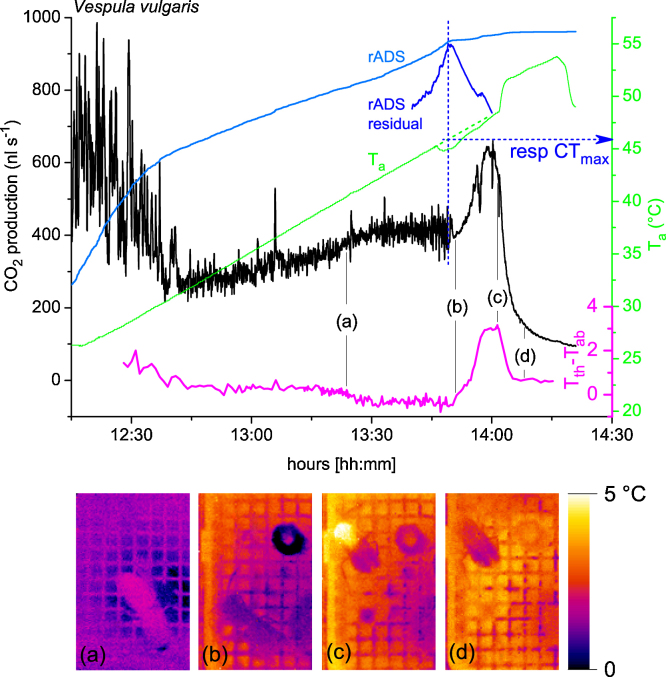
Fig. 9 shows an experimental run to determine the respiratory critical thermal maximum (respiratory CTmax) of a vespine wasp (Vespula vulgaris). Real-time infrared thermography allowed behavioural observation of the insect in the dark and simultaneous body temperature measurement. In the beginning, at an ambient temperature (Ta) of 25 °C, the wasp was weakly active and showed discontinuous respiration (large variations of the CO2 signal). As Ta reached 30 °C, she calmed down and showed cyclic respiration (medium variations of the CO2 signal). During the further increase of ambient temperature (‘ramping’ [40]) CO2 turnover increased. At a Ta of 45.9 °C respiratory control failed and the stigmata could no longer be closed (no cyclic variations of the CO2 signal). An elegant possibility to determine the exact point of loss of respiratory control is to determine the absolute difference sum of CO2 production in a first step (rADS, light blue curve in Fig. 9). It is a measure of the cumulative dynamic variability [40]. In a second step a regression line was calculated through the rADS signal values from 10 min before to 10 min after the estimated CTmax value. The reversal point of rADS residuals (difference between rADS curve and regression line; Fig. 9, arbitrary units) helped to determine the exact point of the respiratory critical thermal maximum.
In the experiment shown in Fig. 9 the Ta curve shows a slight depression 2 min 46 s before the respiratory CTmax was reached. Using the original Ta curve would have led to an underestimation of the respiratory CTmax by ∼1 °C. Real-time infrared thermography showed the reason of this temperature depression (see thermogram (b) in Fig. 9). The wasp had regurgitated a droplet of liquid and smeared it on the thermocouple sensor envelope (see dark ring in upper right edge of the thermogram). The evaporative cooling of this liquid caused the temperature depression.
After the respiratory CTmax had been reached, the wasp showed a considerable increase of CO2 emission (post mortal peak [40]). One could argue that this peak resulted from a general failure of cellular metabolism and stigma opening upon death, and the following ‘washout’ (diffusion) of the rest of CO2 from the wasp body. Infrared thermography revealed the true reason of this CO2 peak. After the respiratory CTmax had been reached the wasps started final thoracic heating with their flight muscles (see thermogram (c) and temperature curve in Fig. 9), despite the high temperature in the measurement chamber. We suggest that this occurred because of a malfunction of nervous centres controlling activation of the flight muscles, e.g. pattern generators in the ventral nerve cord or higher order nervous centres [52].
4. Conclusions
Our investigations demonstrate the benefits of a combined use of infrared thermography with respiratory measurements in insect ecophysiological research. Infrared thermography enables investigation of behaviour and thermoregulation without any behavioural impairment. Comparison with flow-through measurements of O2 consumption or CO2 production brings new insights into the mechanisms governing energetic optimisation of foraging in honeybees and wasps. Our description of an experimental setup even allows the repeated, simultaneous determination of energy turnover and thermoregulatory behaviour in the same individual. In determinations of insect respiratory critical thermal limits, the combined use of thermography and respirometry makes possible a thorough interpretation of respiratory traces.
Acknowledgements
The research was funded by the Austrian Science Fund (FWF): P20802-B16, P16584-B06. We thank J. Vollmann, M. Bodi, M. Brunnhofer and S. Schmaranzer, and the students of the ‘Modul Ökophysiologie der Tiere’ for help with part of the experiments.
Contributor Information
Anton Stabentheiner, Email: anton.stabentheiner@uni-graz.at.
Helmut Kovac, Email: he.kovac@uni-graz.at.
References
- 1.Stabentheiner A., Schmaranzer S. Thermographic determination of body temperatures in honey bees and hornets: calibration and applications. Thermology. 1987;2:563–572. [Google Scholar]
- 2.Schmaranzer S., Stabentheiner A. Variability of the thermal behavior of honeybees on a feeding place. J. Comp. Physiol. B. 1988;158:135–141. [Google Scholar]
- 3.Schmaranzer S., Stabentheiner A. Berührungslose Körpertemperaturmessung bei Insekten. Biologie in unserer Zeit. 1991;21:260–262. [Google Scholar]
- 4.Kovac H., Stabentheiner A., Schmaranzer S. Thermoregulation of water foraging honeybees—balancing of endothermic activity with radiative heat gain and functional requirements. J. Insect Physiol. 2010;56:1834–1845. doi: 10.1016/j.jinsphys.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovac H., Stabentheiner A. Thermoregulation of foraging honeybees on flowering plants: seasonal variability and influence of radiative heat gain. Ecol. Entomol. 2011;36:686–699. doi: 10.1111/j.1365-2311.2011.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stabentheiner A., Pressl H., Th Papst, Hrassnigg N., Crailsheim K. Endothermic heat production in honeybee winter clusters. J. Exp. Biol. 2003;206:353–358. doi: 10.1242/jeb.00082. [DOI] [PubMed] [Google Scholar]
- 7.Stabentheiner A., Kovac H., Brodschneider R. Honeybee colony thermoregulation—regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS ONE. 2010;5:e8967. doi: 10.1371/journal.pone.0008967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich B. Berlin; Springer: 1993. The Hot Blooded Insects. [Google Scholar]
- 9.Bishop J.A., Armbruster W.S. Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Funct. Ecol. 1999;13:711–724. [Google Scholar]
- 10.Stabentheiner A. Individuelle und soziale Thermoregulation der Honigbiene. Entomol. Austriaca. 2005;12:13–22. [Google Scholar]
- 11.Schmolz E., Hoffmeister D., Lamprecht I. Calorimetric investigations on metabolic rates and thermoregulation of sleeping honeybees (Apis mellifera carnica) Thermochim. Acta. 2002;382:221–227. [Google Scholar]
- 12.Stabentheiner A., Vollmann J., Kovac H., Crailsheim K. Oxygen consumption and body temperature of active and resting honeybees. J. Insect Physiol. 2003;49:881–889. doi: 10.1016/S0022-1910(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 13.Kovac H., Stabentheiner A., Hetz S.K., Petz M., Crailsheim K. Respiration of resting honeybees. J. Insect Physiol. 2007;53:1250–1261. doi: 10.1016/j.jinsphys.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esch H. Über die Körpertemperaturen und den Wärmehaushalt von Apis mellifica. Z. vergl. Physiol. 1960;43:305–335. [Google Scholar]
- 15.Heinrich B. Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. J. Exp. Biol. 1979;80:217–229. [Google Scholar]
- 16.Stone G.N., Willmer P.G. Endothermy and temperature regulation in bees: a critique of grab and stab measurement of body temperature. J. Exp. Biol. 1989;143:211–223. [Google Scholar]
- 17.Roberts S.P., Harrison J.F. Mechanisms of thermal stability during flight in the honeybee, Apis mellifera. J. Exp. Biol. 1999;202:1523–1533. doi: 10.1242/jeb.202.11.1523. [DOI] [PubMed] [Google Scholar]
- 18.Woods W.A., Jr., Heinrich B., Stevenson R.D. Honeybee flight metabolic rate: does it depend upon air temperature? J. Exp. Biol. 2005;208:1161–1173. doi: 10.1242/jeb.01510. [DOI] [PubMed] [Google Scholar]
- 19.S. Schmaranzer, Die Körpertemperaturen belasteter Bienen im Fesselflug und Beobachtungen frei beweglicher Tiere mit Hilfe der Thermovision, Dissertation, University of Graz, 1984.
- 20.Stabentheiner A., Hagmüller K. Sweet food means ‘hot dancing’ in honey bees. Naturwissenschaften. 1991;78:471–473. [Google Scholar]
- 21.Stabentheiner A., Kovac H., Hagmüller K. Thermal behavior of round and wagtail dancing honeybees. J. Comp. Physiol. B. 1995;165:433–444. [Google Scholar]
- 22.Stabentheiner A. Thermoregulation of dancing bees: thoracic temperature of pollen and nectar foragers in relation to profitability of foraging and colony need. J. Insect Physiol. 2001;47:385–392. doi: 10.1016/s0022-1910(00)00132-3. [DOI] [PubMed] [Google Scholar]
- 23.Kroder S., Samietz J., Stabentheiner A., Dorn S. Body temperature of the parasitic wasp Pimpla turionellae (L.) (Hymenoptera) during host location by vibrational sounding. Physiol. Entomol. 2008;33:17–24. doi: 10.1111/j.1365-3032.2007.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamprecht I., Maierhofer C., Röllig M. A thermographic promenade through the Berlin Botanic Garden. Thermochim. Acta. 2006;446:4–10. [Google Scholar]
- 25.Fahrenholz L., Lamprecht I., Schricker B. Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B. 1989;159:551–560. [Google Scholar]
- 26.Schmolz E., Lamprecht I., Schricker B. Calorimetric investigations on social thermogenesis in the hornet Vespa crabro. Thermochim. Acta. 1993;229:173. [Google Scholar]
- 27.Schmolz E., Lamprecht I., Schricker B. A method for continous direct calorimetric measuments of energy metabolism in intact hornet (Vespa crabro) and honeybee (Apis mellifera) colonies. Thermochim. Acta. 1995;251:293–301. [Google Scholar]
- 28.Lamprecht I., Schmolz E. Calorimetry goes afield. Thermochim. Acta. 2000;355:95–106. [Google Scholar]
- 29.Balderrama N.M., Almeida de B. L.O., Núñez J.A. Metabolic rate during foraging in the honeybee. J. Comp. Physiol. B. 1992;162:440–447. doi: 10.1007/BF00258967. [DOI] [PubMed] [Google Scholar]
- 30.Moffat L., Núñez J.A. Oxygen consumption in the foraging honeybee depends on the reward rate at the food source. J. Comp. Physiol. B. 1997;167:36–42. doi: 10.1007/s003600050045. [DOI] [PubMed] [Google Scholar]
- 31.Lighton J.R.B. Oxford University Press; New York: 2008. Measuring Metabolic Rates. [Google Scholar]
- 32.Moffatt L. Changes in the metabolic rate of the foraging honeybee, effect of the carried weight or of the reward rate. J. Comp. Physiol. A. 2000;186:299. doi: 10.1007/s003590050430. [DOI] [PubMed] [Google Scholar]
- 33.Moffatt L. Metabolic rate and thermal stability during honeybee foraging at different reward rates. J. Exp. Biol. 2001;204:759–766. doi: 10.1242/jeb.204.4.759. [DOI] [PubMed] [Google Scholar]
- 34.Harrison J.F., Fewell J.H., Roberts S.P., Hall H.G. Achievement of thermal stability by varying metabolic heat production in flying honeybees. Science. 1996;274:88–90. doi: 10.1126/science.274.5284.88. [DOI] [PubMed] [Google Scholar]
- 35.Seeley T.D. Honey bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 1994;34:51–62. [Google Scholar]
- 36.Coelho J.R. The effect of thorax temperature on force production during tethered flight in the honeybee (Apis mellifera) drones, workers and queens. Physiol. Zool. 1991;64:823–835. [Google Scholar]
- 37.Underwood B. Thermoregulation and energetic decisionmaking by the honeybees Apis cerana, Apis dorsata and Apis laboriosa. J. Exp. Biol. 1989;157:19–34. [Google Scholar]
- 38.Stabentheiner A., Kovac H. Energetik und Thermoregulation sammelnder Honigbienen. Entomol. Austriaca. 2009;16:152–154. [Google Scholar]
- 39.Seymour R.S., White C.R., Gibernau M. Endothermy of dynastine scarab beetles (Cyclocephala colasi) associated with pollination biology of a thermogenic arum lily (Philodendron solimoesense) J. Exp. Biol. 2009;212:2960–2968. doi: 10.1242/jeb.032763. [DOI] [PubMed] [Google Scholar]
- 40.Lighton J.R., Turner R.J. Thermolimit respirometry: an objective assessment of critical thermal maxima in two sympatric desert harvester ants, Pogonomyrmex rugosus and P. californicus. J. Exp. Biol. 2004;207:1903–1913. doi: 10.1242/jeb.00970. [DOI] [PubMed] [Google Scholar]
- 41.Lighton J.R.B., Halsey L.G. Flow-through respirometry applied to chamber systems: pros and cons, hints and tips. Comp. Biochem. Physiol. Part A. 2011;158:265–275. doi: 10.1016/j.cbpa.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Petz M., Stabentheiner A., Crailsheim K. Respiration of individual honeybee larvae in dependence on age and ambient temperature. J. Comp. Physiol. B. 2004;174:511–518. doi: 10.1007/s00360-004-0439-z. [DOI] [PubMed] [Google Scholar]
- 43.Hetz S.K., Bradley T.J. Insects breath discontinuously to avoid oxygen toxicity. Nature. 2005;433:516–519. doi: 10.1038/nature03106. [DOI] [PubMed] [Google Scholar]
- 44.Kaiser A., Hartzendorf S., Wobschall A., Hetz S.K. Modulation of cyclic CO2 release in response to endogenous changes of metabolism during pupal development of Zophobas rugipes (Coleoptera: Tenebrionidae) J. Insect Physiol. 2010;56:502–512. doi: 10.1016/j.jinsphys.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 45.M. Hartbauer, A., Stabentheiner, H. Römer, Signalling plasticity and energy saving in a tropical bushcricket. J. Comp. Physiol. A 198, in press, doi:10.1007/s00359-011-0700-3. [DOI] [PMC free article] [PubMed]
- 46.Sparrow E.M., Cess R.D. Brooks/Cole Pub. Comp.; Belmont, California: 1970. Radiation Heat Transfer. [Google Scholar]
- 47.Kovac H., Stabentheiner A. Effect of food quality on the body temperature of wasps (Paravespula vulgaris) J. Insect Physiol. 1999;45:183–190. doi: 10.1016/s0022-1910(98)00115-2. [DOI] [PubMed] [Google Scholar]
- 48.Blatt J., Roces F. Haemolymph sugar levels in foraging honeybees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurement of maximal rates of trehalose synthesis. J. Exp. Biol. 2001;204:2709–2716. doi: 10.1242/jeb.204.15.2709. [DOI] [PubMed] [Google Scholar]
- 49.Schmaranzer S. Thermoregulation of water collecting honey bees (Apis mellifera) J. Insect Physiol. 2000;46:1187–1194. doi: 10.1016/s0022-1910(00)00039-1. [DOI] [PubMed] [Google Scholar]
- 50.Kovac H., Schmaranzer S. Thermoregulation of honeybees (Apis mellifera) foraging in spring and summer at different plants. J. Insect Physiol. 1996;42:1071–1076. [Google Scholar]
- 51.Dyer C.D., Seeley T.D. Interspecific comparison of endothermy in honeybees (Apis): deviations from the expected size-related patterns. J. Exp. Biol. 1987;127:1–26. [Google Scholar]
- 52.H. Käfer, H., Kovac, A. Stabentheiner, Resting metabolism and critical thermal maxima of vespine wasps (Vespula sp.), J. Insect Physiol. 58, doi:10.1016/j.jinsphys.2012.01.015, in press. [DOI] [PMC free article] [PubMed]