Abstract
Aims
Pharmacological-challenge magnetic resonance imaging (phMRI) is powerful new tool enabling researchers to map the central effects of neuroactive drugs in vivo. To employ this technique pre-clinically, head movements and the stress of restraint are usually reduced by maintaining animals under general anaesthesia. However, interactions between the drug of interest and the anaesthetic employed may potentially confound data interpretation. NMDA receptor (NMDAR) antagonists used widely to mimic schizophrenia have recently been shown to interact with the anaesthetic halothane. It may be the case that neural and cerebrovascular responses to NMDAR antagonists are dependent on the types of anaesthetic used.
Methodology
We compared the phMRI response to NMDAR antagonist ketamine in rats maintained under α-chloralose to those under isoflurane anaesthesia. A randomized placebo/vehicle controlled design was used in each of the anaesthetic groups.
Results
Changes in the anaesthetic agent resulted in two very different profiles of activity. In the case of α-chloralose, positive activations in cortical and sub-cortical structures reflected a response which was similar to patterns seen in healthy human volunteers and metabolic maps of conscious rats. However, the use of isoflurane completely reversed such effects, causing widespread deactivations in the cortex and hippocampus.
Conclusion
This study provides initial evidence for a drug-anesthetic interaction between ketamine and isoflurane that is very different from responses to α-chloralose-ketamine.
Keywords: Anaesthesia, anesthesia, NMDA, ketamine, schizophrenia, α-chloralose, isoflurane, phMRI, fMRI, BOLD, rat
1. INTRODUCTION
The neuronal activity induced by acute drug challenges retains close spatial and temporal relationships with the central hemodynamic response that can be measured by pharmacological-challenge magnetic resonance imaging (phMRI) (Leslie et al., 2000). In recent years, this method has been applied to study the central effects of drugs on neurotransmitter pathways both in human and animal models. However, for most pre-clinical experiments it is usually necessary to maintain animals under general anaesthesia to minimize head movements and stress induced by restraint. Under these conditions, phMRI responses to the drug of interest can vary considerably compared to the awake state (Luo et al., 2007; Skoubis et al., 2006; Zhang et al., 2000), as anaesthetics will likely alter the properties of neuronal activity and neurovascular coupling, due to their action on different receptors (Alkire et al., 2008). Previous studies have demonstrated changes to end tidal carbon dioxide (ETCO2) or hyperventilation may rapidly alter CO2 levels in the blood, leading to an overall decrease in the haemodynamic response (Gollub et al., 1998; Weckesser et al., 1999). Similarly, cerebral haemodynamics may be affected by large and rapid changes in mean arterial blood pressure (MABP), due to a breakdown in the auto regulatory mechanisms that control cerebral blood flow (CBF) (Gozzi et al., 2007a; Luo et al., 2003a; Tuor et al., 2002; Wang et al., 2006). However, for many anaesthetics, the target receptors or neurotransmitters remain unknown, making it difficult to predict the influence these agents have on underlying neural circuits following pharmacological stimulation (Steward et al., 2005).
N-methyl D-aspartate (NMDA) receptor associated ion-channel blockers such as ketamine or phencyclidine (PCP) induce schizophrenia-like symptoms in healthy volunteers (Adler et al., 1999) and exacerbate symptoms in stabilized patients (Lahti et al., 1995; Malhotra et al., 1997). These clinical observations provide the strongest line of evidence linking schizophrenia to disruptions in NMDA mediated glutamate neurotransmission (Olney et al., 1995). This has prompted researchers to explore non-invasive imaging techniques as a means of linking brain regions to behaviours and to identify the neurofunctional basis of NMDAR antagonists. In a recent phMRI study performed by this group, we assessed the acute effects of ketamine challenge in healthy volunteers, and identified areas of increased blood-oxygen-level-dependent (BOLD) signal in the posterior cingulate, thalamus and temporal cortex (Deakin et al., 2008). Consistent increases in cerebral blood flow (CBF) have also been reported in the same areas using positron emission tomography (PET) (Breier et al., 1997; Langsjo et al., 2003; Vollenweider et al., 1997). In the rat, similarities in the brain regions activated by ketamine have been reported by Littlewood et al. (2006) and more recently Gozzi et al. (2007b) and Risterucci et al. (2005), who examined the effects of PCP using relative cerebral blood volume (rCBV) and perfusion imaging techniques. However, NMDA ligands such as PCP and ketamine may have actions on other neurotransmitters at high doses potentially limiting their usefulness as models of schizophrenia (Kapur et al., 2002; Seeman et al., 2005). In a systematic investigation of the sign and spatiotemporal response pattern to PCP, Gozzi et al. (2008b) recently uncovered dramatic drug-anaesthetic interactions in rats anaesthetised by halothane inhalation, demonstrating complete reversal of PCP’s effects at higher challenge doses or halothane concentration. It may be the case that neural and cerebrovascular responses to NMDAR antagonists are dependent on the types of anaesthetic used.
The goal of the present study was to investigate the BOLD response to an acute ketamine challenge in rats anaesthetised by intravenous α-chloralose and isoflurane inhalation. These two anaesthetic agents are routinely used in phMRI, and have been successfully utilized to map the central haemodydnamic response to a range of neuroactive drugs.
2. MATERIALS AND METHODS
2.1 Animal Care
All experiments were approved by the U.K. Home Office governing animal welfare and protection. The protocols were also reviewed and consented to by a local ethical review committee at the University of Manchester. Studies were performed in vivo on male Sprague-Dawley rats [N=30, weight (mean ± S.D.) 283 ± 29g; Charles River, U.K.]. Animals were housed in groups of four per cage with free access to standard rat chowder and tap water. Room temperature (21 ± 2°C), relative humidity (45 ± 10%), and dark/light cycles (12h each) were controlled automatically. After arrival, rats were allowed to acclimatise for at least 5 days prior to beginning the experiments.
2.2 Anaesthesia
Animal preparations followed requirements of intravenous or volatile anaesthetic regimes (see section 2.3). Briefly, rats were anaesthetized with 3% isoflurane in 30%:70% O2:N2O gas mixture (0.3:0.7 l/min), a PE50 cannula was inserted subcutaneously for drug challenges and the tail was cannulated for delivery of intravenous anaesthetic. Switching anaesthesia to intravenous α-chloralose, administration of a 60 mg/kg i.v bolus was followed by continuous infusion at 30 mg/kg/h through the tail vein as described previously (Dodd et al., 2010; Stark et al., 2006). When using inhalation anaesthesia, the maintenance isoflurane level was set to 1.5% with identical delivery gas mixtures and flow rates. (The light level of anaesthesia achieved with this concentration assured normoventilation). After surgery, rats were moved to a customized stereotactic holder and secured using ear and bite bars to prevent head movement. The body temperature of all rats was maintained within the range of 37±1°C using a water heating system incorporated in the stereotactic MRI cradle. Ancillary bench experiments monitored cardiovascular parameters (heart rate and blood pressure) under all conditions used in this study. For this assessment, mean arterial blood pressure (MABP) was monitored continually by means of a blood pressure transducer (AD Instruments, U.K.) inserted in the right femoral artery. Blood samples were also extracted via the catheter to monitor arterial blood gases (VetScan/i-Stat blood analyzer, Abbott, Birmingham, U.K.) and percentage oxygen saturation was measured by pulse oximetry. At the end of the experiments, animals were euthanized with an overdose of anaesthetic followed by cervical-spine dislocation.
2.3 Study Design
A total of 30 rats (230-330g) were randomly assigned to one of four challenge arms:
Isoflurane anaesthesia, challenged with placebo (vehicle saline 1ml/kg s.c.)(N=6)
Isoflurane anaesthesia, challenged with ketamine (30mg/kg s.c.)(N=6)
α-chloralose anaesthesia, challenged with placebo (vehicle saline 1ml/kg s.c.)(N=6)
α-chloralose anaesthesia, challenged with ketamine (30mg/kg s.c.)(N=6)
Ancillary bench experiments were performed in a fifth group of animals (N=6) to assess the effects of anaesthesia on cardiovascular parameters during BOLD signal measurement. For all the experiments, drug challenges were administered at a rate of 1ml/min followed by 500μl saline to flush the line. Ketamine (Sigma-Aldrich, UK) was prepared from its salt compound and dissolved in saline. The dose employed here is sub-anaesthetic, and has previously been shown to elicit robust increases in glutamatergic neurotransmission (Moghaddam et al., 1997) and localised neurometabolic (2-deoxyglucose) responses in freely moving rodents (Duncan et al., 1998; Miyamoto et al., 2000).
2.4 MRI Acquisition
MRI data were acquired using a Magnex 7T system (Magnex Scientific) interfaced to an SMIS console (Surrey Medical Imaging Systems) with a custom-made radiofrequency transmit/receive birdcage coil. For each individual, T2-weighted anatomical images were acquired using a fast spin echo (FSE) sequence (TR=2000ms, TE=32ms, FOV = 40mm, 256×256 matrix, 11 contiguous 1mm slices), followed by an optimized T2*-weighted BOLD-sensitive time-series with the same spatial coverage but a lower resolution (TR=272ms, TE=15ms, 128×128 matrix, flip angle = 90°). The resulting in-plane voxel dimension was 312.5 × 312.5μm, with a temporal resolution of 70 seconds per scan. Ketamine or vehicle challenge was administered at volume 18, giving ~20 minutes of baseline for subsequent analysis.
2.5 Data Analysis
The data were analyzed in SPM5 governed by the framework of the general linear model (GLM). The BOLD time-series were realigned using the first image as a reference and co-registered to the anatomical image. Individual subjects in each study were spatially normalized by mapping their anatomical images to a stereotaxic rat brain MRI template (Schwarz et al., 2006) and applying the resulting transformation matrix to the accompanying time-series. The functional data were smoothed to a FWHM 0.8mm (~2.5mm × 2.5mm in-plane voxel dimensions), and multiplied by a brain parenchyma mask from the template set to remove extra-cranial and CSF contributions.
First-level analysis was performed using the pseudo-block analysis technique first described by McKie et al. (2005). This method enables accurate assessment of the drug-related changes by challenge-phMRI in human (Deakin et al., 2008) and animal systems (Dodd et al., 2010; Stark et al., 2006). Briefly, for each subject, a series of six successive time-bins (~10 minutes per block) were entered into the SPM design matrix, comparing each time block after injection to the pre-drug baseline period. After regression analysis, the series of effect size maps generated for each of the time-bins forms the input data for the secondlevel analysis. A random-effects repeated-measures 2×6 factorial ANOVA analysis then compared the effects of ketamine vs vehicle for the two independent anaesthetic groups. Statistical parametric maps were thresholded using a significance value of p<0.05 corrected for false-discovery rate (FDR). Volume of interest (VOI) analysis was conducted using a 3D digital reconstruction of a rat brain atlas (Paxinos and Watson, 1998) co-registered with the MRI template (Schwarz et al., 2006). The mean area-under-curve (AUC) from significant clusters were extracted by SPM toolbox Mars BaR (Brett et al., 2002).
3. RESULTS AND DISCUSSION
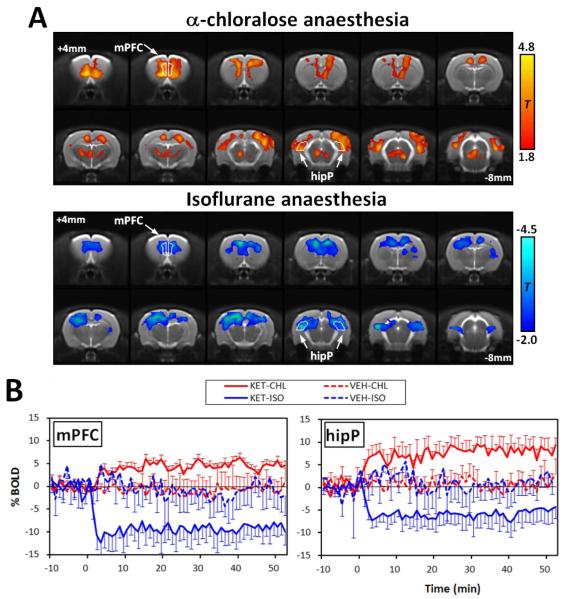
The two anaesthetic regimes produced extreme differences in sign and amplitude of the BOLD response to acute ketamine challenge. Images displaying the overall effects of ketamine versus vehicle are shown in Figure 1A. The maps indicate that under α-chloralose anaesthesia, only positive BOLD signal changes were present, with activations localised to discrete cortical and subcortical structures of the rat brain. Conversely, animals under isoflurane anaesthesia exhibited no statistically significant positive effects, but instead demonstrated large clusters of deactivation that were distributed across much of the cortex. Time-courses for the ketamine-induced BOLD responses are illustrated in Figure 1B.
Figure 1.
(A) Statistical parametric maps indicating the two characteristic responses after acute ketmaine challenge. Maps were calculated by comparing ketamine versus vehicle in α-chloalose and isoflurane anaesthetic groups. T-statistic thresholding is reported on the right for comparison. (B) Plots showing the temporal response profiles from two representative brain regions. These are the group mean time courses from the peak responding voxels in the ROI. Time zero indicates the point of injection.
(Abbreviations; Cg, cingulated; mPFC, medial prefrontal cortex; ctxSS; somatosensory cortex; hipP, posterior hippocampus)?
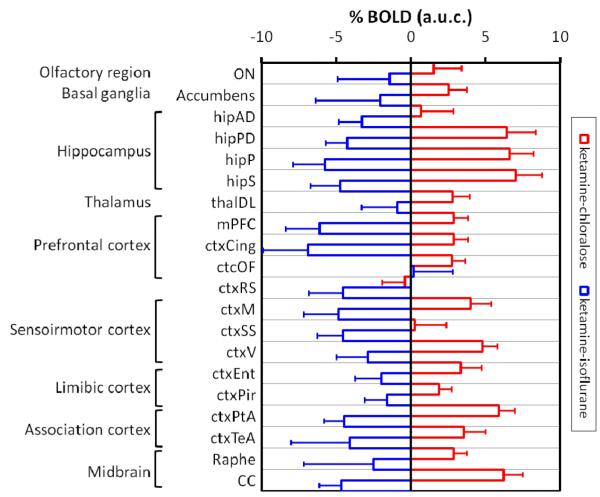
Plots of the signal changes show a similar time-dependent effect to ketamine in all the regions inspected (e.g medial prefrontal and posterior hippocampus), with a rapid increase or decrease in BOLD typically reaching the maxima or minima 5-6 minutes after ketamine administration. In non-activated regions no significant alterations in BOLD were observed with respect to vehicle control. The magnitude of the positive BOLD signal changes were region-dependent, with the largest response found in specific sub-regions of the cortex and hippocampus. Negative responses displayed a similar variation in magnitude across the examined regions, exhibiting only slightly stronger foci of deactivation in the prefrontal cortex. These effects are summarized in Figure 2.
Figure 2. Magnitude of the BOLD signal changes in significant VOIs.
The histograms represent mean area-under-curve (A.U.C) of all post injection time-points, with error bars indicating 90% confidence intervals. Abbreviations: ON, olfactory nucleus; hipAD, antero-dorsal hippocampus; hipPD, postero-dorsal hippocampus; hipS, hippocampus subicculum; ctxOF, orbitofrontal cortex; ctxRS, retrosplenial cortex; ctxM, motor cortex; ctxEnt, entorhinal cortex; ctxPir, piriform cortex; ctxTeA; temporal association cortex; CC, corpus callosum.
Cardiovascular parameters from the anaesthetic groups, prior to and following ketamine administration are displayed in the Supplementary Information (Figure S1). With isoflurane anaesthesia, ketamine induced only transient increases in heart rate (+45bpm, peaking 8min post-injection) and temporarily lowered BP (−19mmHg, 3min post-injection). However, this effect returned to the pre-injection baseline (364±14 bpm / 95±5mmHg) within 30mins, and did not correlate with the observed BOLD signal changes. Under α-chloralose anaesthesia, ketamine produced sustained decreases in both heart rate and BP (−28 bpm/-17mmHg, from baseline 385±21/93±9mmHg) lasting the entire duration of the study. Arterial blood gases paO2 and paCO2 were found to be within physiological range for both anaesthetic groups (20-50mmHg for paCO2, >80mmHg for paO2). These values are also reported in the Supplementary Information (Table S2).
In this study, we describe the use of phMRI to investigate the effects of an intravenous and a volatile anaesthetic on the ketamine-induced BOLD response. As a qualitative assessment of the changes in neuronal activity, we found dramatic differences in the sign and anatomical distribution of the BOLD signal that were dependent on the anaesthetics employed. Under α-chloralose, strictly positive BOLD changes were detected in precise cortical and subcortical structures of the rat brain, with varying effect sizes. However, for isoflurane anaesthesia, no positive BOLD changes were detected and instead, patterns of deactivation (negative BOLD response) were predominant. These results indicate an interaction between ketamine and isoflurane that completely reverses the phMRI signature of interest.
The use of anaesthesia is a potential confound that can affect the sign and distribution of the pharmacological stimulation. In the case of ketamine and isoflurane, we have found strong evidence for a direct or indirect drug-anaesthetic interaction which is specific to the experimental conditions of the study. The anaesthetic level at which these effects occur [1.5 minimal alveolar concentration (MAC)] are typical of many phMRI and fMRI studies employing volatile anaesthetics, suggesting that the anaesthetic concentrations achieved when using spontaneous breathing protocols may not be suitable in the investigation of NMDAR anatagonists. Consistent with these findings, Gozzi et al. have shown that the NMDAR antagonist PCP, displays a remarkably similar pattern of deactivation when maintained under high levels of halothane anaesthesia (i.e. 1.0 MAC) (Gozzi et al., 2008b). However, activations following an infusion of PCP were successfully observed at lower levels of halothane [0.8 MAC], although animals required careful ventilation and the use of muscle relaxants to control subject movement (Gozzi et al., 2008a; Gozzi et al., 2007b; Gozzi et al., 2008b).
Unlike commonly used inhalants, α-chloralose is known to preserve cerebrovascular reactivity and neuronal excitability. Here we observed specific BOLD increases in cortical and subcortical areas of the rat brain that were more closely paralleled to the central effects of NMDAR antagonists in freely moving animals and conscious humans. For instance, metabolic maps of increased 2-deoxyglucose (2-DG) uptake in rodents injected with ketamine and MK-801, showed considerable similarity to the positive response observed in this study (Duncan et al., 1998; Miyamoto et al., 2000). A further likeness can also been drawn from CBF measurements following ketamine administration using [14C]-iodoantipyrine (Cavazzuti et al., 1987). In healthy human volunteers, we have previously explored the functional effects of ketamine using BOLD phMRI (Deakin et al., 2008), and identified increased areas of activation in the posterior cingulate, thalamus, and temporal cortex. For rats under the α-chloralose regime, very similar patterns of activation were observed, with apparent minimal involvement from the anaesthesia. Altogether, these findings indicate a significant overlap between the positive BOLD response pattern obtained with α-chloralose to regions activated by ketamine in humans and metabolic maps of conscious animals. Furthermore, in awake and chloral hydrate anaesthetized animals (actions similar to α–chloralose), the neurophysiological effects of NMDAR antagonists (MK-801, PCP) have been shown to induce an increase in neuronal spiking rate (multi unit activity) (Homayoun et al., 2005; Kargieman et al., 2007; Suzuki et al., 2002), which would be consistent with a positive BOLD response. Consequently, the use of intravenous α-chloralose could offer a suitable alternative to gaseous anaesthetic agents when probing the neurofunctional effects of NMDAR antagonists.
The question remains as to whether systemic effects of ketamine may be differentially modulated by the anaesthetic agent which in turn affects the central BOLD response. Large changes in cardiac output or blood gases could alter vascular reactivity in the brain and this could lead to changes in the regional BOLD response to ketamine challenge. We did indeed observe a sustained reduction in blood pressure and heart rate when ketamine was administered to α-chloralose-anaesthetized rats, but it is difficult to understand how such a hypotensive effect could reverse the BOLD response compared to using an anaesthetic regime (isoflurane) in which ketamine had no sustained systemic effects. It has been shown that systemically-induced increases of MABP, of similar magnitude, do not alter the BOLD effect (Luo et al., 2003b). It should also be noted that the magnitude of the hypotensive changes are well within the range of effective auto regulation (<30mmHg), whereby abrupt manipulation of blood pressure can be homeostatically compensated without significant depression of the central BOLD response (Wang et al., 2006). It is notable that there were no effects on respiration rate or blood gases following infusion of ketamine under either anaesthetic regime. In conclusion, we do not favour an explanation based on systemic effects of ketamine causing the differential BOLD responses in the brain under the different anaesthetic regimes.
How anaesthesia affects neurovascular coupling and metabolism remains a controversial issue. In general, haloether volatile agents, such as isoflurane, greatly potentiate γ-aminobutyric acid (GABAA) receptors and have modest effects on NMDA receptors (de Sousa et al., 2000; Eger et al., 2006; Harrison et al., 1993; Kelly et al., 2007; Lin et al., 1992; Ogata et al., 2006; Solt et al., 2006; Yamakura et al., 2000). Previous studies have indicated that the MAC of isoflurane can be significantly decreased following gradual infusion of ketamine or MK-801 (Eger et al., 2006; Kuroda et al., 1993; Pascoe et al., 2007). As lower MAC values represent a more potent volatile anesthetic, it is possible that ketamine-induced disinhibition of glutamate neurones is being over-ridden by the action of isoflurane in enhancing GABAA-receptor-mediated synaptic inhibition and decreasing excitatory NMDA cation currents. This would cause neuronal hyperpolarization that reduces cell excitability and likely contributes to the negative effects of this anaesthetic on the ketamine-induced BOLD signal. Whether or not this speculation is correct, it is clear that there is some cross-reactivity or synergism of these two compounds in some brain regions that complicates the effects of the challenge drug.
4. CONCLUSION
To conclude, this study has indicated the potential for a drug-anaesthetic interaction between ketamine and isoflurane, whereby a complete reversal of the BOLD response can occur. With α-chloralose, the response pattern was strictly positive, resembling the activations from human neuroimaging studies and metabolic maps in conscious rats. In the future, studies such as this may be important in identifying the correct challenge dose and anaesthetic level for preclinical phMRI. In addition, we have described how comparisons with other experimental modalities can help validate phMRI data.
Supplementary Material
Fig. S1. Effects of anaesthesia on cardiovascular parameters following acute ketamine challenge.
Graphs indicate changes to heart rate/ MABP (A), respiration rate / percentage O2 saturation (B) (mean ± SEM).
Table S2. Blood gas measurements performed prior to and after ketamine chellenge.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Katie Murray for technical assistance. This work was supported by the award of a Capacity-Building Studentship to DJH from the Medical Research Council (MRC), by a grant from NARSAD to CdG and by the EU-sponsored New Mood project.
Footnotes
COMPETING INTERESTS The authors have no competing of interest to declare.
REFERENCES
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–9. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox 8th International Conference on Functional Mapping of the Human Brain.2002. p. 16. [Google Scholar]
- Cavazzuti M, Porro CA, Biral GP, Benassi C, Barbieri GC. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987;7:806–11. doi: 10.1038/jcbfm.1987.138. [DOI] [PubMed] [Google Scholar]
- de Sousa SLM, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmacomagnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–64. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Williams SR, Luckman SM. Functional magnetic resonance imaging and c-Fos mapping in rats following a glucoprivic dose of 2-deoxy-d-glucose. Journal of Neurochemistry. 2010;113:1123–1132. doi: 10.1111/j.1471-4159.2010.06671.x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Leipzig JN, Mailman RB, Lieberman JA. Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Research. 1998;812:65–75. doi: 10.1016/s0006-8993(98)00926-3. [DOI] [PubMed] [Google Scholar]
- Eger EI, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV, Sonner JM. Contrasting roles of the N-Methyl-d-Aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesthesia & Analgesia. 2006;102:1397–1406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, Makris N, Guimaraes A, Riorden J, Campbell T, Foley M, Hyman SE, Rosen B, Weisskoff R. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Ceolin L, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. A multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magnetic Resonance Imaging. 2007a;25:826–833. doi: 10.1016/j.mri.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Herdon H, Schwarz A, Bertani S, Crestan V, Turrini G, Bifone A. Pharmacological stimulation of NMDA receptors via co-agonist site suppresses fMRI response to phencyclidine in the rat. Psychopharmacology (Berl) 2008a;201:273–84. doi: 10.1007/s00213-008-1271-z. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A. Differential Effects of Antipsychotic and Glutamatergic Agents on the phMRI Response to Phencyclidine. Neuropsychopharmacology. 2007b;33:1690–1703. doi: 10.1038/sj.npp.1301547. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Crestan V, Bifone A. Drug-anaesthetic interaction in phMRI: the case of the psychotomimetic agent phencyclidine. Magnetic Resonance Imaging. 2008b;26:999–1006. doi: 10.1016/j.mri.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Molecular Pharmacology. 1993;44:628–632. [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. Journal of Neurophysiology. 2005;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–44. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci USA. 2007;104:14843–8. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EW, Solt K, Raines DE. Volatile aromatic anesthetics variably impact human γ-Aminobutyric acid type A receptor function. Anesthesia & Analgesia. 2007;105:1287–1292. doi: 10.1213/01.ane.0000282829.21797.97. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Strebel S, Rafferty C, Bullock R. Neuroprotective doses of N-Methyl-D-Aspartate receptor antagonists profoundly reduce the Minimum Alveolar Anesthetic Concentration (MAC) for Isoflurane in rats. Anesthesia & Analgesia. 1993;77:795–800. doi: 10.1213/00000539-199310000-00025. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V, Sipila H, Kurki T, Silvanto M, Scheinin H. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:614–23. doi: 10.1097/00000542-200309000-00016. [DOI] [PubMed] [Google Scholar]
- Leslie RA, James MF. Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends in Pharmacological Sciences. 2000;21:314–318. doi: 10.1016/s0165-6147(00)01507-8. [DOI] [PubMed] [Google Scholar]
- Lin LH, Chen LL, Zirrolli JA, Harris RA. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: lack of involvement of intracellular calcium. Journal of Pharmacology and Experimental Therapeutics. 1992;263:569–578. [PubMed] [Google Scholar]
- Littlewood CL, Jones N, O’Neill MJ, Mitchell SN, Tricklebank M, Williams SC. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology (Berl) 2006;186:64–81. doi: 10.1007/s00213-006-0344-0. [DOI] [PubMed] [Google Scholar]
- Luo F, Li Z, Treistman SN, Kim YR, King JA, Fox GB, Ferris CF. Confounding effects of volatile anesthesia on CBV assessment in rodent forebrain following ethanol challenge. J Magn Reson Imaging. 2007;26:557–63. doi: 10.1002/jmri.21083. [DOI] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li SJ. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magnetic Resonance in Medicine. 2003a;49:264–270. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li SJ. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn Reson Med. 2003b;49:264–70. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A. Clozapine blunts N-Methyl--Aspartate Antagonist-induced psychosis: A study with Ketamine. Biological Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- McKie S, Del-Ben C, Elliott R, Williams S, del Vai N, Anderson I, Deakin JF. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 2005;180:680–6. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology. 2000;22:400–12. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-Methyl-d-Aspartate receptors expressed in xenopus oocytes. Journal of Pharmacology and Experimental Therapeutics. 2006;318:434–443. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Pascoe PJ, Ilkiw JE, Craig C, Kollias-Baker C. The effects of ketamine on the minimum alveolar concentration of isoflurane in cats. Vet Anaesth Analg. 2007;34:31–9. doi: 10.1111/j.1467-2995.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Jeanneau K, Schoppenthau S, Bielser T, Kunnecke B, von Kienlin M, Moreau JL. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology (Berl) 2005;180:724–34. doi: 10.1007/s00213-005-2204-8. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Danckaert A, Reese T, Gozzi A, Paxinos G, Watson C, Merlo-Pich EV, Bifone A. A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: Application to pharmacological MRI. NeuroImage. 2006;32:538–550. doi: 10.1016/j.neuroimage.2006.04.214. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Tallerico T. Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry. 2005;10:877–83. doi: 10.1038/sj.mp.4001682. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Hradil V, Chin CL, Luo Y, Fox GB, McGaraughty S. Mapping brain activity following administration of a nicotinic acetylcholine receptor agonist, ABT-594, using functional magnetic resonance imaging in awake rats. Neuroscience. 2006;137:583–91. doi: 10.1016/j.neuroscience.2005.08.072. [DOI] [PubMed] [Google Scholar]
- Solt K, Eger EI, Raines DE. Differential modulation of human N-Methyl-d- Aspartate Receptors by structurally diverse general anesthetics. Anesthesia & Analgesia. 2006;102:1407–1411. doi: 10.1213/01.ane.0000204252.07406.9f. [DOI] [PubMed] [Google Scholar]
- Stark JA, Davies KE, Williams SR, Luckman SM. Functional magnetic resonance imaging and c-Fos mapping in rats following an anorectic dose of mchlorophenylpiperazine. Neuroimage. 2006;31:1228–37. doi: 10.1016/j.neuroimage.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Steward CA, Marsden CA, Prior MJ, Morris PG, Shah YB. Methodological considerations in rat brain BOLD contrast pharmacological MRI. Psychopharmacology (Berl) 2005;180:687–704. doi: 10.1007/s00213-005-2213-7. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience. 2002;114:769–779. doi: 10.1016/s0306-4522(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magnetic resonance imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F] fluorodeoxyglucose (FDG) Eur Neuropsychopharmacol. 1997;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, Tuor UI. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Weckesser M, Posse S, Olthoff U, Kemna L, Dager S, Müller-Gärtner HW. Functional imaging of the visual cortex with bold-contrast MRI: Hyperventilation decreases signal response. Magnetic Resonance in Medicine. 1999;41:213–216. doi: 10.1002/(sici)1522-2594(199901)41:1<213::aid-mrm31>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels: comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–1101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen AH, Avison MJ, Gerhardt GA, Gash DM. Functional MRI of apomorphine activation of the basal ganglia in awake rhesus monkeys. Brain Res. 2000;852:290–6. doi: 10.1016/s0006-8993(99)02243-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of anaesthesia on cardiovascular parameters following acute ketamine challenge.
Graphs indicate changes to heart rate/ MABP (A), respiration rate / percentage O2 saturation (B) (mean ± SEM).
Table S2. Blood gas measurements performed prior to and after ketamine chellenge.