Abstract
The understanding of how environmental factors regulate toxic secondary metabolite production in cyanobacteria is important to guarantee water quality. Very little is known on the regulation of toxic secondary metabolite production in benthic cyanobacteria. In this study the physiological regulation of the production of the toxic heptapeptide microcystin (MC) and the non-toxic related peptide nostophycin (NP) in the benthic cyanobacterium Nostoc sp. strain 152 was studied under contrasting environmental conditions. I used a 2k levels factorial design, where k is the number of four factors that have been tested: Reduction in temperature (20 vs. 12°C), irradiance (50 vs. 1 μmol · m−2 · s−1), P-PO4 (144 vs. 0.14 μM P-PO4), N-NO3 (5.88 mM vs. N-NO3 free). While the growth rate was reduced more than hundred fold under most severe conditions of temperature, irradiance, and phosphate reduction the production of MC and NP never ceased. The MC and NP contents per cell varied at maximum 5- and 10.6-fold each, however the physiological variation did not outweigh the highly significant linear relationship between the daily cell division rate and the MC and NP net production rates. Surprisingly the MC and NP contents per cell showed a maximum under P-PO4 reduced and irradiance reduced conditions. Both intra- and extracellular MC and NP concentrations were negatively related to P-PO4 and irradiance. It is concluded that the proximate factor behind maximal cellular MC and NP contents is physiological stress.
Keywords: dissolved toxins, eutrophication, Microcystis, Planktothrix, soluble reactive phosphorus
Introduction
Cyanobacteria are well known for their production of a multitude of highly toxic and/or allelopathic compounds. The toxic compounds include various cyclic peptides (the hepatotoxic microcystins) and alkaloids (the potent neurotoxins and the hepatotoxic cylindrospermopsin), which have been studied both from a toxicological and a biological perspective (Chorus and Bartram 1999, Hudnell 2008). Several hypotheses have been formulated to explain the physiological regulation and the biological function of these toxic molecules, particularly of the microcystins (MCs, Dittmann et al. 2001, Kaebernick and Neilan 2001, Kehr et al. 2006, Schatz et al. 2007, and others). For example most frequently cited hypotheses include functions related to allelopathy such as feeding deterrence and inhibition of other (eukaryotic) algae, and functions related to primary metabolism such as iron scavenging, regulation of photosynthesis and quorum-sensing like cell-cell communication processes (see Kaebernick and Neilan 2001, Schatz et al. 2007, Leao et al. 2009 for more recent reviews). Typically planktonic cyanobacteria (Anabaena, Microcystis, Planktothrix) have been investigated for the regulation of toxin production under various environmental conditions (Sivonen and Jones 1999). Chemical structures of MCs produced by benthic cyanobacteria, e.g. Nostoc sp. have been reported already during the 1990ies (Sivonen et al. 1992, Beattie et al. 1998). The genus Nostoc is common in both terrestrial and aquatic habitats typically growing on sediments or stones in the littoral or in running water (Komarek and Anagnostidis 1989, Dodds et al. 1995). Only recently increasing evidence on the worldwide abundance of Nostoc sp. as a MC-producing organism has been reported (Oksanen et al. 2004, Mohamed et al. 2006, Wood et al. 2008, Bajpai et al. 2009, Oudra et al 2009, Genuario et al. 2010). Indeed it has been suggested that cyanobacteria growing on the sediments in reservoirs constitute a significant source of MC (Izaguirre et al. 2007) and should be included in routine monitoring for the presence of MC in raw water used for drinking water purification (Hurtado et al. 2008) or irrigation (Mohamed et al. 2006). In addition to MCs a number of other bioactive compounds have been described in Nostoc sp. Prominently, the depsipeptides cryptophycins, which show strong cytotoxic effects as tubulin polymerization inhibitors, have been discovered during screening tests for bioactive activity (Golakoti et al. 1995). Other Nostoc sp. strains have been studied for their production of allelopathic compounds. For example nostocyclamide, a cyclic hexapeptide produced by Nostoc inhibits growth in algae and bacteria (Jüttner et al. 2001). Becher et al. (2009) described nostocarboline that - functionally similar to anatoxin-a(s) - is an inhibitor of acetylcholinesterase and the first serine protease inhibitor of an alkaloid structure that has been described. Hirata et al. (1996) described nostocine A, a violet pigment that occurred in the medium and inhibited the growth of various algae and cultured plants. Similarly, muscoride A an oxazol alkaloid peptide was reported to show weak antibacterial activity (Nagatsu et al. 1995). Gromov et al. (1991) described cyanobacterin from Nostoc linckia, which is non-toxic to mice, however effective against Synechococcus at a concentration of 1 mg · L−1.
The strain Nostoc sp. 152 produces several microcystin structural variants (Namikoshi et al. 1990, Sivonen et al. 1992) and at least one another cyclic peptide, nostophycin (NP) (Fujii et al. 1999). The MCs are cyclic heptapeptides that are defined by the presence of the ß-amino acid residue (2S, 3S, 8S, 9S)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid (Adda) that is characteristic to the MC family (Diehnelt et al. 2006). While the MCs produced by Nostoc sp. 152 all show a structural modification in the Adda side chain, resulting in acetylated and demethylated MC structural variants (Sivonen et al. 1992) they all have been shown to retain their toxicity when compared to MC-LR (Sivonen et al. 1990). Similarly to MC, NP is a cyclic hexapeptide that also contains a ß-amino acid residue (2S,3R,5R)-3-amino-2,5-dihydroxy-8-phenyloctanoic acid (Ahoa) and so far NP has been described to occur only in this strain. Since the MCs and NP have a beta-amino acid and the occurrence of two D-amino acids in common it has been suggested that their synthesis is related (Fujii et al. 1999). However, in contrast to the toxin MC, NP showed no bioactivity (20 μg · ml−1) against several microorganisms (Aspergillus, Candida, Staphylococcus, Bacillus and Escherichia) but weakly cytotoxic activity against lymphocytic mouse leukemia (Fujii et al. 1999). In this study the physiological regulation of both MC and the related NP was studied under a regime of contrasting temperature, irradiance, and nutrient conditions. It is shown that both MC and NP were constitutively produced but the cellular content was significantly increased under physiological stress conditions. The extent of the modulation, however, was not high enough to prevent a linear correlation between both MC and NP production rate and the growth rate.
Material and Methods
Growth experiments
All experiments were performed with the axenic strain Nostoc VAUCHER ex BORNET et FLAHAULT strain PCC9237 (Nostoc sp. strain 152 originally isolated from Lake Sääksjärvi, Finland in 1986, Sivonen et al. 1990). Nostoc strain 152 was grown in O2 medium (144 μM P-PO4, 5.88 mM N-NO3, Van Liere and Mur 1978). I used a 2k levels factorial design, where k is the number of four factors that have been tested: Temperature reduction (20°C vs. 12°C), irradiance reduction (50 μmol · m−2 · s−1 vs. 1 μmol), P-PO4 reduction (0.14 μM P-PO4 vs. 144 μM), N-NO3 free conditions (5.88 mM N-NO3 vs. N-NO3 free). Cultures were illuminated from below and shaken once each day. The light intensity was determined using a quantum sensor (T. and J. Crump, Scientific instruments, Rayleigh, NC) outside the culture flasks. Glassware used for the phosphate reduction treatment was washed with sulphuric acid (10%, v/v) and deionised water to eliminate any possible external phosphate contamination. The design had 16 possible combinations that were tested in triplicate subsequently during one year.
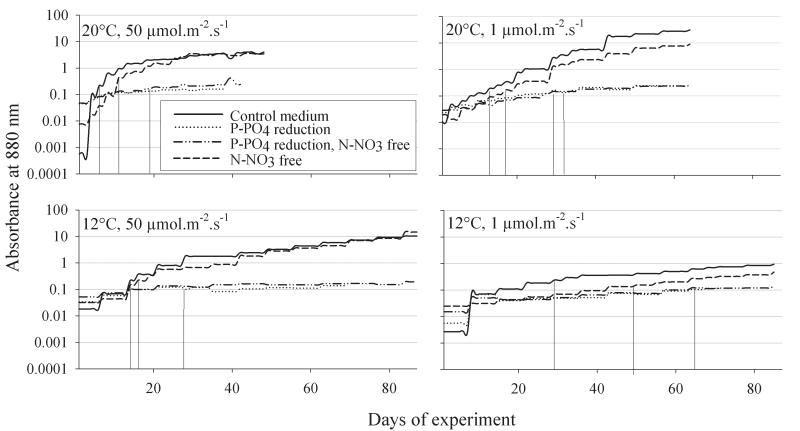
In order to adapt the cells to the experimental conditions precultures for a minimal duration of three months were established and the growth was monitored by measuring absorbance at 880 nm (5 cm irradiance path) measured in an spectrophotometer. At the start of each growth experiment precultures were diluted down to OD 0.01 (23,000 ± 2,400 (1 SE) cells · mL−1) in a total volume of 800 ml and aliquots of 100 ml were filled in eight 250 ml Erlenmeyer flasks to achieve optimal growth. Batch cultures were monitored every other day with the exception of extremely slow growing cultures under P-PO4 reduction and/or at 12°C (Fig. 1). A relatively wide variation in OD among treatments at 20°C, 50 μmol · m−2 · s−1 (0.001-0.05) and at 12°C, 1 μmol · m−2 · s−1 (0.003-0.02) was observed at day 1 subsequent to the inoculation at day 0 (after two days). It is speculated that cell lysis subsequent to the inoculation can explain the extremely low OD readings at day 1 in some treatments. Cells were harvested at OD 0.10 – 0.15 at two consecutive sampling dates. Depending on the average growth rate the two sampling dates were 2-3 days (at 20°C) or 2-6 days (12°C) apart. From these dates cellular growth rates (day−1) and MC and NP production rates (day−1) were calculated using the formula, r = (lnN2 – lnN1)/Δt, where N1,2 were the cell concentrations or peptide concentrations at consecutive sampling days (t0, t1) and Δt was the interval in days. Cells were fixed in 2% formaldehyde and counted using DAPI staining under an epifluorescence microscope according to standard techniques (Porter and Feig 1980). Samples were filtered onto pre weighed glass fibre filters (BMC Ederol, Vienna), dried at room temperature in a vacuum centrifuge, reweighed and stored frozen at −20°C.
Fig. 1.
Growth of axenic strain Nostoc strain 152 under high irradiance (50 μmol · m−2 · s−1) and low irradiance (1 μmol · m−2 · s−1) conditions and two different temperatures (12°C, 20°C) and P-PO4 reduced and N-NO3 free conditions. Growth has been monitored from day 1 by reading absorbance at 880 nm (mean value of three parallels) subsequent to inoculation. Vertical lines drawn from the growth curve down to the x-axis indicate the days of first harvest (t0).
Extracellular peptides were collected from the filtrate using solid phase extraction (SPE) via tC18 cartridges (Waters, Sep-Pak Vac 1cc (100 mg) according to manufacturer’s instructions. Cartridges were stored at −20°C. Peptides were eluted in 1 ml 90% MeOH and extracellular extracts were stored at −20°C. Pilot experiments dissolving NP in O2 medium and re-collecting NP using SPE showed a recovery rate of 103 ± 2.4 (SE) %. The effective recovery of MC-LR (85.6 ± 12.6%) by using the same technique was shown during a previous study (Kosol et al. 2009).
To extract intracellular MC and NP, cells on filters (2 mg of dry weight) were extracted in 50% MeOH (v/v) after ultrasonification for 10 min. Extracts were shaken for 30 min, centrifuged at 16,000×g and the clear supernatant was evaporated to dryness in a vacuum centrifuge at room temperature. This procedure was repeated three times and the extracts were finally combined. Pilot experiments showed 99% extraction efficiency after three repetitions and optimal yields using sonification at room temperature compared without sonfication and/or extraction on ice.
For HPLC analysis the samples were redissolved in 600 μL MeOH (50%, v/v), centrifuged at 16,000×g and the cleared supernatant injected into HPLC-DAD. MCs and NP were detected at 210 nm using a linear gradient from 20% (v/v) acetonitrile (0.05% (v/v) TFA) to 50% on a LiChrospher® 100, ODS, 5 mm, LiChroCART® 250-4 cartridge system (Agilent, Vienna). NP (M+H 889, Fujii et al. 1999) eluted at 39 min and MCs eluted at 40-44 min (M+H 1009, 1023, 1037, Namikoshi et al. 1990). NP was linearly detected from an area of 10,000 mAU (milli absorbance units) down to 100 mAU. Quantification was achieved using a calibrated standard provided by the University of Dundee (Louise Morrison, Geoffrey Codd). The calibration curve was y = 1.6054x (n = 8, R2 = 1.0), where y is the area recorded in mAU at 210 nm and x is the amount of NP injected (in ng). The concentration of MCs were determined as concentration equivalents of [Mdha, MeAsp]-MC-LR (Calbiochem). The linear regression line was y = 1.471x (n = 8, R2 = 0.999), where y is the peak area at 210 nm and x is the injected amount of MC-LR standard (ng).
Multiple linear regression analysis was used to test the relationship between the days until harvest at OD 0.1, the number of cells/heterocysts per filament, intra- and extracellular MC and NP contents per cell and the influence of temperature, irradiance, P-PO4 and N-NO3 concentrations as independent variables. A forward stepwise analysis was employed selecting for the independent variable for inclusion that makes the most significant unique (or additional) contribution to the prediction of the data. Calculations were performed using SPSS 15.0 for Windows and the F value to enter the respective model was set default (p < 0.05). The data were log transformed in order to fulfil the assumptions of normality, constant variance and multicollinearity.
Results
Growth characteristics under the experimental conditions
In all 16 treatments Nostoc strain 152 showed a significant increase in cell numbers from the day of inoculation (OD880 0.01) until the day of first harvest (OD880 = 0.1, min-mean-max, 5-29-76 fold increase in cell numbers, Fig. 1). The number of days to reach OD880 = 0.1, however, differed significantly between treatments and they were at maximum 10.9-fold higher under P-PO4 reduced conditions (1 μmol · m−2 · s−1, 12°C), i.e. 70 ± 3 (1 SE) days when compared with optimal conditions (20°C, 50 μmol · m−2 · s−1) in the full O2 medium (6 ± 0 days), (Table 1). The cellular growth rate (ind−1 · day−1) as calculated from cell numbers counted at the first (t0) and the consecutive day (t1) of cell harvest differed widely, i.e. 0.003 ± 0.03 under P-PO4 reduced and N-NO3 free conditions (12°C, 50 μmol · m−2 · s−1) vs. 0.49 ± 0.11 under optimal conditions in the full medium (Table 1). Using multiple regression analysis all four variables (i.e. the reduction in temperature, irradiance, P-PO4 and N-NO3) were included in the forward stepwise method and in total explained 89% of the variation that was observed among the days until first harvest (t0, Table 2). As expected temperature and irradiance reduction had the strongest influence on the reduction in cellular growth rate while P-PO4 reduction and N-NO3 still contributed significantly.
Table 1.
Time until day of first harvest (t0), growth rates and cell numbers (mean ± 1SE) of axenic strain Nostoc strain 152 grown under high irradiance (50 μmol · m-2 · s−1) and low irradiance (1 μmol · m−2 · s−1) conditions and two different temperatures (12°C, 20°C) and phosphorus reduced and nitrate free conditions. t0, t1 = consecutive sampling days (2-3 days (20°C) or 2-6 days (12°C) apart)
| Time until day of first harvest (in days) |
Cells · Fil−1 | Heterocysts · Fil−1 |
Vegetative cells . heterocyst · −1 |
growth rate · day−1 | 106 cells · ml−1, t0 | 106 cells · ml−1, t1 | ||
|---|---|---|---|---|---|---|---|---|
| 20°C, 50 μmol · m−2 · s−1 | Full medium | 6±0 | 33±0.4 | 1.2±0.2 | 29±2 | 0.493±0.11 | 1.296±0.125 | 3.535±0.584 |
| P-PO4 reduced | 15±1 | 24±4.7 | 0.2±0.1 | 277±209 | 0.049±0.03 | 0.436±0.042 | 0.487±0.069 | |
| P-PO4 reduced, N-NO3 free | 25±4 | 29.6±2.1 | 0.7±0.2 | 133±93 | 0.041±0.04 | 0.527±0.43 | 0.543±0.096 | |
| N-NO3 free | 8±1 | 30.6±1.3 | 1.6±0.2 | 19±1 | 0.374±0.08 | 1.539±0.503 | 3.008±0.642 | |
| 20°C, 1 μmol · m−2· s−1 | Full medium | 13±0 | 34.8±0.4 | 0.8±0.1 | 46±3 | 0.170±0.04 | 0.658±0.095 | 1.003±0.217 |
| P-PO4 reduced | 26±2 | 36.8±2.4 | 0.2±0 | 302±60 | 0.130±0.02 | 0.486±0.155 | 0.616±0.17 | |
| P-PO4 reduced, N-NO3 free | 27±2 | 35.2±1.7 | 0.7±0.1 | 52±3 | 0.027±0.08 | 0.566±0.032 | 0.636±0.134 | |
| N-NO3 free | 19±3 | 35.3±1.4 | 1.8±0.2 | 20±1 | 0.177±0.02 | 0.704±0.105 | 1.128±0.067 | |
| 12°C, 50 μmol · m−2 · s−1 | Full medium | 15±1 | 35.7±0.1 | 1.2±0.1 | 31±1 | 0.286±0.05 | 1.030±0.273 | 2.018±0.314 |
| P-PO4 reduced | 36±3 | 14.9±5.6 | 0.1±0 | 245±107 | 0.053±0.01 | 0.185±0.015 | 0.219±0.011 | |
| P-PO4 reduced, N-NO3 free | 36±3 | 34.9±1.1 | 0.9±0.1 | 42±2 | 0.003±0.03 | 0.354±0.097 | 0.360±0.098 | |
| N-NO3 free | 18±3 | 35.7±0.6 | 1.9±0 | 19±0.4 | 0.172±0.01 | 1.256±0.108 | 2.160±0.366 | |
| 12°C, 1 μmol · m−2 s−1 | Full medium | 31±2 | 33.5±2.8 | 0.5±0 | 74±10 | -0.0321±0.05 | 0.144±0.028 | 0.132±0.005 |
| P-PO4 reduced | 70±3 | 28.5±0.9 | 0.3±0 | 111±14 | 0.037±0.05 | 0.215±0.055 | 0.249±0.041 | |
| P-PO4 reduced, N-NO3 free | 68±2 | 32.3±0.1 | 0.6±0.1 | 62±8 | 0.062±0.03 | 0.238±0.029 | 0.300±0.01 | |
| N-NO3 free | 56±4 | 26.2±2.3 | 1±0 | 26±1 | 0.054±0.04 | 0.515±0.013 | 0.686±0.119 |
Cell growth was negative in two of three parallels as measured during the t0-t1 interval (3 days).
Fil = filament
Table 2.
Parameters of multiple linear regression analysis used to test the influence of temperature (T), irradiance (I), P-PO4 and N-NO3 concentrations as independent variables (Var) on the days until harvest (at OD880nm = 0.1), number of cells per filament, number of heterocysts per filament, intra- and extracellular MC and NP contents per cell. The regression was logy = B0 + B1 × logX1 + B2 × logX2 + B3 × logX3 + B4 × logX4, where X1-4 is the corresponding (log10 transformed) real value to be multiplied by the regression coefficients B1-B4.
| Var1 | Var2 | Var3 | Var4 | R2 Var1 | R2 Var2 | R2 Var3 | R2 Var4 | B0 | B1 | B2 | B3 | B4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days until day of first harvest | T | I | P-PO4 | N-NO3 | 0.39 | 0.64 | 0.87 | 0.89 | 2.291 | −0.037 | −0.006 | −0.002 | −0.016 |
| Cells · fil−1 | P-PO4 | I | 0.05 | 0.098 | 1.468 | 0.001 | −0.002 | ||||||
| Heterocysts · fil−1 | P-PO4 | N-NO3 | 0.38 | 0.61 | −0.304 | 0.004 | −0.077 | ||||||
| Microcystin | |||||||||||||
| MC fg· cell−1 | I | P-PO4 | 0.38 | 0.48 | 2.156 | −0.006 | −0.001 | ||||||
| Total MC fg· cell−1 | I | P-PO4 | T | 0.28 | 0.46 | 0.51 | 2.404 | −0.005 | −0.001 | −0.011 | |||
| Diss MC fg· cell−1 | P-PO4 | T | 0.24 | 0.47 | 2.001 | −0.003 | −0.039 | ||||||
| %diss MC | T | P-PO4 | I | 0.27 | 0.38 | 0.44 | 1.621 | −0.028 | −0.001 | 0.003 | |||
| Nostophycin | |||||||||||||
| NP fg· cell−1 | P-PO4 | I | T | 0.39 | 0.69 | 0.72 | 2.057 | −0.003 | −0.008 | 0.012 | |||
| Total NP fg· cell−1 | P-PO4 | I | T | 0.55 | 0.75 | 0.77 | 2.24 | −0.004 | −0.007 | 0.011 | |||
| Diss NP fg· cell−1 | P-PO4 | T | I | 0.67 | 0.73 | 0.78 | 1.56 | −0.006 | 0.025 | −0.005 | |||
| %diss NP | P-PO4 | I | T | 0.33 | 0.39 | 0.43 | 1.346 | −0.002 | 0.003 | 0.009 | |||
Nostoc strain 152 formed long filaments under optimal or near optimal growth conditions while filaments broke apart under P-PO4 reduced conditions. On average the number of cells per filament were 31 ± 0.9 (min 6, max 50). The number of cells per filament was found more variable under P-PO4 reduced conditions, at maximum it was reduced to 14.9 ± 5.6 at 50 μmol · m−2 · s−1 (12°C). The multiple regression approach revealed P-PO4 and irradiance reduction as significant predictor variables, however, the explained variability was low (Table 2). On average one filament contained 0.9 ± 0.1 heterocysts (min 0, max 3). The formation of heterocysts depended on P-PO4 and N-NO3 reduction only (R2 = 0.61). When compared to the growth conditions in the full medium at 50 μmol · m−2 · s−1 (20°C) the number of heterocysts per filament as well as the ratio of vegetative cells/heterocysts was increased by 0.8-1.6 fold under conditions of N-NO3 reduction. However, it was decreased to 0.07-0.23 fold under conditions of P-PO4 reduction. From the considerable variation in the growth rate as well as the ratio of vegetative cells/heterocysts that was found it is concluded that environmental conditions both limiting and non-limiting to growth were observed under the experimental design.
Cellular content of microcystin and nostophycin under the experimental conditions
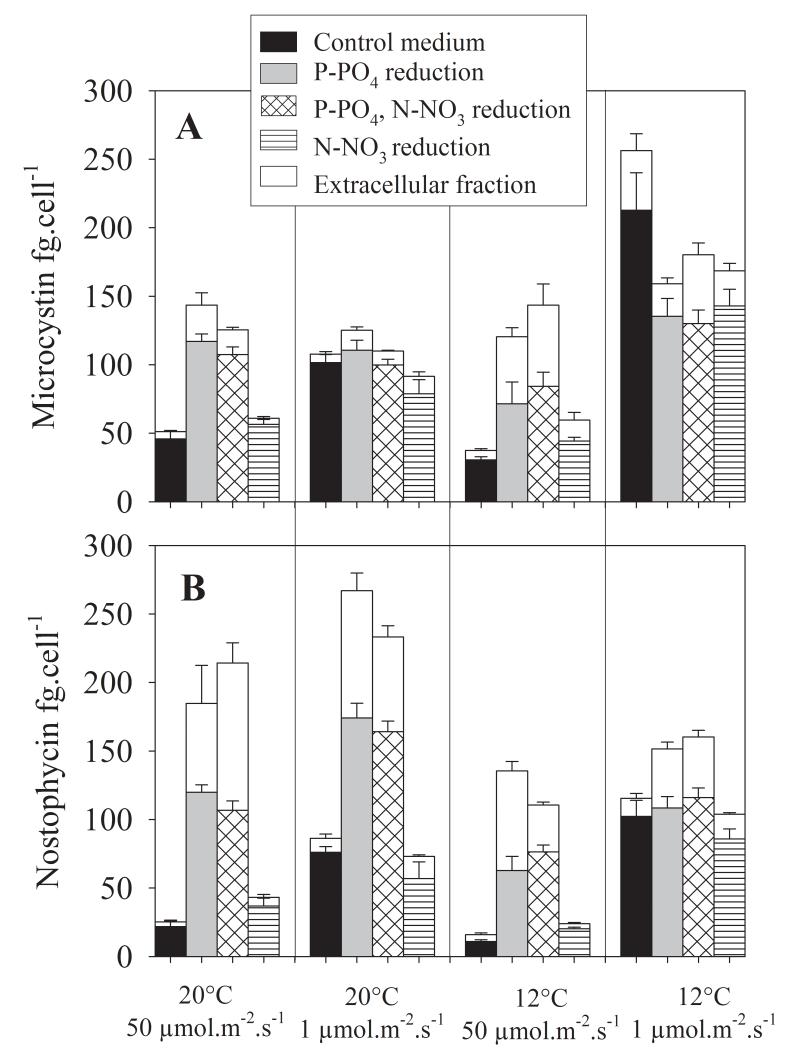
In general the net production of MC and NP continued under all experimental conditions. Taking all data together no difference between the average total content including both intra- and extracellular MC (119 ± 7 fg · cell−1) and NP (120 ± 8 fg · cell−1) was detected (Paired t-test, p = 0.87, df = 94). In contrast the percentage of the average amount of extracellular MC (19 ± 1.5 %) was lower when compared with the average amount of extracellular NP (29 ± 1.5 %), (Paired t-test, p < 0.001, df = 94). Vice versa the average intracellular MC content (98 ± 5 fg · cell−1) was significantly higher than the average intracellular NP content (83 ± 5 fg · cell−1), (Paired t-test, p < 0.001, df = 95, Suppl. Table 2).
When compared with optimal growth at 50 μmol · m−2 · s−1 (20°C) in the full O2 medium the total (intra- and extracellular) MC and NP contents varied at maximum 5-fold and 10-fold, each. According to multiple regression analysis the variables P-PO4, irradiance and temperature reduction had the strongest influence on the variation as observed among the intra-/extracellular MC and NP contents (Table 2). In contrast to its effect on growth, the factor N-NO3 reduction was never included as a predictor variable by the multiple regression analysis. The majority of the regression coefficients were negative implying significant negative relationships between predictor variables and the respective dependent variable (see Suppl. Table 1 for Pearson product-moment correlation coefficients between log10-transformed variables).
The total MC cellular contents were 2.2-5.0-fold increased under irradiance reduced conditions, while P-PO4 reduction led to 2.2-3.6-fold increase in the cellular MC content (Fig. 2). Accordingly for both intra- and extracellular MC contents the factors irradiance, P-PO4 and temperature reduction were found to best predict the variation that was recorded. Irradiance, P-PO4 reduction and temperature were all negatively related to MC. The reduction in P-PO4 also had the most significant effect on total NP net production. When compared with optimal conditions (50 μmol · m−2 · s−1, 20°C, full O2 medium) the total NP cellular contents were 4.4-10.6-fold higher under conditions of P-PO4 reduction at any irradiance and temperature condition or their combination. For the total NP cellular content, and both intra- and extracellular NP contents P-PO4 reduction and to a less extent irradiance and temperature reduction were identified as predictor variables explaining the variation that was observed (Suppl. Table 2, Fig. 2). Both P-PO4 reduction and irradiance were significantly negatively related to NP while temperature showed a significant positive relationship. In summary P-PO4 reduction and reduced irradiance led to a pronounced increase in the cellular MC and NP contents.
Fig. 2.
Mean (+1SE) concentration of intra- and extracellular (A) MC and (B) NP contents (fg · cell−1) of Nostoc strain 152 under four different environmental conditions and their combinations. High irradiance (50 μmol · m−2 · s−1) and low irradiance (1 μmol · m−2 · s−1) conditions, two different temperatures (12°C, 20°C) and P-PO4 reduced and N-NO3 free conditions. For each treatment combination the extracellular fraction is shown on the top of each column (in white).
Nostophycin and microcystin net production rates
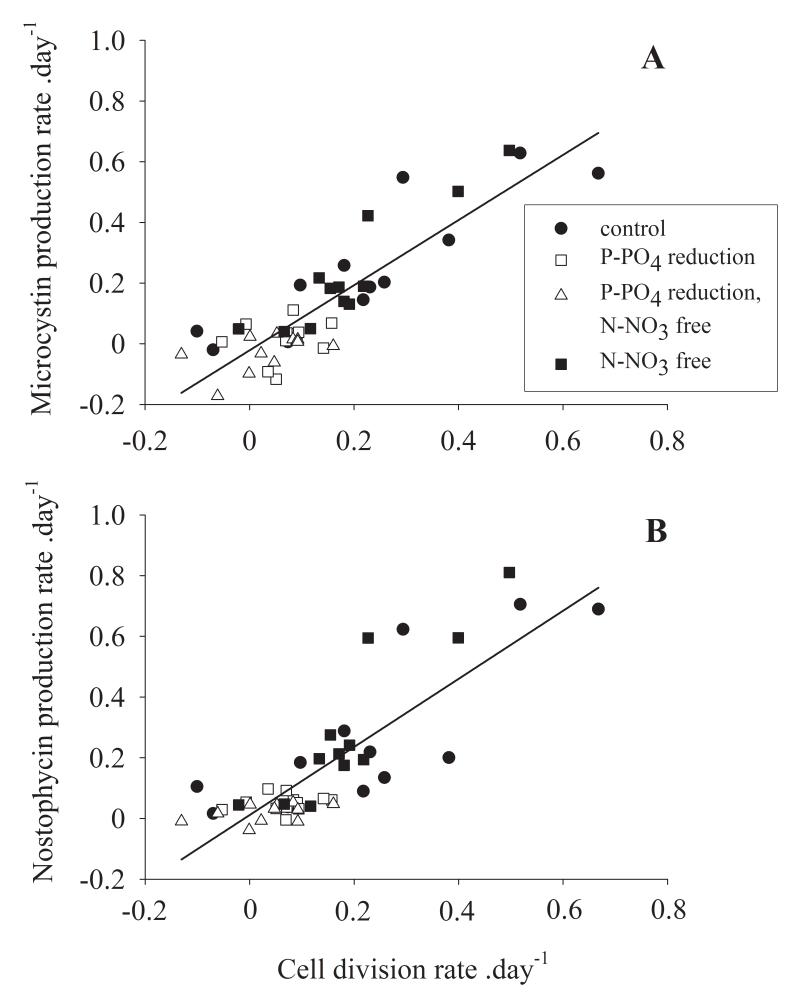
Because of the large physiological variation that was found it was of interest to find out whether cell numbers could still be used as a variable to predict MC and NP net production in water. Taking the data from all experiments together peptide net production rates were plotted against the cell production rates and analysed by using linear regression analysis. The highest MC and NP production rates were observed in the most rapidly growing cultures. For both peptides a significant linear relationship between the daily production of biovolume (cell growth) and the daily production of MC or NP was found (Fig. 3): MC, y = −0.02 + 1.07x (R2 = 0.76), NP, y = 0.01 + 1.12x (R2 = 0.7), where y, x were the log10-transformed peptide production rates (day−1) and cellular growth rates (day−1), each. Notably the slope of both regression curves was close to one and the origin of the regression curves was close to zero. In summary, for both peptides, the physiologically induced variation among cellular peptide contents did not outweigh the overall influence of the daily cellular growth rate on peptide net production.
Fig. 3.
Relationship between the cellular division rate (day−1) and (A) the net MC production rate and (B) the net NP production rate (day−1). The regression curves were fitted by a linear regression calculation and least square approximation. The P-PO4 reduced treatments are indicated by open symbols, the closed symbols indicate the full medium and N-NO3 free medium. For details on regression curves see text.
Discussion
According to this study the highest MC and NP contents per cell were observed under severe growth limiting conditions, which were mostly due to P-PO4 limitation. During the experiments Nostoc strain 152 was grown under P-PO4 and N-NO3 reduction conditions at 12°C under both irradiance levels for more than twelve months (including the time of pre-culturing). Therefore the cells were considered to be acclimatized to the experimental conditions. The concentration of 0.144 μM of P-PO4 is corresponding to the lower threshold used to define the mesotrophic state of surface water (Vollenweider and Kerekes 1982). During the experiments mostly linear growth of the cells under the various treatments has been observed (Fig. 1). This implies that the measured growth rates (Table 1) do not correspond to the exponential growth phase but rather indicate an average growth rate as observed under the various treatment conditions. Accordingly a slow growing culture can be expected to show an increase in MC and NP production if the maximum intrinsic growth rate would have been recorded, for example as realized under continuous culture conditions. On the other hand the maxima of the cellular growth rates as reported in this study correspond with the growth rates as determined for other planktonic MC-producing cyanobacteria under continuous culture conditions (Long et al. 2001, Wiedner et al. 2003, Tonk et al. 2005). The 5-fold variation on cellular MC contents (31-213 fg MC cell−1) observed during this study compares with the variations reported from continuous cultures (3-fold; Long et al. 2001; 2-fold, Wiedner et al. 2003; 5-fold, Tonk et al. 2005). For the planktonic genera Anabaena, Microcystis and Planktothrix rather similar cellular MC contents have been reported, i.e. < 1-2 mg of MC g−1 of dry weight in Anabaena (Rapala et al. 1997), 0.02 −0.53 mg of MC g−1 dry weight of Microcystis sp. (Saker et al. 2005), 1.7 ± 0.3 (max 4.5) mg of MC g−1 dry weight and 1.2 ± 0.2 (max 4.5) mg of MC g−1 dry weight for Planktothrix agardhii and P. rubescens, each (Kosol et al. 2009). In contrast, for Nostoc sp. MC contents have been reported that are lower when compared with planktonic cyanobacteria (0.139 mg of MC g−1 of dry weight, Oudra et al. 2009, 0.025 mg of MC g−1 of dry weight, Bajpai et al. 2009, 0.2 mg of MC g−1 of dry weight, Oksanen et al. 2004). In this study cellular MC and NP production levels have been found that compare with those reported for Microcystis and Planktothrix (Wiedner et al. 2003, Tonk et al. 2005). Recently Kaasalainen et al. (2009) reported 0.4 mg of MC g−1 of dry weight for Nostoc strain UK18 that has been isolated from the lichen Peltigera leucophlebia. Consequently, it is concluded that there is not a principal difference in MC content between planktonic and benthic MC-producing genera.
It is striking, however that in contrast to Microcystis and Planktothrix the cellular MC content in Nostoc strain 152 was negatively related to light availability. While for the genera Microcystis and Planktothrix a 3-4 fold higher cellular MC content at 50 μmol · m−2 · s−1 when compared with one μmol · m−2 · s−1 has been reported (Wiedner et al. 2003, Tonk et al. 2005), the cellular contents of both MC and NP of Nostoc strain 152 were highest under conditions of one μmol · m−2 · s−1. It is speculated that this principal difference might be caused by the non-planktonic origin of the strain 152. Although Nostoc strain 152 has been isolated from a water bloom (Sivonen et al. 1990) it is likely that it originated indeed from soil due to wash-out after precipitation for the following reasons: (i) When collected, the water bloom sample was dominated by Aphanizomenon flos-aquae. The mouse bioassay of the bloom sample indicated that it was nontoxic (50% lethal dose, intraperitoneally, mouse, > 1,500 mg kg−1) implying that the abundance of Nostoc sp. strain 152 in the plankton community could not be high. (ii) The same acetylated and demethylated MC structural variants (ADMAdda variants, Sivonen et al. 1992) as found in Nostoc strain 152 have been identified from a Nostoc strain originally isolated from lichens (Oksanen et al. 2004) and in Nostoc sp. occurring in cyanobacterial mats of Antarctica (Wood et al. 2008). With one exception (Planktothrix agardhii, Laub et al. 2002) the occurrence of the rare ADMAdda MC variants has only been reported from Nostoc sp. (Beattie et al. 1998, Oksanen et al. 2004). Therefore it is most likely that Nostoc strain 152 originated from a benthic habitat, as it is well known that Nostoc sp. occurs as epilithic algae on stones or as epipelic algae on sediments.
Notably, although the P-PO4 reduced and irradiance reduced conditions significantly increased the MC content per cell up to 5-fold and the NP content per cell up to 10-fold, the physiologically induced variation did not outweigh the general relationship between cell division and the MC and NP net production rates. In their seminal paper, Orr and Jones (1998) concluded that for individual strains of cyanobacteria MC net production depends primarily on the cellular growth rate, while environmental conditions influence MC production rather indirectly via the cellular growth rate. Basically the Orr and Jones hypothesis was built on the overall observation that - although MCs and other toxins such as anatoxin a are clearly secondary metabolites - environmental factors may affect their content in cyanobacteria but only within a range of less than an order of magnitude (Sivonen and Jones 1999). As the hypothesis of a non-inducible continuous production of cyanotoxins has never been disproved, the monitoring approach to use cyanobacterial biovolume as a proxy to estimate MC production in surface water has become more widely accepted (e.g. Bartram et al. 1999, Rogalus and Watzin 2008).
In summary the results show that although the synthesis of MC and NP is clearly regulated in response to low P-PO4 concentrations, and low irradiance and temperature conditions, the net production rate of both compounds is related to the cell division process, respectively population growth. This implies that the synthesis of both peptides is highly integrated into the primary metabolism. While this has been found for planktonic genera such as Anabaena, Microcystis and Planktothrix, it has never been reported for benthic cyanobacteria. From Nostoc a surprising type of physiological regulation of MC production in response to the environment has been observed. Caution is needed when the type of regulation as observed in one single strain/species is extrapolated to other strains or more distantly related MC-producing cyanobacteria.
Supplementary Material
Acknowledgements
I am grateful to Anika Stracke, Josef Knoblechner, Gertraud Roidmayr, Eva Schober for their technical assistance in the laboratory. I am most grateful to Louise Morrison (University of Dundee) for preparing the NP standard. The Nostoc strain PCC9237 was provided by Nicole Tandeau de Marsac (Institute Pasteur, Paris). Thomas Rohrlack (NIVA, Oslo) identified the MCs by means of LC-MS. I am grateful to two anonymous reviewers for their critical comments. This study was supported through the EU project PEPCY (Contract No QLRT-2001-02634) and the Austrian Science Fund (P20231).
Abbreviations
- DAPI
4′,6-Diamidino-2-phenylindol
- HPLC-DAD
high performance liquid chromatography coupled to diode array detection
- LC-MS
Liquid chromatography coupled to mass spectrometry
- MC
microcystin
- NP
nostophycin
References
- Bajpai R, Sharma NK, Lawton LA, Edwards C, Rai AK. Microcystin producing cyanobacterium Nostoc sp BHU001 from a pond in India. Toxicon. 2009;53:587–90. doi: 10.1016/j.toxicon.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Bartram J, Burch M, Falconer IR, Jones G, Kuiper-Goodman T. Situation assessment, planning and management. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. WHO, E & FN Spon; London, UK: 1999. pp. 179–234. [Google Scholar]
- Beattie KA, Kaya K, Sano T, Codd GA. Three dehydrobutyrine-containing microcystins from Nostoc. Phytochemistry. 1998;47:1289–92. doi: 10.1021/np980047m. [DOI] [PubMed] [Google Scholar]
- Becher PG, Baumann HI, Gademann K, Jüttner F. The cyanobacterial alkaloid nostocarboline: an inhibitor of acetylcholinesterase and trypsin. J. Appl. Phycol. 2009;21:103–10. [Google Scholar]
- Chorus I, Bartram J. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. WHO, E & FN Spon; London: 1999. p. 416. [Google Scholar]
- Diehnelt CW, Dugan NR, Peterman SM, Budde WL. Identification of microcystin toxins from a strain of Microcystis aeruginosa by liquid chromatography introduction into a hybrid linear ion trap-fourier transform ion cyclotron resonance mass spectrometer. Anal. Chem. 2006;78:501–12. doi: 10.1021/ac051556d. [DOI] [PubMed] [Google Scholar]
- Dittmann E, Erhard M, Kaebernick M, Scheler C, Neilan BA, von Döhren H, Börner T. Altered expression of two light-dependent genes in a microcystin-lacking mutant of Microcystis aeruginosa PCC 7806. Microbiology. 2001;147:3113–19. doi: 10.1099/00221287-147-11-3113. [DOI] [PubMed] [Google Scholar]
- Dodds WK, Gudder DA, Mollenhauer D. The ecology of Nostoc. J. Phycol. 1995;31:2–18. [Google Scholar]
- Fujii K, Sivonen K, Kashiwagi T, Hirayama K, Harada K-I. Nostophycin, a novel cyclic peptide from the toxic cyanobacterium Nostoc sp. 152. J. Org. Chem. 1999;64:5777–82. [Google Scholar]
- Genuario DB, Silva-Stenico ME, Welker M, Moraes LAB, Fiore MF. Characterization of a microcystin and detection of microcystin synthetase genes from a Brazilian isolate of Nostoc. Toxicon. 2010;55:846–54. doi: 10.1016/j.toxicon.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Golakoti T, Ogino J, Heltzel CE, Le Husebo T, Jensen C, Larsen L, Patterson G, Moore R, Mooberry SL, Corbett TH, Valeriotes FA. Structure determination, conformational analysis, chemical stability studies, and antitumor evaluation of the cryptophycins. Isolation of 18 new analogs from Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1995;117:12030–49. [Google Scholar]
- Gromov BV, Vepritskiy AA, Titova1 NN, Mamkayeva KA, Alexandrova OV. Production of the antibiotic cyanobacterin LU-1 by Nostoc linckia CALU 892 (cyanobacterium) J. Appl. Phycol. 1991;3:55–59. [Google Scholar]
- Hirata K, Nakagami H, Takashina J, Mahmud T, Kobayashi M, In Y, Ishida T, Miyamoto K. Novel violet pigment, nostocine A, an extracellular metabolite from cyanobacterium Nostoc spongiaeforme. Heterocycles. 1996;43:1513–19. [Google Scholar]
- Hudnell H. Proceedings of the interagency, international symposium on cyanobacterial harmful algal blooms (ISOC-HAB): State of the science and research needs; 2007; Berlin: Springer-Verlag; p. 924. Advances in Experimental Medicine and Biology. [Google Scholar]
- Hurtado I, Aboal M, Zafra E, Campillo D. Significance of microcystin production by benthic communities in water treatment systems of arid zone. Wat. Res. 2008;42:1245–53. doi: 10.1016/j.watres.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Izaguirre G, Jungblut AD, Neilan BA. Benthic cyanobacteria (Oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in Southern California. Wat. Res. 2007;41:492–98. doi: 10.1016/j.watres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Jüttner F, Todorova A, Walch N, Von Philipsborn W. Nostocyclamide M: a cyanobacterial cyclic peptide with allelopathic activity from Nostoc 31. Phytochemistry. 2001;57:613–19. doi: 10.1016/s0031-9422(00)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaebernick M, Neilan BA. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol. Ecol. 2001;35:1–9. doi: 10.1111/j.1574-6941.2001.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Kaasalainen U, Jokela J, Fewer DP, Sivonen K, Rikkinen J. Microcystin production in the tripartite cyanolichen Peltigera leucophlebia. Mol. Plant Microbe Interact. 2009;22:695–702. doi: 10.1094/MPMI-22-6-0695. [DOI] [PubMed] [Google Scholar]
- Kehr JC, Zilliges Y, Springer A, Disney MD, Ratner DD, Bouchier C, Seeberger PH, de Marsac NT, Dittmann E. A mannan binding lectin is involved in cell-cell attachment in a toxic strain of Microcystis aeruginosa. Mol. Microbiol. 2006;59:893–906. doi: 10.1111/j.1365-2958.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- Komarek J, Anagnostidis K. Modern approach to the classification-system to cyanophytes 4 – Nostocales. Arch. Hydrobiol./Suppl. Algol. Stud. 1989;82:247–345. [Google Scholar]
- Kosol S, Schmidt J, Kurmayer R. Variation in peptide net production and growth among strains of the toxic cyanobacterium Planktothrix spp. Eur. J. Phycol. 2009;44:49–62. [Google Scholar]
- Laub J, Henriksen P, Brittain SM, Wang J, Carmichael WW, Rinehart KL, Moestrup O. [ADMAdda5]-microcystins in Planktothrix agardhii strain PH-123 (cyanobacteria) - importance for monitoring microcystins in the environment. Environ. Toxicol. 2002;17:351–57. doi: 10.1002/tox.10042. [DOI] [PubMed] [Google Scholar]
- Leao PN, Vasconcelos MTSD, Vasconcelos VM. Allelopathy in freshwater cyanobacteria. Crit. Rev. Microbiol. 2009;35:271–82. doi: 10.3109/10408410902823705. [DOI] [PubMed] [Google Scholar]
- Long BM, Jones GJ, Orr PT. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Appl. Environ. Microbiol. 2001;67:278–83. doi: 10.1128/AEM.67.1.278-283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed ZA, El-Sharouny HM, Ali WSM. Microcystin production in benthic mats of cyanobacteria in the Nile River and irrigation canals, Egypt. Toxicon. 2006;47:584–90. doi: 10.1016/j.toxicon.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Nagatsu A, Kajitani H, Sakakibara J. Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett. 1995;36:4097–100. [Google Scholar]
- Namikoshi M, Rinehart K, Sakai R, Sivonen K, Carmichael W. Structures of three new cyclic heptapeptide hepatotoxins produced by the cyanobacterium (blue-green alga) Nostoc sp. strain 152. J. Org. Chem. 1990;55:6135–39. [Google Scholar]
- Oksanen I, Jokela J, Fewer DP, Wahlsten M, Rikkinen J, Sivonen K. Discovery of rare and highly toxic microcystins from lichen-associated cyanobacterium Nostoc sp strain IO-102-I. Appl. Environ. Microbiol. 2004;70:5756–63. doi: 10.1128/AEM.70.10.5756-5763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr PT, Jones GJ. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998;43:1604–14. [Google Scholar]
- Oudra B, Andaloussi MDE, Vasconcelos VM. Identification and quantification of microcystins from a Nostoc muscorum bloom occurring in Ouka Meden River (High-Atlas Mountains of Marrakech, Morocco) Environ. Monit. Assess. 2009;149:437–44. doi: 10.1007/s10661-008-0220-y. [DOI] [PubMed] [Google Scholar]
- Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–48. [Google Scholar]
- Rapala J, Sivonen K, Lyra C, Niemelä SI. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 1997;63:2206–12. doi: 10.1128/aem.63.6.2206-2212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalus MK, Watzin MC. Evaluation of sampling and screening techniques for tiered monitoring of toxic cyanobacteria in lakes. Harmful Algae. 2008;7:504–14. [Google Scholar]
- Saker M, Fastner J, Dittmann E, Christiansen G, Vasconselos V. Variation between strains of the cyanobacterium Microcystis aeruginosa isolated from a Portugese river. J. Appl. Microbiol. 2005;99:749–57. doi: 10.1111/j.1365-2672.2005.02687.x. [DOI] [PubMed] [Google Scholar]
- Schatz D, Keren Y, Vardi A, Sukenik A, Carmeli S, Börner T, Dittmann E, Kaplan A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007;9:965–70. doi: 10.1111/j.1462-2920.2006.01218.x. [DOI] [PubMed] [Google Scholar]
- Sivonen K, Carmichael WW, Namikoshi M, Rinehart KL, Dahlem AM, Niemela SI. Isolation and characterization of hepatotoxic microcystin homologs from the filamentous fresh-water cyanobacterium Nostoc sp strain-152. Appl. Environ. Microbiol. 1990;56:2650–57. doi: 10.1128/aem.56.9.2650-2657.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivonen K, Namikoshi M, Evans WR, Färdig M, Carmichael WW, Rinehart KL. Three new microcystins, cyclic heptapeptide hepatotoxins, from Nostoc sp. strain 152. Chem. Res. Toxicol. 1992;6:464–69. doi: 10.1021/tx00028a003. [DOI] [PubMed] [Google Scholar]
- Sivonen K, Jones G. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. WHO, E & FN Spon; London, UK: 1999. pp. 41–112. [Google Scholar]
- Tonk L, Visser P, Christiansen G, Dittmann E, Sneldfer E, Wiedner C, Mur L, Huisman J. The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005;71:5177–81. doi: 10.1128/AEM.71.9.5177-5181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Liere L, Mur LR. Light-limited cultures of the blue-green alga Oscillatoria agardhii. Mitt. Internat. Verein. Limnol. 1978;21:158–67. [Google Scholar]
- Vollenweider R, Kerekes J. Eutrophication of waters. Monitoring, assessment and control. OECD Cooperative programme on monitoring of inland waters (Eutrophication control), Environmental Directorate, OECD; Paris: 1982. p. 154. [Google Scholar]
- Wiedner C, Visser P, Fastner J, Metcalf JS, Codd GA, Mur LR. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003;69:1475–81. doi: 10.1128/AEM.69.3.1475-1481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Mountfort D, Selwood AI, Holland PT, Puddick J, Cary SC. Widespread distribution and identification of eight novel microcystins in antarctic cyanobacterial mats. Appl. Environ. Microbiol. 2008;74:7243–51. doi: 10.1128/AEM.01243-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.