Abstract
Objective
To study the clinical characteristics, treatment and prognosis of patients with solitary fibrous tumor of the pleura (SFTP) in order to improve the diagnostic accuracy and treatment of SFTP.
Methods
A retrospective analysis of clinical and imaging features, serum biochemical changes, pathological characteristics and treatment follow-up results was conducted for 40 SFTP patients from January 1998 to March 2010.
Results
A chest CT diagnosis was conducted for 63.6% of the patients; the unenhanced and contrast-enhanced CT findings were significantly different among the cohort (P<0.01). Patients lacking a uniform MRI internal signal comprised 88.9% of the group, 66.7% showed T1 isointensity, 33.3% demonstrated T1 hypointensity, 44.4% showed T2 hypointensity and 44.4% had slight hypointensity. The follow-up time was between 2 and 116.9 months, with an average of 35 months; postoperative adjuvant therapy was not administered. Currently, all the studied patients are alive, with no metastasis or recurrence.
Conclusion
Chest CT is a valuable tool for diagnosis and surgical decisions, and the efficacy of MRI examination is comparable to chest CT. The majority of cases originated in the visceral pleural; for these, surgical resection is an effective treatment, and the prognosis is generally favorable.
Key words : Solitary fibrous tumor of the pleura, clinical features, imaging, pathology, treatment
Introduction
Solitary fibrous tumor (SFT) is a rare type of soft tissue tumor (1). According to a 2006 review by Robinson, only about 800 cases have been reported in the literature since 1931 (2). The histological features of SFT were first described in detail by Wagner in 1870, but it was not until 1931 that Klemperer and Rabin officially classified it as an independent disease (3). Historically, SFT had long been considered a localized pleural mesothelioma, but in 1979, Scharifker and Kaneko showed that SFT originated in subserosal mesenchymal tissue (4); this finding has gradually been accepted by most scholars. In the past, the SFT nomenclature was rather disorganized. Terms such as localized fibroma, localized mesothelioma, solitary fibrous mesothelioma, localized fibrous mesothelioma, subcutaneous fibroma, subserosal fibroids and others have been reported in previous studies and considered to be solitary fibrous tumors (5,6). According to the 2002 publication by the WHO titled "Pathology & Genetics of Tumors of Soft tissue and Bone" (7), SFT is defined as a mesenchymal tumor that may have fibroblastic characteristics and clear peripheral vascular tumor-like branching blood vessels. Those classified as fibroblastic/myofibroblastic tumors may represent an intermediate type (i.e., occasionally metastatic) and intermediate malignancy. SFT is often misdiagnosed because the disease lacks obvious clinical features, imaging findings are often nonspecific and knowledge among medical professionals is lacking (8-11). This study retrospectively analyzed data for 40 SFTP patients admitted to our hospital with the aim of augmenting clinicians' awareness and improving the diagnostic and therapeutic accuracy for this disease.
Patients and methods
Case selection and clinical data
Inclusion criteria: clinical and pathological data integrity and a final pathological diagnosis of SFTP. Data for 40 SFT patients treated in the Department of Thoracic Surgery, People's Liberation Army General Hospital was collected from January 1998 to March 2010. Clinical features, imaging characteristics, serum biochemical changes, surgical procedures and pathology results were recorded. Imaging and pathological data were examined and confirmed by radiology or pathology physicians as well as the deputy director and other experts, and the final data were reported.
Follow-up
Follow-ups were conducted by telephone. The follow-up times ranged from 2 to 116.9 months, with a median of 65.5 months. Follow-up records included the patients' survival status and recurrence of notable preoperative physical symptoms found in the examination after surgery. Other treatment regimens administered after the operation were recorded, and the patients' recurrence status after the operation was also noted; if a relapse occurred, the post-treatment situation was studied in detail.
Statistical analysis
The analyzed data are presented as means ± standard deviations. The chi-squared test was used for rate comparisons, and average values for the two groups were compared using a t-test. P<0.05 indicates a statistically significant difference. The above analyses were conducted with the SPSS Statistics v17.0 software.
Results
Patient demographics and clinical features
Among the 40 patients, there were 17 males and 23 females, a male to female ratio of 1:1.35. The patients were aged 17-78 years, with a median age of 48 years, and 30 patients (75%) were more than 40 years old. Twenty patients in the group did not have any symptoms, but chest masses were found during an examination, and these patients subsequently received treatment. Fourteen patients showed simple chest symptoms. Two cases had simple joint symptoms. One case had both chest symptoms (cough) and joint symptoms (right knee joint swelling). One case showed chest symptoms (chest tightness) and clubbed fingers. One case showed chest symptoms (chest pain, chest tightness and cough) and early morning confusion. One case had long-term right upper-quadrant discomfort and fullness. All these patients were receiving treatment for the symptoms. Table 1 lists the related conditions of five patients with paraneoplastic syndrome. There were 17 patients (42.5%) with chest symptoms, for whom the average value of the maximum tumor diameter was 15.34 cm. The analysis showed that the presence of symptoms was correlated with the maximum tumor diameter (P<0.01).
Table 1. Clinical data of five patients with paraneoplastic syndrome.
| Number | Gender | Age | Symptoms or signs | Tumor origin | Pedunculated or not |
Size (cm3) | Malignancy | Others |
|---|---|---|---|---|---|---|---|---|
| 1 |
Female |
64 |
Chest tightness, cough, and clubbed fingers |
(Right) parietal pleura |
Wide base |
18×14×12 |
Benign |
Invasion of the lung parenchyma |
| 2 |
Male |
43 |
Can not extend (keep straight) his right elbow |
(Lower left) visceral pleura |
Yes |
17×15×10 |
Benign |
Tumor necrosis and hemorrhage |
| 3 |
Female |
30 |
Swelling and percussive pain of the right knee |
(Left) parietal pleura |
Wide base |
5.5×4×4 |
Malignant |
none |
| 4 |
Female |
59 |
Chest pain, chest tightness, cough, and early morning confusion |
(Right upper) visceral pleura |
Wide base |
20×19×10 |
Benign |
Invasion of the lung parenchyma |
| 5 | Male | 73 | Swelling of the right knee |
(Lower right) visceral pleura | Yes | 5×4.5×3.5 | Benign | none |
Imaging data
Chest X-ray was used to examine 33 cases. In 31 cases, clear pleural space-occupying signs or nodules (93.9% positive rate) were found in the posteroanterior chest film, whereas the other 2 cases showed normal chest X-ray results. Chest CT scans were used to examine 33 patients (82.5%); 11 patients (33.3%) were determined to have fibroids, according to the imaging data. Ten cases (30.3%) were diagnosed as benign (indeterminate), 5 patients (15.2%) did not receive a diagnosis and 3 cases (9.1%) had mesothelioma. There was 1 case each of thymoma, neurogenic tumor, giant lymph node hyperplasia and lung cancer. Twenty-two cases were definitively diagnosed with a CT scan (66.7%), among which 21 cases showed mild or greater enhancement (95.5%) and 11 cases showed uneven enhancement (accounting for 52.4% of the enhanced cases). Sixteen cases were reported with average unenhanced and enhanced CT values (Figure 1), as shown in Table 2 Unenhanced CT values and enhanced CT values were significantly different (P<0.01). The incidence of maximum tumor diameter less than 10 cm and greater than or equal to 10 cm in the uniformity of tumor CT density were significantly different (P<0.01).
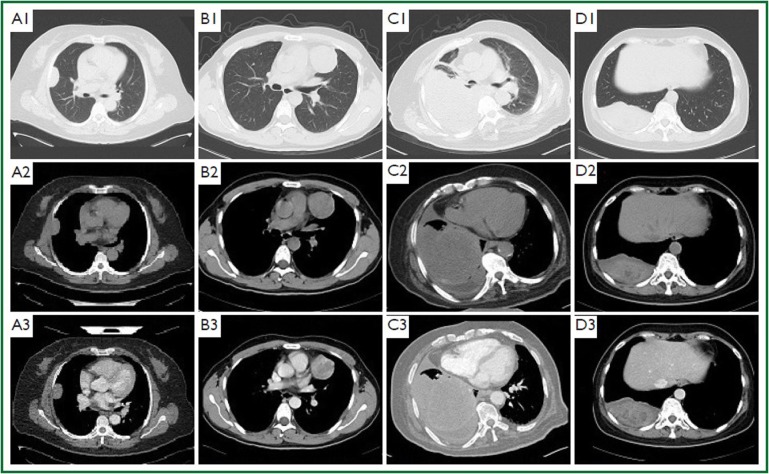
Figure 1.
Plain and contrast-enhanced chest computed tomography (CT) for four patients with SFTP. A1-A3. The patient is a middle-aged woman. An oval-shaped mass on the right lateral chest wall protrudes into the lung, with its wide base connected to the thoracic wall. The mass has smooth margin and uniform density, with a size of 5 cm × 2 cm and a CT value of about 35 HU. The mass was slightly enhanced after contrast material administration, with a CT value of 43-51 HU. B1-B3. The patient is a young men. A round-like mass is observed on the left upper mediastinum. It has a size of 4.7 cm × 5.5 cm, smooth margin, and a CT value of 31-47 HU. Inhomogeneous enhancement was observed following contrast medium administration, with a CT value of 55-90 HU. C1-C3. The patient is an old man. A large soft tissue mass is visible in the middle and lower portions of the right side of thoracic cavity. The mass has a size of about 15 cm × 9 cm, inhomogeneous density, and punctate calcification, with a mean CT value of about 25HU. Inhomogeneous enhancement was observed following contrast medium administration, with a CT value of about 40 HU. Compression atelectasis of the right lower lobe is seen. A curved line of effusion is visible in the right pleural cavity. The mediastinum slightly extends to the left, and the lymph nodes in mediastinum and behind the trachea and superior vena cava enlarge. D1-D3. The patient is a young woman. A spindle-shaped soft-tissue shadow is seen on the right posterior chest wall, with a size of about 5 cm × 10 cm, uneven density, and a CT value of about 34-50 HU. Calcified plaque is observed inside the lesions, whose margins are surrounded by low-density fluid. Fat lines exist on the mass and chest wall, showing a tight relationship with the diaphragma. The mass was slightly enhanced after administration of contrast material, with a CT value of about 36-59 HU.
Table 2. Comparison of the plain and contrast-enhanced CT findings in 16 patients.
| Mean ± Standard deviation | P | |
|---|---|---|
| Plain CT values (HU) |
37.0225 ± 6.64079 |
<0.001 |
| Cotrast-enhanced CT values (HU) | 60.2288 ± 26.68830 |
Nine patients received a chest MRI examination (both unenhanced and enhanced scans) (Figure 2). Among the unenhanced scans, 8 cases lacked internal signal uniformity (88.9%), whereas 1 case showed a uniform internal signal. The T1 phase tended to be isointense or hypointense, with 6 isointense cases (66.7%) and 3 hypointense cases (33.3%). For the T2 phase, 4 cases (44.4%) were hypointense, 4 cases (44.4%) were slightly hypointense and 1 case (11.1%) was mixed isointense-hypointense.
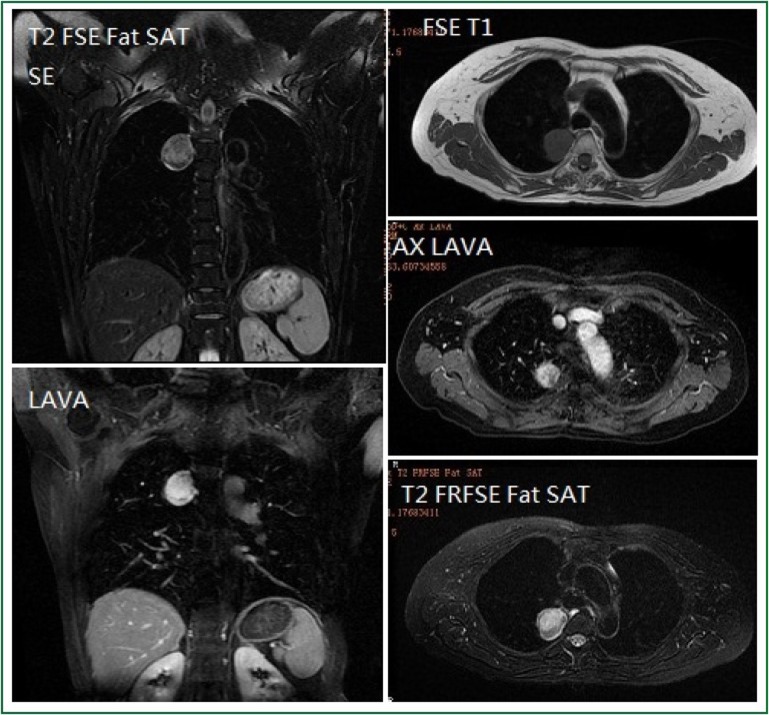
Figure 2.
Chest MRI findings of a middle-aged female patient. A round-like lesion with long T1 and T2 signals at the upper posterior portion of the right mediastinum (near the third thoracic vertebra) is observed. The lesion has a size of 2.9 cm × 3.1 cm × 3.5 cm, clearly defined margin, and inhomogeneous internal signal intensity. The lesion shows slightly high signal intensity on DWI. Dynamic enhancement imaging shows that the lesion is slightly enhanced in the arterial phase, and such enhancement becomes even more obvious in the portal venous phase and delayed phase; meanwhile, inhomogeneous internal enhancement is also observed.
Related laboratory indices
Among the 39 SFTP cases, blood sugar, potassium, sodium and blood chloride were measured in 33 patients before and after surgery. The preoperative examinations revealed 1 case of high blood sugar and 3 cases of low blood sugar. Blood glucose and serum potassium levels showed no significant difference before and after surgery (P>0.05), whereas serum sodium and serum chloride showed significant changes after surgery (P<0.05 and P<0.01, respectively).
Pathological data
Of the 40 cases in this group, 39 tissue samples underwent a routine pathological examination and immunohistochemical analysis. Twenty-seven cases originated in the visceral pleura, of which 2 were malignant (7.4%). Tumors protruding the lung surface were found in 25 cases, with 2 cases of cancer growing inside the lung and below the pleura. Eight cases originated in the parietal pleura; 4 of these cases (50.0%) were malignant, and 2 cases originated in the right mediastinal pleura. Four cases originated in the interstitial cells of the lung parenchyma, and 1 of these (25%) was malignant. The SFTP malignancy rate for cases originating in the non-visceral pleura was 41.7%, which was 5.6-fold higher than for SFTP originating in the visceral pleural. Table 3 lists the pulmonary invasion statuses for 5 patients. Table 4 lists the related statuses of 6 wide-base tumor cases. Table 5 lists the features of 8 patients with malignant SFTP. Figure 3 show the typical cytology and immunohistochemical features of SFTP.
Table 3. Clinical features of five patients with tumor invasion of the lung parenchyma.
| Case | Gender | Age | Symptoms and signs | Tumor size (cm3) | Tumor origin | Malignancy |
|---|---|---|---|---|---|---|
| 1 |
Male |
78 |
Chest tightness and cough |
30×25×20 |
Parietal pleura |
Fair |
| 2 |
Male |
32 |
None |
6×5.5×4.5 |
Visceral pleura |
Fair |
| 3 |
Female |
64 |
Chest tightness and clubbed fingers |
18×14×12 |
Parietal pleura |
Fair |
| 4 |
Male |
67 |
Cough |
4×4×3.5 |
Invasion of the lung parenchyma |
Malignancy |
| 5 | Female | 59 | Chest pain, chest tightness, cough, and hypoglycemia | 20×19×10 | Visceral pleura | Fair |
Table 4. Clinical data of six patients with tumors with a wide base.
| Case |
Gender |
Age |
Symptoms and signs |
Tumor size (cm3) |
Tumor origin |
Margin |
Malignancy |
| 1 |
Female |
33 |
Chest pain |
5×4.5×3.5 |
Parietal pleura |
Clearly defined |
Fair |
| 2 |
Female |
64 |
Chest tightness and clubbed fingers |
18×14×12 |
Parietal pleura |
Clearly defined |
Fair |
| 3 |
Female |
45 |
None |
3×3×2.5 |
Visceral pleura |
Unclear |
Fair |
| 4 |
Female |
30 |
Joint swelling |
5.5×4×4 |
Parietal pleura |
Unclear |
Malignancy |
| 5 |
Female |
59 |
Chest pain, chest tightness, cough, and hypoglycemia |
20×19×10 |
Visceral pleura |
Clearly defined |
Fair |
| 6 | Male | 48 | Cough | 25×17×9 | Parietal pleura | Unclear | Malignancy |
Table 5. Clinical and pathological features of eight patients with malignant SFTP.
| Case | Gender | Age | Signs | Tumor origin | Size (cm3) | Margin | Wide base | Others |
|---|---|---|---|---|---|---|---|---|
| 1 |
Male |
67 |
Cough |
Parenchymal of lower left lung |
4×4×3.5 |
Clearly defined |
No |
None |
| 2 |
Male |
48 |
None |
Left parietal pleura |
20×10×9 |
Clearly defined |
No |
None |
| 3 |
Female |
30 |
Joint swelling |
Left mediastinal pleura |
5.5×4×4 |
Clearly defined |
Yes |
None |
| 4 |
Male |
69 |
None |
Right visceral pleura |
9.5×7.5×5 |
Unclear |
No |
None |
| 5 |
Female |
44 |
Chest tightness and short of breath |
— |
— |
— |
— |
— |
| 6 |
Female |
60 |
Chest pain |
Lower left visceral pleura |
11×11×6 |
Unclear |
No |
Necrosis |
| 7 | Male | 48 | Cough | Left mediastinal pleura | 25×17×9 | Unclear | Yes | Mucinous change |
“Others” refers to conditions including internal hemorrhage, local necrosis, cystic change, mucinous change, internal spaces, or hyaline degeneration. E5 did not receive surgical treatment and therefore no relevant data are available.
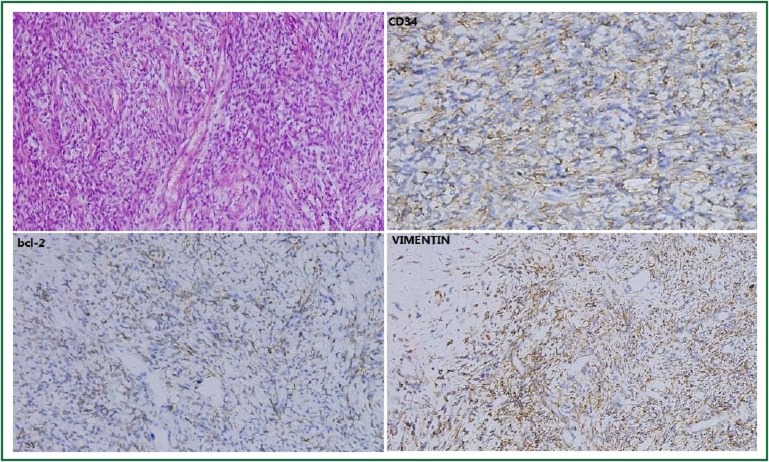
Figure 3.
The tumor cells and intercellular collagen fibers proliferated without obvious direction or structure, which corresponded to the so-called patternless. Immunohistochemical analysis revealed positivity for CD34 (++), bcl-2(+++), and VIMENTIN (++) in tumor cells.
Preoperative biopsy, surgical treatment and prognosis
Fourteen patients received a puncture biopsy (11 cases were biopsied in this hospital), and 9 cases were confirmed as SFTP. Three cases biopsied in other hospitals had not undergone immunohistochemistry analysis; thus, these diagnoses were not confirmed. The puncture biopsies conducted in this hospital showed an 81.8% positive rate, and 2 puncture biopsy cases in this hospital could not be confirmed as SFTP due to an insufficient biopsy specimen obtained. One case was diagnosed as benign fibroblastic proliferative lesion, and another case was diagnosed as spindle cell tumor. Thirty-nine patients received and completed radical surgical treatment. The surgical resection rate was 100%, with no serious complications, and these patients were discharged after 1-2 weeks. Eight patients were determined to have hypervascular tumors with vascular embolization from the preoperative imaging studies. Twenty-four patients (61.5%) received conventional open thoracotomy, 9 cases (23.1%) underwent simple thoracoscopic surgery and 6 cases (15.4%) received video-assisted thoracoscopic surgery. Twenty-nine patients (74.4%) underwent simple tumor resection, 6 patients (15.4%) underwent lung cancer resection and a partial lobectomy and 4 cases (10.3%) received a lobectomy. Among the lobectomy patients, 1 case showed an extremely large right pleural tumor (30 cm × 25 cm × 20 cm) pressing on the lower right lobe and causing compression atelectasis. During the tumor separation procedure, the lower right lobe was severely damaged, and the lower right lobe was removed during tumor resection; an upper lobe resection was performed in 1 case due to the tumor being located in the parenchyma near the hilum in the right upper lobe, with a high risk of simple partial tumor resection and lung resection. One case showed a very large tumor (20 cm × 19 cm × 10 cm) exerting a high degree of pressure, and the middle lobe of the right lung had consequent atelectasis and consolidation; the middle lobe of the right lung was therefore removed for this case. In 1 case, the middle lobe of the right lung was resected due to the middle lobe vein being located near the tumor.
Follow-up
The author conducted telephone follow-up interviews with 40 patients; 11 cases (27.5%) were lost, primarily due to changes in phone number. The specific patient situations are illustrated in Table 3. The follow-up duration was 2-116.9 months, with a median of 65.5 months. Four patients with malignancy were followed up for 116.9, 34.3, 9.5 and 1.8 months, respectively. Among the 25 patients with benign tumors, the follow-up duration ranged from 3.3 to 96.2 months, with an average of 34.1 months; 9 patients were followed up for more than 36 months. For 29 cases, no other adjuvant treatment had been administered after the surgery at the time of the final follow-up; all these patients are currently alive, with no metastasis, recurrence or relapse.
Discussion
Previous studies have reported the age of SFTP patients to be between 5-87 years (12), and a higher incidence of SFTP among elderly patients has been reported. This study retrospectively analyzed 40 SFTP patients aged from 17 to 78 years, with 75% of the cohort being 40 years of age and older. Consistent with previous reports, SFTP was mostly reported as a benign tumor. This may be related to its characteristic slow growth and development, the delayed presence of clinical symptoms and its atypical nature. The incidence of SFTP is currently thought to be comparable between men and women. In this study, there were 23 female and 17 male patients, a male to female ratio of 1:1.35. We reviewed 157 cases of pleural solitary fibrous tumor reported in the literature between 2000 and 2009, among which 71 cases were males and 86 cases females, a male to female ratio of 1:1.21. This demonstrates that the incidence of SFTP was greater in females than males. SFTP patients are often asymptomatic; most cases are discovered during a physical examination. Among the 40 cases of this group, 20 patients (50%) showed no symptoms but were found to have SFTP upon a physical examination, which is consistent with reports in the literature indicating that the percent of SFTP patients showing symptoms was between 40-50%. Seventeen cases in this group had chest symptoms. The average maximum diameter of the tumor specimens was 15.34 cm, and 12 tumors (70.6%) had maximum diameters larger than or equal to 10 cm. Twenty-four cases had an average maximum tumor diameter less than 10 cm, and chest symptoms were reported in 5 of these patients (20.8%). Eight cases had a maximum tumor diameter of 10-20 cm, and 4 of these cases (50%) showed symptoms. Of 8 cases with tumor diameters larger than or equal to 20 cm, 7 (87.5%) showed symptoms. These data indicate that tumor size and the occurrence of chest symptoms are closely related: the larger the tumor, the more prone the patient is to presenting with chest symptoms. The literature has reported that chest symptoms were often reported in patients with a maximum tumor diameter greater than 10 cm (13), whereas this study indicates that chest symptoms are more prevalent in patients with a maximum tumor diameter greater than 20 cm. Arthritis-like symptoms and clubbing can occur in SFTP patients, which is considered hypertrophic osteoarthropathy (HOA). This cohort included 3 patients with arthritis-like symptoms.
Imaging is essential for SFTP diagnosis and treatment. Benign tumors have smooth edges and clear boundaries. Interestingly, after multiple imaging studies, changes in the tumor's location can be detected; this is often related to benign SFTP connecting with pleural tissue through the pedicle. Lewis et al. reported this phenomenon in 1985 (14); this finding has some significance for SFTP diagnosis and surgery, but the specific impacts of this behavior were not well known then. Malignant SFTP mostly grows in a wide base, spread along the chest wall or with a deep invasion into lung tissue. It is generally thought that CT and MRI tests are valuable in distinguishing benign from malignant SFTP. Benign tumors show encapsulation and a clear boundary and often have pedunculated growth; in contrast, malignant tumors tend to show hemorrhaging, necrosis, organ infiltration, recurrence and metastasis, among other signs. All patients in this study underwent a routine preoperative chest X-ray examination; the positive diagnosis rate of the chest X-ray examination in this hospital was 93.9%. Tumors were not discovered in 2 cases. In 1 of these cases, the tumor was located on the right side of the chest and had a size of 3.55 cm × 8.76 cm; this tumor appeared spindle-shaped and was close to the chest wall. In another case, the tumor was located in the right lower mediastinum. This tumor was about 5 cm × 4 cm × 3 cm in size and was covered by the heart shadow. Chest X-ray examinations had a relatively high positive diagnosis rate, and this type of examination is convenient, inexpensive and suitable for routine screening and postoperative follow-up tracking. Among 33 patients who received chest CT examination, 11 (33.3%) were considered to have fibroids, and 10 cases (30.3%) were considered benign (indeterminate). The diagnostic accuracy rate of CT imaging reached 63.6%. de Perrot et al. reported that in an unenhanced scan, the tumor CT value was usually between 25 and 40 HU (13). Unenhanced and enhanced CT scans were used for 16 patients in this group. The CT values were significantly different for different tumor sizes, which is consistent with SFT histologically being a hypervascular tumor (15).
Inside the tumor, heterogeneous components may be caused by hemorrhage, necrosis and cystic masses, leading to heterogeneity in the internal density of the CT scan. An increase in heterogeneity was observed when the CT values increased, which is called the “map-like enhancement mode” (16).
CT can be used to make a preliminary judgment about whether a tumor is benign or malignant, and it is invaluable for choosing the surgical approach and estimating procedural difficulty. It was believed in the past that MRI was more valuable than CT for diagnosing SFTP, but analysis of 9 patients in this group who received either a chest MRI or CT examination showed that MRI showed a comparable efficacy to CT in diagnosing SFTP, and the cost of an MRI examination is higher than that of CT. Therefore, MRI should not replace CT for SFTP diagnosis. SFTP patients can occasionally have low blood sugar, a phenomenon that is seen more often in patients with malignant SFTP and is known as Doege-Potter syndrome. Doege-Potter syndrome is believed to be a type of non-insulin-dependent hypoglycemia, which can accompany a variety of interstitial tumors, including SFTP, as well as some epithelial and hematological tumors (1). We found 3 cases of low blood sugar that were detected before surgery. However, when statistical tests were conducted to analyze blood sugar values before and after surgery, the results showed no significant difference between the two time points. We speculated that SFTP-related decreased blood sugar maybe due to a variety of independent factors, such as the amount of IGF-II production and the consumption of sugar by the tumor, among others. Most SFTP patients show normal blood sugar levels, but SFTP patients with hypoglycemia require clinical attention. Delayed treatment may have serious and unexpected consequences and can even lead to death. Serum potassium, sodium and chloride levels showed no abnormalities before or after surgery. Preoperative and postoperative serum potassium levels were not significantly different, but preoperative and postoperative serum sodium and serum chloride levels were significantly different. No previous studies have reported that SFTP causes changes in serum electrolytes. We speculate that the patients' serum sodium and chloride levels differed before and after the operation due to postoperative aseptic inflammation, fasting and fluid intake rather than SFTP-induced pathophysiological changes.
SFTP specimens are generally spherical, spherical-like or ellipsoidal. The literature has reported tumor sizes ranging from several centimeters to tens of centimeters. Whereas benign tumors mostly have intact membranes, malignant tumors often show cystic degeneration and necrosis, which are important indicators of malignancy. Most SFTP cases originate from the visceral pleura, fewer originate from the parietal pleura and tumors rarely originate from the lung parenchyma. In this group, 8 cases were found to originate in the parietal pleura, 4 cases showed wide bases and 3 cases were malignant. Generally, it is believed that SFTP originating from the visceral pleura is mostly benign, and these tumors are usually attached to the pleura by a pedicle, with only a small number of wide bases. Those originating from the parietal pleura often have wide bases and a large volume. A substantial proportion of these latter tumors showed malignant tendency. Cardillo et al. reported that in 55 cases of SFTP, 7 were derived from non-visceral pleura, of which 3 cases were malignant (43%) (17).
In contrast, of 48 cases originating from the visceral pleura, only 4 were malignant (8%). For the patients in the present study, among 6 cases of tumors with wide bases, only 2 were malignant. Of the 7 cases of malignant SFTP, only 3 tumors had wide bases, which is inconsistent with the previously reported incidence of tumors with wide bases that were prone to malignancy. Currently, SFTP is primarily diagnosed with pathological microscopy and especially using immunohistochemistry. The immunohistochemical studies reported here did not show significant differences between the malignant and benign cases.
SFTP is generally difficult to diagnose preoperatively because its clinical features and imaging findings do not tend to show abnormalities. Chest X-ray or CT examinations indicate that when a single large oval shadow is seen in the thoracic cavity, an SFTP diagnosis should be considered (18). However, diagnostic confirmation depends on postoperative pathological and immunohistochemical examinations of the specimens (9-11). The necessity of a preoperative puncture biopsy remains controversial. Suter et al. believe that a puncture biopsy cannot obtain sufficient tissue for cytological analysis and tends to result in a high false negative rate (19). In addition, for tumors with a rich blood supply, a puncture biopsy is accompanied by a risk of bleeding. However, Weynand et al. used immunohistochemistry to definitively diagnose 5 SFTP patients before surgery (20). In the present analysis of 40 patients, 14 received a puncture biopsy (11 cases were biopsied in this hospital), and the puncture biopsy positive rate in this hospital was 81.8%.
We can conclude that puncture biopsy is a valuable tool for diagnosing SFTP; the key is to obtain a sufficient amount of biopsy tissue for successful immunohistochemical staining. Complete surgical resection is the first-line treatment choice, and it is the only demonstrated effective treatment. Most patients are cured after a complete surgical resection. Thirty-nine patients in this study received surgery, with a 100% resection rate and complete removal of the tumor. There were no serious postoperative complications, and patients were discharged in 1-2 weeks. For benign SFTP cases, extended resection is not recommended. Tumors such as those originating from the pedicle visceral pleura do not typically require further action or an entire lung lobe wedge resection. For tumors invading the parenchyma, it is recommended that after the tumor is removed, a partial or complete lung lobe resection should then be conducted. For malignant SFTP, local extension of the lung and pleura removal are necessary. For tumors originating in the parietal pleura, removal of the thoracic fascia is recommended if possible, and the surgeon can consider removing the chest wall if invasion is detected. The choice of surgical approach is primarily based on tumor size and the difficulty of removal. Standard open-chest surgery, video-assisted thoracoscopic surgery (VATS) and thoracoscopic surgery are potential treatment options (21). If complete resection of SFTP is achieved, further radiotherapy and chemotherapy are not necessary. According to the follow-up results in the present study, 28 patients who underwent complete tumor removal did not receive adjuvant radiotherapy or chemotherapy (including those patients with malignancies). The maximum follow-up duration was 9.6 years. Metastasis and recurrence have not yet occurred. Postoperative radiotherapy or chemotherapy should be administered for those patients who did not receive a complete resection. Some scholars suggest using doxorubicin and clacarbazin as chemotherapeutic agents, but the specific effects of these treatment modalities requires further study.
In conclusion, SFTP occurs more often in the elderly, and half of the patients are asymptomatic. Chest CT is a valuable tool for diagnosis and surgical decisions, and the efficacy of MRI examination is comparable to chest CT. The majority of cases originated in the visceral pleural; for these, surgical resection is an effective treatment, and the prognosis is generally favorable.
Footnotes
No potential conflict of interest.
References
- 1.Saha SP. CT signs of solitary fibrous tumors of the pleura. J Thorac Dis. 2010;2:4-5 [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006;13:264-269 [DOI] [PubMed] [Google Scholar]
- 3.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48:63-74 [DOI] [PubMed] [Google Scholar]
- 4.Scharifker D, Kaneko M. Localized fibrous "mesothelioma" of pleura (submesothelial fibroma): a clinicopathologic study of 18 cases. Cancer. 1979;43:627-635 [DOI] [PubMed] [Google Scholar]
- 5.Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997;81:116-121 [DOI] [PubMed] [Google Scholar]
- 6.Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology. 1997;31:568-576 [DOI] [PubMed] [Google Scholar]
- 7.In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Orgnization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: ARCPress;2002.
- 8.Luciano C, Francesco A, Giovanni V, Federica S, Cesare F. CT signs, patterns and differential diagnosis of solitary fibrous tumors of the pleura. J Thorac Dis. 2010;2:21-25 [PMC free article] [PubMed] [Google Scholar]
- 9.Milano MT, Singh DP, Zhang H. Thoracic malignant solitary fibrous tumors: A population-based study of survival. J Thorac Dis. 2011;3:99-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardissone F. Thoracic malignant solitary fibrous tumors: Prognostic factors and long-term survival. J Thorac Dis. 2011;3:84-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langman G. Solitary fibrous tumor: A pathological enigma and clinical dilemma. J Thorac Dis. 2011;3:86-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Ji Y, Shan F, Guo W, Ding J, Ge D. Solitary fibrous tumor of the pleura: an analysis of 13 cases. World J Surg. 2008;32:1663-1668 [DOI] [PubMed] [Google Scholar]
- 13.de Perrot M, Kurt AM, Robert JH, Borisch B, Spiliopoulos A. Clinical behavior of solitary fibrous tumors of the pleura. Ann Thorac Surg. 1999;67:1456-1459 [DOI] [PubMed] [Google Scholar]
- 14.Lewis MI, Horak DA, Yellin A, Rotter A, Belman MJ, Benfield JR. The case of the moving intrathoracic mass. Pedunculated benign localized pleural mesothelioma. Chest. 1985;88:897-898 [DOI] [PubMed] [Google Scholar]
- 15.Asai K, Suzuki K, Shimota H, Takahashi T, Asano K, Kazui T. Solitary fibrous tumor of the pleura with hemothorax at the thoracic apex. Jpn J Thorac Cardiovasc Surg. 2003;51:434-437 [DOI] [PubMed] [Google Scholar]
- 16.Cardinale L, Allasia M, Ardissone F, et al. CT features of solitary fibrous tumour of the pleura: experience in 26 patients. Radiol Med (Torino). 2006;111:640-650 [DOI] [PubMed] [Google Scholar]
- 17.Cardillo G, Facciolo F, Cavazzana AO, Capece G, Gasparri R, Martelli M. Localized (solitary) fibrous tumors of the pleura: an analysis of 55 patients. Ann Thorac Surg. 2000;70:1808-1812 [DOI] [PubMed] [Google Scholar]
- 18.Cardinale L. CT Imaging of Solitary fibrous tumour of the pleura (SFTP):Typical Patterns and Pitfalls. J Thorac Dis. 2011. Epub ahead of print In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suter M, Gebhard S, Boumghar M, Peloponisios N, Genton CY. Localized fibrous tumours of the pleura: 15 new cases and review of the literature. Eur J Cardiothorac Surg. 1998;14:453-459 [DOI] [PubMed] [Google Scholar]
- 20.Weynand B, Noël H, Goncette L, Noirhomme P, Collard P. Solitary fibrous tumor of the pleura: a report of five cases diagnosed by transthoracic cutting needle biopsy. Chest. 1997;112:1424-1428 [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Cai C, Wang D, et al. Video-assisted thoracoscopic surgery (VATS) for patients with solitary fibrous tumors of the pleura. J Thorac Oncol. 2010;5:240-243 [DOI] [PubMed] [Google Scholar]