Introduction
Bevacizumab is a monoclonal antibody that binds and inactivates all isoforms of VEGF to inhibit angiogenesis and tumor growth and proliferation. It can improve outcomes in patients with sarcoma (1). Bevacizumab has several notable adverse effects including bleeding, hypertension, heart failure, proteinuria, thromboembolism and gastrointestinal (GI) perforation, an uncommon but serious adverse effect (2-4). However, bilateral SP occurring after bevacizumab-containing chemotherapy had never been reported in the literature. Herein, we present a case of bilateral pneumothorax occurring after bevacizumab-containing chemotherapy.
Case report
A 23-year-old man had a complaint of swelling and intermittent pain in the right thigh for two months before admission to the Surgical Department of our hospital. Physical examination showed significant swelling of the right thigh. Whole body PET/CT (Figure 1) demonstrated a large heterogeneous lesion located in the right iliac foss and no evidence of lung metastasis. Right inguinal lymph node biopsy was low-grade fibrosarcoma. Bevacizumab (5 mg/kg) and DP (docetaxol 75 mg/m2, cisplatin 75 mg/m2) every 21days were prescribed as first-line chemotherapy. The right thigh swelling was better than before. The patient had sudden-onset chest pain and dyspnea 13 days after the third cycle of Bevacizumab plus DP (day 55 after initial chemotherapy). Physical examination showed decreased breath sounds of the chest. Compared with the previous chest radiograph (Figure 2), a bilateral pneumothorax was disclosed (Figure 3). The pneumothorax resolved completely after chest tube drainage. The chest tube was removed 3 days later. In the fourth chemotherapy, bevacizumab was not used and pneumothorax was not occurred. However, in the fifth chemotherapy, we add the bevacizumab again, chest radiograph showed right pneumothorax a week later after bevacizumab (Figure 4). The pneumothorax resolved completely after chest tube drainage. The chest tube was removed 7 days later and the follow-up radiograph did not show recurrence of the pneumothorax. At the same time, we never use the bevacizumab again.
Figure 1.
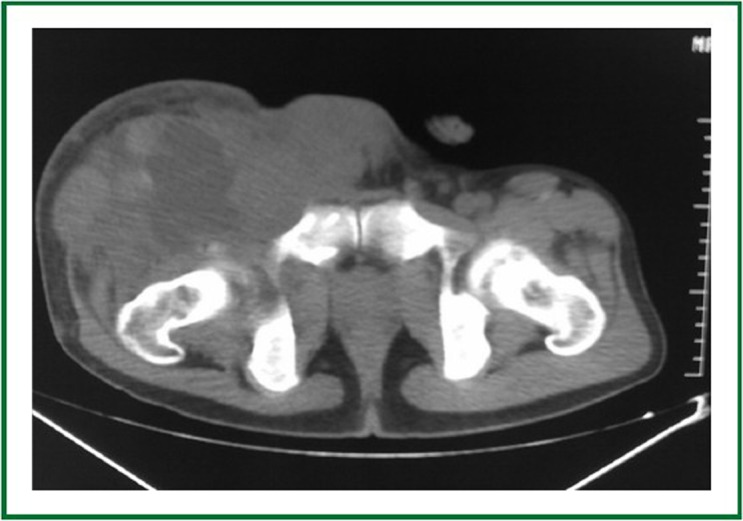
Whole body PET/CT demonstrated a large heterogeneous lesion located in the right iliac foss.
Figure 2.
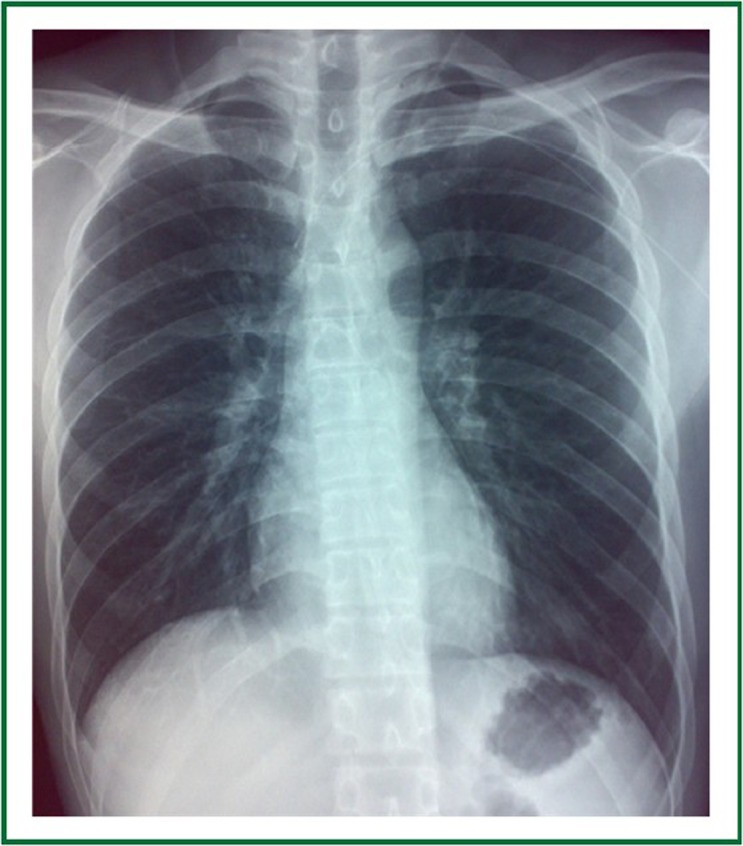
Chest radiography before treatment shows no lung metastases.
Figure 3.
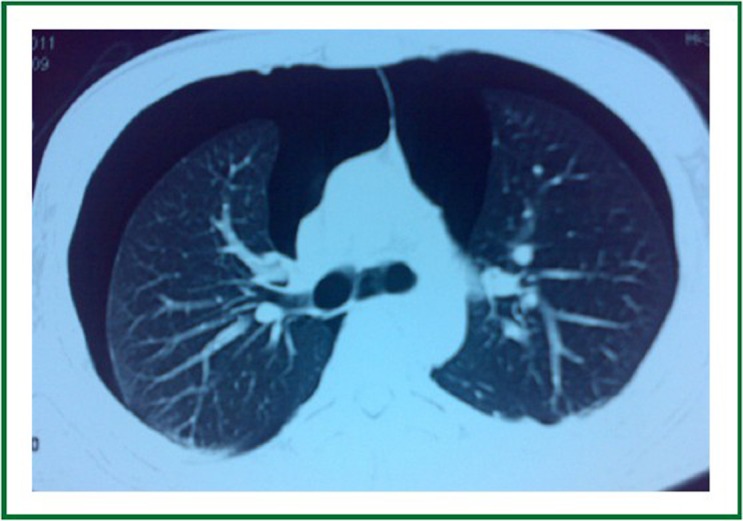
Chest CT shows bilateral pneumothorax after three cycles of bevacizumab containing chemotherapy.
Figure 4.
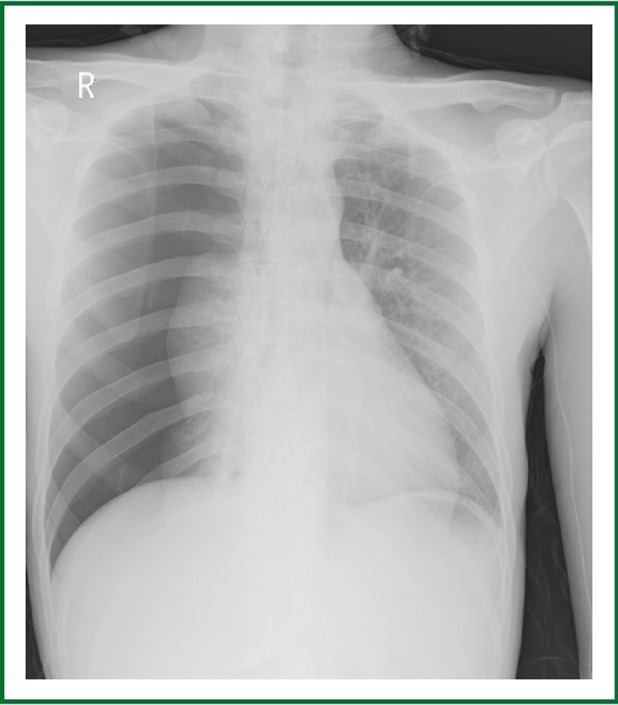
Chest radiography shows right pneumothorax after the fifth chemotherapy.
Discussion
Bevacizumab has several notable adverse effects including bleeding, hypertension, heart failure, proteinuria, thromboembolism and gastrointestinal (GI) perforation and so on, but secondary SP especial bilateral SP after bevacizumab-containing therapies is a rare. Sarcomas appear to be the malignancy most often associated with SP (5,6). There were some sarcoma patients with bilateral SP during chemotherapy where pulmonary metastases were not detectable. This suggests that SP was either a coincidence or rather a complication of very small metastases adjacent to the pleura (7,8). In this case, when we used bevacizumab, SP was occurred, and when we did not use bevacizumab, there was not SP. On this basis of the above, we speculate that the pneumothorax in our patient was likely an adverse effect of the bevacizumab.
The risk factors for bevacizumab associated pneumothorax are unclear. In this case, metastatic lesions could not be noted in the initial chest radiography and CT, we presume that micrometastases were occurred before it could be detected by imaging studies. Several theories regarding possible mechanisms of the bilateral penumothourax in sarcomas have been put forward. First, the rupture of a subpleural bleb in a patient with an underlying chronic pulmonary disease is possible. Second, tumor nodules may act as ball valves to produce a partial bronchiolar obstruction and hyperinflation of alveoli. The rupture of an emphysematous bulla in an overexpanded portion of the lung produces a pneumothorax (9).
Early detection of lung metastases is difficult, and Furrer et al. suggested that imaging studies such as chest radiography and CT are suboptimal for detecting micro-lesions and that sometimes pneumothorax could be the first and only evidence for metastases (10).
The pneumothorax after bevacizumab therapy was easily treated with a small caliber chest tube. A small caliber chest tube is considered as effective in management of an uncomplicated pneumothorax as a large caliber chest tube (11), and it can avoid a large wound and decrease the risk of bleeding from a large caliber chest tube, which is especially important due to the concerns of bleeding and delayed wound healing with bevacizumab therapy.
Footnotes
No potential conflict of interest.
References
- 1.Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22:351-355 [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842-1847 [DOI] [PubMed] [Google Scholar]
- 3.Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm. 2009;66:999-1013 [DOI] [PubMed] [Google Scholar]
- 4.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559-568 [DOI] [PubMed] [Google Scholar]
- 5.Smevik B, Klepp O. The risk of spontaneous pneumothorax in patients with osteogenic sarcoma and testicular cancer. Cancer. 1982;49:1734-1737 [DOI] [PubMed] [Google Scholar]
- 6.Biran H, Dgani R, Wasserman JP, Weissberg D, Shani A. Pneumothorax following induction chemotherapy in patients with lung metastases: a case report and literature review. Ann Oncol. 1992;3:297-300 [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Park KU, Nah DY, Won KS. Bilateral spontaneous pneumothorax during cytotoxic chemotherapy for angiosarcoma of the scalp: a case report. J Korean Med Sci. 2003;18:277-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markman M, Meek AG, Wingard JR. Spontaneous pneumothorax during adjuvant chemotherapy for soft-tissue sarcoma. Johns Hopkins Med J. 1981;148:264-265 [PubMed] [Google Scholar]
- 9.Lawton PA, Knowles S, Karp SJ, Suvana SK, Spittle MF. Bilateral pneumothorax as a presenting feature of metastatic angiosarcoma of the scalp. Br J Radiol. 1990;63:132-134 [DOI] [PubMed] [Google Scholar]
- 10.Furrer M, Althaus U, Ris HB. Spontaneous pneumothorax from radiographically occult metastatic sarcoma. Eur J Cardiothorac Surg. 1997;11:1171-1173 [DOI] [PubMed] [Google Scholar]
- 11.Conces DJ, Jr, Tarver RD, Gray WC, Pearcy EA. Treatment of pneumothoraces utilizing small caliber chest tubes. Chest. 1988;94:55-57 [DOI] [PubMed] [Google Scholar]


