Introductory Paragraph
Tomato (Solanum lycopersicum) is a major crop plant and a model system for fruit development. Solanum is one of the largest angiosperm genera1 and includes annual and perennial plants from diverse habitats. We present a high quality genome sequence of domesticated tomato, a draft sequence of its closest wild relative, S. pimpinellifolium2, and compare them to each other and to potato (S. tuberosum). The two tomato genomes show only 0.6% nucleotide divergence and signs of recent admixture, but show >8% divergence from potato, with nine large and several smaller inversions. In contrast to Arabidopsis, but similar to soybean, tomato and potato, small RNAs map predominantly to gene-rich chromosomal regions, including gene promoters. The Solanum lineage has experienced two consecutive genome triplications: one that is ancient and shared with rosids, and a more recent one. These triplications set the stage for the neofunctionalization of genes controlling fruit characteristics, such as colour and fleshiness.
Main Text
The genome of the inbred tomato cultivar ‘Heinz 1706’ was sequenced and assembled using a combination of Sanger and “next generation” technologies (Supplementary Section 1). The predicted genome size is ~900 Mb, consistent with prior estimates3, of which 760 Mb were assembled in 91 scaffolds aligned to the 12 tomato chromosomes, with most gaps restricted to pericentromeric regions (Fig. 1A; Supplementary Fig. 1). Base accuracy is approximately one substitution error per 29.4 kb and one indel error per 6.4 kb. The scaffolds were linked with two BAC-based physical maps and anchored/oriented using a high-density genetic map, introgression line mapping and BAC fluorescence in situ hybridisation (FISH).
Figure 1.

A. Multi-dimensional topography of tomato chromosome 1 (chromosomes 2-12 are shown in Supplementary Figure 1).
(a) Left: contrast-reversed, DAPI-stained pachytene chromosome; centre and right: FISH signals for repeat sequences on diagrammatic pachytene chromosomes: TGR1 purple, TGR4 blue, telomere repeat red, Cot 100 DNA (including most repeats) green. (b) Frequency distribution of recombination nodules representing crossovers on 249 chromosomes. Red stars mark 5 cM intervals starting from the end of the short arm (top). Scale is in micrometers. (c) FISH-based locations of selected BACs (horizontal blue lines on left). (d) Kazusa F2-2000 linkage map. Blue lines to the left connect linkage map markers on the (c) BAC-FISH map, (e) heat maps and (f) DNA pseudomolecule. (e) From left to right: linkage map distance (cM/Mb, turquoise); repeated sequences (% nucleotides/500 kb, purple); genes (% nucleotides/500 kb, blue); chloroplast insertions; RNA-Seq reads from leaves and breaker fruits of S. lycopersicum and S. pimpinellifolium (number of reads/500 kb, green and red, respectively); microRNA genes (transcripts per million/500 kb, black); small RNAs (thin horizontal black lines, sum of hits-normalized abundances). Horizontal grey lines represent gaps in the pseudomolecule (f). (f) DNA pseudomolecule consisting of nine scaffolds. Unsequenced gaps (approximately 9.8 Mb, Supplementary Table 13) are indicated by white horizontal lines. Tomato genes identified by map-based cloning (Supplementary Table 14) are indicated on the right. For more details, see legend to Supplementary Figure 1.
B. Syntenic relationships in the Solanaceae.
COSII-based comparative maps of potato, eggplant, pepper and Nicotiana with respect to the tomato genome (Supplementary section 4.5, Supplementary Fig. 14). Each tomato chromosome is assigned a different colour and orthologous chromosome segment(s) in other species are shown in the same colour. White dots indicate approximate centromere locations. Each black arrow indicates an inversion relative to tomato and “+1”indicates a minimum of one inversion. Each black bar beside a chromosome indicates translocation breakpoints relative to tomato. Chromosome lengths are not to scale, but segments within chromosomes are.
C. Tomato-potato syntenic relationships.
Dot plot of tomato and potato genomic sequences based on collinear blocks Supplementary Section 4.1). Red and blue dots represent gene pairs with statistically significant high and low ω (Ka/Ks) in collinear blocks, which average Ks≤0.5, respectively. Green and magenta dots represent genes in collinear blocks which average 0.5<Ks≤1.5 and Ks>1.5, respectively. Yellow dots represent all other gene pairs. Blocks circled in red are examples of pan-eudicot triplication. Inserts represent schematic drawings of BAC-FISH patterns of cytologically demonstrated chromosome inversions (also in Supplementary Fig. 15).
The genome of S. pimpinellifolium (accession LA1589) was sequenced and assembled de novo using Illumina short reads, yielding a 739 Mb draft genome (Supplementary Section 3). Estimated divergence between the wild and domesticated genomes is 0.6% (5.4M SNPs distributed along the chromosomes (Fig. 1A, Supplementary Fig. 1)). Tomato chromosomes consist of pericentric heterochromatin and distal euchromatin, with repeats concentrated within and around centromeres, in chromomeres and at telomeres (Fig. 1A, Supplementary Fig. 1). Substantially higher densities of recombination, genes and transcripts are observed in euchromatin, while chloroplast insertions (Supplementary Sections 1.22-1.23) and conserved miRNA genes (Supplementary Section 2.9) are more evenly distributed throughout the genome. The genome is highly syntenic with those of other economically important Solanaceae (Fig. 1B). Compared to the genomes of Arabidopsis4 and sorghum5, tomato has fewer high-copy, full-length LTR retrotransposons with older average insertion ages (2.8 versus 0.8 mya) and fewer high-frequency k-mers (Supplementary Section 2.10). This supports previous findings that the tomato genome is unusual among angiosperms by being largely comprised of low-copy DNA6,7.
The pipeline used to annotate the tomato and potato8 genomes is described in Supplementary Section 2. It predicted 34,727 and 35,004 protein-coding genes, respectively. Of these, 30,855 and 32,988, respectively, are supported by RNA-Seq data, and 31,741 and 32,056, respectively, show high similarity to Arabidopsis genes (Supplementary section 2.1). Chromosomal organisation of genes, transcripts, repeats and sRNAs is very similar in the two species (Supplementary Figures 2-4). The protein coding genes of tomato, potato, Arabidopsis, rice and grape were clustered into 23,208 gene groups (≥2 members), of which 8,615 are common to all five genomes, 1,727 are confined to eudicots (tomato, potato, grape and Arabidopsis), and 727 are confined to plants with fleshy fruits (tomato, potato and grape) (Supplementary Section 5.1, Supplementary Fig. 5). Relative expression of all tomato genes was determined by replicated strand-specific Illumina RNA-Seq of root, leaf, flower (2 stages) and fruit (6 stages) in addition to leaf and fruit (3 stages) of S. pimpinellifolium (Supplementary Table 1).
sRNA sequencing data supported the prediction of 96 conserved miRNA genes in tomato and 120 in potato, a number consistent with other plant species (Fig. 1A, Supplementary Figures 1 and 3, Supplementary Section 2.9). Among the 34 miRNA families identified, 10 are highly conserved in plants and similarly represented in the two species, whereas other, less conserved families are more abundant in potato. Several miRNAs, predicted to target TIR-NBS-LRR genes, appeared to be preferentially or exclusively expressed in potato (Supplementary Section 2.9).
Supplementary section 4 deals with comparative genomic studies. Sequence alignment of 71 Mb of euchromatic tomato genomic DNA to their potato8 counterparts revealed 8.7% nucleotide divergence (Supplementary Section 4.1). Intergenic and repeat-rich heterochromatic sequences showed more than 30% nucleotide divergence, consistent with the high sequence diversity in these regions among potato genotypes8. Alignment of tomato-potato orthologous regions confirmed 9 large inversions known from cytological or genetic studies and several smaller ones (Fig. 1C). The exact number of small inversions is difficult to determine due to the lack of orientation of most potato scaffolds. 18,320 clearly orthologous tomato-potato gene pairs were identified. Of these, 138 (0.75%) had significantly higher than average non-synonymous (Ka) versus synonymous (Ks) nucleotide substitution rate ratios (ω), suggesting diversifying selection, whereas 147 (0.80%) had significantly lower than average ω, suggesting purifying selection (Supplementary Table 2). The proportions of high and low ω between sorghum and maize (Zea mays) are 0.70% and 1.19%, respectively, after 11.9 Myr of divergence9, suggesting that diversifying selection may have been stronger in tomato-potato. The highest densities of low-ω genes are found in collinear blocks with average Ks >1.5, tracing to a genome triplication shared with grape (see below) (Fig. 1C, Supplementary Fig. 6, Supplementary Table 3). These genes, which have been preserved in paleo-duplicated locations for more than 100 Myr10,11 are more constrained than ‘average’ genes and are enriched for transcription factors and genes otherwise related to gene regulation (Supplementary Tables 3-4).
Sequence comparison of 32,955 annotated genes in tomato and S. pimpinellifolium revealed 6,659 identical genes and 3,730 with only synonymous changes. A total of 22,888 genes had non-synonymous changes, including gains and losses of stop codons with potential consequences for gene function (Supplementary Tables 5-7). Several pericentric regions, predicted to contain genes, are absent or polymorphic in the broader S. pimpinellifolium germplasm (Supplementary Table 8, Supplementary Fig. 7). Within cultivated germplasm, particularly among the small-fruited cherry tomatoes, several chromosomal segments are more closely related to S. pimpinellifolium than to ‘Heinz 1706’ (Supplementary Figures 8-9), supporting previous observations on recent admixture of these gene pools due to breeding12. ‘Heinz 1706’ itself has been reported to carry introgressions from S. pimpinellifolium13, traces of which are detectable on chromosomes 4, 9, 11 and 12 (Supplementary Table 9).
Comparison of the tomato and grape genomes supports the hypothesis that a whole-genome triplication affecting the rosid lineage occurred in a common eudicot ancestor11 (Fig. 2B). The distribution of Ks between corresponding gene pairs in duplicated blocks suggests that one polyploidisation in the solanaceous lineage preceded the rosid-asterid (tomato-grape) divergence (Supplementary Fig. 10).
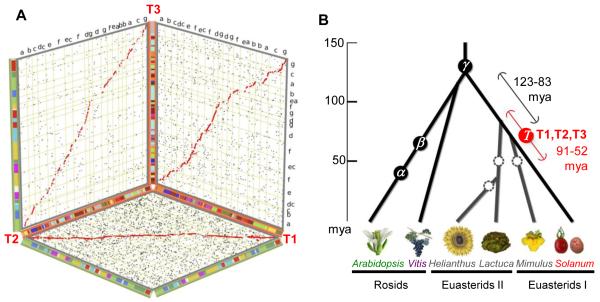
Figure 2. The Solanum whole genome triplication.
A. Based on alignments of multiple tomato genome segments to single grape genome segments, the tomato genome is partitioned into three non-overlapping ‘subgenomes’ (T1, T2, T3), each represented by one axis in the 3D plot. The ancestral gene order of each subgenome is inferred according to orthologous grape regions, with tomato chromosomal affinities shown by red-shaded (inner) bars. Segments tracing to pan-eudicot triplication (γ) are shown by green-shaded (outer) bars with colours representing the seven putative pre-γ eudicot ancestral chromosomes10, also coded a-g.
B. Speciation and polyploidisation in eudicot lineages. Confirmed whole-genome duplications and triplications are shown with annotated circles, including “T” (this paper) and previously discovered events α, β, γ10,11,14. Dashed circles represent one or more suspected polyploidies reported in previous publications that need further support from genome assemblies27,28. Grey branches indicate unpublished genomes. Black and red error bars bracket, respectively, the likely timings of divergence of major asterid lineages and of “T”. The post-“T” subgenomes, designated T1, T2, and T3, are further detailed in Supplementary Fig. 10.
Comparison to the grape genome also reveals a more recent triplication in tomato and potato. While few individual tomato/potato genes remain triplicated (Supplementary Tables 10-11), 73% of tomato gene models are in blocks that are orthologous to one grape region, collectively covering 84% of the grape gene space. Among these grape genomic regions, 22.5% have one orthologous region in tomato, 39.9% have two, and 21.6% have three, indicating that a whole genome triplication occurred in the Solanum lineage, followed by widespread gene loss. This triplication, also evident in potato (Supplementary Fig. 11) is estimated at 71 (+/-19.4) mya based on Ks of paralogous genes (Supplementary Fig. 10), and therefore predates the ~7.3 mya tomato-potato divergence. Based on alignments to single grape genome segments, the tomato genome can be partitioned into three non-overlapping ‘subgenomes’ (Fig. 2A). The number of euasterid lineages that have experienced the recent triplication remains unclear and awaits complete euasterid I and II genome sequences. Ks distributions show that euasterids I and II, and indeed the rosid-asterid lineages, all diverged from common ancestry at or near the pan-eudicot triplication (Fig. 2B), suggesting that this event may have contributed to formation of major eudicot lineages in a short period of several million years14, partially explaining the explosive radiation of angiosperm plants on earth15.
Supplementary section 5 reports on the analysis of specific gene families. Fleshy fruits (Supplementary Fig. 12) are an important means of attracting vertebrate frugivores for seed dispersal16. Combined orthology and synteny analyses suggest that both genome triplications added new gene family members that mediate important fruit-specific functions (Fig. 3). These include transcription factors and enzymes necessary for ethylene biosynthesis (RIN, CNR, ACS) and perception (LeETR3/NR, LeETR4)17, red light photoreceptors influencing fruit quality (PHYB1/PHYB2) and ethylene- and light-regulated genes mediating lycopene biosynthesis (PSY1/PSY2). Several cytochrome P450 subfamilies associated with toxic alkaloid biosynthesis show contraction or complete loss in tomato and the extant genes show negligible expression in ripe fruits (Supplementary Section 5.4).
Figure 3. Whole genome triplications set the stage for fruit-specific gene neofunctionalisation.
The genes shown represent a fruit ripening control network regulated by transcription factors (MADS-RIN, CNR) necessary for production of the ripening hormone ethylene, the production of which is regulated by ACC synthase (ACS). Ethylene interacts with ethylene receptors (ETRs) to drive expression changes in output genes, including phytoene synthase (PSY), the rate-limiting step in carotenoid biosynthesis. Light, acting through phytochromes, controls fruit pigmentation through an ethylene-independent pathway. Paralogous gene pairs with different physiological roles (MADS1/RIN, PHYB1/PHYB2, ACS2/ACS6, ETR3/ETR4, PSY1/PSY2), were generated during the eudicot (γ, black circle) or the more recent, Solanum (T, red circle) triplications. Complete dendrograms of the respective protein families are shown in Supplementary Figures 16 and 17.
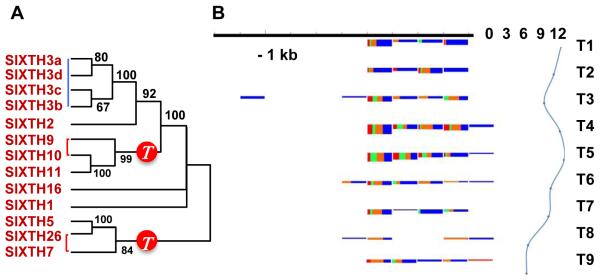
Fruit texture has profound agronomic and sensory importance and is controlled in part by cell wall structure and composition18. More than 50 genes showing differential expression during fruit development and ripening encode proteins involved in modification of wall architecture (Fig. 4A and Supplementary Section 5.7). For example, a family of xyloglucan endotransglucosylase-/hydrolases (XTHs) has expanded both in the recent whole genome triplication and through tandem duplication. One of the triplicated members, SlXTH10, shows differential loss between tomato and potato (Fig. 4A, Supplementary Table 12), suggesting genetically driven specialisation in the remodelling of fruit cell walls.
Figure 4. The tomato genome allows systems approaches to fruit biology.
A. Xyloglucan transglucosylase-hydrolases (XTHs) differentially expressed between mature green and ripe fruits (Supplementary Section 5.7). These XTH genes and many others are expressed in ripening fruits and are linked with the Solanum triplication, marked with a red circle on the phylogenetic tree. Red lines on the tree denote paralogs derived from the Solanum triplication, and blue lines are tandem duplications.
B. Developmentally regulated accumulation of sRNAs mapping to the promoter region of a fruit-regulated cell wall gene (Pectin acetylesterase, Solyc08g005800). Variation of abundance of sRNAs (left) and mRNA expression levels from the corresponding gene (right) over a tomato fruit developmental series (T1 – bud, T2 – flower, T3 – fruit 1- 3mm, T4 – fruit 5-7mm, T5 – fruit 11-13mm, T6 – fruit mature green, T7 – breaker, T8 – breaker+3days, T9 – breaker+7days). The promoter regions are grouped in 100nt windows. For each window the size class distribution of sRNAs is shown (21 – red, 22 – green, 23 – orange, 24 – blue). The height of the box corresponding to the first time point shows the cumulative sRNA abundance in log scale. The height of the following boxes is proportional to the log offset fold change (offset = 20) relative to the first time point. The expression profile of the mRNA is shown in log2 scale.
Similar to soybean and potato and in contrast to Arabidopsis, tomato sRNAs map preferentially to euchromatin (Supplementary Fig. 2). sRNAs from tomato flowers and fruits19 map to 8,416 gene promoters. Differential expression of sRNAs during fruit development is apparent for 2,687 promoters, including those of cell wall-related genes (Fig. 4B) and occurs preferentially at key developmental transitions (e.g. flower to fruit, fruit growth to fruit ripening, Supplementary Section 2.8).
The genome sequences of tomato, S. pimpinellifolium and potato provide a starting point for comparing gene family evolution and sub-functionalization in the Solanaceae. A striking example is the SELF PRUNING (SP) gene family, which includes the homolog of Arabidopsis FT, encoding the mobile flowering hormone florigen20 and its antagonist SP, encoding the ortholog of TFL1. Nearly a century ago, a spontaneous mutation in SP spawned the “determinate” varieties that now dominate the tomato mechanical harvesting industry21. The genome sequence has revealed that the SP family has expanded in the Solanum lineage compared to Arabidopsis, driven by the Solanum triplication and tandem duplication (Supplementary Fig. 13). In potato, SP3D and SP6A control flowering and tuberisation, respectively22, whereas SP3D in tomato, known as SINGLE FLOWER TRUSS, similarly controls flowering, but also drives heterosis for fruit yield in an epistatic relationship with SP23,24,25. Interestingly, SP6A in S. lycopersicum is inactivated by a premature stop codon, but remains functionally intact in S. pimpinellifolium. Thus, allelic variation in a subset of SP family genes has played a major role in the generation of both shared and species-specific variation in Solanaceous agricultural traits.
The genome sequences of tomato and S. pimpinellifolium also provide a basis for understanding the bottlenecks that have narrowed tomato genetic diversity: the domestication of S. pimpinellifolium in the Americas, the export of a small number of accessions to Europe in the 16th Century, and the intensive breeding that followed. Charles Rick pioneered the use of trait introgression from wild tomato relatives to increase genetic diversity of cultivated tomatoes26. Introgression lines exist for seven wild tomato species, including S. pimpinellifolium, in the background of cultivated tomato. The genome sequences presented here and the availability of millions of SNPs will allow breeders to revisit this rich trait reservoir and identify domestication genes, providing biological knowledge and empowering biodiversity-based breeding.
Methods Summary
A total of 21 Gb of Roche/454 Titanium shotgun and matepair reads and 3.3 Gb of Sanger paired-end reads, including ~200,000 BAC and fosmid end sequence pairs, were generated from the ‘Heinz 1706’ inbred line (Supplementary Sections 1.1-1.7), assembled using both Newbler and CABOG and integrated into a single assembly (Supplementary Sections 1.17-1.18). The scaffolds were anchored using two BAC-based physical maps, one high density genetic map, overgo hybridization and genome-wide BAC FISH (Supplementary Sections 1.8-1.16 and 1.19). Over 99.9% of BAC/fosmid end pairs mapped consistently on the assembly and over 98% of EST sequences could be aligned to the assembly (Supplementary Section 1.20). Chloroplast genome insertions in the nuclear genome were validated using a matepair method and the flanking regions were identified (Supplementary Sections 1.22-1.24). Annotation was carried out using a pipeline based on EuGene that integrates de novo gene prediction, RNA-Seq alignment and rich function annotation (Supplementary Section 2). To facilitate interspecies comparison, the potato genome was re-annotated using the same pipeline. LTR retrotransposons were detected de novo with the LTR-STRUC program and dated by the sequence divergence between left and right solo LTR (Supplementary Section 2.10). The genome of S. pimpinellifolium was sequenced to 40x depth using Illumina paired end reads and assembled using ABySS (Supplementary Section 3). The tomato and potato genomes were aligned using LASTZ (Supplementary Section 4.1). Identification of triplicated regions was done using BLASTP, in-house generated scripts and three way comparisons between tomato, potato and S. pimpinellifolium using MCscan (Supplementary Sections 4.2-4.4). Specific gene families/groups (genes for ascorbate, carotenoid and jasmonate biosynthesis, cytochrome P450s, genes controlling cell wall architecture, hormonal and transcriptional regulators, resistance genes) were subjected to expert curation/analysis, (Supplementary Section 5). PHYML and MEGA were used to reconstruct phylogenetic trees and MCSCAN was used to infer gene collinearity (Supplementary Section 5.2).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by: Argentina: INTA and CONICET. Belgium: Flemish Institute for Biotechnology and Ghent University. China: The State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences; Ministry of Science and Technology (2006AA10A116, 2004CB720405, 2006CB101907, 2007DFB30080) Ministry of Agriculture (“948” Program: 2007-Z5); National Natural Science Foundation (36171319); Postdoctoral Science Foundation (20070420446); European Union: FP6 Integrated Project EU-SOL PL 016214; France: Institute National de la Recherche Agronomique and Agence Nationale de la Recherche; Germany: the Max Planck Society; India: Department of Biotechnology, Government of India; Indian Council of Agricultural Research; Italy: Ministry of Research (FIRB-SOL, FIRB-Parallelomics, ItaLyco and GenoPOM projects); Ministry of Agriculture (Agronanotech and Biomassval projects); FILAS foundation; ENEA; CNR-ENEA project L. 191/2009; Japan: Kazusa DNA Research Institute Foundation and National Institute of Vegetable and Tea Science; Korea: KRIBB Basic Research Fund and Crop Functional Genomics Research Center (CFGC), MEST; Netherlands: Centre for BioSystems Genomics, Netherlands Organization for Scientific Research; Spain: Fundación Genoma España; Cajamar; FEPEX; Fundación Séneca; ICIA; IFAPA; Fundación Manrique de Lara; Instituto Nacional de Bioinformatica; UK: BBSRC grant BB/C509731/1; DEFRA; SEERAD; USA: NSF (DBI-0116076; DBI-0421634; DBI-0606595; IOS-0923312; DBI-0820612; DBI-0605659; DEB-0316614; DBI 0849896 and MCB 1021718); USDA (2007-02773 and 2007-35300-19739); USDA-ARS.
We acknowledge the Potato Genome Sequencing Consortium for sharing data prior to publication; potato RNA-Seq data provided by C.R. Buell from the NSF funded Potato Genome Sequence and Annotation project; tomato RNA-Seq data by the USDA-funded SolCAP project, N. Sinha and J. Maloof (UC Davis); the Amplicon Express team (Pullman, WA, USA) for BAC pooling services; construction of the Whole Genome Profiling™ (WGP) physical map was supported by EnzaZaden, RijkZwaan, Vilmorin & Cie, and Takii & Co. Keygene N.V. owns patents and patent applications covering its AFLP® and Whole Genome Profiling technologies; AFLP and Keygene are registered trademarks of Keygene N.V.
The following individuals are also acknowledged for their contribution to the work described: Jongsun Park, Biao Wang, Chengfeng Niu, Di Liu, Francesco Cojutti, Silvia Pescarolo, Alessandro Zambon, Gong Xiao, Jianjun Chen, Jinfeng Shi, Lei Zhang, Liping Zeng, Mario Caccamo, Dan Bolser, David Martin, Mireia Gonzalez, Patricia A. Bedinger, Paul A. Covey, Purnima Pachori, Renato Rodriguez Pousada, Sana Hakim, Sarah Sims, Vincent Cahais, Wenbo Long, Xincheng Zhou, Yiqi Lu, Waleed Haso, Cathy Lai, Stephanie Lepp, Cass Peluso, Homa Teramu, Hannah De Jong, Raphael Lizarralde, Eliel Ruiz May, and Zi Li. Marc Zabeau is thanked for his support and encouragement and Sandra van den Brink for her secretarial support. We dedicate this work to the late Professor Charles Rick (U. C. Davis) who pioneered tomato genetics, collection of wild germplasm and the distribution of seed and knowledge.
LIST OF TOMATO GENOME CONSORTIUM AUTHORS
Kazusa DNA Research Institute: Shusei Sato (Principal Investigator)1, Satoshi Tabata (Principal Investigator)1, Hideki Hirakawa1, Erika Asamizu1, Kenta Shirasawa1, Sachiko Isobe1, Takakazu Kaneko1, Yasukazu Nakamura1, Daisuke Shibata1, Koh Aoki1; 454 Life Sciences, a Roche company: Michael Egholm2, James Knight2; Amplicon Express Inc.: Robert Bogden3; Beijing Academy of Agriculture and Forestry Sciences: Changbao Li413; BGI-Shenzhen: Yang Shuang5, Xun Xu5, Shengkai Pan5, Shifeng Cheng5, Xin Liu5, Yuanyuan Ren5, Jun Wang5; BMR-Genomics SrL: Alessandro Albiero6, Francesca Dal Pero6, Sara Todesco6; Boyce Thompson Institute for Plant Research: Joyce Van Eck7, Robert M. Buels7, Aureliano Bombarely7, Joseph R. Gosselin7, Minyun Huang7, Jonathan A. Leto7, Naama Menda7, Susan Strickler7, Linyong Mao7, Shan Gao7, Isaak Y. Tecle7, Thomas York7, Yi Zheng7, Julia T. Vrebalov7, JeMin Lee7, Silin Zhong7, Lukas A. Mueller (Principal Investigator)7; Centre for BioSystems Genomics: Willem J. Stiekema8; Centro Nacional de Análisis Genómico (CNAG): Paolo Ribeca9, Tyler Alioto910; China Agricultural University: Wencai Yang11; Chinese Academy of Agricultural Sciences: Sanwen Huang (Principal Investigator)12, Yongchen Du (Principal Investigator)12, Zhonghua Zhang12, Jianchang Gao12, Yanmei Guo12, Xiaoxuan Wang12, Ying Li12, Jun He12; Chinese Academy of Agricultural Sciences: Chuanyou Li (Principal Investigator)13, Zhukuan Cheng (Principal Investigator)13, Jianru Zuo (Principal Investigator)13, Jianfeng Ren13, Jiuhai Zhao13, Liuhua Yan13, Hongling Jiang13, Bao Wang13, Hongshuang Li13, Zhenjun Li13, Fuyou Fu13, Bingtang Chen13, Bin Han (Principal Investigator)14, Qi Feng14, Danlin Fan14, Ying Wang (Principal Investigator)15, Hongqing Ling (Principal Investigator)16, Yongbiao Xue (Principal Investigator)17; Cold Spring Harbor Laboratory and United States Department of Agriculture - Agricultural Research Service: Doreen Ware (Principal Investigator)18, W. Richard McCombie (Principal Investigator)18, Zachary B. Lippman (Principal Investigator)18, Jer-Ming Chia18, Ke Jiang18, Shiran Pasternak18, Laura Gelley18, Melissa Kramer18; Colorado State University: Lorinda K. Anderson19, Song-Bin Chang20, Suzanne M. Royer19, Lindsay A. Shearer19, Stephen M. Stack (Principal Investigator)19; Cornell University: Jocelyn K. C. Rose21, Yimin Xu21, Nancy Eannetta21, Antonio J. Matas21, Ryan McQuinn21, Steven D. Tanksley (Principal Investigator)21; Genome Bioinformatics Laboratory GRIB -- IMIM/UPF/CRG: Francisco Camara22, Roderic Guigó22, Stephane Rombauts23, Jeffrey Fawcett23, Yves Van de Peer (Principal Investigator)23; Hebrew University of Jerusalem: Dani Zamir24; Heilongjiang Academy of Agricultural Sciences: Chunbo Liang25; Helmholtz Center for Health and Environment: Manuel Spannagl26, Heidrun Gundlach26, Remy Bruggmann26, Klaus Mayer (Principal Investigator)26; Henan Agricultural University: Zhiqi Jia27; Huazhong Agricultural University: Junhong Zhang28, Zhibiao Ye28; Imperial College London: Gerard J Bishop (Principal Investigator)29, Sarah Butcher (Principal Investigator)29, Rosa Lopez-Cobollo29, Daniel Buchan29, Ioannis Filippis29, James Abbott29; Indian Agricultural Research Institute: Rekha Dixit30, Manju Singh30, Archana Singh30, Jitendra Kumar Pal30, Awadhesh Pandit30, Pradeep Kumar Singh30, Ajay Kumar Mahato30, Vivek Dogra30, Kishor Gaikwad30, Tilak Raj Sharma30, Trilochan Mohapatra30, Nagendra Kumar Singh (Principal Investigator)30; INRA Avignon: Mathilde Causse31; INRA Bordeaux: Christophe Rothan32; INRA Toulouse: Thomas Schiex (Principal Investigator)33, Céline Noirot33, Arnaud Bellec34, Christophe Klopp35, Corinne Delalande36, Hélène Berges34, Jérôme Mariette35, Pierre Frasse36, Sonia Vautrin34; Institut National Polytechnique de Toulouse: Mohamed Zouine36, Alain Latché36, Christine Rousseau36, Farid Regad36, Jean-Claude Pech36, Murielle Philippot36, Mondher Bouzayen (Principal Investigator)36; Instituto de Biología Molecular y Celular de Plantas (CSIC-UPV): Pierre Pericard37, Sonia Osorio37, Asunción Fernandez del Carmen37, Antonio Monforte37, Antonio Granell (Principal Investigator)37; Instituto de Hortofruticultura Subtropical y Mediterránea (IHSM-UMA-CSIC): Rafael Fernandez-Muñoz38; Instituto Nacional de Tecnología Agropecuaría (IB-INTA) and Consejo Nacionalde Investigaciones Científicas y Técnicas (CONICET): Mariana Conte39, Gabriel Lichtenstein39, Fernando Carrari (Principal Investigator)39; Italian National Res Council, Institute for Biomedical Technologies: Gianluca De Bellis (Principal Investigator)40, Fabio Fuligni40, Clelia Peano40; Italian National Res Council, Institute of Plant Genetics, Research Division Portici: Silvana Grandillo41, Pasquale Termolino41; Italian National Agency for New technologies, Energy and Sustainable Development: Marco Pietrella4243, Elio Fantini42, Giulia Falcone42, Alessia Fiore42, Giovanni Giuliano (Principal Investigator)42, Loredana Lopez44, Paolo Facella44, Gaetano Perrotta44, Loretta Daddiego44; James Hutton Institute: Glenn Bryan (Principal Investigator)45; Joint IRB-BSC program on Computational Biology: Modesto Orozco46, Xavier Pastor46, David Torrents4647; Keygene N.V.: Marco G. M. van Schriek48, Richard M.C. Feron48, Jan van Oeveren48, Peter de Heer48, Lorena daPonte48, Saskia Jacobs-Oomen48, Mike Cariaso48, Marcel Prins48, Michiel J.T. van Eijk (Principal Investigator)48, Antoine Janssen48, Mark J.J. van Haaren48; Korea Research Institute of Bioscience and Biotechnology: Sung-Hwan Jo49, Jungeun Kim49, Suk-Yoon Kwon49, Sangmi Kim49, Dal-Hoe Koo49, Sanghyeob Lee49, Cheol-Goo Hur49; Life Technologies: Christopher Clouser50, Alain Rico51; Max Planck Institute for Plant Breeding Research: Asis Hallab52, Christiane Gebhardt52, Kathrin Klee52, Anika Jöcker52, Jens Warfsmann52, Ulrike Göbel52; Meiji University: Shingo Kawamura53, Kentaro Yano53; Montana State University: Jamie D. Sherman54; NARO Institute of Vegetable and Tea Science: Hiroyuki Fukuoka (Principal Investigator)55, Satomi Negoro55; National Institute of Plant Genome Research: Sarita Bhutty56, Parul Chowdhury56, Debasis Chattopadhyay (Principal Investigator)56; Plant Research International: Erwin Datema57, Sandra Smit57, Elio G.W.M. Schijlen57, Jose van de Belt57, Jan C. van Haarst57, Sander A. Peters57, Marjo J. van Staveren57, Marleen H.C. Henkens57, Paul J.W. Mooyman57, Thamara Hesselink57, Roeland C.H.J. van Ham (Principal Investigator)4857; Qingdao Agricultural University: Guoyong Jiang58; Roche Applied Science: Marcus Droege59; Seoul National University: Doil Choi (Principal Investigator)60, Byung-Cheol Kang60, Byung Dong Kim60, Minkyu Park60, Seungill Kim60, Seon-In Yeom60, Yong-Hwan Lee61, Yang-Do Choi62; Shandong Academy of Agricultural Sciences: Guangcun Li63, Jianwei Gao64; Sichuan University: Yongsheng Liu65, Shengxiong Huang65; Sistemas Genomicos: Victoria Fernandez-Pedrosa66, Carmen Collado66, Sheila Zuñiga66; South China Agricultural University: Guoping Wang67; Syngenta Biotechnology: Rebecca Cade68, Robert A. Dietrich68; The Genome Analysis Centre: Jane Rogers (Principal Investigator)69; The Natural History Museum: Sandra Knapp70; United States Department of Agriculture - Agricultural Research Service, Robert W. Holley Center and Boyce Thompson Institute for Plant Research: Zhangjun Fei (Principal Investigator)771, Ruth A. White771, Theodore W. Thannhauser71, James J. Giovannoni (Principal Investigator)72171; Universidad de Malaga-Consejo Superior de Investigaciones Cientificas: Miguel Angel Botella72, Louise Gilbert72; Universitat Pompeu Fabra: Ramon Gonzalez73; University of Arizona: Jose Luis Goicoechea74, Yeisoo Yu74, David Kudrna74, Kristi Collura74, Marina Wissotski74, Rod Wing (Principal Investigator)74; University of Bonn: Heiko Schoof (Principal Investigator)75; University of Delaware:Blake C. Meyers (Principal Investigator)76, Aishwarya Bala Gurazada76, Pamela J. Green76; University of Delhi South Campus: Saloni Mathur77, Shailendra Vyas77, Amolkumar U. Solanke77, Rahul Kumar77, Vikrant Gupta77, Arun K. Sharma77, Paramjit Khurana77, Jitendra P. Khurana (Principal Investigator)77, Akhilesh K. Tyagi (Principal Investigator)77; University of East Anglia, School of Biological Sciences: Tamas Dalmay (Principal Investigator)78; University of East Anglia, School of Computing Sciences: Irina Mohorianu79; University of Florida: Brandon Walts80, Srikar Chamala80, W. Brad Barbazuk80; University of Georgia: Jingping Li81, Hui Guo81, Tae-Ho Lee81, Yupeng Wang81, Dong Zhang81, Andrew H. Paterson (Principal Investigator)81, Xiyin Wang (Principal Investigator)8182, Haibao Tang8183; University of Naples “Federico II”: Amalia Barone84, Maria Luisa Chiusano84, Maria Raffaella Ercolano84, Nunzio D’Agostino84, Miriam Di Filippo84, Alessandra Traini84, Walter Sanseverino84, Luigi Frusciante (Principal Investigator)84; University of Nottingham: Graham B. Seymour (Principal Investigator)85; University of Oklahoma: Mounir Elharam86, Ying Fu86, Axin Hua86, Steven Kenton86, Jennifer Lewis86, Shaoping Lin86, Fares Najar86, Hongshing Lai86, Baifang Qin86, Chunmei Qu86, Ruihua Shi86, Douglas White86, James White86, Yanbo Xing86, Keqin Yang86, Jing Yi86, Ziyun Yao86, Liping Zhou86, Bruce A. Roe (Principal Investigator)86; University of Padua: Alessandro Vezzi87, Michela D’Angelo87, Rosanna Zimbello87, Riccardo Schiavon87, Elisa Caniato87, Chiara Rigobello87, Davide Campagna87, Nicola Vitulo87, Giorgio Valle (Principal Investigator)87; University of Tennessee Health Science Center: David R Nelson88; University of Udine: Emanuele De Paoli89; Wageningen University: Dora Szinay9091, Hans H. de Jong (Principal Investigator)90, Yuling Bai91, Richard G.F. Visser91, René M. Klein Lankhorst (Principal Investigator)92; Wellcome Trust Sanger Institute: Helen Beasley93, Karen McLaren93, Christine Nicholson93, Claire Riddle93; Ylichron SrL: Giulio Gianese94
Footnotes
AUTHOR CONTRIBUTIONS
AAL, ABA, ABE, ABG, ABO, AFI, AGR, AHA, AHU, AJA, AJM, AJO, AKM, AKS, AKT, AMO, APA, ARI, ASI, ATR, AUS, AVE, BAR, BAW, BCK, BCM, BDK, BFQ, BMW, BTC, CBL, CCL, CCO, CDE, CGE, CGH, CHR, CKL, CLI, CLR, CMQ, CNI, CNO, CPE, CRI, CYL, DCA, DCH, DDW, DHK, DIC, DKU, DLF, DOZ, DRN, DSH, DSZ, DTO, DWB, DZA, EAS, ECA, EDA, EDP, EFA, EGS, FCA, FDP, FEA, FFU, FRE, FYF, FYU, FZN, GBS, GDB, GEB, GFA, GGI, GHE, GIG, GJB, GLI, GPE, GUL, GVA, GWA, HBE, HBT, HDJ, HEB, HFU, HGU, HHI, HLA, HLJ, HSC, HSL, IFI, IMO, IYT, JAL, JCA, JDS, JDW, JEK, JFA, JFR, JGA, JHE, JHZ, JJG, JKN, JKP, JKR, JLE, JLG, JLI, JLL, JMA, JMC, JPK, JRG, JRO, JTV, JUW, JVB, JVE, JVH, JVO, JWA, JYI, JZH, KAO, KCO, KGA, KJI, KKL, KMA, KML, KQW, KSH, KYA, LAM, LAS, LDA, LDP, LGE, LHY, LKA, LLO, LMA, LOG, LPX, MAB, MAF, MAV, MBO, MCC, MCO, MDA, MDB, MDF, MDR, MEG, MEL, MHM, MHU, MKP, MLC, MOR, MPH, MPI, MRE, MSI, MSP, MVS, MWI, MZO, NDA, NEA, NKS, NME, NVI, PCH, PDH, PFA, PFR, PJM, PKH, PKS, PPE, PRI, PTE, QFE, RAD, RAW, RBO, RBR, RCR, RDI, RFE, RGO, RGU, RHS, RKU, RLC, RMB, RMC, RMF, RMK, RSC, RVH, RWI, RZI, SAP, SBC, SBH, SCH, SDT, SGA, SGR, SHH, SHJ, SHL, SHU, SIK, SIS, SIY, SJO, SKA, SKE, SKP, SMA, SMK, SMR, SMS, SNE, SOS, SPA, SPL, SSA, SSM, SST, STO, SUR, SVA, SVY, SXH, SYK, SZH, SZU, TAL, TDA, THE, THL, TKA, TMO, TRS, TSC, TWT, TYO, UGO, VDO, VGU, VYF, WBB, WRM, WSA, WYA, XLI, XPA, XWA, XXU, XXW, YBA, YBX, YDU, YGU, YHL, YLI, YNA, YPW, YRE, YSH, YXU, YYU, YZH, ZBL, ZFE, ZJI, ZJL, ZYA, ZYE, ZZH were involved in data generation and/or analysis. AAL, AGR, AHP, AKT, AVE, BAR, CCO, CYL, DCH, DIC, DRN, DSZ, DWA, DZA, ECA, EDA, EDP, EGS, FEA, GBS, GEB, GHE, GIG, GJB, GVA, HDJ, HHI, HSC, IFI, JFR, JJG, JKR, JLE, JLG, JMC, JPK, JTV, JVE, KJI, KMA, LAM, LKA, MAB, MBO, MCO, MKP, MLC, MPI, MRE, MSP, MVE, MZO, NKS, RAW, RMK, RVH, RWI, SAP, SDT, SGR, SIK, SIY, SKN, SMR, SMS, SPA, SSA, SSM, TDA, TMO, TRS, TSC, WBB, YVP, YXU, ZBL, ZFE wrote the manuscript. AGR, AHU, AJA, AKS, AKT, ALA, AVE, BAR, BCM, BHA, CGH, CRO, CYL, DCH, DIC, DTO, DWA, DZA, FEA, FZN, GBS, GDB, GEB, GIG, GJB, GVA, GYJ, HDJ, HFU, HQL, HSC, JCP, JDW, JJG, JPK, JRO, JRZ, JVE, JWG, KMA, LAM, LFR, MAB, MBO, MCA, MPR, MSP, MVE, MVH, MVS, PJG, PKH, RAD, RGU, RGV, RHS, RMK, RVH, RWI, SAB, SDT, SHU, SMR, SMS, SPL, SSA, STA, TDA, TSC, VYF, WJS, WRM, XPA, YBX, YDC, YDU, YOX, YSL, YVP, YWA, ZBL, ZKC designed experiments, supervised data generation/analysis and managed subprojects/tasks.
The authors declare no competing financial interests.
REFERENCES
- 1.Frodin DG. History and concepts of big plant genera. Taxon. 2004;53:753–776. [Google Scholar]
- 2.Peralta IE, Knapp S, Spooner DM. Taxonomy of tomatoes: A revision of wild tomatoes (Solanum section Lycopersicon) and their outgroup relatives in sections Juglandifolia and Lycopersicoides. Systematic Botany Monographs. 2008;84:1–186. [Google Scholar]
- 3.Michaelson MJ, Price HJ, Ellison JR, Johnston JS. Comparison of plant DNA contents determined by Feulgen microspectrophotometry and laser flow cytometry. American journal of botany. 1991:183–188. [Google Scholar]
- 4.The_Arabidopsis_Genome_Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 5.Paterson AH, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 6.Zamir D, Tanksley SD. Tomato genome is comprised largely of fast- evolving, low copy-number sequences. Molecular and General Genetics MGG. 1988;213:254–261. [Google Scholar]
- 7.Peterson DG, Pearson WR, Stack SM. Characterization of the tomato (Lycopersicon esculentum) genome using in vitro and in situ DNA reassociation. Genome. 1998;41:346–356. [Google Scholar]
- 8.Xu X, et al. Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- 9.Swigonova Z, et al. Close split of sorghum and maize genome progenitors. Genome Res. 2004;14:1916–1923. doi: 10.1101/gr.2332504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaillon O, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- 11.Tang H, et al. Synteny and collinearity in plant genomes. Science. 2008;320:486–488. doi: 10.1126/science.1153917. [DOI] [PubMed] [Google Scholar]
- 12.Ranc N, Munos S, Santoni S, Causse M. A clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (solanaceae) BMC Plant Biol. 2008;8:130. doi: 10.1186/1471-2229-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozminkowski R. Pedigree of variety Heinz 1706. Rep Tomato Genet Coop. 2004;54:26. [Google Scholar]
- 14.Moore MJ, Soltis PS, Bell CD, Burleigh JG, Soltis DE. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc Natl Acad Sci U S A. 2010;107:4623–4628. doi: 10.1073/pnas.0907801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockey RA, Graham SW, Crane PR. Introduction to the Darwin special issue: The abominable mystery. Am J Bot. 2009;96:3–4. doi: 10.3732/ajb.0800402. [DOI] [PubMed] [Google Scholar]
- 16.Howe HF, Smallwood J. Ecology of seed dispersal. Annual review of ecology and systematics. 1982:201–228. [Google Scholar]
- 17.Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 18.Vicente AR, Saladie M, Rose JKC, Labavitch JM. The linkage between cell wall metabolism and fruit softening: looking to the future. Journal of the Science of Food and Agriculture. 2007;87:1435–1448. [Google Scholar]
- 19.Mohorianu I, et al. Profiling of short RNAs during fleshy fruit development reveals stage-specific sRNAome expression patterns. Plant J. 2011;67:232–246. doi: 10.1111/j.1365-313X.2011.04586.x. [DOI] [PubMed] [Google Scholar]
- 20.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 21.Rick CM. The tomato. Scientific American. 1978;239:67–76. [Google Scholar]
- 22.Navarro C, et al. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature. 2011;478:119–122. doi: 10.1038/nature10431. [DOI] [PubMed] [Google Scholar]
- 23.Lifschitz E, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci U S A. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger U, Lippman ZB, Zamir D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat Genet. 2010;42:459–463. doi: 10.1038/ng.550. [DOI] [PubMed] [Google Scholar]
- 25.Pnueli L, et al. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development. 1998;125:1979–1989. doi: 10.1242/dev.125.11.1979. [DOI] [PubMed] [Google Scholar]
- 26.Rick CM. Hybridization between Lycopersicon esculentum and Solanum pennellii: Phylogenetic and Cytogenetic Significance. Proc Natl Acad Sci U S A. 1960;46:78–82. doi: 10.1073/pnas.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker MS, et al. Multiple paleopolyploidizations during the evolution of the Compositae reveal parallel patterns of duplicate gene retention after millions of years. Mol Biol Evol. 2008;25:2445–2455. doi: 10.1093/molbev/msn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aagaard JE, Willis JH, Phillips PC. Relaxed selection among duplicate floral regulatory genes in Lamiales. J Mol Evol. 2006;63:493–503. doi: 10.1007/s00239-005-0306-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.