Abstract
Cytidine-5’-diphosphocholine (citicoline) has a variety of cognitive enhancing, neuroprotective, and neuroregenerative properties. In cocaine-addicted individuals, citicoline has been shown to increase brain dopamine levels and reduce cravings. The effects of this compound on appetite, food cravings, and brain responses to food are unknown. We compared the effects of treatment with citicoline (500 mg/day versus 2000 mg/day) for six weeks on changes in appetite ratings, weight, and cortico-limbic responses to images of high calorie foods using functional magnetic resonance imaging (fMRI). After six weeks, there was no significant change in weight status, although significant declines in appetite ratings were observed for the 2000 mg/day group. The higher dose group also showed significant increases in functional brain responses to food stimuli within the amygdala, insula, and lateral orbitofrontal cortex. Increased activation in these regions correlated with declines in appetite ratings. These preliminary findings suggest a potential usefulness of citicoline in modulating appetite, but further research is warranted.
Keywords: Citocoline, Cytidine-5’-diphosphocholine, FMRI, Neuroimaging, Food, Appetite, Orbitofrontal Cortex, Insula, Amygdala
The epidemic of obesity is one of the most pressing health concerns of the 21st century (Mokdad, et al., 1999). The factors that lead to poor appetite control, excessive weight gain, and obesity are multifaceted, but neuroscience research is making headway into clarifying the neuro-cognitive systems involved in regulating appetite and food intake. Hormones such as insulin, leptin, and ghrelin on the homeostatic functions of the hypothalamus have long been known to mediate appetitive responses, but recent evidence suggests that these hormones may also have direct effects on dopamine neurons, which in turn may have a more immediate and direct effect on the motivation to eat and the reward value of food (Figlewicz & Benoit, 2008; Palmiter, 2007). Because of its primary involvement in reward and motivation, the dopamine system is implicated in a variety of substance abuse/addictive behavior disorders such as cocaine addiction and pathological gambling (Dalley, et al., 2007; Goodman, 2008). The involvement of the dopamine system in appetite and eating behavior suggests that dopaminergic-reward models of craving and substance-dependence may potentially apply to the regulation of food intake (Avena, Bocarsly, Rada, Kim, & Hoebel, 2008; Avena, Rada, & Hoebel, 2008; Thanos, Michaelides, Piyis, Wang, & Volkow, 2008).
In cocaine addicted individuals, preliminary evidence suggests that it may be possible to reduce drug cravings through the administration of cytidine-5’-diphosphocholine (citicoline) (Renshaw, Daniels, Lundahl, Rogers, & Lukas, 1999). Furthermore, one recent randomized, placebo-controlled study found that cocaine dependent outpatients treated with citicoline were less likely to screen positive for cocaine by the end of the trial (Brown, Gorman, & Hynan, 2007). Citicoline, which is marketed as a nutritional supplement and widely available in health food stores, is essentially a complex organic molecule that has been shown to have a variety of cognitive enhancing, neuroprotective, and neuroregenerative properties (Ozay, et al., 2007; Parisi, et al., 2008; Secades & Lorenzo, 2006), although findings are far from conclusive (Cohen, et al., 2003; Fioravanti & Buckley, 2006). Some evidence suggests that citicoline may affect the dopamine system (Secades & Lorenzo, 2006), thereby altering the reward value of stimuli. As a nucleotide molecule, citicoline is involved in cellular metabolism and biosynthesis of phospholipids (Conant & Schauss, 2004). When taken orally, exogenous citicoline undergoes hydrolysis in the small intestine, where it is absorbed as choline and cytidine (Weiss, 1995). Because it is water-soluble, citicoline is rapidly hydrolyzed and absorbed into the blood stream, demonstrating over 90-percent bioavailability (Conant & Schauss, 2004; D'Orlando & Sandage, 1995). Once absorbed, choline and cytidine are circulated throughout the body and become available to a variety of biosynthetic systems, and readily cross the blood-brain barrier where they are synthesized once again into citicoline (Rao, Hatcher, & Dempsey, 1999). It has been suggested that exogenous administration of citicoline can help preserve endogenous choline reserves and minimize cell membrane phospholipid catabolism, a process that may occur when the demand for acetylcholine exceeds available stores of endogenous choline (D'Orlando & Sandage, 1995; Weiss, 1995). Citicoline is believed exert a variety of effects on the central nervous system via synthesis of acetylcholine and phosphatidylcholine (D'Orlando & Sandage, 1995), restoration of membrane phospholipid components such as cardiolipin (Rao, Hatcher, & Dempsey, 2001) and sphingomyelin (Adibhatla & Hatcher, 2002), and enhancement of neurotransmitters such as norepinephrine and dopamine (Agut, Coviella, & Wurtman, 1984; Agut, Ortiz, & Wurtman, 2000; Lopez, Coviella, Agut, & Wurtman, 1986; Petkov, Stancheva, Tocuschieva, & Petkov, 1990). A number of studies have suggested that citicoline administration has several effects on the dopamine system, including increasing the levels of dopamine in neural tissues (Agut, et al., 2000; Rejdak, Toczolowski, Solski, Duma, & Grieb, 2002), increasing dopamine receptor densities (Gimenez, Raich, & Aguilar, 1991), and neuroprotection of dopamine neurons (Radad, Gille, Xiaojing, Durany, & Rausch, 2007). The effects of citicoline on the dopamine-reward system in conjunction with preliminary evidence of its effectiveness at reducing cravings in cocaine users (Renshaw, et al., 1999) raises the possibility that citicoline may also have the potential to affect appetite and food cravings.
Citicoline has been studied extensively in recent years for a variety of cognitive enhancing and neuroprotective functions, but there have been no investigations into the potential effects of this compound on appetite and cerebral responses to food. Therefore, we conducted a preliminary evaluation of two different doses of citicoline (500 mg/day vs 2000 mg/day) administered daily for six weeks on changes in appetite and cortico-limbic responses to images of high calorie foods during functional magnetic resonance imaging (fMRI). It was hypothesized that the higher of the two doses of citicoline would be associated with greater declines in appetite ratings and increased activation within brain regions that are involved in inhibitory control, satiation, and withdrawal responses relative to the lower dose, and that these changes in brain activation would predict appetite changes. We focused our analyses on three regions based on previous research suggesting that they are particularly important in appetite. First, we focused on the lateral orbitofrontal cortex, a region that functions as part of the gustatory cortex and is often activated in studies using appetizing food images (Schienle, Schafer, Hermann, & Vaitl, 2008; Simmons, Martin, & Barsalou, 2005). Furthermore, the lateral orbitofrontal cortex is involved in behavioral control and has been shown to be more active when an individual feels sated (Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001) and when stimuli are perceived as less rewarding (Kringelbach & Rolls, 2004). Therefore, this region was selected for specific study. Second, we focused on the insular cortex, as evidence suggests that it is involved in visceral bodily sensations such as those that occur during disgust responses (Stark, et al., 2007; Wright, He, Shapira, Goodman, & Liu, 2004) and interoceptive awareness of somatic states (Craig, 2002), and is commonly activated in studies showing photographs of appetizing foods (Porubska, Veit, Preissl, Fritsche, & Birbaumer, 2006; Siep, et al., 2008; Simmons, et al., 2005). Finally, because the amygdala is often involved in responses to food stimuli and appetite (Killgore, et al., 2003; LaBar, et al., 2001), particularly in obese individuals (Stoeckel, et al., 2008), we also hypothesized that this region would be affected by administration of citicoline.
Methods
Participants
Sixteen healthy adults (8 men; 8 women; 12 right-handed by self-report) ranging from 40 to 57 years (M = 47.3, SD = 5.4) were recruited from the community of Belmont, MA. At intake, the Body Mass Index (BMI) of participants ranged from 20.1 to 38.6 (M = 25.3, SD = 5.2). Volunteers were screened for a wide range of potential medical, psychiatric, and health concerns and only those participants that were deemed to be in good medical and psychiatric health were included. Participants had normal or corrected-normal vision (with contact lenses). The present study was conducted under the guidelines of the McLean Hospital Institutional Review Board. All participants provided written informed consent and were given a small financial compensation for their participation.
Study Design
Participants completed two interview/functional imaging scanning sessions separated by six weeks. At the first visit, participants completed a medical and psychiatric interview and several questionnaires about food and lifestyle preferences, and were asked to rate their typical appetite on a 10-point Likert scale from 1 (never hungry) to 10 (always hungry). Following the interview and questionnaires, participants underwent an fMRI scan to examine responses to images of high calorie foods. Participants were scanned at approximately the same time of day to minimize circadian influences. No attempts were made to restrict food intake prior to the scans and participants were allowed to follow their normal diets. In an open label design, participants were randomly assigned to one of two conditions, a Low Dose or a High Dose administration of citicoline (Cognizin™, Kyowa Hakko Kogyo Co., Ltd, Japan). Eight participants (4 male, 4 female) were assigned to consume the Low Dose (i.e., one 500 mg capsule/day) of citicoline over the intervening six week period, while the other eight participants were assigned to consume the High Dose (i.e., four 500 mg capsules/day) during the same time period. Participants were contacted by telephone twice per week to improve compliance and to allow for reporting of any adverse effects. Participants returned to the neuroimaging center to repeat the questionnaires and fMRI scanning procedure after six weeks of treatment. Changes in appetite ratings and weight were calculated for each participant by subtracting scores at Visit 1 from those at Visit 2.
Imaging Methods
Functional images were acquired on a Siemens Trio whole body 3T MRI scanner equipped with a quadrature RF head coil (TR = 3 sec, TE = 30 msec, flip angle = 90 degrees). Fifty images per slice were collected over 35 to 41 coronal slices (5 mm thick, 0 skip) with a 20 cm field of view and a 64 × 64 acquisition matrix (in-plane resolution = 3.125 × 5 × 3.125 mm) using a single-shot, gradient pulse-echo sequence. To allow the scanner to reach a steady-state, three dummy images were acquired at the start of each functional scan and discarded from analysis. The participant’s head was secured using foam padding.
Stimulation Paradigms
The stimulation paradigm has been described in detail in several previous reports (Killgore, et al., 2003; Killgore & Yurgelun-Todd, 2005a, 2005b, 2007). In brief, participants were scanned while viewing a series of colorful visual images that included both high-calorie foods (e.g., cheeseburgers, hot dogs, french-fries, ice cream, cake, cookies) and control images of non-food objects with similar visual complexity, texture, and color (e.g., rocks, shrubs, bricks, trees, flowers). The stimulation paradigm was 150 seconds in duration, and comprised 5 alternating 30-second periods (i.e., control, high-calorie, control, high-calorie, control). Each alternating block consisted of ten photographs (2500 msec stimulus presentation and a 500 msec inter-stimulus interval). Stimuli were controlled by a Macintosh computer running Psyscope (Macwhinney, Cohen, & Provost, 1997) and were back-projected onto a screen placed at the rear of the scanner. Participants viewed the stimuli via a mirror mounted on the head coil. The same stimuli were presented at baseline and again following six weeks of treatment.
Image Processing and Analysis
Data were preprocessed and analyzed in SPM99 (Friston, et al., 1995). Images were motion corrected, convolved into the standard MNI space, smoothed using an isotropic Gaussian kernel (full width half maximum [FWHM] = 6 mm), and resliced to 2×2×2 mm voxels using sinc interpolation. Data analysis was completed in two stages. At the first stage, contrast images were constructed to evaluate activation specific to viewing the high-calorie food images relative to the nonfood control images. Within-subject contrast images were also created to determine regions of change between Visit 1 and Visit 2. In the second, or “random-effects” level of analysis, these change images were entered into a between groups t-test to compare the effects of Low vs. High dose citicoline. The change images were also entered into a simple linear regression model in SPM99 with appetite change scores entered as the covariate of interest. Three region of interest (ROI) masks were created using the WFU Pickatlas utility (Maldjian, Laurienti, Kraft, & Burdette, 2003) to restrict analyses to only pre-specificed areas. Based on previous research showing that food images are associated with activation of the amygala (Killgore, et al., 2003; Killgore & Yurgelun-Todd, 2005b; Siep, et al., 2008; Stoeckel, et al., 2008), insula (Siep, et al., 2008; Simmons, et al., 2005; Stoeckel, et al., 2008), and lateral orbitofrontal cortex (OFC)(Killgore & Yurgelun-Todd, 2006; Simmons, et al., 2005; Stoeckel, et al., 2008), these three regions were analyzed using the published anatomical atlas of Tzourio-Mazoyer and colleagues (Tzourio-Mazoyer, et al., 2002). Because these three ROIs were predicted a priori to be affected by citicoline and to show functional changes with appetite ratings, we used a statistical threshold of p < .05, k = 10 contiguous voxels. Exploratory whole brain analyses were undertaken at a more stringent threshold of p < .001, k = 10 for the direct contrasts between baseline and post-treatment and the correlation analyses.
Results
Appetite Ratings and Weight
Self-rated appetite declined significantly between Visit 1 (M = 6.8, SD = 1.5) and Visit 2 (M = 6.1, SD = 1.5) for the sample as a whole, t(15) = -2.83, p = .02. The mean change scores for both groups declined between visits, but the magnitude of decline only reached significance for the High Dose group (M = −0.88, SD = 0.83), t(7) = −2.97, p = .02, while the decline for the Low Dose group did not (M = −0.38, SD = 0.92), t(7) = −1.16, p = .29. Between group comparison of these changes did not reach statistical significance, however, t(14) = 1.14, p = .27. Similarly, there was no significant change in weight from Visit 1 to Visit 2 for the low (M = −6.4 lbs, SD = 11.0, t(6) = −1.55, p = .17) or high (M = −0.57 lbs, SD = 3.8, t(6) = −0.40, p = .71) dose groups and the magnitude of weight change did not differ between the two groups, t(13) = 1.55, p = .15.
Dose Group Comparisons
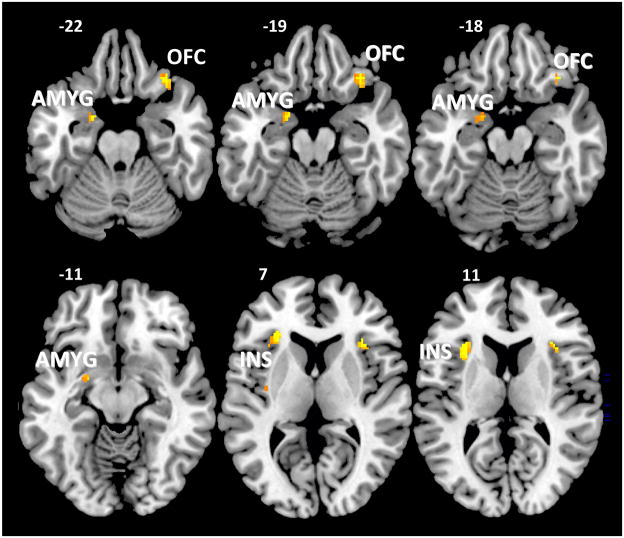
The effects of High vs. Low Dose citicoline on changes in brain activation were compared for the three ROIs. As evident in Figure 1, the High Dose group showed significantly greater between-visit increases in activation within the left amygdala (T = 2.25, 40 voxels, MNI coordinates: x = −20, y = 0, z = −22), bilateral insula (T = 3.59, 92 voxels, MNI coordinates: x = −28, y = 32, z = 6; T = 3.49, 25 voxels; MNI coordinates: x = 34, y = 22, z = 10; T = 1.99, 10 voxels, MNI coordinates: x = −36, y = −10, z = 6), and right lateral orbitofrontal cortex (T = 2.76, 41 voxels, MNI coordinates: x = 34, y = 30, z = −22) relative to the Low Dose group. In contrast, there were no ROIs where Low Dose citicoline produced greater change than High Dose citicoline. In contrast to the ROIs, exploratory whole brain comparisons revealed that only one region, located within the right cerebellum (T = 4.35, 10 voxels, MNI coordinates: x = 30, y = −56, z = −24), showed significantly greater change in activation in the High Dose group relative to the Low Dose group. In contrast, there were no regions that showed greater pre-post changes in activation in the Low Dose group relative to the High Dose group for the exploratory whole brain analysis.
Figure 1.
Axial slices showing significantly greater increases in functional activation after six weeks of treatment with High Dose citicoline (2000 mg/day) versus the Low Dose (500 mg/day) treatment during the food perception task. Activation is shown only within the three regions of interest, including the left amygdala (AMYG), bilateral insula (INS), and right lateral orbitofrontal cortex (OFC).
Correlation Between Brain Activation and Appetite Changes
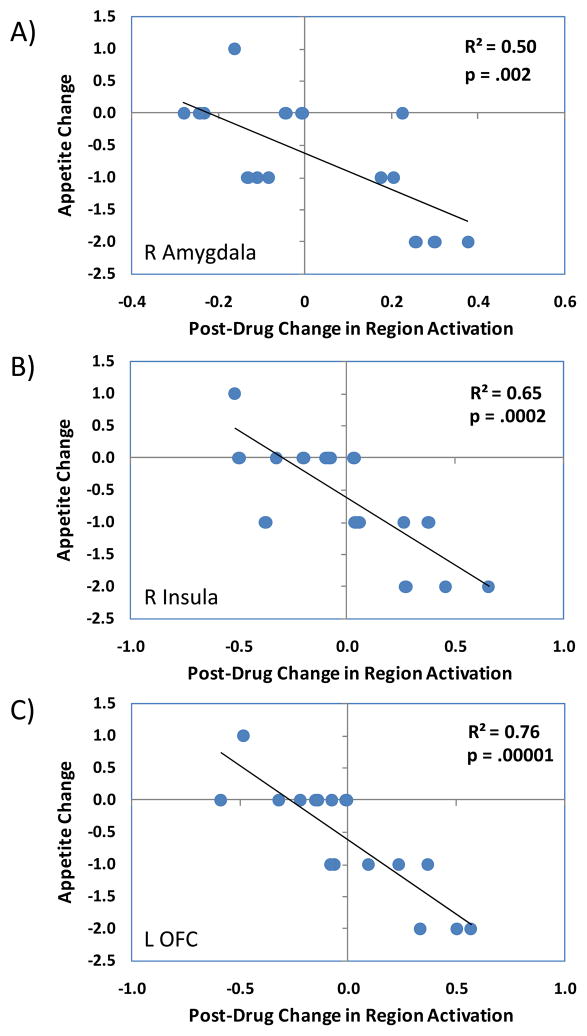
Changes in regional brain activation from Visit 1 to Visit 2 were used to predict corresponding changes in appetite ratings. As evident in Figure 2, changes in ROI activation when viewing high-calorie food images were associated with changes in appetite between the two visits. Specifically, participants that showed the greatest increase in the task-related activation of the right amygdala (T = 3.76, 146 voxels, x = 28, y = −2, z = −24), bilateral insula (T = 5.09, 865 voxels, x = 36, y = 12, z = 4; T = 4.36, 22 voxels, x = −28, y = 22, z = −20; T = 3.75, 624 voxels, x = −42, y = 10, z = −6), and left lateral orbitofrontal cortex (T = 6.63, 532 voxels, x = −36, y = 22, z = −16; T = 4.22, 549, voxels, x = 26, y = 24, z = −14) tended to show the greatest declines in appetite ratings between the two visits. The correlations were similar for the Low Dose (i.e., right amygdala r = −.63, p = .085; right insula r = −.89, p = .003; left OFC r = −.91, p = .002) and High Dose (i.e., right amygdala r = −.91, p = .002; right insula r = −.70, p = .051; left OFC r = −.88, p = .004) groups. Exploratory analysis of the correlations at the whole brain level revealed no regions showing positive correlations between changes in brain activation and changes in appetite ratings, but did show a number of negatively correlated clusters where increased brain activation between the two testing sessions was associated with decreased appetite ratings. These regions included inferior orbitofrontal cortex, thalamus, and insula, among others (see Table 1).
Figure 2.
The scatterplots show the correlations between changes in brain activation in response to the food perception task and changes in appetite ratings following six weeks of treatment with citicoline in the maximally correlated voxel for A) the right amygdala, B) right insula, and C) left lateral orbitofrontal cortex (see text for MNI coordinates).
Table 1.
Whole Brain Analysis showing Regions where Increased Brain Activation from Pre-Post Citicoline Administration was Correlated with Significant Declines in Appetite Ratings.
| Region | Number of Voxels | x | y | z | SPM {t} |
|---|---|---|---|---|---|
| R Postcentral Gyrus | 71 | 24 | −32 | 54 | 7.40 |
| L Inferior Orbitofrontal Gyrus | 54 | −36 | 22 | −16 | 6.63 |
| L Rolandic Operculum | 37 | −42 | 4 | 16 | 5.72 |
| R Superior Frontal Gyrus | 20 | 20 | −10 | 52 | 5.53 |
| L Inferior Orbitofrontal Gyrus | 19 | −44 | 30 | −4 | 5.42 |
| L Superior Temporal Gyrus | 35 | −52 | −6 | −2 | 5.39 |
| L Thalamus | 39 | −10 | −10 | 0 | 5.20 |
| L Thalamus | 73 | −16 | −22 | 12 | 5.17 |
| R Insula | 35 | 36 | 12 | 4 | 5.09 |
| R Insula | 14 | 26 | 24 | −12 | 5.02 |
| R Insula | 11 | 34 | −24 | 2 | 4.97 |
| L Middle Cingulate Gyrus | 10 | −12 | −6 | 46 | 4.79 |
| R Precentral Gyrus | 10 | 26 | −18 | 72 | 4.77 |
| L Middle Orbitofrontal Gyrus | 13 | −32 | 54 | −4 | 4.75 |
| R Thalamus | 26 | 8 | −8 | 8 | 4.69 |
| R Middle Cingulate Gyrus | 13 | 12 | −14 | 32 | 4.66 |
| R Rolandic Operculum | 34 | 62 | 6 | 6 | 4.49 |
| L Superior Medial Frontal Gyrus | 10 | −8 | 54 | 8 | 4.42 |
P < .001 (uncorrected), k = 10.
Discussion
These preliminary findings suggest that citicoline administration was associated with a modest but significant decline in appetite ratings for the group as a whole. High-Dose citicoline (i.e., 2000 mg/day) for six weeks was associated with a significant decline in appetite ratings from baseline, whereas no significant effect was observed for the Low Dose (i.e., 500 mg/day), and no changes were evident in weight status. Because the appetite effect was only significant in the High Dose group, it raises the possibility of a dose-dependent effect of citicoline on appetite suppression. Such findings are consistent with animal studies linking citicoline to increases in dopamine (Agut, et al., 2000; Rejdak, et al., 2002) and human evidence suggesting that citicoline may be effective at reducing aspects of craving in cocaine-dependent individuals (Renshaw, et al., 1999). However, given the preliminary nature of these findings and the lack of significant between-group differences in appetite suppression or weight change, further research that includes larger samples and a placebo control group will be necessary to determine the magnitude and reliability of the effects of citicoline on appetite.
Previous studies using fMRI have shown that visual perception of images of appetizing foods are generally associated with increased activation in a broad network of cortical and limbic regions, including the orbitofrontal cortex, medial prefrontal cortex, amygdala, hippocampus, ventral striatum, insula, and cingulate gyrus (Killgore, et al., 2003; Killgore & Yurgelun-Todd, 2005b; Siep, et al., 2008; Stoeckel, et al., 2008), but activation of these regions is highly dependent upon a number of factors including weight (Killgore & Yurgelun-Todd, 2005a; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008; Stoeckel, et al., 2008), mood (Killgore & Yurgelun-Todd, 2006, 2007), eating disorder diagnostic status (Santel, Baving, Krauel, Munte, & Rotte, 2006; Schienle, et al., 2008), and immediate hunger or nutritional state of the individual (Cornier, Von Kaenel, Bessesen, & Tregellas, 2007; Fuhrer, Zysset, & Stumvoll, 2008; Siep, et al., 2008). For the present study, we focused our analyses on three regions that are often associated with cerebral responses to food. These included the lateral orbitofrontal cortex, insular cortex, and amygdala.
In the present study, we found that citicoline administration was associated with dose-dependent changes in functional brain responses to high calorie foods between the two visits. Compared to the Low Dose of citicoline, the High Dose was associated with increased activation within the right lateral orbitofrontal cortex ROI during visual perception of high calorie foods. Medial aspects of the orbitofrontal cortex have been associated with reward processing (Kringelbach & Rolls, 2004) and this region tends to be activated in during perception of appetizing food stimuli (Rolls & McCabe, 2007; Schienle, et al., 2008). In contrast, activation in the lateral orbitofrontal regions has been associated with punishment experiences (Kringelbach & Rolls, 2004), satiety, and the desire to stop eating (Hinton, et al., 2004; Killgore & Yurgelun-Todd, 2006; Small, et al., 2001). When sated, images of normally appetizing foods produce increased activation of the lateral orbitofrontal cortex (Santel, et al., 2006). When considered in light of these previous studies, the present findings suggest that the High Dose treatment may have led to appetite changes by increasing the responsiveness of this region to images of calorie-rich and high-fat foods, though this speculation will require further study. High doses of citicoline were also associated with greater activation increases in bilateral insula and the left amygdala in response to the high-calorie food images. Activation of these regions has been associated with anticipation of aversive experiences and visual perception of unpleasant images in previous research (Nitschke, Sarinopoulos, Mackiewicz, Schaefer, & Davidson, 2006), and the insula has frequently been implicated in the experience and perception of disgust (Stark, et al., 2007; Wright, et al., 2004) and interoceptive awareness of visceral/somatic states (Craig, 2002). The present findings suggest that treatment with the High Dose of citicoline produced significantly greater increases in left amygdala activation than the Low Dose treatment. Previous research has suggested that visual perception of foods, regardless of calorie content, appears to be associated with amygdala activation (Killgore, et al., 2003). Elevated activation within the amygdala is often associated with negative affective experiences, such as conditioned fear (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998) or perception of unpleasant or negatively valenced emotional stimuli (Stark, et al., 2007). Again, while speculative and in need of further study, these findings tentatively suggest that citicoline may affect appetite by increasing responsiveness of these regions.
Finally, it was hypothesized that change in activation of the three cerebral regions of interest between Visit 1 to Visit 2 would correlate with appetite changes over this same period. This hypothesis was supported, as increased activation within each of the three regions was significantly predictive of reduced appetite by the end of the study. In other words, appetite ratings declined most extensively for those individuals that showed the greatest increases in activation within the amygdala, insular cortex, and lateral orbitofrontal cortex in response to high calorie food images over the six-week period. Findings for the insula and orbitofrontal cortex were further confirmed in the exploratory whole brain analysis. Because activation in these paralimbic regions is often associated with negative affect (Markowitsch, Vandekerckhovel, Lanfermann, & Russ, 2003), aversive perceptions (Stark, et al., 2007; Wright, et al., 2004), and behavioral inhibition (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004), increased activation in these regions might indicate that the food images were being perceived as less rewarding and potentially more aversive than at baseline and therefore led to reduced desire to consume food.
Although our hypothesis was based on limited evidence that citicoline may affect the dopamine system (Agut, et al., 2000; Gimenez, et al., 1991; Radad, et al., 2007; Rejdak, et al., 2002), it is possible that the changes observed here in the High Dose group may have resulted from properties of citicoline other than its effects on the dopamine system. Citicoline has a number of mechanisms of action, including functioning as a precursor of phospholipid and acetylcholine synthesis (Conant & Schauss, 2004; D'Orlando & Sandage, 1995), enhancement of the release of other neurotransmitters such as norepinephrine (Lopez, et al., 1986), counteracting the buildup of beta-amyloid protein and cellular apoptosis in the hippocampus (Alvarez, Sampedro, Lozano, & Cacabelos, 1999), and repair of neuronal membranes via increased synthesis of phospholipid components including cardiolipin (Rao, et al., 2001) and sphingomyelin (Adibhatla & Hatcher, 2002). Growing evidence suggests that citicoline may have neuroprotective effects following stroke or other brain injuries and may enhance cognitive performance in patients suffering from degenerative dementias such as Alzheimer’s and Parkinson’s Diseases (Conant & Schauss, 2004). Thus, the mechanisms of action and potential neural systems affected by citicoline are numerous and remain to be fully elucidated. Further research will be necessary to determine the specific appetite systems affected by citicoline and whether this compound shows clinical efficacy at changing appetite or weight status.
We present these findings as preliminary, fully mindful of the limitations inherent in a non-placebo controlled design with a relatively small sample size. Furthermore, because this was an open label trial and participants were aware of the treatment and dosage they received, it is possible that their expectations may have affected their responses to the questionnaires or the stimuli. Future studies would benefit from the use of double blind crossover designs and these findings will need to be replicated with larger samples. It should also be reiterated that despite the decline in appetite ratings, no change in actual weight was noted. However, it is possible that the change in appetite was gradual and that a six week trial may not have been adequate to be expressed in changes in weight. Trials extending for longer durations may clarify this issue. An additional factor to be considered is the potential influence that body mass may play in the effects of citicoline on brain responses, as previous research suggests that body mass is related to brain responses to food images (Killgore & Yurgelun-Todd, 2005a). BMI was not controlled or manipulated in the present study due to the small sample size and limited degrees of freedom, but future investigations should consider the role of this variable in food perception. To avoid the effects of hunger on brain activation, no attempts were made to restrict food intake prior to the scans, but this may have also had some effect on brain responses. Future studies will need to examine the interaction of citicoline and hunger on brain responses to food stimuli. Finally, it is not possible to rule out exposure and habituation effects in the present study, as participants viewed the same stimuli on both occasions. However, this is unlikely in light of the six-week intervening period between the scans and our finding that most participants, particularly those in the High Dose group actually showed increased activation rather than a reduction, arguing against habituation to the food stimuli. In light of the numerous neuroprotective and health promoting effects, high tolerability, and low side-effect profile of citicoline, these tentative findings are intriguing and warrant further research into the efficacy of this substance as a potential supplement for modulating appetite.
Acknowledgments
This study was supported by Kyowa Hakko Kogyo Co., Ltd., JAPAN and by NIDA 1R01 DA020269 (DYT).
Footnotes
DISCLOSURES
PFR and DYT serve as consultants to and TK and YK are employees of Kyowa Hakko Kogyo Co., Ltd., JAPAN
References
- Adibhatla RM, Hatcher JF. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res. 2002;70(2):133–139. doi: 10.1002/jnr.10403. [DOI] [PubMed] [Google Scholar]
- Agut J, Coviella IL, Wurtman RJ. Cytidine(5')diphosphocholine enhances the ability of haloperidol to increase dopamine metabolites in the striatum of the rat and to diminish stereotyped behavior induced by apomorphine. Neuropharmacology. 1984;23(12A):1403–1406. doi: 10.1016/0028-3908(84)90080-7. [DOI] [PubMed] [Google Scholar]
- Agut J, Ortiz JA, Wurtman RJ. Cytidine (5')diphosphocholine modulates dopamine K(+)-evoked release in striatum measured by microdialysis. Ann N Y Acad Sci. 2000;920:332–335. doi: 10.1111/j.1749-6632.2000.tb06944.x. [DOI] [PubMed] [Google Scholar]
- Alvarez XA, Sampedro C, Lozano R, Cacabelos R. Citicoline protects hippocampal neurons against apoptosis induced by brain beta-amyloid deposits plus cerebral hypoperfusion in rats. Methods Find Exp Clin Pharmacol. 1999;21(8):535–540. doi: 10.1358/mf.1999.21.8.794835. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94(3):309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Gorman AR, Hynan LS. A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. J Clin Psychopharmacol. 2007;27(5):498–502. doi: 10.1097/JCP.0b013e31814db4c4. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Browndyke JN, Moser DJ, Paul RH, Gordon N, Sweet L. Long-term citicoline (cytidine diphosphate choline) use in patients with vascular dementia: neuroimaging and neuropsychological outcomes. Cerebrovasc Dis. 2003;16(3):199–204. doi: 10.1159/000071116. [DOI] [PubMed] [Google Scholar]
- Conant R, Schauss AG. Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature. Altern Med Rev. 2004;9(1):17–31. [PubMed] [Google Scholar]
- Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86(4):965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- D'Orlando KJ, Sandage BW., Jr Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury. Neurol Res. 1995;17(4):281–284. doi: 10.1080/01616412.1995.11740327. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: Update 2008. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin Interv Aging. 2006;1(3):247–251. doi: 10.2147/ciia.2006.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;5:189–201. [Google Scholar]
- Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16(5):945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- Gimenez R, Raich J, Aguilar J. Changes in brain striatum dopamine and acetylcholine receptors induced by chronic CDP-choline treatment of aging mice. Br J Pharmacol. 1991;104(3):575–578. doi: 10.1111/j.1476-5381.1991.tb12471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol. 2008;75(1):266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20(5):1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005a;16(8):859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol. 2005b;47(4):377–397. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. International Journal of Eating Disorders. 2006;39(5):357–363. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Positive affect modulates activity in the visual cortex to images of high calorie foods. Int J Neurosci. 2007;117(5):643–653. doi: 10.1080/00207450600773848. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Lopez I, Coviella G, Agut J, Wurtman RJ. Effect of cytidine(5')diphosphocholine (CDP-choline) on the total urinary excretion of 3-methoxy-4-hydroxyphenylglycol (MHPG) by rats and humans. J Neural Transm. 1986;66(2):129–134. doi: 10.1007/BF01260908. [DOI] [PubMed] [Google Scholar]
- Macwhinney B, Cohen J, Provost J. The PsyScope experiment-building system. Spat Vis. 1997;11(1):99–101. doi: 10.1163/156856897x00113. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhovel MM, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39(4–5):643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. JAMA. 1999;282(16):1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29(1):106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ozay R, Bekar A, Kocaeli H, Karli N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surg Neurol. 2007;68(6):615–622. doi: 10.1016/j.surneu.2006.12.054. discussion 622. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Parisi V, Coppola G, Centofanti M, Oddone F, Angrisani AM, Ziccardi L, et al. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res. 2008;173:541–554. doi: 10.1016/S0079-6123(08)01137-0. [DOI] [PubMed] [Google Scholar]
- Petkov VD, Stancheva SL, Tocuschieva L, Petkov VV. Changes in brain biogenic monoamines induced by the nootropic drugs adafenoxate and meclofenoxate and by citicholine (experiments on rats) Gen Pharmacol. 1990;21(1):71–75. doi: 10.1016/0306-3623(90)90598-g. [DOI] [PubMed] [Google Scholar]
- Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32(3):1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Xiaojing J, Durany N, Rausch WD. CDP-choline reduces dopaminergic cell loss induced by MPP(+) and glutamate in primary mesencephalic cell culture. Int J Neurosci. 2007;117(7):985–998. doi: 10.1080/10623320600934341. [DOI] [PubMed] [Google Scholar]
- Rao AM, Hatcher JF, Dempsey RJ. CDP-choline: neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res. 1999;58(5):697–705. [PubMed] [Google Scholar]
- Rao AM, Hatcher JF, Dempsey RJ. Does CDP-choline modulate phospholipase activities after transient forebrain ischemia? Brain Res. 2001;893(1–2):268–272. doi: 10.1016/s0006-8993(00)03280-7. [DOI] [PubMed] [Google Scholar]
- Rejdak R, Toczolowski J, Solski J, Duma D, Grieb P. Citicoline treatment increases retinal dopamine content in rabbits. Ophthalmic Res. 2002;34(3):146–149. doi: 10.1159/000063658. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Daniels S, Lundahl LH, Rogers V, Lukas SE. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology (Berl) 1999;142(2):132–138. doi: 10.1007/s002130050871. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rolls ET, McCabe C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci. 2007;26(4):1067–1076. doi: 10.1111/j.1460-9568.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Santel S, Baving L, Krauel K, Munte TF, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114(1):138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-Eating Disorder: Reward Sensitivity and Brain Activation to Images of Food. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006;28(Suppl B):1–56. [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Stark R, Zimmermann M, Kagerer S, Schienle A, Walter B, Weygandt M, et al. Hemodynamic brain correlates of disgust and fear ratings. Neuroimage. 2007;37(2):663–673. doi: 10.1016/j.neuroimage.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56(9):637–660. doi: 10.1016/0024-3205(94)00427-t. [DOI] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15(15):2347–2351. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]