Abstract
Dynamics of the actin cytoskeleton in the trabecular meshwork play a crucial role in the regulation of trabecular outflow resistance. The actin filament disruptors and Rho kinase inhibitors affect the dynamics of the actomyosin system by either disrupting the actin filaments or inhibiting the Rho kinase-activated cellular contractility. Both approaches induce similar morphological changes and resistance decreases in the trabecular outflow pathway, and thus both have potential as antiglaucoma medications. Although the drugs might induce detrimental changes in the cornea following topical administration, lower drug concentrations in larger volumes as used clinically, but not higher drug concentrations in smaller volumes as used experimentally, could minimize corneal toxicity. Additionally, developments of trabecular meshwork-specific actin filament disruptors or Rho kinase inhibitors, prodrugs and new drug-delivery methods might avoid the drugs’ toxicity to the cornea. Gene therapies with cytoskeleton-modulating proteins may mimic the effects of the cytoskeleton-modulating agents and have the potential to permanently decrease trabecular outflow resistance.
Keywords: actin filament disruptor, actomyosin, cytoskeleton, glaucoma, intraocular pressure, outflow facility, Rho kinase inhibitor, trabecular meshwork
The glaucomas are a group of ophthalmologic disorders responsible for visual impairment. They are the leading cause of irreversible blindness worldwide. In 2010, there were approximately 60.5 million people worldwide with glaucoma, of whom approximately 74% had primary open-angle glaucoma (although ~half of the glaucoma cases in Asian regions were angle-closure glaucoma), and approximately 14% were blind from the disease. The glaucoma population could increase to approximately 80 million over the next 10 years [1]. Generally, primary open-angle glaucoma is characterized by progressive optic neuropathy usually associated with relatively elevated (i.e., higher than the optic nerve can tolerate on a chronic basis) intraocular pressure (IOP) consequent to abnormally high flow resistance in the trabecular outflow pathway. At present, the only effective approach available to treat glaucoma is to reduce IOP. Although surgical treatments may decrease IOP in glaucomatous eyes, pharmacological treatments are typically the first choices, since surgeries carry risks such as infection, cataracts and hypotony, and many surgical patients eventually must resume topical therapy owing to loss of effectiveness. Medications currently used to reduce IOP clinically include aqueous humor secretory inhibitors (e.g., β-adrenergic receptor antagonists, α2-adrenergic agonists and carbonic anhydrase inhibitors), uveoscleral-outflow enhancers (e.g., prostaglandin analogs), and indirect trabecular-outflow enhancers (e.g., cholinergic drugs). However, secretory suppression may affect supplies of oxygen and nutrients to the nonvascularized tissues, such as the cornea, lens and trabecular meshwork (TM; the major structure of the trabecular outflow pathway), and prostaglandin analogs do not substantially improve the trabecular outflow (the main route of aqueous humor outflow in the human eye and the predominant site of the abnormal outflow resistance in glaucoma). Although cholinergic drugs indirectly elevate the trabecular outflow by contracting the ciliary muscle and deforming the TM, their effects on the pupil and accommodation limit their clinical use. Additionally, epinephrine-like drugs, which work on both the trabecular and uveoscleral outflow routes, are now seldom used clinically because of their local and systemic side effects. Thus, there are no TM-selective outflow enhancers in current common clinical use. Therefore, the development of novel TM-selective outflow enhancers is important for glaucoma therapy.
Autoregulation of trabecular outflow resistance in the eye may play an important role in the maintenance of physiological levels of IOP. The basis for this regulation is unknown, but multiple cellular and associated factors, such as contractility of both the TM cells and ciliary muscle, pore formation in the inner wall of the Schlemm’s canal (SC), amount and composition of extracellular matrix (ECM) in the TM, and morphology and volume of TM cells, may be involved. A variety of endogenous biochemical reactions may modulate these cellular characteristics and extracellular environments to regulate outflow resistance through different receptor-mediated signaling pathways. Rearrangement of the actin cytoskeleton due to changes in IOP-induced TM cell stress may be particularly important [2]. For instance, the stretch of TM cells induced by differential pressure between the anterior chamber and the lumen of SC may signal the rearrangement of the actomyosin system in the cells and induce alterations of cell morphology, contractility and adhesive interactions, and ECM turnover or synthesis. These alterations may lead to changes in the geometry of the drainage pathway, and paracellular and transcellular permeability, which could increase or decrease outflow resistance and, in turn, maintain a certain hydraulic conductivity across the TM and a corresponding IOP level. Glaucomatous trabecular tissue has shown ‘disordered’ actin architecture [3], which could potentially affect the biomechanical properties of the outflow pathway tissues and/or the cell’s responses to changes in the biomechanical properties induced by elevated IOP. Cytoskeleton-modulating agents (e.g., the actin filament disruptors and Rho kinase inhibitors) may act directly on the TM and decrease trabecular outflow resistance by regulating dynamics of the actin cytoskeleton or actomyosin contractility [4,5], which may mimic the normal physiological function and be employed to therapeutically reduce IOP to levels tolerated by the optic nerve. In this article, we review and compare changes of the actin cytoskeleton induced by actin filament disruptors and Rho kinase inhibitors in cultured human TM cells, and decreases of the trabecular outflow resistance following the two categories of agents in organ-cultured anterior segments of enucleated animal or postmortem human eyes and living animal eyes. Possibilities of these agents for future use as antiglaucoma medications are also discussed.
Perturbations of the actomyosin system
The actomyosin system in eukaryotic cells is composed of actin microfilaments and associated proteins, primarily including focal adhesions, adheren cell–cell junctions and bundles of microfilaments (stress fibers) [6]. F-actin, formed by the ATP-dependent assembly of the G-actin monomer, is the major component of microfilaments, but other actin-associated proteins modulate its organization. Stress fibers are important for many cellular functions, such as cell morphogenesis, wound healing and cell cycle progression, and are capable of contractility that is crucial for regulating focal adhesions and remodeling the ECM [7]. Since stress fibers from neighboring cells are physically connected through discontinuous adherens junctions, integrity of stress fibers is also important for adheren cell–cell junctions [8,9]. The polymerization and depolymerization cycle of stress fibers can be affected by actin filament disruptors or Rho kinase inhibitors.
Effects of actin filament disruptors on the actomyosin system
Since the 1970s, the effects of several actin filament disruptors on the actin cytoskeleton have been studied in several types of cultured cells, including human TM cells. Fungal metabolites, the cytochalasins, directly disrupt actin microfilaments by capping the filaments and preventing their elongation, thereby affecting a wide variety of actin-dependent cellular events, such as cell morphogenesis, motility and endocytosis [10]. Latrunculins, macrolides isolated from the marine sponge Negombata (formerly Latrunculia) magnifica, sequester monomeric G-actin by binding to the monomer and therefore preventing monomer addition to filament ends, leading to the disassembly of actin microfilaments and morphological changes in cells [11]. In cultured human TM cells, cytochalasins (e.g., cytochalasin B or D) and latrunculins (e.g., latrunculin A or B) induce cell retracting and rounding, intercellular separation, and destruction of stress fibers and associated proteins [12–14]. Swinholides (e.g., swinholide A), the dimeric macrolides isolated from the marine sponge Theonella swinhoei, sever actin microfilaments but stabilize actin in a dimeric form, leading to changes in cell morphology similar to those following latrunculins [15,16]. Although the increase of G-actin after latrunculin A inhibits actin synthesis, whereas the formation of dimeric actin after swinholide A reduces G-actin and, in turn, enhances actin synthesis, both similarly decrease the level of F-actin [16], suggesting that the decrease of F-actin and consequent depolymerization of stress fibers is the key factor in the drug-induced cellular morphological changes. The stress fiber depolymerization induced by cytochalasins or latrunculins also inhibits smooth muscle and nonmuscle cell contractility in vitro and/or in vivo [17–19], perhaps by affecting the dynamic equilibrium between F-actin and G-actin [20], and/or disrupting the contractile apparatus in cells (Figure 1).
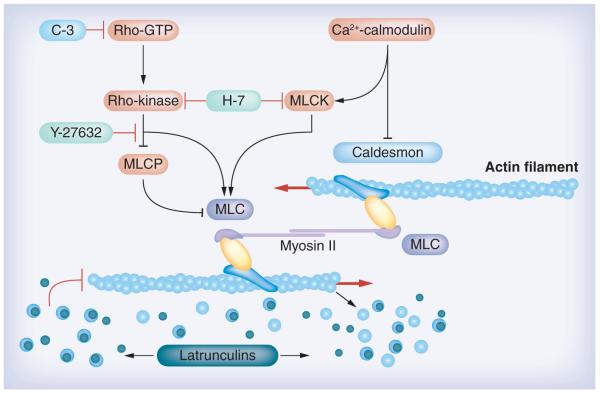
Figure 1. Targets where agents (or proteins) can disrupt the actin cytoskeleton and enhance outflow facility.
Rho kinase inhibitors, including the nonselective Rho kinase inhibitor H-7 and the specific Rho kinase inhibitor Y-27632 (and other specific Rho kinase inhibitors as indicated in the article), block the Rho cascade, inhibiting actomyosin contraction and disrupting actin stress fibers. H-7 also blocks MLCK, which may affect myosin light-chain phosphorylation and, in turn, interfere with actin–myosin interactions. Actin filament disruptors latrunculins (e.g., latrunculins A and B) sequester monomeric G-actin leading to microfilament disassembly. The cytoskeleton-modulating proteins caldesmon and C3 affect the actin cytoskeleton similar to the compounds as indicated above. Caldesmon negatively regulates actin–myosin interactions, and C3 blocks the Rho cascade similar to Rho kinase inhibitors.
Modified with permission from a figure in [87] © Elsevier. The figure was originally created by Alexander Bershadsky.
Effects of Rho kinase inhibitors on the actomyosin system
Conversely, the inhibition of cellular contractility may induce actin microfilament depolymerization. Smooth muscle and non-muscle cell contractions are primarily regulated by the increase in the intracellular Ca2+ concentration and subsequent phosphorylation of the myosin light chain (MLC) by Ca2+-calmodulin-dependent MLC kinase (MLCK). In the absence of an obligatory change in the concentration of intracellular Ca2+, the contractions can be enhanced by G-protein-mediated Ca2+ sensitization, in which Rho kinase plays a key role [21]. Additionally, PKC is also involved in the Ca2+ -independent cellular contraction [22]. H-7, a nonselective serine–threonine kinase inhibitor, reduces actomyosin-driven contractility and leads to deterioration of the microfilaments and perturbation of their membrane anchorage, and loss of stress fibers and focal adhesions [23–25]. Although H-7 inhibits multiple protein kinases including Rho kinase, MLCK and PKC, it may decrease actomyosin-driven contraction primarily by inhibiting Rho kinase, since the inhibition constant (Ki) value for H-7 to inhibit Rho kinase (0.45 μM) is much lower than that for it to inhibit MLCK (170 μM) or PKC (7.7 μM) [26]. Therefore, H-7 may be considered as a nonselective Rho kinase inhibitor. The Rho kinase system plays a crucial role in maintaining sustained contraction in cells [27], which promotes the formation of stress fibers and focal adhesions [7]. The levels of mRNA for Rho kinase and Rho kinase substrates are higher in the TM than in the ciliary muscle in both the monkey and human eye [28]. The more specific Rho kinase inhibitors Y-27632 and Y-39983 relax the phorbol myristate acetate and/or carbachol-induced contractions in isolated bovine or monkey TM strips [28,29]. Y-27632 and H-1152 (another specific Rho kinase inhibitor) reduce basal MLC phosphorylation in cultured human TM cells, leading to changes in cell shape, depolymerization of actin stress fibers and loss of focal adhesions [30–32]. All these support the idea that Rho kinase is a key regulator in cellular contractility and focal adhesion, and stress fiber formations in nonmuscle cells including TM cells (Figure 1).
Comparisons of actomyosin changes induced by actin filament disruptors & Rho kinase inhibitors
Although the actin filament disruptors and the Rho kinase inhibitors affect the actin cytoskeleton by different mechanisms, they basically induce similar cytoskeletal changes including depolymerization of stress fibers, separation of adherens cell–cell junctions and focal adhesions, and changes in cell morphology [13,14,24,25,30,33,34]. These similarities could be due to a possible crosstalk between the Rho signaling system and the actomyosin system that allows the inhibition of one system to affect the other [35]. However, there are also differences in the cytoskeletal changes induced by the two categories of cytoskeletal agents. In human TM cells, β-catenin-rich intercellular adheren junctions appear more sensitive to latrunculins, while focal adhesions are more sensitive to H-7 [13,25,33]. Cytochalasin D (25 μM) and latrunculin A (0.25 μM) produce a complete rounding of cells along with cell–cell separation, cell detachment and almost complete disappearance of stress fibers in the cultured human TM cells. H-7 (50 μM) and Y-27632 (25 μM) induce cell–cell separation and a stellate appearance in cells. Although most stress fibers disappear after H-7 or Y-27632, some of them still remain at the peripheral region of the cell body. Additionally, treatment of human TM cells with cytochalasin D or latrunculin A leads to significant activation of matrix metalloproteinase (MMP)-2, increases in the expression of membrane type-1 MMP, and decreases the protein levels of the MMP inhibitors. In contrast, treatment with Y-27632 or H-7 does not exert significant effects on MMP-2 activation and membrane type-1 MMP expression [12]. The effects of cytochalasin D and latrunculin A on MMP-2 suggests a mechanistic connection between actin cytoskeletal organization and MMP-2 activation in TM cells. Since MMPs play an important role in the turnover of ECM components, the cytochalasin D- and latrunculin A-induced activation of MMP-2 may decrease the amount of ECM in the TM. It is not yet clear whether H-7 and Y-27632 may increase the activation of MMP-2 at concentrations higher than those indicated above. However, Rho kinase inhibition may affect ECM synthesis, because sustained activation of Rho GTPase activity increases expression of ECM proteins and cytokines in TM cells [36,37]. This suggests a potential molecular interplay between acto myosin cytoskeletal tension and ECM synthesis regulated by the Rho signaling pathway.
Functional & structural changes of the TM
The TM consists of arrays of collagen beams covered by endothelial-like cells, with loose ECM occupying the spaces between the cells of the adjacent beams. The outermost juxtacanalicular (JXT) or cribriform region has no collagenous beams, but rather several cell layers immersed in a loose web of ECM fibrils. The adjacent SC is a continuous endothelium-lined channel that drains aqueous humor to the general venous circulation. Although the exact location of the major resistance barrier to trabecular outflow is not clear, there is no doubt that nearly all the resistance resides in the JXT tissue of the TM and the adjacent inner wall of SC [38,39]. Physiologically, cells in the JXT–SC region are in a contracted state, which is required to maintain the actin filament-related structures in the outflow pathway and, conversely, can be affected by depolymerization of the actin filaments [7]. Since the contractility and the actin filament-related structures (e.g., adhesions to neighboring cells and to the ECM) of TM/SC cells play important roles in the regulation of trabecular outflow resistance, actin filament disruptors or Rho kinase inhibitors may decrease the outflow resistance (e.g., decreasing IOP or increasing outflow facility) by relaxing and expanding the meshwork and/or separating cell–cell and cell–ECM adhesions in the JXT tissue and the inner wall of the SC [4]. Additionally, a decrease in the amount and composition of the ECM in the JXT region may also play an important role in the drug-induced resistance reduction [36,37].
Changes in outflow facility & IOP after actin filament disruptors
Actin filament disruptors cytochalasins B and D, and latrunculin A and B significantly increase outflow facility in living monkeys [40–43]. Latrunculin A or B administered intracamerally or topically to monkey eyes, increase outflow facility by up to two- to four-fold in a dose-dependent manner without generating ciliary muscle contraction [34,42]. In organ-cultured anterior segments from enucleated porcine eyes or postmortem human eyes, latrunculin B significantly increases outflow facility by 60–70% [33,44]. Single and/or multiple topical treatments with latrunculin A or B also significantly decrease IOP in the normotensive monkey eye. In the experiment with latrunculin B, IOP decreased from 19.3 to 16.4, 18.8 to 15.7, or 17.0 to 13.5 mmHg, after a single topical treatment with either 0.005, 0.01 or 0.02% dose, respectively, with the maximal IOP reduction (adjusted for baseline and contralateral control eye) being 2.5, 2.7 or 3.1 mmHg, respectively, which was observed at hours 3–6. After multiple twice-daily treatments with a 0.005% or 0.01% dose, the maximal IOP reduction (−3.2 or −4.4 mmHg, respectively) occurred at hours 3–4 on day 5, and some IOP effects might last for 24 h [42,43]. In a recent clinical trial with glaucoma patients, 0.02 or 0.05% topical latrunculin B decreased IOP by 3.8 or 3.9 mmHg from baseline (22.9 or 23.5 mmHg), respectively, after the first instillation. Twice-daily treatments with 0.02% latrunculin B decreased IOP by 5.4 mmHg from baseline, with a maximal 12-h IOP reduction of 4 mmHg after adjustment for baseline and contralateral control eye occurring on day 3 [45].
Changes in outflow facility & IOP after Rho kinase inhibitors
The nonselective Rho kinase inhibitor H-7, administered intracamerally or topically to living monkey eyes, doubles outflow facility and decreases IOP [24,46]. Similar to the outflow facility enhancement after latrunculins, the H-7-increased outflow passes through the trabecular outflow pathway, independent of ciliary muscle contraction [47]. In organ-cultured anterior segments of porcine, human or monkey eyes, H-7 also significantly increases outflow facility [33,48,49]. A single or multiple topical treatment of H-7 dose-dependently decreases IOP by up to 4 mmHg in the normotensive monkey eye (average baselines: ~12–17 mmHg) and ~6 mmHg in the glaucomatous monkey eye (average baseline: ~34 mmHg) [46]. More specific Rho kinase inhibitors, such as Y-27632, Y-39983, HA-1077, H-1152 and INS117548, increase outflow facility and/or decrease IOP in living rabbits and monkeys and/or enucleated animal eyes, similar to H-7 [30–32,50–53]. In a Phase I clinical trial, a single instillation of the specific Rho kinase inhibitor SNJ-1656 dose-dependently decreased IOP in healthy volunteers with a maximal IOP reduction of 3 mmHg at 4 h after a 0.1% dose, and repeated instillations of the drug induced greater IOP reductions compared with a single dose [54]. In a Phase II clinical trial, the specific Rho kinase inhibitor AR-12286 produced significant IOP reductions in a dose-dependent manner in subjects with elevated IOP, with peak effects occurring 2–4 h after dosing. The largest IOP reductions were produced by 0.25% AR-12286 after twice-daily dosing (up to −6.8 mmHg; 28%). The 0.25% concentration had a long duration of effect, with a daily afternoon dosing producing highly significant IOP reduction throughout the following day −5.4 to −4.2 mmHg) [55]. Other dosing regimens suggested an even greater IOP reduction, with a final IOP <10 mmHg in a significant number of ocular normotensive subjects.
Comparisons of TM morphological changes after actin filament disruptors & Rho kinase inhibitors
Cytoskeletal changes in TM and SC cells induced by actin filament disruptors or Rho kinase inhibitors cause or contribute to the drug-increased trabecular outflow facility in live monkey eyes and organ-cultured human/animal anterior segments [24,33,34,44]. Consistent with cytoskeletal changes in cultured cells, morphological changes in the TM of the live eye and the organ-cultured anterior segment after the two categories of drugs are also qualitatively similar.
Electron microscopy of live monkey eyes has revealed massive ‘ballooning’ of the JXT region following latrunculin B treatment, leading to a substantial expansion of the space between the inner wall of SC and the trabecular collagen beams without observable separations between inner wall cells and between the inner wall endothelium and the subendothelial cell layer. The inner wall cells of SC after latrunculin B are substantially extended [56]. In postmortem human eyes, the facility increase is accompanied by increased paracellular pores in the inner wall, with only very modest rarefaction of the JXT tissue and separation of the inner wall of SC from JXT tissue [44]. Although the magnitude of the facility increase and morphologic changes are much less in the postmortem human eye than in the live monkey eye, perhaps owing to differences in species and/or experimental conditions, the facility increase and the extent of inner wall separation from the JXT region in the former are both qualitatively similar to that in the latter [44,56].
Similarly, the H-7-induced increase in outflow facility in the live monkey eye is associated with cellular relaxation and drainage-surface expansion of the TM and SC, accompanied by loss of ECM. The inner wall cells of SC become highly extended, yet cell–cell junctions are maintained [57,58]. Gold tracers infused intracamerally in the H-7-treated eye distributes in a more uniform manner, with tracer labeling along >80% of the inner wall, compared with the sparse tracer foci occupying 10–20% of the inner wall length in the control eyes [57]. A recent morphological study with the enucleated bovine eye indicates that the inner wall of SC and the JXT connective tissue in Y-27632-treated eyes are significantly distended compared with those in control eyes, with discernible separation between the basal lamina of the inner wall and the ECM of the JXT connective tissue [59], which is similar to the change in the live monkey eye after latrunculin B [56]. However, in the enucleated monkey eye after Y-27632, the expansion of the JXT region is primarily due to separations of cell–cell and cell–ECM adhesions within the JXT region [60]. In both the enucleated bovine and monkey eyes, the average percent effective filtration length of the inner wall (filtration length/total length) is two- to three-fold larger in Y-27632-treated eyes than in controls, with the distribution of fluorescent microspheres used in the perfusion experiments being more uniform and extensive in the former than in the latter. A significant positive correlation is found between the effective filtration length of the inner wall and the separation length of the inner wall (length exhibiting separations between the JXT connective tissue and the inner wall) in both species, suggesting that the structural correlate to the increase in outflow facility after Y-27632 is the meshwork relaxation and/or intercellular space expansion in the JXT region [59,60].
It has been speculated that the pores of the inner wall may cause a funneling effect in which aqueous humor flows preferentially through the regions of the JXT tissue near the pores [61]. This hydrodynamic interaction between the inner wall endothelium and the JXT tissue may lead to a resistance greater than that generated by either tissue alone [38]. Therefore, although no apparent cell–cell separations are seen in the inner wall of SC in the perfused live monkey eye following both the actin filament disruptors and the Rho kinase inhibitors, the drug-induced meshwork relaxation and/or the substantial expansion of the space between the inner wall and the trabecular collagen beams and/or within the JXT region may significantly decrease outflow resistance by regulating the funneling effect [38]. The traditional indirect trabecular outflow enhancer pilocarpine does not affect actin cytoskeleton in the TM. However, it indirectly expands the TM and induces a loose arrangement of the cribriform region, with many open pathways and empty spaces adjacent to the inner wall endothelium, by contracting the ciliary muscle [62]. These morphological changes in overall TM architecture after pilocarpine appear qualitatively similar to the TM changes induced by actin filament disruptors or Rho kinase inhibitors. Additionally, the ECM deposited in the spaces of the JXT region may also contribute to trabecular outflow resistance [63]. The TM expansion by cytoskeleton-modulating agents during perfusion is often accompanied by loss of ECM, perhaps due to the drug-induced washout. However, actin filament disruptors and Rho kinase inhibitors themselves may affect the ECM turnover and/or synthesis [12,36,37], which could also play an important role in the drug-induced IOP reduction during multiple chronic treatments.
Side effects of the potential antiglaucoma medications
The effects of the actin filament disruptors or Rho kinase inhibitors on the actin cytoskeleton are reversible in live monkey eyes after a single maximal facility-effective dose or multiple lower dose treatment(s) with the drug, and in cultured human TM cells incubated with different doses of the drug for 24 h [13,25,34,58], suggesting that these cytoskeleton-modulating agents might have no permanent toxic effects to cells and tissues in treatments for a short period or at low doses. However, although actin filament disruptors and Rho kinase inhibitors affect the actin cytoskeleton by different mechanisms, they induce similar disorganization of the actin cytoskeleton system [12,13,25]. Therefore, multiple treatments with maximal facility-effective doses of both categories of the drugs in the long term might induce detrimental structural and functional changes in ocular tissues adjacent to the TM, especially the cornea, which will be exposed to a higher concentration of topical drugs.
Side effects of actin filament disruptors
In addition to inducing transient morphological changes in TM/SC cells, cytochalasin B administered intracamerally to the monkey eye also induces similar cellular changes in the anterior ciliary processes, iris pigmented epithelium and corneal endothelium [64]. In the cultured rabbit cornea, cytochalasin B dose-dependently induces a progressive increase in both the degree and rate of corneal swelling, accompanied by progressive change in endothelial cell shape [65]. Latrunculin A administered topically to the monkey eye transiently alters the corneal endothelial morphology and disturbs anterior segment barrier functions, which induce temporary increases of corneal endothelial permeability, anterior chamber protein concentration, aqueous humor formation, and ciliary epithelial and iridovascular endothelial permeability [42]. However, topical latrunculin B has a milder effect on corneal endothelial permeability compared with latrunculin A, and has essentially no effect on aqueous humor formation in the monkey eye [42]. Submaximal concentration of latrunculin B administered intracamerally to monkey eyes does not change the morphology of the corneal endothelium [56]. Although a single treatment with 20 μl of 0.02% topical latrunculin B significantly increases central and peripheral corneal thickness of the monkey eye [42], multiple topical treatments with 20 μl of 0.01% solution of the drug, which significantly increases outflow facility and decreases IOP, do not change the central corneal thickness [43]. In the clinical trial indicated above, latrunculin B is generally well tolerated, although adverse events, such as mild ocular redness, irritation and a transient, clinically insignificant increase (≤2.5%) in central corneal thickness at the two highest doses (0.02 and 0.05%), are noted [45]. Additionally, latrunculin B in outflow facility-increasing concentrations has no effect on retinal vascular permeability by vitreous fluorophotometry and fluorescein angiography, or electrophysiology by measurements of photopic full-field electroretinograms (ffERGs), multifocal electroretinograms (mfERGs) and scotopic full-field electroretinograms (sERGs) in the live monkey eye [66].
Side effects of Rho kinase inhibitors
Similar to latrunculin B, a single maximal facility-effective topical dose of H-7(~15%; 20 μl) essentially does not change the aqueous humor formation and corneal endothelium transfer coefficient in the monkey eye, although it transiently increases the protein concentration in the anterior chamber, the rate of entry of intravenous fluorescein into the aqueous humor and cornea, and the corneal thickness. A maximal facility-effective intracameral dose of H-7 (300 μM) does not induce structural changes in the corneal endothelium or ciliary epithelium by light or electron microscopy [67]. Multiple lower doses of topical H-7 (5%; 20 μl), which significantly increases outflow facility and decreases IOP, do not change the corneal thickness [46]. H-7 in the vitreous at 300 μM does not change the retinal physiology of the monkey eye [66]. Y-39983 (0.003–0.1%) administered topically to the rabbit eye and monkey eye does not affect the corneal surface, anterior chamber, lens, vitreous or retina by slit-lamp examination, although it induces conjunctival hyperemia and punctate subconjunctival hemorrhage following frequent treatments. No significant findings of toxicity are detected on histologic examinations of these ocular tissues. Analysis of retinal electrophysiology reveals no abnormalities in the drug-treated eyes of both species [51]. In clinical trials of the specific Rho kinase inhibitors SNJ-1656 and AR-12286, there are no significant abnormal findings except conjunctival hyperemia in the drug-treated eyes by slit-lamp examination [54,55].
Dissociative effects of low doses of actin filament disruptors & Rho kinase inhibitors on the TM & cornea
Evidence has suggested that low doses of actin filament disruptors and Rho kinase inhibitors administered intracamerally or topically may increase outflow facility effectively without meaningfully affecting the cornea and other ocular tissues [43,46,56,67]. Possible mechanisms for the dissociative effects of low doses of those agents on the TM and cornea could be due to the different architecture and physiologic milieu of the two tissues. The TM is a suspended multilayered tissue, in which JXT cells have no real basement membrane. When the actomyosin system is disorganized by actin filament disruptors or Rho kinase inhibitors, the TM can be readily distorted and distended by fluid flow down the pressure gradient between the anterior chamber and SC. However, the corneal endothelium in the live monkey and human eye is a single cell layer on a well-defined basement membrane and ECM structure (Descemet’s membrane and stroma) with much less fluid flow across it than the TM, and thus less easily distended or distorted even when the contractile apparatus, and consequently, cellular adhesions are weakened by the drugs. Inner wall cells of SC have only a thin, diaphanous, discontinuous basement membrane, so that the inner wall endothelium may not be as strong as the corneal endothelium, but stronger than the JXT cells.
Biomolecular differences between the TM and cornea, and different molecular targets or mechanisms for different agents may also be involved in the dissociative effects. In a recent study, Y-27632 has been found to promote proliferation as well as adhesion of monkey corneal endothelial cells and inhibit their apoptosis, in contrast to the results reported previously in various other types of cells [68]. This unexpected result may suggest that the Rho kinase signaling is cell type-dependent [69,70], and that Rho kinase inhibitors might not significantly affect the actin cytoskeleton in the corneal endothelium as they do in the TM. Additionally, decreased Na+/K+ ATPase activity or pump density in the corneal endothelium induces corneal edema, which often occurs in the cornea of diabetics [71]. PKC inhibitors might induce corneal hydration via inhibiting insulin’s positive effect on the Na+/K+ ATPase activity and pump function of the corneal endothelium [72], mimicking diabetic corneas. As we know, the more specific Rho kinase inhibitor Y-27632 seems to have a more potent outflow-facility effect and less corneal side effects than the non-selective Rho kinase inhibitor H-7, while the broad-spectrum protein kinase inhibitor staurosporine seems to have a more potent outflow facility effect than Y-27632 and more severe corneal side effects than H-7 [24,52,67,73]. Based on Ki or IC50 values, the rank order potency of the inhibitory effect on Rho kinase for the three drugs is staurosporine » Y-27632 > H-7 [26,74], while that on PKC is staurosporine » H-7 > Y-27632 [26,75]. Therefore, one may reasonably hypothesize that the outflow-facility effects of these protein kinase inhibitors might be primarily related to their Rho kinase inhibition, and the corneal side effects might be primarily related to their PKC inhibition. Actually, the specificities of the current specific protein kinase inhibitors are more or less relative. Low doses of the so-called specific Rho kinase inhibitors may be more specific to Rho kinase than high doses of the drugs, which may explain the dissociative effects of low doses of these agents on the TM and cornea.
Different actin filament disruptors may also affect the TM and cornea differently. Latrunculin B has a similar structure and actin-disrupting activity in cultured cells compared with latrunculin A, but the effect of latrunculin B on the morphology and actin organization in hamster fibroblasts is less potent than that of latrunculin A [11]. Additionally, latrunculin B, but not latrunculin A, is slowly inactivated by an as yet unknown serum component, so that after 48 h of exposure to a maximal latrunculin B dose, cells completely recover, exhibiting a well-developed system of stress fibers [11]. Interestingly, unlike in the cultured hamster fibroblasts, latrunculin B is ten-times more potent in reducing outflow resistance compared with latrunculin A in living monkeys (e.g., 0.2 μM latrunculin B has a similar or greater effect on outflow facility to or than 2 μM latrunculin A) [34,41]. Clarification of the differences between the two latrunculins may facilitate development of more TM-selective and less cornea-affective actin filament disruptors for glaucoma therapy.
Future studies of the TM-selective outflow enhancers & gene therapy
Many studies as described above have confirmed that actin filament disruptors and the Rho kinase inhibitors may decrease trabecular outflow resistance by affecting dynamics of the actin cytoskeleton or actomyosin contractility in the TM. Therefore, the two categories of actin cytoskeletal agents may be good candidates for TM-selective antiglaucoma medications. However, although lower drug concentrations in larger volumes as used clinically (e.g., ~50 μl), but not higher drug concentrations in smaller volumes as used experimentally (e.g., ~20 μl) [42,67], could be a good strategy to minimize corneal toxicity without significantly sacrificing the effect of the drug on the TM, the potential cornea toxicity is still an obstacle to the use of higher concentrations of the drugs topically for a greater outflow-facility increase, especially for older glaucomatous eyes that may be at greater risk owing to their reduced number of corneal endothelial cells and the cumulative effects of years of corneal epithelial and endothelial exposure to topical antiglaucoma drugs and preservatives. In recent clinical trials with the actin filament disruptor latrunculin B and several Rho kinase inhibitors, although the Rho kinase inhibitor AR-12286 seems to have promising efficacy and tolerability [55], the balance between efficacy and tolerability in choices of drug dosages is still a general challenge for developing TM-selective outflow enhancers with cytoskeleton-modulating agents [5,45]. Therefore, detection of TM-specific receptors that may respond to specific actin filament disruptors, development of more selective and more potent Rho kinase inhibitors that may have a stronger outflow effect but less corneal toxicity, and development of prodrugs that may have no cytoskeletal effects until reaching the TM, might be useful measures to avoid the corneal side effect of topical ophthalmic solutions of these agents. Additionally, since only approximately 1% of the topically administered drug may enter the anterior chamber [24], a much higher drug concentration of ophthalmic solution than necessary for increasing outflow facility (if applied directly to the TM) must be used for topical treatments. Therefore, novel drug-delivery methods, which have a much higher bioavailability than traditional topical application and might deliver the drug directly to the target tissues, may reduce the corneal side effects of cytoskeleton-modulating drugs. For example, long-term controlled drug delivery with drug-coated/encapsulated biodegradable microneedles and implanted drug-delivery devices may allow facility-effective-only doses of cytoskeleton-modulating drugs to slowly enter the TM and SC without going through the cornea or even the anterior chamber [76]; nanocapsules carrying drugs may reduce the adverse effects of drugs on the cornea by avoiding direct corneal exposure to the drug during permeation [76,77].
It had been hypothesized that one-time treatment with a cytoskeleton-modulating agent might change the meshwork cytoskeleton and wash out excess or pathologic resistance-increasing ECM that had accumulated in the TM of glaucomatous eyes over many years. This might decrease trabecular outflow resistance for a long time, since reaccumulation of the pathologic resistance-increasing ECM might also need many years [78]. However, since the excess or pathologic ECM is not the only factor that increases trabecular outflow resistance and the cytoskeletal changes induced by cytoskeleton-modulating agents are reversible, such a ‘pharmacologic trabeculocanalotomy’ may not be achieved by simply using those agents. Modern molecular genetic technology raises the possibility of overexpressing or underexpressing cytoskeleton-modulating proteins to ‘set’ the outflow tissues to a different ‘performance’ level genetically [79–82]. Gene therapies with cytoskeleton-modulating proteins, such as caldesmon, exoenzyme C3 transferase and dominant-negative RhoA, have been successfully tested in cultured TM cells and organ-cultured anterior segments of human or monkey eyes. Caldesmon is a multifunctional ubiquitous regulator of the actin cytoskeleton that regulates myosin II activity by blocking its interaction with actin. Exoenzyme C3 transferase may affect actin–myosin interactions by inhibiting Rho-GTP at the beginning of the Rho activation cascade (Figure 1). Overexpression of caldesmon or C3 transferase in cultured human TM cells induces cellular relaxation and consequent microfilament depolymerization, mimicking the effect of Rho kinase inhibitors on the actomyosin system [83–85]. Expression of dominant-negative RhoA also results in similar cellular changes as those following substantial inhibition of MLC phosphorylation by Rho kinase inhibitors [86]. Gene-transfer approaches that may mimic actin filament disruptors remain to be identified. However, the cytoskeleton-modulating proteins indicated above all secondarily disrupt stress fibers. As expected, outflow facility in organ-cultured human or monkey eyes has been dramatically increased following overexpression of these genes [83,85,86]. All the gene therapy studies described above suggest that, similar to pharmacological approaches, gene therapy may also disrupt the actomyosin system in the TM and, in turn, increase trabecular outflow facility by regulating expressions of cytoskeleton-modulating proteins. The potential long-term or permanent decrease of outflow resistance by genetic approaches with cytoskeleton-modulating proteins may mimic the hypothesized ‘pharmacologic trabeculocanalotomy’.
Expert commentary
The trabecular outflow pathway is the main drainage apparatus of the aqueous humor in the human eye. Elevated IOP in open-angle glaucoma is consequent to abnormally high-flow resistance in the trabecular outflow pathway. At present, the only effective approach available to treat glaucoma is to reduce IOP, either surgically or pharmacologically. Since surgeries carry risks for complications, pharmacological treatments are typically the first choices. Currently, there are no medications that directly decrease trabecular outflow resistance in clinical use. Actin filament disruptors and Rho kinase inhibitors are potential TM-selective outflow enhancers. Although the two categories of the drugs disorganize the actin cytoskeleton network in TM/SC cells by different mechanisms (disrupting the actin filaments or inhibiting Rho kinase), both of them similarly and directly decrease trabecular outflow resistance. However, further studies are needed to facilitate the transition of the TM-selective outflow enhancers from laboratory to clinic.
Five-year view
Currently, the actin filament disruptor latrunculin B and some Rho kinase inhibitors are in clinical trials [45,54,55]. The specific Rho kinase inhibitor AR-12286 appears to have very promising efficacy and tolerability. It is estimated that more candidates for TM-selective outflow enhancers will be developed within the next 5 years. However, before any new drug-delivery system can be used to replace traditional topical eye-drop administration in glaucoma therapy, identification of less toxic and highly facility-effective cytoskeleton-modulating agents will still be key in the development of the TM-selective outflow enhancers.
Key issues.
Developments of antiglaucoma medications that directly decrease trabecular outflow resistance are important to current clinical practice.
The actin filament disruptor latrunculin B, which depolymerizes stress fibers by sequestering monomeric G-actin, significantly decreases trabecular outflow resistance, independent of ciliary muscle contraction.
Rho kinase inhibitors, which disorganize the actomyosin system by inhibiting Rho-activated cellular contractility, significantly decrease trabecular outflow resistance similar to latrunculin B.
Structural changes related to the decrease of outflow resistance induced by the two categories of drugs similarly include meshwork relaxation and expansion, cell–cell and cell–extracellular matrix adhesion separations, and extracellular matrix washout, but no discernible breaks or cell separations in the inner wall endothelium.
The cytoskeletal effects of these drugs may have detrimental effects on the cornea and other ocular tissues adjacent to the trabecular meshwork.
Developments of trabecular meshwork-specific actin filament disruptors or Rho kinase inhibitors, prodrugs and new drug-delivery methods might avoid the toxicity of the drug to the cornea.
Genetic approaches to over- or under-express cytoskeleton-modulating proteins may have the potential to permanently decrease trabecular outflow resistance.
Acknowledgements
This article was supported by grants from the US National Eye Institute (EY002698 and EY016665), Research to Prevent Blindness, the Wisconsin Alumni Research Foundation, and the Ocular Physiology Research and Education Foundation.
Footnotes
Financial & competing interests disclosure The studies related to H-7 and latrunculins from the authors’ laboratory in this article arose, in whole or in part, from direct costs funded by the NIH (EY002698 and EY016665). The University of Wisconsin–Wisconsin Alumni Research Foundation holds a patent related to latrunculin B and H-7; accordingly, P Kaufman has a proprietary interest. P Kaufman also serves as a consultant for and receives an honorarium from Alcon Laboratories, Inc., Allergan, Inc., Altheos, Inc., Amakem Therapeutics, Bausch & Lomb, Inc., Johnson & Johnson, Merck, Inc., Pfizer, Inc., QLT, and Santen. P Kaufman also received grants, royalty and/or travel financial support from Santen, WARF, Pfizer and Alcon.
No writing assistance was utilized in the production of this manuscript.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1998;39(8):1361–1371. [PubMed] [Google Scholar]
- 3.Read AT, Chan DW, Ethier CR. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp. Eye Res. 2007;84(1):214–226. doi: 10.1016/j.exer.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp. Eye Res. 2009;88(4):713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandell KJ, Kudelka MR, Wirostko B. Rho kinase inhibitors for treatment of glaucoma. Expert Rev. Ophthalmol. 2011;6(6):611–622. doi: 10.1586/eop.11.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger B, Yehuda-Levenberg S, Bershadsky AD. Molecular interactions in the submembrane plaque of cell–cell and cell–matrix adhesions. Acta Anat. (Basel) 1995;154(1):46–62. doi: 10.1159/000147751. [DOI] [PubMed] [Google Scholar]
- 7.Hirata H, Tatsumi H, Sokabe M. Dynamics of actin filaments during tension-dependent formation of actin bundles. Biochim. Biophys. Acta. 2007;1770(8):1115–1127. doi: 10.1016/j.bbagen.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Millán J, Cain RJ, Reglero-Real N, et al. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk MM. Adherens junctions remain dynamic. BMC Biol. 2010;8:34. doi: 10.1186/1741-7007-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherlach K, Boettger D, Remme N, Hertweck C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010;27(6):869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 11.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins – novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton. 1989;13(3):127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 12.Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2007;48(5):2105–2114. doi: 10.1167/iovs.06-1089. [DOI] [PubMed] [Google Scholar]
- 13.Cai S, Liu X, Glasser A, et al. Effect of latrunculin-A on morphology and actin-associated adhesions of cultured human trabecular meshwork cells. Mol. Vis. 2000;6:132–143. [PubMed] [Google Scholar]
- 14.McKee CT, Wood JA, Shah NM, et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials. 2011;32(9):2417–2423. doi: 10.1016/j.biomaterials.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubb MR, Spector I, Bershadsky AD, Korn ED. Swinholide A is a microfilament disrupting marine toxin that stabilizes actin dimers and severs actin filaments. J. Biol. Chem. 1995;270(8):3463–3466. doi: 10.1074/jbc.270.8.3463. [DOI] [PubMed] [Google Scholar]
- 16.Lyubimova A, Bershadsky AD, Ben-Ze’ev A. Autoregulation of actin synthesis responds to monomeric actin levels. J. Cell. Biochem. 1997;65(4):469–478. [PubMed] [Google Scholar]
- 17.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J. Physiol. 1999;519(Pt 3):829–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederholt M, Dörschner N, Groth J. Effect of diuretics, channel modulators and signal interceptors on contractility of the trabecular meshwork. Ophthalmologica. 1997;211(3):153–160. doi: 10.1159/000310783. [DOI] [PubMed] [Google Scholar]
- 19.Peterson JA, Tian B, Geiger B, Kaufman PL. Latrunculin-A causes mydriasis and cycloplegia in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1999;40(3):631–638. [PubMed] [Google Scholar]
- 20.Shaw L, Ahmed S, Austin C, Taggart MJ. Inhibitors of actin filament polymerisation attenuate force but not global intracellular calcium in isolated pressurised resistance arteries. J. Vasc. Res. 2003;40(1):1–10. doi: 10.1159/000068940. [DOI] [PubMed] [Google Scholar]
- 21.Iizuka K, Yoshii A, Samizo K, et al. A major role for the Rho-associated coiled coil forming pretein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br. J. Pharmacol. 1999;128(4):925–933. doi: 10.1038/sj.bjp.0702864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harnett KM, Biancani P. Calcium-dependent and calcium-independent contractions in smooth muscles. Am. J. Med. 2003;115(Suppl. 3A):24S–30S. doi: 10.1016/s0002-9343(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 23.Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil. Cytoskeleton. 1994;29(4):321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- 24.Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch. Ophthalmol. 1998;116(5):633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Cai S, Glasser A, et al. Effect of H-7 on cultured human trabecular meshwork cells. Mol. Vis. 2001;7:145–153. [PubMed] [Google Scholar]
- 26.Uehata M, Ishizaki T, Satoh H, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389(6654):990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 27.Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J. Cell Biol. 2001;153(3):569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima E, Nakajima T, Minagawa Y, Shearer TR, Azuma M. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J. Pharm. Sci. 2005;94(4):701–708. doi: 10.1002/jps.20285. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal R, Choritz L, Schlott S, et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp. Eye Res. 2005;80(6):837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Honjo M, Tanihara H, Inatani M, et al. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001;42(1):137–144. [PubMed] [Google Scholar]
- 31.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 32.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp. Eye Res. 2005;80(2):197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 33.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest. Ophthalmol. Vis. Sci. 1999;40(1):74–81. [PubMed] [Google Scholar]
- 34.Peterson JA, Tian B, Bershadsky AD, et al. Latrunculin-A increases outflow facility in the monkey. Invest. Ophthalmol. Vis. Sci. 1999;40(5):931–941. [PubMed] [Google Scholar]
- 35.Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am. J. Physiol. Heart Circ. Physiol. 2009;296(4):H917–H926. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 2008;295(5):C1057–C1070. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2010;298(3):C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 2009;88(4):656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 2009;88(4):648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1982;23(5):646–650. [PubMed] [Google Scholar]
- 41.Peterson JA, Tian B, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp. Eye Res. 2000;70(3):307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- 42.Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins’ effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2000;41(7):1749–1758. [PubMed] [Google Scholar]
- 43.Okka M, Tian B, Kaufman PL. Effect of low-dose latrunculin B on anterior segment physiologic features in the monkey eye. Arch. Ophthalmol. 2004;122(10):1482–1488. doi: 10.1001/archopht.122.10.1482. [DOI] [PubMed] [Google Scholar]
- 44.Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest. Ophthalmol. Vis. Sci. 2006;47(5):1991–1998. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin. Ophthalmol. 2011;5:667–677. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian B, Wang RF, Podos SM, Kaufman PL. Effects of topical H-7 on outflow facility, intraocular pressure, and corneal thickness in monkeys. Arch. Ophthalmol. 2004;122(8):1171–1177. doi: 10.1001/archopht.122.8.1171. [DOI] [PubMed] [Google Scholar]
- 47.Tian B, Gabelt BT, Peterson JA, Kiland JA, Kaufman PL. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Invest. Ophthalmol. Vis. Sci. 1999;40(1):239–242. [PubMed] [Google Scholar]
- 48.Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm’s canal cells and outflow facility in anterior segments of human eyes. Invest. Ophthalmol. Vis. Sci. 2004;45(7):2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- 49.Hu Y, Gabelt BT, Kaufman PL. Monkey organ-cultured anterior segments: technique and response to H-7. Exp. Eye Res. 2006;82(6):1100–1108. doi: 10.1016/j.exer.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Honjo M, Inatani M, Kido N, et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch. Ophthalmol. 2001;119(8):1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 51.Tokushige H, Inatani M, Nemoto S, et al. Effects of topical administration of y-39983, a selective Rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest. Ophthalmol. Vis. Sci. 2007;48(7):3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- 52.Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res. 2005;80(2):215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Peterson WM, Lampe J, Navratil T, et al. Topical administration of a novel and potent Rho kinase (ROK) inhibitor INS117548 alters the actin cytoskeleton, effectively lowers IOP, and is well tolerated on the ocular surface. Invest. Ophthalmol. Vis. Sci. 2008;49 E-abstract 3816. [Google Scholar]
- 54.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008;126(3):309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- 55.Williams RD, Novack GD, van Haarlem T, Kopczynski C. AR-12286 Phase 2a Study Group. Ocular hypertensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol. 2011;152(5):834–841. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp. Eye Res. 2006;82(2):236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Sabanay I, Gabelt BT, Tian B, Kaufman PL, Geiger B. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Arch. Ophthalmol. 2000;118(7):955–962. [PubMed] [Google Scholar]
- 58.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp. Eye Res. 2004;78(1):137–150. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by Rho-kinase inhibition with Y-27632 in bovine eyes. Exp. Eye Res. 2008;86(2):271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Z, Zhang Y, Freddo TF, Gong H. Similar hydrodynamic and morphological changes in the aqueous humor outflow pathway after washout and Y27632 treatment in monkey eyes. Exp. Eye Res. 2011;93(4):397–404. doi: 10.1016/j.exer.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest. Ophthalmol. Vis. Sci. 1992;33(5):1670–1675. [PubMed] [Google Scholar]
- 62.Lutjen-Drecoll E, Wiendl H, Kaufman PL. Acute and chronic structural effects of pilocarpine on monkey outflow tissues. Trans. Am. Ophthalmol. Soc. 1998;96:171–191. [PMC free article] [PubMed] [Google Scholar]
- 63.Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 2009;88(4):676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Svedbergh B, Lütjen-Drecoll E, Ober M, Kaufman PL. Cytochalasin B-induced structural changes in the anterior ocular segment of the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1978;17(8):718–734. [PubMed] [Google Scholar]
- 65.Kaye GI, Fenoglio CM, Hoefle FB, Fischbarg J. Studies on the cornea. IX. Physiologic and morphologic effects of cytochalasin B on endothelium of rabbit corneas perfused in vitro. J. Cell Biol. 1974;61(2):537–543. doi: 10.1083/jcb.61.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiland JA, Miller CL, Kim CB, et al. Effect of H-7 and Lat-B on retinal physiology. Curr. Eye Res. 2006;31(5):441–455. doi: 10.1080/02713680600672185. [DOI] [PubMed] [Google Scholar]
- 67.Tian B, Sabanay I, Peterson JA, Hubbard WC, Geiger B, Kaufman PL. Acute effects of H-7 on ciliary epithelium and corneal endothelium in monkey eyes. Curr. Eye Res. 2001;22(2):109–120. doi: 10.1076/ceyr.22.2.109.5529. [DOI] [PubMed] [Google Scholar]
- 68.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest. Ophthalmol. Vis. Sci. 2009;50(8):3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 69.Olson MF. Applications for ROCK kinase inhibition. Curr. Opin. Cell Biol. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coleman ML, Marshall CJ, Olson MF. Ras and Rho GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell Biol. 2004;5(5):355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- 71.Whikehart DR. The inhibition of sodium, potassium-stimulated ATPase and corneal swelling: the role played by polyols. J. Am. Optom. Assoc. 1995;66(6):331–333. [PubMed] [Google Scholar]
- 72.Hatou S, Yamada M, Akune Y, et al. Role of insulin in regulation of Na+-/K+-dependent ATPase activity and pump function in corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2010;51(8):3935–3942. doi: 10.1167/iovs.09-4027. [DOI] [PubMed] [Google Scholar]
- 73.Tian B, Gabelt BT, Kaufman PL. Effect of staurosporine on outflow facility in monkeys. Invest. Ophthalmol. Vis. Sci. 1999;40(5):1009–1011. [PubMed] [Google Scholar]
- 74.Turner MS, Fen-Fen-Lin, Trauger JW, Stephens J, LoGrasso P. Characterization and purification of truncated human Rho-kinase II expressed in Sf-21 cells. Arch. Biochem. Biophys. 2002;405(1):13–20. doi: 10.1016/s0003-9861(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 75.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 76.Weiner AL, Gilger BC. Advancements in ocular drug delivery. Vet. Ophthalmol. 2010;13(6):395–406. doi: 10.1111/j.1463-5224.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 77.Calvo P, Sanchez A, Martinez J, et al. Polyester nanocapsules as new topical ocular delivery systems for cyclosporin A. Pharm. Res. 1996;13(2):311–315. doi: 10.1023/a:1016015803611. [DOI] [PubMed] [Google Scholar]
- 78.Kaufman PL. Pharmacologic trabeculocanalotomy. Facilitating aqueous outflow by assaulting the meshwork cytoskeleton, junctional complexes, and extracellular matrix. Arch. Ophthalmol. 1992;110(1):34–36. doi: 10.1001/archopht.1992.01080130036022. [DOI] [PubMed] [Google Scholar]
- 79.Borrás T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. 2006;8(5):589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 80.Khare PD, Loewen N, Teo W, et al. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol. Ther. 2008;16(1):97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- 81.Barraza RA, Rasmussen CA, Loewen N, et al. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum. Gene Ther. 2009;20(3):191–200. doi: 10.1089/hum.2008.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 2010;51(1):236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabelt BT, Hu Y, Vittitow JL, et al. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp. Eye Res. 2006;82(6):935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Grosheva I, Vittitow JL, Goichberg P, et al. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp. Eye Res. 2006;82(6):945–958. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol. Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 86.Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol. Vis. 2005;11:288–297. [PubMed] [Google Scholar]
- 87.Gabelt BT, Kaufman PL. Production and flow of aqueous humor. In: Levin LA, Nilsson SFE, Ver Hoeve J, Wu SM, editors. Adler’s Physiology of the Eye. 11th Edition Elsevier Inc.; Edinburgh, UK: 2011. pp. 274–307. [Google Scholar]