Abstract
Current techniques to describe atrial function are limited by their load dependency and hence do not accurately reflect intrinsic mechanical properties. To assess the impact of atrial fibrillation on atrial function, combined pressure-volume relationships (PVR) measured by conductance catheters were used to evaluate the right (RA) and left (LA) atrium in 12 isoflurane-anesthetized pigs. Biatrial PVR were recorded over a wide range of volumes during transient caval occlusion at baseline sinus rhythm (SR), after onset of rapid atrial pacing (RAP), after 1 h of RAP, after conversion to SR, and after 1 h of recovery. Cardiac output decreased by 16% (P = 0.008) with onset of RAP. Mean LA and RA pressures increased by 21 and 40% (P < 0.001), respectively, and remained elevated during the entire recovery period. RA reservoir function increased from 51 to 58% and significantly dropped to 43% after resumption of SR (P = 0.017). Immediately after RAP, a right shift of LA end-systolic PVR-intercept for end-systolic volume required to generate an atrial end-systolic pressure of 10 mmHg (24.4 ± 4.9 to 28.1 ± 5.2 ml, P = 0.005) indicated impaired contractility compared with baseline. Active LA emptying fraction dropped from 17.6 ± 7.5 to 11.7 ± 3.7% (P < 0.001), LA stroke volume and ΔP/Δtmax/P declined by 22% (P = 0.038 and 0.026, respectively), while there was only a trend to impaired RA systolic function. Stiffness quantified by the ratio of pressure to volume at end-diastole was increased immediately after RAP only in the RA (P = 0.020), but end-diastolic PVR shifted rightward in both atria (P = 0.011 LA, P = 0.045 RA). These data suggest that even short periods of RAP have a differential impact on RA and LA function, which was sustained for 1 h after conversion to SR.
Keywords: arrhythmia, atrium, contractility, pressure, tachyarrhythmias
abnormalities of atrial performance are clinically relevant and are present in many pathological conditions of the heart. Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide with an increase in our aging population (19). It results in a loss of booster pump function in the atrium, which might contribute to the significant morbidity and mortality secondary to hemodynamic compromise and tachycardia-induced cardiomyopathy in some patients. Due to this fact, most attention has been given to the impaired left atrial (LA) contractility during AF (24, 28). However, atrial diseases in which LA compliance is markedly reduced are associated with impaired LA filling while increase in diastolic stiffness resulting in increased LA pressure can maintain stroke volume in the absence of atrial booster pump function, indicating that the changes in mechanical atrial function during AF might be much more complex (5, 22).
Many previous studies focused on the evaluation of atrial function after the treatment of AF, including catheter ablation or surgery, and revealed a decremental effect of variable degrees of those procedures (7, 29). The Cox-Maze procedure (CMP) was introduced for the surgical treatment of AF in 1987, and several modifications led to the currently used ablation-assisted CMP-IV. Since then, we have shown that both the booster pump and reservoir function of the LA were compromised after the CMP compared with preoperative values (36). However, to better delineate the effects of surgery or catheter ablation on atrial function, it first is necessary to understand how AF itself alters atrial hemodynamics. While previous studies described atrial stunning after electrical cardioversion, it remains uncertain if this was caused by the arrhythmia or the intervention (6). Data on the effect of AF on atrial function after spontaneous restoration of normal sinus rhythm (NSR) are limited, and a comparison with baseline before the onset of the arrhythmia is rarely possible. Moreover, right atrial (RA) function has not been extensively studied so far.
The assessment of atrial function has been difficult because of its amorphous architecture and the complex interplay that occurs between the atrium, ventricle, and venous circulation. Previously used techniques have included echocardiography, angiography, and magnetic resonance imaging (MRI). Those techniques are limited by either load dependency, geometric assumptions, poor boundary delineation, or by the lack of capability to thoroughly assess the function of both atria at the same time.
To simultaneously evaluate both LA and RA function independently from atrial loading conditions, conductance catheters were used in this study to assess biatrial mechanics. This technique was originally designed for hemodynamic measurements of the ventricle, but modifications developed by our institution allowed for accurate and reproducible measurement of volume and volume changes of the atria (3, 10, 11, 21, 36).
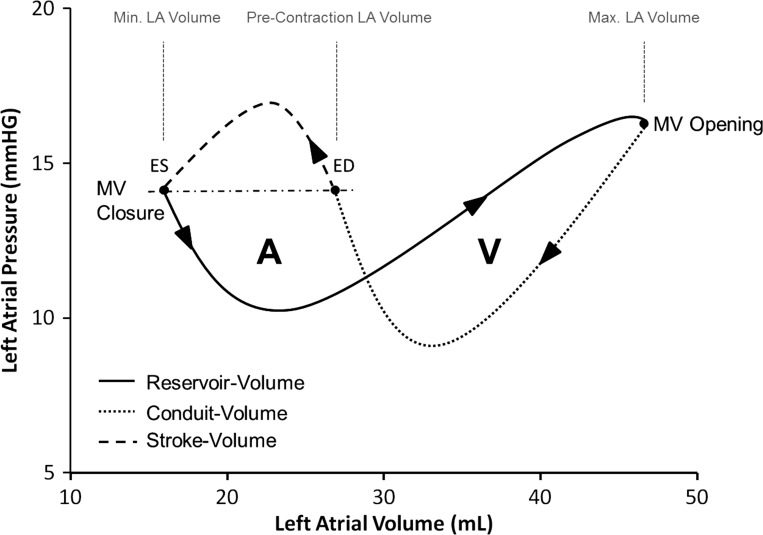
The goal of this study was to investigate the hemodynamic impact of AF on RA and LA during rapid atrial pacing and after restoration of NSR in an acute pig model, using combined pressure-volume relationships (Fig. 1).
Fig. 1.
Typical figure-of-eight pattern of the atrial pressure-volume relationship. The A-loop represents active atrial contraction and proceeds in a counterclockwise fashion. The V-loop represents passive atrial reservoir function and proceeds in a clockwise manner over time. Left atrial (LA) end-diastole (ED) was defined as the time point at which LA pressure corresponds to LA end-systolic (ES) pressure. MV, mitral valve.
METHODS
Twelve male domestic pigs weighing 81–94 kg were used in this study. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85–23, revised 1985). The study was approved by the Washington University School of Medicine Animal Studies Committee.
Surgical Preparation
The animals were premedicated with ketamine hydrochloride (15 mg/kg im), intubated, and anesthetized with supplemental inhalational 2–4% isoflurane. Pulse oximetry, surface electrocardiogram (ECG), and arterial blood gases were monitored throughout the experiment. Arterial blood pressure was measured invasively at the femoral artery. Supplemental oxygen and sodium bicarbonate were administered as necessary to maintain a normal acid-base balance and arterial oxygen tension between 100 and 200 mmHg. Potassium and Ca2+ were substituted as needed to maintain their physiological range, and Ringer-lactate solution was administered intermittently. The animals were fully heparinized (350 U/kg iv), and the activated clotting time was maintained at >250 s throughout the study.
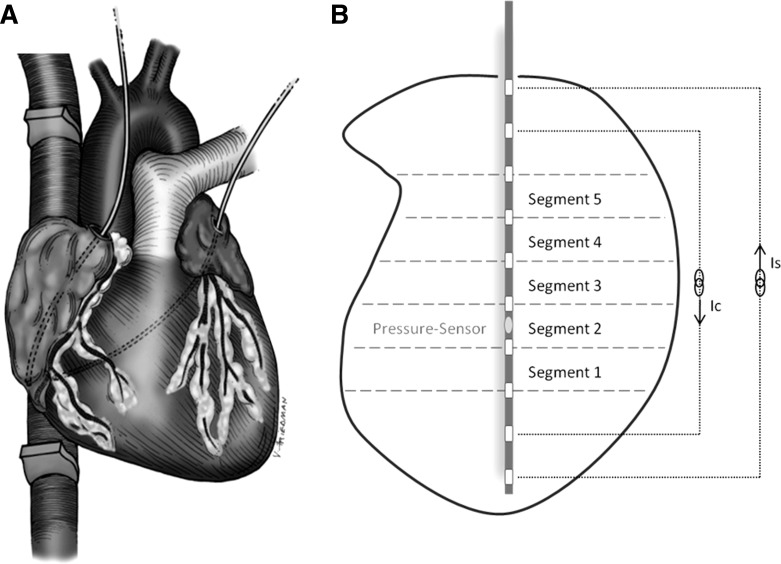
A median sternotomy was performed leaving the pericardium intact. Ultrasonic flow probes (14- to 18-mm perivascular probes with a T402 Flowmeter; Transonic Systems, Ithaca, NY) were placed around the superior (SVC) and inferior (IVC) vena cava ∼1 cm from the caval-atrial junction to obtain accurate measurement of RA inflow. A tourniquet was placed around the SVC and IVC to allow transient caval occlusion for preload alteration during data acquisition. A small pericardial incision was made over the RA free wall, the LA appendage, and the atrioventricular groove to permit instrumentation. A vascular loop was placed around the coronary sinus to allow for temporary occlusion during data collection. A bipolar pacing electrode was sewn on the RA free wall, and a 5-Fr combined pressure-volume conductance catheter (Millar SPR-766; Millar Instruments, Houston, TX) was introduced through a purse string suture at the RA appendage. This conductance catheter was positioned along the long axis of the RA with its tip resting at the inferior caval-atrial junction (Fig. 2A). A second 5-Fr combined pressure-volume catheter (Millar SPR-766) was inserted in the LA through a purse-string suture at the LA appendage. This conductance catheter was positioned along the long axis of the LA by directing the tip of the catheter toward the orifice of the inferior right pulmonary vein. The catheters measured online atrial pressure and atrial chamber conductance using dual-field technology with 10 electrodes being 4 mm apart (Fig. 2B). They were custom designed because we found this spacing to be most appropriate for the atria, as opposed to conventional catheters designed for the ventricle chamber. The tourniquets around the venae cavae and the catheters were tunneled, and the sternum was loosely approximated. The conductance catheters were connected to two signal conditioner processors (Sigma 5DF; CD Leycom, Zoetermeer, The Netherlands) to convert instantaneous conductance measurements into relative RA and LA volume as described previously and summarized below (11, 21, 27, 31, 36, 38). At the conclusion of the study, the animals were killed, and accurate positioning of the conductance catheters was confirmed.
Fig. 2.
A: intraoperative setting: conductance catheters were placed in the right and left atrium with the tips resting at the inferior caval-atrial junction and the orifice of the right inferior pulmonary vein, respectively. Ultrasonic flow probes were placed around the superior and inferior vena cava. B: total atrial volume was divided into 5 segments. Conductance was measured using dual-field technology. Ic/Is, current yielding dual field tachnology.
Experimental Protocol
To evaluate the changes of atrial function during rapid atrial pacing and after restoration of NSR, baseline data including pressure-volume relations were initially recorded over a wide range of physiological filling pressure with the animals being in sinus rhythm. Rapid atrial pacing was then started with a cycle length of 70 ms to simulate AF. This resulted in irregular capture of the ventricles with a mean ventricular heart rate of 145 ± 39 beats/min. Pressure and volume data were obtained right after the initiation of rapid atrial pacing and after 1 h of pacing. After 1 h, rapid atrial pacing was stopped, and hemodynamic measurements, including pressure-volume relations, were taken over a wide range of physiological filling pressures right after restoration of NSR and 1 h after reconversion. All animals reconverted spontaneously into NSR within 6 min after rapid atrial pacing was stopped. However, three animals required temporary atrial pacing for 5, 5, and 6 min, respectively, due to initial sinus node suppression.
Data Acquisition
The RA and LA pressure-volume relations were acquired simultaneously throughout the study. During each data acquisition run, ECG, arterial pressure, SVC and IVC flow, RA and LA pressure, as well as RA and LA conductance signals were acquired at 200 Hz and processed with custom-designed software. The RA and LA pressure signals were differentiated with respect to time to calculate instantaneous RA ΔP/Δt and LA ΔP/Δt. Normalization of ΔP/Δt was performed (ΔP/Δt/P) throughout the cardiac cycle to yield less load-dependent values than raw ΔP/Δt. To minimize the effect of intrathoracic pressure variation, the sternum was readapted, and the respirator was temporarily interrupted at end-expiration during data collection for 10–15 s. After steady-state data were obtained for 3–5 s, slow, progressive bicaval occlusion was performed to generate RA and LA pressure-volume loops over a wide physiological range of filling pressure. Data acquisition runs were repeated in triplicate, and sufficient time (2–5 min) was allowed between the runs for hemodynamic recovery.
Data Analyses
Atrial volume.
Total atrial volume was divided into five segmental volumes as shown in Fig. 2B. Segmental conductance signals (G) were combined to yield the total volume (V) using the following equation:
where L is the interelectrode distance, t is time, ρ is blood resistivity, α is the conductance gain factor, i is the segment number, and Gc is the parallel conductance constant (31). To ensure volume sampling accurately reflects changes in atrial (vs. caval or pulmonary vein) volume, each volume segment was individually assessed for its synchronization and phase relationship to the other segments. Conductance catheters have previously been used to quantify RA and LV volume (4, 8, 21, 23, 27, 36). Relative RA conductance-derived volume changes have previously been shown to correlate highly to relative volume changes calculated with caval flow analysis (r = 0.97, r2 = 0.94, P < 0.001) (21). Absolute left atrial conductance-derived volumes have also been shown to correlate with three-dimensional MRI-derived chamber volumes (r = 0.83, r2 = 0.69, P < 0.02) (36).
Atrial elastance (contractility).
Atrial end-diastole (ED) was defined as the time of tricuspid/mitral valve opening, corresponding to the time of maximum atrial volume and the atrial V-wave. Atrial end-systole (ES) was defined for each atrium as the time of minimum atrial volume after the ECG P-wave. Active atrial emptying fraction represents the volume ejected during atrial contraction as a percentage of atrial preload and is a load-dependent measure of the atrial kick (ES volume − ED volume/ED volume) (36). Atrial contractility was assessed in a load-independent manner by using the atrial end-systolic pressure-volume relation (ESPVR; chamber elastance) as previously described (1, 10, 13). Atrial end-systolic pressure (APA-ES) and volume (AVA-ES) points were determined for each cardiac cycle during preload reduction, and, by means of least-squares linear regression, a straight line was fitted to the following equation:
where EA-ES (mmHg/ml) and V0 (ml) are the slope (atrial chamber elastance) and volume-axis intercept, respectively.
To prevent problems with linear interpolation of a possible curvilinear relationship beyond the range of data recorded (which can lead to negative values for the volume-axis intercept), the end-systolic volume required to generate an atrial end-systolic pressure of 10 mmHg (V10) was calculated (25, 37). This parameter incorporates changes in both the slope and the volume-axis intercept of the ESPVR. Therefore, V10 can reliably be used as a single variable to quantify atrial contractility (ESPVR-intercept V10) (25). An increase in V10 indicates a greater end-systolic volume is required to obtain an atrial end-systolic pressure of 10 mmHg, reflecting impaired atrial contractility.
Atrial stiffness.
Static atrial stiffness was defined as the slope of the atrial end-diastolic pressure-volume relation (EDPVR; chamber stiffness). Atrial end-diastolic pressure (APA-ED) and volume (AVA-ED) points were determined at the time of maximum atrial volume (corresponding to atrioventricular valve opening) for each cardiac cycle during preload reduction. By least-square linear regression, a straight line was fitted to the following equation:
where atrial stiffness (ml/mmHg) and VA-ED are the slope and atrial diastolic equilibrium volume, respectively. In general, the diastolic pressure-volume curve is quite linear when analyzed within the physiological range as long as the volume change is small enough to utilize Taylor expansion (38). However, across the entire spectrum of pressures, the relationship is more accurately represented with the nonlinear, exponential equation: P = α × eβV + c, where α, β, and c are the fitted coefficients and compliance is quantified as 1/β (ml/mmHg). Compliance is reported with the V10 intercept for each atrium, which, as described above for systolic function, can be used as a single variable to describe the atrial end-diastolic pressure-volume curve.
RA reservoir and conduit function.
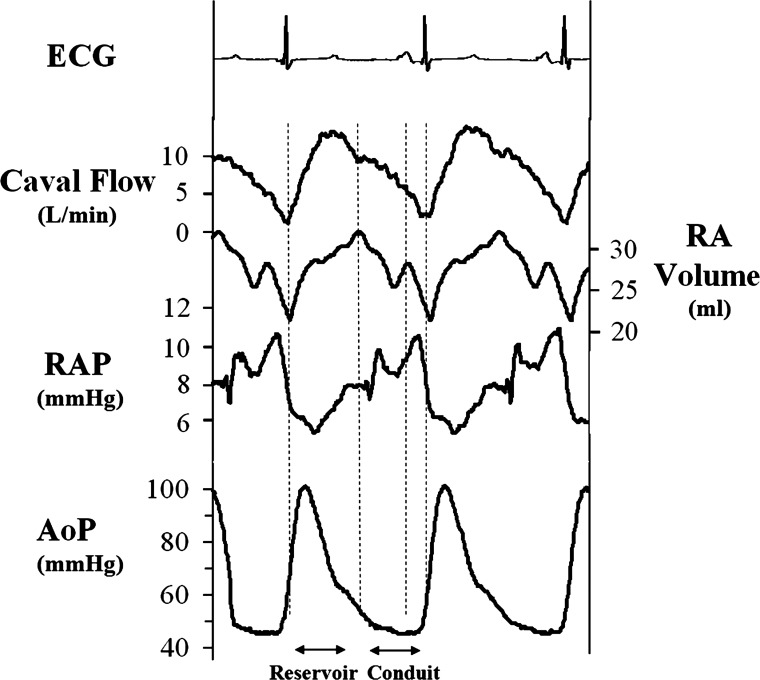
Reservoir and conduit function of the RA were calculated by integrating RA inflow (combined SVC and IVC flow) during right ventricular systole (reservoir) and right ventricular diastole (conduit) (14, 15, 26). Because the tricuspid valve is closed during right ventricular systole, all blood that enters from the venae cavae during this period is reserved in the atrium. Thus, RA reservoir volume equals integrated RA inflow from the ECG R-wave to the time of maximum RA volume, which corresponds to the time of tricuspid valve opening (15). RA conduit volume equals integrated RA inflow during right ventricular filling when the tricuspid valve is open. Total conduit volume was separated into an early component, from the time of maximum RA volume to the beginning of atrial contraction (A-wave, time of maximum RA ΔP/Δt), and a late component from the A-wave to the end of right ventricular filling (ECG R-wave). The late conduit component quantifies blood entry into the atrium against the force of the atrial kick (Fig. 3). Integrated RA inflow volume during reservoir and conduit phases was divided by total RA inflow volume (stroke volume) to yield reservoir as well as early and late conduit function as a percentage of total RA inflow. During rapid atrial pacing, volume changes were analyzed over a steady-state period of 5 s. A distinction between early and late conduit function was not applicable in this condition due to the absence of atrial contractility or A-wave, respectively. Right ventricular cardiac output was quantified by integrating RA inflow during the cardiac cycle (stroke volume) and multiplying by heart rate. We have previously found this to correlate 1:1 with left ventricular cardiac output calculated with aortic flow, realizing in the current preparation, however, that if there are differential changes in RA and LA function, cardiac output of the right and left heart may not correlate on a beat-to-beat basis.
Fig. 3.
Typical hemodynamic data recorded from the right atrium (RA). The reservoir phase extends from the electrocardiogram (ECG) R-wave to the time of maximum RA volume (tricuspid valve opening). The early conduit phase extends from the time of maximum RA volume to the A-wave (maximum RA ΔP/Δt) and the late conduit phase from the A-wave to the end of right ventricular filling (subsequent ECG R-wave). RAP, right atrial pressure; AoP, aortic pressure.
Statistical Analysis
All data are reported as means ± SD unless otherwise specified. Hemodynamic data are reported as averages of three consecutive steady-state beats. Data obtained during baseline, rapid atrial pacing, and restored NSR were compared using repeated-measure ANOVA, and, when indicated by a significant F-statistic (P < 0.05), differences were isolated using Fischer's protected least-significant difference test. All statistical analyses were performed using SigmaStat (2.03; SPPS, Chicago, IL).
RESULTS
Hemodynamic Effects of Rapid Atrial Pacing on Atrial Function
Table 1 summarizes the hemodynamic changes from baseline during rapid atrial pacing and during restored NSR.
Table 1.
Hemodynamic changes during RAP and after resumption of sinus rhythm
| Parameters | Baseline NSR | RAP Onset | P Value* | RAP for 1 h | P Value* | NSR Post-RAP | P Value* | NSR 1 h Post-RAP | P Value* |
|---|---|---|---|---|---|---|---|---|---|
| Heart rate, beats/min | 90 ± 11 | 154 ± 32 | <0.001 | 145 ± 39 | <0.001 | 101 ± 13 | 0.152 | 96 ± 14 | 0.434 |
| Cardiac output, l/min | 5.5 ± 1.1 | 4.6 ± 0.9 | 0.008 | 4.4 ± 1.2 | 0.002 | 5.9 ± 1.3 | 0.242 | 5.0 ± 1.0 | 0.100 |
| Stroke volume, ml | 61.5 ± 11.2 | 30.9 ± 8.1 | <0.001 | 32.2 ± 10.9 | <0.001 | 58.8 ± 13.5 | 0.596 | 52.8 ± 12.9 | 0.006 |
| Mean AoP, mmHg | 62.3 ± 8.6 | 46.3 ± 8.8 | <0.001 | 45.2 ± 10.1 | <0.001 | 57.4 ± 12.6 | 0.064 | 49.6 ± 9.6 | <0.001 |
| Mean LA pressure, mmHg | 11.9 ± 2.8 | 14.4 ± 2.0 | <0.001 | 14.3 ± 1.9 | <0.001 | 14.4 ± 2.4 | <0.001 | 13.6 ± 2.9 | 0.006 |
| Mean RA pressure, mmHg | 8.6 ± 1.3 | 12.0 ± 1.6 | <0.001 | 12.9 ± 1.4 | <0.001 | 11.3 ± 1.9 | <0.001 | 11.8 ± 2.1 | <0.001 |
| Maximum LA volume, ml | 31.0 ± 6.1 | 26.6 ± 6.6 | 0.010 | 29.3 ± 6.1 | 0.246 | 35.5 ± 6.7 | <0.001 | 38.2 ± 8.5 | <0.001 |
| Maximum RA volume, ml | 31.5 ± 7.3 | 33.6 ± 6.6 | 0.098 | 33.9 ± 6.7 | 0.062 | 38.1 ± 5.3 | <0.001 | 38.6 ± 4.7 | <0.001 |
| RA reservoir volume, ml | 30.9 ± 15.3 | 15.7 ± 5.8 | <0.001 | 18.9 ± 5.8 | 0.002 | 25.0 ± 11.3 | 0.086 | 22.6 ± 10.4 | 0.019 |
Values are means ± SD. RAP, rapid atrial pacing; NSR, normal sinus rhythm; AoP, aortic pressure; LA, left atrial; RA, right atrial.
vs. Baseline.
Cardiac output and mean arterial pressure decreased significantly by 16% (P = 0.008) and 26% (P < 0.001), respectively, with the onset of rapid atrial pacing and returned to normal after restoration of NSR (P = 0.242 and 0.064, respectively). During the recovery period of 1 h, cardiac output progressively decreased again from 5.9 ± 1.3 to 5.0 ± 1.0 l/min (P = 0.009), but was not different from baseline (P = 0.100). Mean aortic pressure fell at all later time points, suggesting some time dependence of systemic hemodynamics. Both mean LA and RA pressure significantly increased during rapid atrial pacing by 21% (P < 0.001) and 40% (P < 0.001), respectively, and remained elevated after restoration of NSR compared with baseline (P < 0.001 and 0.001, respectively, Table 1).
Reservoir and conduit function.
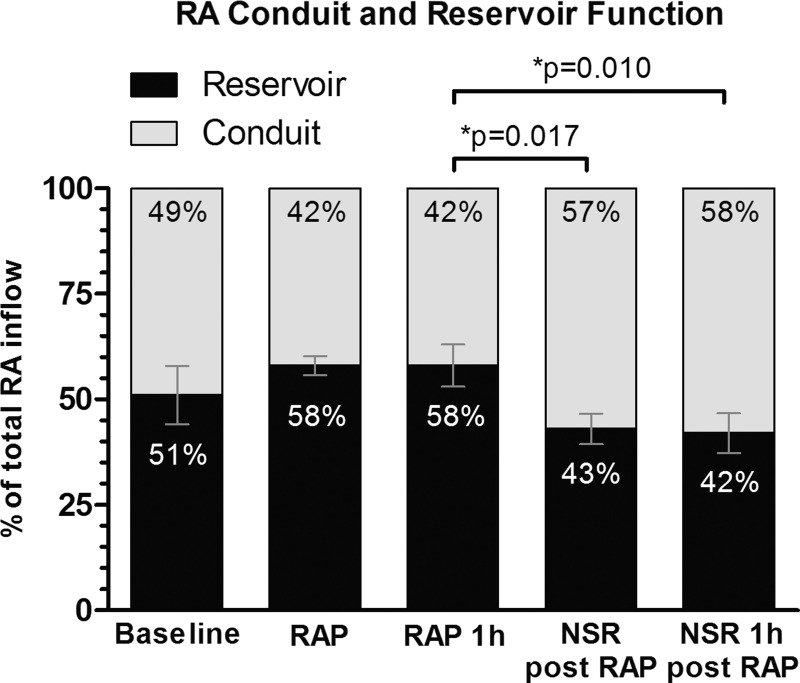
The percentages of reservoir and conduit function of the RA are illustrated in Fig. 4. While reservoir function increased during rapid atrial pacing, it dropped significantly after reconversion to NSR compared with rapid atrial pacing (P = 0.017). There was a significant reduction of RA reservoir volume from 30.9 ± 15.3 ml at baseline to 22.6 ± 10.4 ml at the end of the recovery period (P = 0.019). However, while there was a trend toward a reduced reservoir proportion in the reservoir-conduit relation after rapid atrial pacing compared with baseline, this did not reach significance (P = 0.165).
Fig. 4.
Changes in reservoir and conduit function of the RA. NSR, normal sinus rhythm. Data are shown as means ± SE.
Hemodynamic Changes after Rapid Atrial Pacing
Table 2 summarizes the hemodynamic changes of RA and LA function during sinus rhythm calculated from the recorded combined pressure-volume relations after 1 h of rapid atrial pacing and 1 h after reconversion. Typical RA and LA pressure-volume loops at baseline and after restoration of NSR are illustrated in Fig. 5.
Table 2.
Hemodynamic changes after 1 h of RAP
| Parameters | Baseline NSR | NSR Post-RAP | P Value* | NSR 1 h Post-RAP | P Value* | P Value‡ |
|---|---|---|---|---|---|---|
| LA active emptying fraction†, % | 17.6 ± 7.5 | 11.7 ± 3.7 | <0.001 | 10.2 ± 4.3 | <0.001 | 0.465 |
| RA active emptying fraction†, % | 11.4 ± 4.8 | 10.7 ± 4.0 | 0.499 | 10.7 ± 4.1 | 0.309 | 0.751 |
| LA ESPVR-slope, mmHg/ml | 1.64 ± 0.69 | 1.59 ± 0.98 | 0.619 | 1.14 ± 0.63 | 0.306 | 0.561 |
| LA ESPVR-intercept V10, ml | 24.3 ± 4.9 | 28.1 ± 5.2 | 0.005 | 28.7 ± 8.4 | 0.012 | 0.841 |
| r2 | 0.86 ± 0.13 | 0.88 ± 0.06 | 0.25 | 0.92 ± 0.04 | 0.125 | 0.605 |
| RA ESPVR-slope, mmHg/ml | 1.07 ± 0.43 | 1.20 ± 0.41 | 0.455 | 1.35 ± 0.42 | 0.122 | 0.433 |
| RA ESPVR-intercept V10, ml | 33.2 ± 6.3 | 33.5 ± 6.3 | 0.139 | 37.5 ± 5.6 | 0.006 | 0.136 |
| r2 | 0.83 ± 0.09 | 0.84 ± 0.13 | 0.716 | 0.88 ± 0.09 | 0.318 | 0.196 |
| Maximum LA ΔP/Δt/P, s−1 | 12.3 ± 7.1 | 9.6 ± 4.0 | 0.026 | 10.5 ± 3.9 | 0.122 | 0.409 |
| Maximum RA ΔP/Δt/P, s−1 | 12.9 ± 3.6 | 11.6 ± 2.9 | 0.157 | 10.9 ± 2.8 | 0.062 | 0.663 |
| LA EDPVR-slope, mmHg/ml | 1.03 ± 0.54 | 1.53 ± 0.96 | 0.120 | 1.12 ± 0.71 | 0.298 | 0.615 |
| r2 | 0.88 ± 0.07 | 0.90 ± 0.09 | 0.980 | 0.88 ± 0.08 | 0.967 | 0.986 |
| LA compliance, ml/mmHg | 10.6 ± 6.0 | 9.0 ± 5.2 | 0.814 | 12.6 ± 6.2 | 0.213 | 0.145 |
| LA EDPVR-intercept V10, ml | 27.8 ± 5.6 | 32.3 ± 6.1 | 0.011 | 31.9 ± 8.9 | 0.083 | 0.363 |
| RA EDPVR-slope, mmHg/ml | 0.98 ± 0.19 | 1.25 ± 0.43 | 0.261 | 1.16 ± 0.49 | 0.391 | 0.817 |
| r2 | 0.79 ± 0.14 | 0.87 ± 0.06 | 0.214 | 0.89 ± 0.08 | 0.082 | 0.537 |
| RA compliance, ml/mmHg | 8.2 ± 2.6 | 8.5 ± 4.5 | 0.662 | 9.6 ± 3.6 | 0.458 | 0.741 |
| RA EDPVR-intercept V10, ml | 35.2 ± 6.0 | 38.2 ± 7.8 | 0.045 | 38.8 ± 5.9 | 0.004 | 0.188 |
| LA PED/VED, mmHg/ml | 0.53 ± 0.14 | 0.51 ± 0.10 | 0.361 | 0.46 ± 0.12 | 0.039 | 0.247 |
| RA PED/VED, mmHg/ml | 0.35 ± 0.10 | 0.40 ± 0.11 | 0.020 | 0.41 ± 0.12 | 0.013 | 0.921 |
| RA conduit function, %RA inflow | 49 ± 24 | 57 ± 12 | 0.209 | 58 ± 16 | 0.165 | 0.889 |
| Early conduit function, % | 36 ± 20 | 39 ± 14 | 0.523 | 40 ± 23 | 0.466 | 0.929 |
| Late conduit function, % | 14 ± 15 | 18 ± 13 | 0.405 | 18 ± 16 | 0.325 | 0.880 |
Values are means ± SD. ESPVR, end-systolic pressure-volume relation; V10, end-systolic volume required to generate an atrial end-systolic pressure of 10 mmHg; r2, correlation coefficient; EDPVR, end-diastolic pressure volume relation; PED/VED, ratio of pressure to volume at end-diastole.
Calculated from conductance;
vs. baseline; and
NSR post-RAP vs. NSR 1 h post-RAP.
Fig. 5.
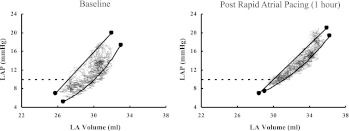
Typical pressure-volume loops of the LA before and 1 h after rapid atrial pacing with a right shift of the end-systolic pressure-volume relation (ESPVR)- and end-diastolic pressure-volume relation (EDPVR)-intercept V10 (end-systolic volume required to generate an atrial end-systolic pressure of 10 mmHg). LAP, left atrial pressure.
Systolic function.
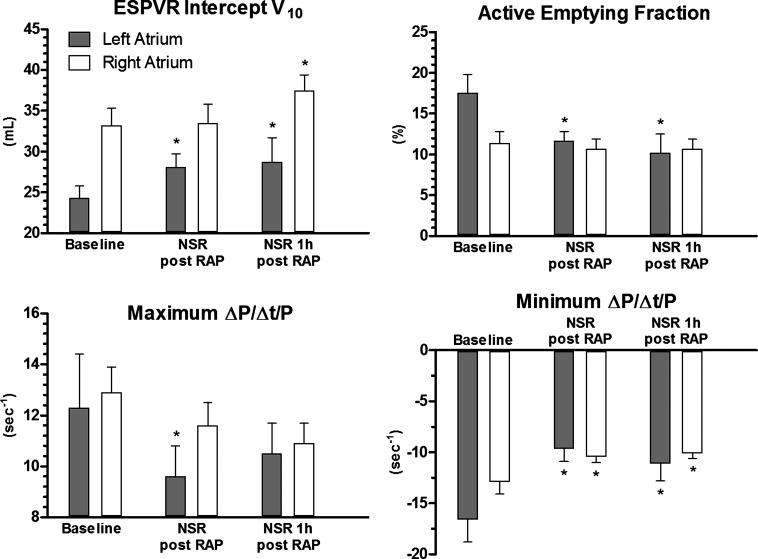
After rapid atrial pacing, there was a right shift for the LA-ESPVR-intercept V10 from 24.4 ± 4.9 to 28.1 ± 5.2 ml (P = 0.005) compared with baseline, indicating a significantly impaired LA contractility after NSR restoration (Fig. 6). This effect persisted for 1 h (28.7 ± 8.4 ml, P = 0.012). For the RA, there was also a notable right shift of the RA-ESPVR-intercept V10 after restoration of NSR (P = 0.139) that became significant only at the end of the 1-h recovery period (33.1 ± 6.3 vs. 37.5 ± 5.6 ml, P = 0.006). LA stroke volume measured by conductance decreased significantly by 22 and 31% at the time of reconversion and 1 h later, respectively (P = 0.038 and 0.006, respectively), and LA active emptying fraction dropped from 17.6 ± 7.5 to 11.7 ± 3.7 and 10.2 ± 4.3%, respectively (P < 0.001 and 0.001, respectively). In contrast, the RA stroke volume or active emptying fraction measured by conductance did not change significantly after rapid atrial pacing (P = 0.184 and 0.499, respectively). Maximum LA ΔP/Δt/P decreased significantly after rapid atrial pacing by 22% (P = 0.026) but almost normalized after 1 h of recovery time (P = 0.122), whereas there was only a trend to a decreased maximum ΔP/Δt/P for the RA after rapid atrial pacing (P = 0.062).
Fig. 6.
Changes of elastance, active emptying fraction, as well as maximum and minimum ΔP/Δt/P of the right and left atrium. RAP, rapid atrial pacing. Data are shown as means ± SE. *P < 0.05.
Diastolic function.
The ratio of pressure to volume at ED (PED/VED), which quantifies static stiffness, changed after rapid atrial pacing compared with baseline. The relation of end-diastolic pressure to volume suggests that the RA was significantly stiffer after rapid atrial pacing with an increase of PED/VED by 12% at the time of reconversion and by 14% 1 h later (P = 0.020 and 0.013, respectively). Static stiffness of the LA did not change after rapid atrial pacing (P = 0.361), but, after 1 h of recovery, the LA presented increasingly dilated with a decrease of PED/VED by 14% (P = 0.039). However, while there was no difference in the linear EDPVR-slope or the load-independent, nonlinear compliance for either atrium, there was a rightward shift in the EDPVR curves. At the time of reconversion, LA nonlinear EDPVR-V10 increased by 16% (P = 0.011), and RA nonlinear EDPVR-V10 increased by 9% (P = 0.045).
DISCUSSION
The mechanical function of the RA and LA is complex and far from being simple passive transport chambers. A quantification of atrial function has been challenging, but its performance can be broadly characterized by three phases (Fig. 1). First, the atria serve as a reservoir by storing pulmonary venous or caval return during ventricular contraction and isovolumetric relaxation. Second, they are a conduit by emptying their content in the ventricles down a pressure gradient after the atrioventricular valves open and passively transferring pulmonary venous and caval blood flow during ventricular diastole. Last, the atria actively contract during the late phase of ventricular diastole and establish final ventricular end-diastolic volume (24, 28). An increase in LA pressure during AF enhances the atrial-ventricular pressure gradient during early ventricular filling in an attempt to maintain ventricular stroke volume. However, the loss of atrial contraction with the onset of AF might overcome this compensatory mechanism and is commonly associated with a reduction in cardiac output as confirmed by this report. Moreover, higher irregular heart rates during AF additionally contribute to hemodynamic impairment.
These reservoir, conduit, and booster pump functions of the atria modulate the transition from continuous venous return to intermittent ventricular filling. Approximately 20% of left ventricular stroke volume is contributed by LA contraction, which becomes significantly important for maintaining cardiac output, especially in the presence of reduced left ventricular compliance (2, 24).
The current study demonstrated that even short periods of rapid atrial pacing have a differential impact on LA and RA function and that these effects are sustained for 1 h beyond reconversion to NSR in this animal model.
In NSR, the multiple deflection morphology of the atrial pressure waveform combined with an essentially monophasic volume waveform results in a complex figure-of-eight pattern of the atrial pressure-volume diagram. It incorporates both, the active (A-loop) and the passive (V-loop) components of atrial function. Because of the random nature of rapid atrial pacing or AF, the contraction patterns of the atria are chaotic in these conditions, and the diagram loosens its typical shape, which depends on preload, fibrillation rate, and afterload.
While previous studies revealed a decreased systolic and diastolic LA function after AF with a decline in booster pump and reservoir function and an augmented conduit function, those reports often lack a baseline before the onset of the arrhythmia or could not distinguish between the impact of AF and the intervention. Echocardiography is a widespread modality to asses LA function and is commonly used in those studies, but it is limited by its load dependency as well as its assumption of prolate ellipsoid geometry, automated boundary detection delay, or inter- and intraobserver variabilities. Thus, it is not able to accurately reflect intrinsic mechanical properties, especially diastolic atrial function (15, 32, 35). Furthermore, it does not allow for the simultaneous evaluation of both atria.
In this study, pressure-volume relationships simultaneously obtained for both atria by conductance catheters using dual-field technology demonstrated a more pronounced effect on LA systolic function after rapid atrial pacing. This was reflected by a decrease in maximum ΔP/Δt/P and active emptying fraction of the LA and confirmed by a right shift of the load-independent ESPVR-intercept V10. Earlier studies suggested atrial stunning due to Ca2+ overload that occurs during AF to be the cause of impaired contractility during the postarrhythmic course (16, 20, 33). However, there was no recovery of LA systolic function and elevated filling pressures within 1 h after cessation of rapid atrial pacing, which would be expected if a transient Ca2+ overload would be the only cause. The different impact on LA and RA systolic function would also suggest differences in the mechanical forces within these two chambers. Parallel shifts of the ESPVR toward larger volumes have been reported in the setting of acute regional ischemia in the ventricle (34), but this is the first study to show this phenomena for the atrium after a short period of rapid atrial pacing, being exclusively valid for the LA only. Available evidence is not conclusive as to whether or not atrial myocytes are ischemic during acute or chronic AF. An irregular heart rhythm with a drop in cardiac output and mean arterial pressure as observed in this study might add to decreased coronary perfusion followed by atrial impairment. Smetnev and coworkers (30) reported myocardial lactate production in patients with AF and normal angiography. Other reports revealed a significant change in coronary flow and coronary flow reserve with the onset of acute AF (17). In context with our findings, this would suggest that the LA is much more vulnerable and affected by a proposed ischemic phase during AF than the RA.
Contrary, impairment of diastolic function was present in both atria, but different in quality. While active relaxation capability reflected by minimum ΔP/Δt/P was reduced in both atria, the passive component PED/VED was significantly increased in the RA after 1 h of rapid atrial pacing, whereas it was unchanged in the LA. This change in stiffness could not be confirmed by a change in the load-independent EDPVR slope for either atrium. Furthermore, changes in EDPVR were inconsistent between animals with either a right shift, an upward shift, or no change. However, it is important to note that, after rapid atrial pacing, the curves extended to higher volumes, a consequence of higher filling pressures found in both atria. Thus a steeper appearance of the slope in this state might just reflect those changes on an intrinsically nonlinear curve. Diastolic dysfunction has been an underestimated sequel of AF or ablation. Unfortunately, it is a complex hemodynamic phenomenon that is related to preload, atrial volumes, and atrial systolic function as well as systolic and diastolic function of the ventricles, which makes interpretation of hemodynamic data challenging. The data obtained for the RA revealed an increase of reservoir function during rapid atrial pacing; however, reservoir function significantly dropped after resumption of NSR, and relaxation as well as PED/VED was impaired. With rapid atrial pacing, there was a reduction in right ventricular cardiac output with no change in heart rate. Absolute RA reservoir volume also decreased in association with the fall in stroke volume, but RA reservoir function, as a percent of total flow, increased. Mean RA pressure also increased, and, while maximum RA volume tended to rise, the change was not significant. Although we were not able to measure diastolic compliance during rapid atrial pacing, the significant rise in atrial pressure without a change in maximum volume is consistent with a fall in atrial compliance.
Although the present study did not allow calculating reservoir function for the LA, relaxation was decreased as well in the LA. Gibson et al. (12) identified atrial diastolic dysfunction as a rare but critical potential complication after catheter ablation causing symptomatic pulmonary hypertension in the absence of mitral valve regurgitation or ventricular dysfunction. Our study could confirm this stiff atrial syndrome in its complete appearance only for the RA. Because systolic function was not impaired in the RA, these changes are not expected to be secondary to systolic abnormalities in this chamber.
Our findings are consistent with earlier reports confirming less impact of surgical ablation on the right booster-pump function than on left atrial systolic properties (9). Moreover, recovery time of systolic function after electrical cardioversion is significantly faster for the RA (18). We also feel that this is consistent with clinical findings. While pulmonary congestion is a recognized symptom in patients with AF, clinical findings of right heart impairment like edema or ascites are more infrequent but present in some patients as well. Although those symptoms seem to be due to impairment of the ventricular function in most patients, another reason might be a differential response of the right and left atrial mechanical properties influencing the complex interaction of the atria and ventricles as reported here. Furthermore, the EDPVR-intercept V10 was different for LA and RA at baseline already, which is consistent with findings in a sham group of our earlier reports, indicating differences of the physiological diastolic mechanical properties between LA and RA (36).
There were several limitations to this study. As with all open-chest physiology studies in anesthetized animals, there are potential difficulties in translating results to the human setting. However, pericardial integrity was maintained through most of the preparations, ventilation was interrupted at end-expiration, and the sternum was readapted for data acquisition to mimic the natural in vivo state. This, of course, does not discount the potential impact of anesthesia and surgical trauma, but we tried to minimize anesthetic changes and surgical trauma following initial placement of monitoring catheters so that their impact would be consistent throughout the study. Additionally, the heart rates of the animals were not exactly the same at baseline and after restoration of NSR, which might have influenced hemodynamics. This could have been avoided by collecting data at a fixed atrial pacing rate. However, determining an appropriate pacing rate was challenging, because the heart rate after reconversion was ∼10% higher than at baseline. A pacing rate of 125 beats/min, which would have been appropriate to cover the heart rate range of all animals in all conditions, was found to already cause tachycardia-induced changes with a significant drop in cardiac output in some animals. The ranges of mean heart rate were not significantly different throughout the NSR states of this study, being in physiological limits between 89 and 100 beats/min, and were felt to reflect best hemodynamic changes important for this study. Furthermore, the significant drop of mean arterial pressure with the onset of rapid atrial pacing in this study might have caused myocardial damage. Those hemodynamic changes reflect the frequently found clinical setting of acute AF caused by the lack of atrial contraction and an accelerated and irregular cardiac rhythm. However, Kochiadakis and coworkers (17) revealed that observed changes in coronary flow and coronary flow reserve were not correlated with changes in blood pressure under the acute onset of AF and suggested that only extreme fluctuation of arterial blood pressure would exceed the bounds of coronary autoregulation. It was rather the irregular rhythm associated with AF that was found to cause a change in coronary vascular resistance and thus decreased coronary circulation. Although our data suggest that rapid atrial pacing has a more decremental impact on the LA than on the RA, a precise calculation for conduit and reservoir function was possible only for the RA. Calculation of LA reservoir function would have required either simultaneous LA and LV volume measurements (36) or pulmonary vein flow probes. We also only quantified RV cardiac output using RA inflow, which under normal conditions should equal LA inflow and LV cardiac output. However, in the variable conditions following resolution of AF with differential impact on RA and LA function, RV cardiac output may not necessarily equal left heart output on a beat-to-beat basis. In addition, there was no control group to independently account for time-dependent changes in atrial properties. In this regard, we did not specifically match fluid administration between animals, but we feel that the ESPVR and exponential diastolic pressure-volume relation provide load-independent measures of LA and RA function, which should at least partially compensate for variations in volume loading. Although cardiac output did not fall in the late stages of the preparation compared with baseline, mean aortic pressure fell at all later time points, suggesting some time dependence of systemic hemodynamics. Last, atrial conductance volumes were not corrected for parallel conductance by means of resistance due to surrounding tissue (31). However, the analytic methods employed in this study were not dependent on absolute volumes and were consistent with previous studies involving the RA (4, 8, 21, 23) and LA (27, 36).
In conclusion, biatrial pressure-volume relationships revealed a significant impact on systolic function after rapid atrial pacing only in the LA, whereas diastolic function was impaired in both atria. While the RA reservoir function declined after rapid atrial pacing, confirming increased stiffness, the LA stiffness was unchanged. These data suggest that even short periods of rapid atrial pacing have a differential impact on RA and LA function and that these effects are sustained for 1 h after conversion to SR in this animal model.
GRANTS
This work was funded, in part, from National Heart, Lung, and Blood Institute Grants R01 HL-032257, R01 HL-085113, T32 HL-07776, and HL-92088.
DISCLOSURES
Dr. Damiano is a consultant for AtriCure and Medtronic and has received research grants from AtriCure, Medtronic, and Estech. Dr. Schuessler receives research support from AtriCure, Estech, and Cardialen and serves on the scientific advisory board of Cardialen. Dr. Weimar is a consultant for AtriCure.
AUTHOR CONTRIBUTIONS
Author contributions: T.W., Y.W., T.K., U.S.L., R.B.S., and R.J.D. conception and design of research; T.W., Y.W., T.K., U.S.L., and R.B.S. performed experiments; T.W., M.R.M., and R.B.S. analyzed data; T.W., M.R.M., R.B.S., and R.J.D. interpreted results of experiments; T.W. and M.R.M. prepared figures; T.W. drafted manuscript; T.W., Y.W., T.K., U.S.L., M.R.M., R.B.S., and R.J.D. edited and revised manuscript; T.W., Y.W., T.K., U.S.L., M.R.M., R.B.S., and R.J.D. approved final version of manuscript.
REFERENCES
- 1. Alexander J, Jr, Sunagawa K, Chang N, Sagawa K. Instantaneous pressure-volume relation of the ejecting canine left atrium. Circ Res 61: 209–219, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 12: 426–440, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Baan J, van der Velde ET, Steendijk P. Ventricular pressure-volume relations in vivo. Eur Heart J 13, Suppl E: 2–6, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Carlson DE, Burchard KW, Gann DS. Right atrial volume during hemorrhage in the dog. Am J Physiol Heart Circ Physiol 250: H1136–H1144, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Dardas PS, Filippatos GS, Tsikaderis DD, Michalis LK, Goudevenos IA, Sideris DA, Shapiro LM. Noninvasive indexes of left atrial diastolic function in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 13: 809–817, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol 23: 307–316, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Feinberg MS, Waggoner AD, Kater KM, Cox JL, Lindsay BD, Perez JE. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation 90: II285–III292, 1994 [PubMed] [Google Scholar]
- 8. Ferguson JJ, 3rd, Miller MJ, Aroesty JM, Sahagian P, Grossman W, McKay RG. Assessment of right atrial pressure-volume relations in patients with and without an atrial septal defect. J Am Coll Cardiol 13: 630–636, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Fleck T, Wolf F, Bader T, Lehner R, Aigner C, Stix G, Wolner E, Wisser W. Atrial function after ablation procedure in patients with chronic atrial fibrillation using steady-state free precession magnetic resonance imaging. Ann Thoracic Surg 84: 1600–1604, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 112: I212–218, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol 288: H2140–H2145, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Gibson DN, Di Biase L, Mohanty P, Patel JD, Bai R, Sanchez J, Burkhardt JD, Heywood JT, Johnson AD, Rubenson DS, Horton R, Gallinghouse GJ, Beheiry S, Curtis GP, Cohen DN, Lee MY, Smith MR, Gopinath D, Lewis WR, Natale A. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 8: 1364–1371, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Hoit BD, Shao Y, Gabel M, Walsh RA. In vivo assessment of left atrial contractile performance in normal and pathological conditions using a time-varying elastance model. Circulation 89: 1829–1838, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Hoit BD, Shao Y, Gabel M, Walsh RA. Influence of pericardium on left atrial compliance and pulmonary venous flow. Am J Physiol Heart Circ Physiol 264: H1781–H1787, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Hoit BD, Shao Y, Tsai LM, Patel R, Gabel M, Walsh RA. Altered left atrial compliance after atrial appendectomy. Influence on left atrial and ventricular filling. Circ Res 72: 167–175, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Kitakaze M, Weisman HF, Marban E. Contractile dysfunction and ATP depletion after transient calcium overload in perfused ferret hearts. Circulation 77: 685–695, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysostomakis SI, Kanoupakis EM, Simantirakis EN, Vardas PE. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J 23: 734–741, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Lehmann G, Horcher J, Dennig K, Plewan A, Ulm K, Alt E. Atrial mechanical performance after internal and external cardioversion of atrial fibrillation: an echocardiographic study. Chest 121: 13–18, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 110: 1042–1046, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Louie EK, Liu D, Reynertson SI, Loeb HS, McKiernan TL, Scanlon PJ, Hariman RJ. “Stunning” of the left atrium after spontaneous conversion of atrial fibrillation to sinus rhythm: demonstration by transesophageal Doppler techniques in a canine model. J Am Coll Cardiol 32: 2081–2086, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Maniar HS, Prasad SM, Gaynor SL, Chu CM, Steendijk P, Moon MR. Impact of pericardial restraint on right atrial mechanics during acute right ventricular pressure load. Am J Physiol Heart Circ Physiol 284: H350–H357, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Mehta S, Charbonneau F, Fitchett DH, Marpole DG, Patton R, Sniderman AD. The clinical consequences of a stiff left atrium. Am Heart J 122: 1184–1191, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Miller MJ, McKay RG, Ferguson JJ, Sahagian P, Nakao S, Come PC, Grossman W. Right atrial pressure-volume relationships in tricuspid regurgitation. Circulation 73: 799–808, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell JH, Shapiro W. Atrial function and the hemodynamic consequences of atrial fibrillation in man. Am J Cardiol 23: 556–567, 1969 [DOI] [PubMed] [Google Scholar]
- 25. Moon MR, DeAnda A, Jr, Daughters GT, 2nd, Ingels NB, Jr, Miller DC. Experimental evaluation of different chordal preservation methods during mitral valve replacement. Ann Thorac Surg 58: 931–944, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Nishikawa Y, Roberts JP, Tan P, Klopfenstein CE, Klopfenstein HS. Effect of dynamic exercise on left atrial function in conscious dogs. J Physiol 481: 457–468, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ota T, Schwartzman D, Francischelli D, Hettrick DA, Zenati MA. Impact of beating heart left atrial ablation on left-sided heart mechanics. J Thoracic Cardiovasc Surg 134: 982–988, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Pagel PS, Kehl F, Gare M, Hettrick DA, Kersten JR, Warltier DC. Mechanical function of the left atrium: new insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiology 98: 975–994, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Rodrigues AC, Scannavacca MI, Caldas MA, Hotta VT, Pisani C, Sosa EA, Mathias W., Jr Left atrial function after ablation for paroxysmal atrial fibrillation. Am J Cardiol 103: 395–398, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Smetnev AS, Bunin Iu A, Nargizian AB, Petrovskii PF, Vakhliaev VD. Characteristics of lactate metabolism in the myocardium of patients with auricular fibrillation. Kardiologiia 23: 70–73, 1983 [PubMed] [Google Scholar]
- 31. Steendijk P, Staal E, Jukema JW, Baan J. Hypertonic saline method accurately determines parallel conductance for dual-field conductance catheter. Am J Physiol Heart Circ Physiol 281: H755–H763, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J 22: 22–36, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Sun H, Chartier D, Leblanc N, Nattel S. Intracellular calcium changes and tachycardia-induced contractile dysfunction in canine atrial myocytes. Cardiovasc Res 49: 751–761, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–H780, 1983 [DOI] [PubMed] [Google Scholar]
- 35. Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol 91: 1079–1083, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Voeller RK, Zierer A, Lall SC, Sakamoto S, Chang NL, Schuessler RB, Moon MR, Damiano RJ., Jr The effects of the Cox maze procedure on atrial function. J Thorac Cardiovasc Surg 136: 1257–1253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yun KL, Niczyporuk MA, Sarris GE, Fann JI, Miller DC. Importance of mitral subvalvular apparatus in terms of cardiac energetics and systolic mechanics in the ejecting canine heart. J Clin Invest 87: 247–254, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Kovacs SJ. The diastatic pressure-volume relationship is not the same as the end-diastolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 294: H2750–H2760, 2008 [DOI] [PubMed] [Google Scholar]